INTRODUCTION
Epilepsy is a chronic neurological condition which affects roughly 50 million people worldwide (Fiest et al., 2017). Epilepsy is a major concern for public health given its significant impact on the physical, social, cultural, and economic aspects of an individual. Research focused on understanding epileptogenesis and the development of more efficacious and safer antiepileptic drugs is thus important. The quest to identify potential, novel therapeutic agents for the management of epilepsy necessitates the use of seizure models.
Veratridine (VTD) is a nonselective activator of voltage-gated sodium channels and calcium channels (Weuring et al., 2020; Zhang et al., 2017). It has been used in various in vitro and in vivo models and has shown to possess seizure-inducing activity in rodents, wild-type zebrafish, and hippocampal slices (Otoom et al., 2006; Singh and Goel, 2016; Weuring et al., 2020). Based on the spontaneous and rhythmic neuronal bursting activity observed in electrophysiological studies on hippocampal CA1 pyramidal neurons and in vivo rodent and zebrafish activity, studies have confirmed that the EEG pattern after VTD administration is comparable to that seen in absence seizures, partial seizures, and epileptic encephalopathies (Otoom and Sequeira, 2011; Singh and Goel, 2016; Weuring et al., 2020). VTD has been commonly applied to study drugs that act on voltage-gated sodium and calcium channels (Atkin et al., 2018; Craig et al., 2020). Therapeutic concentrations of several antiepileptic drugs including valproic acid have been shown to inhibit VTD-induced seizure activity (Otoom and Alkadhi, 2000). In vivo, VTD also produces behavioral effects such as facial automatisms and masticatory jaw movement (Otoom et al., 2006). These behaviors usually culminate in the development of wet dog shakes (WDS). VTD promotes the influx of Na+ and intracellular Ca2+ causing depolarization in the cells (Zhang et al., 2018) and has been applied as a tool for screening potential antiepileptic drugs.
In the development of newer drugs, medicinal herbs have served as a rich source of lead compounds (pradhan and Panchawat, 2018). One such lead compound, eugenol (EUG), is a major constituent of numerous medicinal plants and is extracted from essential oils such as clove and cinnamon oils. It may also be obtained from other plants such as Myristica fragrans (nutmeg). EUG has been employed as a pain reliever in dental practice for several years, with scientifically established analgesic and anesthetic properties (Nejad et al., 2017). It also has been investigated for antioxidant and anticonvulsant actions (Barboza et al., 2018; Ogata et al., 2000). The possible application of EUG in the management of depression, Alzheimer’s, and Parkinson’s diseases has also been explored (Irie, 2006). EUG has also been proposed to have inhibitory action on voltage-gated calcium and sodium, as well as potassium, channels (Chung et al., 2008; Nisar et al., 2021) and anticonvulsant activity against electrical and chemical-induced seizures in pilocarpine and nicotine models (Joushi and Salmani, 2017; Karampour et al., 2017; Yadav et al., 2018).
We, therefore, hypothesize that EUG’s blockade of calcium and sodium currents and anticonvulsant effects in other epilepsy models would make it useful against seizures mediated by VTD’s action on calcium and sodium channels. EUG’s activity against VTD-induced seizures was, therefore, explored in this study.
METHODS
Chemicals
VTD (400 μg/kg), ethosuximide (ETX, 400 mg/kg), sodium valproate (VPA, 200 mg/kg), and EUG (30 and 100 mg/kg) were purchased from Sigma-Aldrich, USA, and dissolved in 1% ethanol. VTD, ETX, and VPA were administered intraperitoneally, while EUG was administered orally. All intraperitoneal drug treatments were administered 30 minutes before administration of VTD, and oral administration was carried out 1 hour before VTD. All drug treatments were carried out by acute administration. The dosing for EUG, VTD, ETX, and VPA was based on previous studies (Barot and Saxena, 2021; Dal Bó et al., 2013; Ramazi et al., 2020).
EEG and WDS recordings
Male Sprague-Dawley rats (250–280 g; n = 7) were obtained from the Departmental Animal Facility. Animals received anesthesia using chloral hydrate (500 mg/kg; i.p.) and buprenorphine (0.1 mg/kg; i.p.), after which they were placed in a stereotaxic frame. Three polyamide-insulated stainless steel electrodes ( p1 Technologies, Roanoke, VA) were constructed and implanted as described by Jeffrey et al. (2014). Anesthesia was discontinued after implantation, and animals were given 7 days for full recovery. A flexible connector cable was then attached to the electrode pedestal and attached to an amplifier (eight-channel) and an analog–digital converter (AD Instruments Ltd., East Sussex, UK). Signals were recorded along a frequency bandwidth of 0.1–1000 Hz. Chart4 software for Windows (AD Instruments Ltd., East Sussex, UK) was used for data recording and analyses for a 60-minute period. Onset time and number of WDS were recorded after VTD administration for a 30-minute period. Animals were also videotaped.
Ethical clearance for the study was given by the Animal Ethics Committee, Kwame Nkrumah University of Science and Technology (KNUST), and experiments were conducted in accordance with the Guiding Principles for the Care and Use of Laboratory Animals.
Sample size was determined using a power analysis with the G*Power software (Heinrich-Heine-Universität Düsseldorf). No explicit criteria were set for the inclusion or exclusion of animals in the groups. Animals were randomly allocated to groups, and treatments were blinded.
Preparation of brain samples
After the experiment, the rats were sacrificed via cervical dislocation and brain tissue samples excised. The cerebral cortex was dissected and synaptosomes were prepared as previously described by Nicholls and Sihra (1986). Synaptosomal pellets were resuspended in an 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer medium (HBM) containing (in mM) HEPES, 10; KCl, 5; NaCl, 140; MgCl2 6H2O, 1; NaHCO3, 5; Na2HPO4, 1.2; and glucose, 10; pH was 7.4. The Bradford assay was then used to determine protein concentration. Synaptosomal pellets (0.5 mg protein) were obtained and stored on ice for further analysis.
Intrasynaptosomal calcium concentration ([Ca2+]i)
Synaptosomes (2 mg/ml) were resuspended in the HBM containing Bovine Serum Albumin (BSA) (16 μM) in the presence of fura-2 (5 μM) and CaCl2 (0.1 mM) and incubated for 30 minutes at 37°C. Fura-2 was loaded and pelleted synaptosomes were resuspended in the HBM with BSA. A 1 ml aliquot was stirred in CaCl2 (1.2 mM), and fluorescence was measured at excitation wavelengths of 340 and 380 nm (with a 505 nm emission wavelength). Data were recorded at 2-second intervals, and [Ca2+]i (nM) was computed as described previously by Grynkiewicz et al. (1985).
Intrasynaptosomal sodium concentration ([Na+]i)
[Na+]I measurements were carried out as described by Cao et al. (2008). Synaptosomes were incubated with a dye-loading buffer (100 μl/well) containing 10 μM (SBFI-AM) Sodium-binding Benzofuran Isophthalate (Invitrogen) and 0.02% Pluronic F-127 (Invitrogen) at 37°C for 1 hours. Sodium-bound SBFI emission was measured at 505 nm (excitation wavelengths: 340 and 380 nm). Fluorescence data were recorded at 5-second intervals, and [Na+]i (nM) was computed using equations described previously by Jabba et al. (2010).
Data analysis
Data were analyzed for statistical differences by one-way analysis of variance (ANOVA), followed by Tukey’s post-hoc test with GraphPad Prism software v 8.0 (San Diego, CA). p ≤ 0.05 was considered statistically significant. Data from all animals were included during data analysis.
RESULTS
WDS
Intraperitoneal VTD at concentrations of 400 μg/kg produced facial automatisms and WDS within 22.29 ± 2.254 minutes. WDS were associated with spike activity on the EEG in some animals (Fig. 1C). The number of WDS was significantly reduced by pretreatment with EUG at 100 mg/kg [Fig. 1B, p =0.0141; F (2, 18) = 5.453] and VPA [Fig. 1B, p = 0.0081; F (2, 18) = 6.368]. All pretreatments did not significantly alter onset times of WDS.
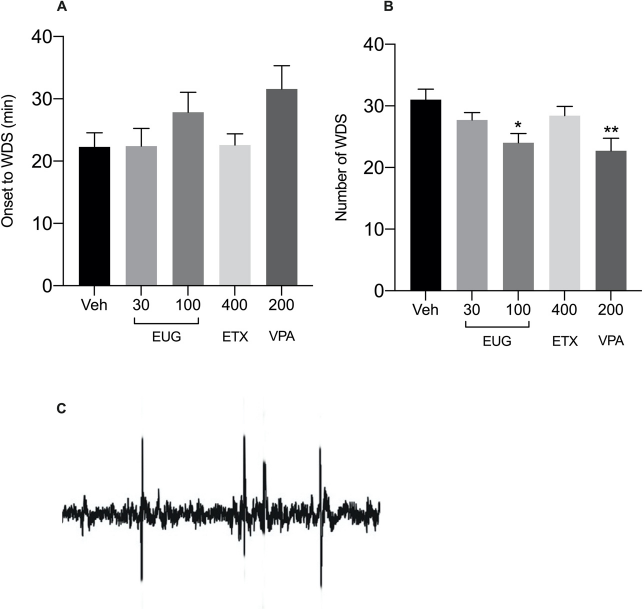 | Figure 1. Onset to WDS (A) and number of WDS (B) induced by intraperitoneal administration of VTD (400 μg/kg) after pretreatment with EUG 30 and 100 mg/kg, p.o., ETX (400 mg/kg; i.p.), and VPA (200 mg/kg; i.p.). (C) Representative EEG spike activity for WDS. Significant differences in comparison with the vehicle-treated group, *p≤0.05; **p≤0.001 (one-way ANOVA, followed by Tukey’s test), n = 7. [Click here to view] |
EEG recordings
Animals experienced frequent quiescent periods which lasted between 1 and 5 minutes, after which they resumed regular activity. The quiescent periods were recurrent every 12–25 minutes. Epileptiform activity was recorded on the EEG during the quiescent periods. Figure 2C shows the seizure activity recorded during quiescent periods in VTD-only-treated rats. In animals pretreated with 200 mg/kg ETX (Fig. 2E) and VPA (Fig. 2F), EEG recording did not display the fast frequency spike activity, similar to vehicle-treated animals (Fig. 2A). Pretreatment with EUG at 100 mg/kg was more effective against inhibition of spike activity (Fig. 2D). No deaths were recorded.
Intrasynaptosomal calcium ([Ca2+]i) and sodium concentrations ([Na+]i)
Table 1 shows the effects of pretreatments of EUG, ETX, and VPA on synaptosomal [Ca2+]i and [Na+]i concentrations. VTD treatment caused a significant [ p = 0.0001; F (5, 36) = 35.72] rise in both [Ca2+]i and [Na+]i concentrations to a plateau level. Rats pretreated with EUG 100 mg/kg had significantly lower synaptosomal [Ca2+]i [ p = 0.0001; F (4, 30) = 127.2] and [Na+]i [ p = 0.0001; F (3, 24) = 30.26] concentrations compared to VTD-only rats. ETX 400 mg/kg significantly prevented a rise in [Ca2+]i [ p = 0.0001; F (2, 18) = 214.7] but failed to prevent a rise in [Na+]i.VPA significantly ( p = 0.0001) inhibited a rise in both [Ca2+]i and [Na+]i concentrations.
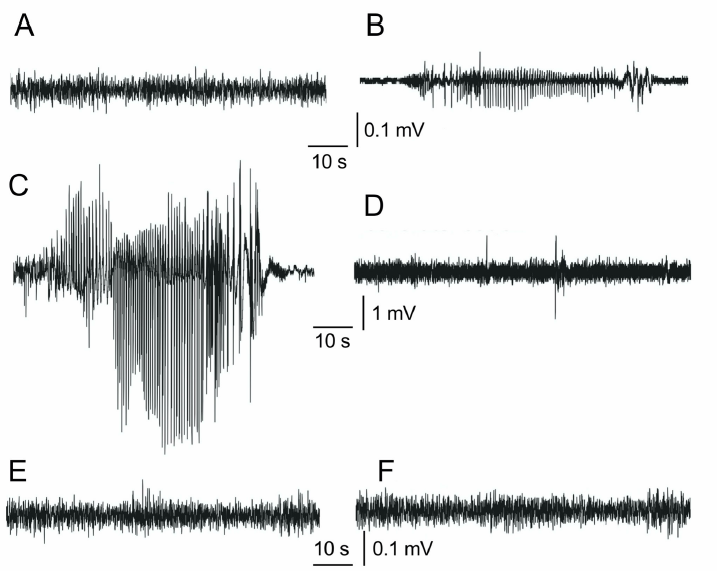 | Figure 2. EEG recordings from (A) vehicle-treated control rats, (B) 30 mg/kg EUG-pretreated rats, (C) VTD-only-treated rats, (D) 100 mg/kg EUG-pretreated rats, (E) ETX-pretreated rats, and (F) VPA-pretreated rats. VTD-only-treated rats presented fast frequency spike activity. [Click here to view] |
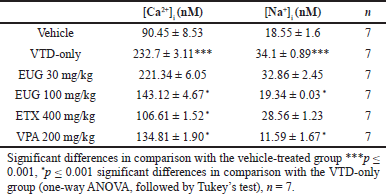 | Table 1. Effect of EUG, ETX, and VPA on Ca2+ and Na+ in rat cerebrocortical synaptosomes. [Click here to view] |
The EEG pattern after VTD administration has been previously confirmed to be comparable to that seen in absence seizures. This has been further supported by the ability of ETX to control VTD-induced seizures (Otoom and Sequeira, 2011). The use of VTD is, therefore, considered a useful animal seizure model for screening antiepileptics, particularly those likely to be effective against absence seizures (Otoom and Sequeira, 2011). Previous findings have also indicated that VTD produces behavioral effects similar to kainic acid-induced effects; kainic acid administration is considered to model complex partial seizures similar to temporal lobe epilepsy in humans. Kainic acid-induced seizures in rodents result in characteristic facial automatisms such as head bobbing and, ultimately, WDS (Lévesque and Avoli, 2013).
In our study, animals receiving VTD exhibited quiescent behaviors with attendant epileptiform activity somewhat similar to that which occurs in absence seizures. In addition, the rats exhibited WDS after about 20 minutes of VTD-only administration. In vivo studies on behavioral effects of VTD show that it produces WDS accompanied by neuronal apoptosis in the hippocampus in a dose-dependent manner (Otoom et al., 2006). Animals exhibiting this behavior experience shakes of the head and the trunk. This behavior has been observed in other partial epilepsy models such as limbic kindling stimulation (Kleinrok and Turski, 1980). Interestingly, the involvement of limbic structures in absence seizures has also been explored (Chan et al., 2004). The limbic circuitry has been shown to be involved in both clinical and experimental atypical absence seizures. Both clinical and experimental atypical absence seizures are commonly associated with severe cognitive impairment, in which the involvement of the thalamohippocampal circuitry has been shown (Chan et al., 2004). Observations of both absence seizure-like effects and WDS in this study may, therefore, be due to an interplay of different brain circuitries (Chan et al., 2004).
Many studies have shown that WDS in rats are mediated by 5-HT2 receptors (Garabadu et al., 2015). The antiepileptic effect of EUG observed as a reduction in the frequency of WDS may, therefore, be associated with interactions with the 5-HT system. Moreover, EUG has been previously reported to reverse and normalize alterations in the serotoninergic system (Garabadu et al., 2015). In this study, EUG may thus be acting by inhibiting the actions of 5-HT that was released due to VTD administration. VTD has previously been shown to increase 5-HT release in the brainstem (Bortolozzi and Artigas, 2003).
Additionally, EUG inhibited epileptiform activity induced with VTD as observed in the EEG. Seizure activity induced by VTD in vivo may be mediated in part by the activity of voltage-gated calcium channels. Other channels such as voltage-gated Na+ channels may be involved; VTD shifts the activation of these channels to negative membrane potentials and delays inactivation (Dorandeu et al., 2017). We, therefore, hypothesize that the efficacy of EUG in reducing absence-like seizures, demonstrated in this study by the inhibition of spike activity, may be related to interaction with voltage-gated calcium channels and/or sodium channels. T-type calcium channels especially have been associated with the pathophysiology of absence seizures. Medications such as ETX and VPA that suppress T-type calcium channels have been shown to be effective in the management of absence seizures (Albuja and Murphy, 2018). Similar efficacies have been observed in this study. Furthermore, EUG’s calcium-modulating activity was confirmed by its ability to inhibit a rise in cytosolic calcium in rat brain synaptosomes in this study. EUG’s efficacy against VTD-induced seizures may, therefore, be additionally due to the attenuation of a rise in cytosolic sodium in rat brains. This further confirms previous reports on EUG’s activity against calcium and sodium ionic influx and currents (Irie, 2006).
CONCLUSION
In conclusion, EUG may, therefore, potentially be used in the management of both complex partial seizures and absence seizures or as an adjunct in the management of these conditions. However, further research is required to ascertain the exact mechanism of EUG in the amelioration of seizures.
DATA AVAILABILITY
All data will be provided on request from the corresponding author.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Albuja AC, Murphy PB. Absence seizure. StatPearls Publishing, Treasure Island, FL, 2018.
Atkin TA, Maher CM, Gerlach AC, Gay BC, Antonio BM, Santos SC, Padilla KM, Rader J, Krafte DS, Fox MA, Stewart GR. A comprehensive approach to identifying repurposed drugs to treat SCN 8A epilepsy. Epilepsia, 2018; 59(4):802–13. CrossRef
Barboza JN, da Silva Maia Bezerra Filho C, Silva RO, Medeiros JV, de Sousa DP. An overview on the anti-inflammatory potential and antioxidant profile of eugenol. Oxid Med Cell Longev, 2018; 2018. CrossRef
Barot J, Saxena B. Therapeutic effects of eugenol in a rat model of traumatic brain injury: a behavioral, biochemical, and histological study. J Traditi Complement Med, 2021; 11(4):318–27. CrossRef
Bortolozzi A, Artigas F. Control of 5-hydroxytryptamine release in the dorsal raphe nucleus by the noradrenergic system in rat brain. Role of α-adrenoceptors. Neuropsychopharmacology, 2003; 28:421–34; doi:10.1038/sj.npp.1300061 CrossRef
Cao Z, George J, Gerwick WH, Baden DG, Rainier JD, Murray TF. Influence of lipid-soluble gating modifier toxins on sodium influx in neocortical neurons. J Pharmacol Exp Ther, 2008; 326:604–13. CrossRef
Chan KF, Jia Z, Murphy PA, Burnham WM, Cortez MA, Snead III OC. Learning and memory impairment in rats with chronic atypical absence seizures. Exp Neurol, 2004; 190:328–36. CrossRef
Chung G, Rhee J, Jung S, Kim J, Oh S. Modulation of CaV2. 3 calcium channel currents by eugenol. J Dent Res, 2008; 87:137–41. CrossRef
Craig RA, Garrison CE, Nguyen PT, Yarov-Yarovoy V, Du Bois J. Veratridine: a janus-faced modulator of voltage-hated sodium ion channels. ACS Chem Neurosci, 2020; 11(3):418–26. CrossRef
Dal Bó W, Luiz AP, Martins DF, Mazzardo-Martins L, Santos AR. Eugenol reduces acute pain in mice by modulating the glutamatergic and tumor necrosis factor alpha (TNF-α) pathways. Fundam Clin Pharmacol, 2013; 27(5):517–25. CrossRef
Dorandeu F, Calas G, Dal Bo G, Fares R. 2017. Chapter 36-Models of chemically-induced acute seizures and epilepsy: toxic compounds and drugs of addiction, in models of seizures and epilepsy. 2nd edition. In: Pitkänen A, Buckmaster PS, Galanopoulou AS, Moshé SL (eds.). Models of seizures and epilepsy, Academic Press, Cambridge, MA, pp 529–51. CrossRef
Fiest KM, Sauro KM, Wiebe S, Patten SB, Kwon CS, Dykeman J, Pringsheim T, Lorenzetti DL, Jetté N. Prevalence and incidence of epilepsy: a systematic review and meta-analysis of international studies. Neurology, 2017; 88(3):296–303. CrossRef
Garabadu D, Shah A, Singh S, Krishnamurthy S. Protective effect of eugenol against restraint stress-induced gastrointestinal dysfunction: potential use in irritable bowel syndrome. Pharm Biol, 2015; 53:968–74. CrossRef
Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem, 1985; 260:3440–50. CrossRef
Irie Y. Effects of eugenol on the central nervous system: its possible application to treatment of alzheimer’s disease, depression, and parkinson’s disease. Current Bioact Compd, 2006; 2:57–66. CrossRef
Jabba SV, Prakash A, Dravid SM, Gerwick WH, Murray TF. Antillatoxin, a novel lipopeptide, enhances neurite outgrowth in immature cerebrocortical neurons through activation of voltage-gated sodium channels. J Pharmacol Exp Ther, 2010; 332:698–709. CrossRef
Jeffrey M, Lang M, Gane J, Chow E, Wu C, Zhang L. Novel anticonvulsive effects of progesterone in a mouse model of hippocampal electrical kindling. Neuroscience, 2014; 257:65–75. CrossRef
Joushi S, Salmani ME. Effect of eugenol on lithium-pilocarpine model of epilepsy: behavioral, histological, and molecular changes. Iran J Basic Med Sci, 2017; 20(7):745.
Karampour NS, Arzi A, Namazifar S. Eugenol efficacy in preventing nicotine-induced seizures in mice. Natl J Physiol Pharm Pharmacol, 2017; 7(11):1C–5. CrossRef
Kleinrok Z, Turski L. Kainic acid-induced wet dog shakes in rats. Naunyn Schmiedeberg Arch Pharmacol, 1980; 314:37–46. CrossRef
Lévesque M, Avoli M. The kainic acid model of temporal lobe epilepsy. Neurosci Biobehav Rev, 2013; 37:2887–99. CrossRef
Nejad SM, Özgüne? H, Ba?aran N. Pharmacological and toxicological properties of eugenol. Turkish J Pharm Sci, 2017; 14(2):201. CrossRef
Nicholls DG, Sihra TS. Synaptosomes possess an exocytotic pool of glutamate. Nature, 1986; 321:772–3. CrossRef
Nisar MF, Khadim M, Rafiq M, Chen J, Yang Y, Wan CC. Pharmacological properties and health benefits of eugenol: a comprehensive review. Oxid Med Cell Longev, 2021; 2021. CrossRef
Ogata M, Hoshi M, Urano S, Endo T. Antioxidant activity of eugenol and related monomeric and dimeric compounds. Chem Pharm Bull, 2000; 48:1467–9. CrossRef
Otoom S, Sequeira RP. Veratridine induced absence like-seizure in the freely moving rats: a study correlating the behavioural findings with the electrophysiological activities. Neuroendocrinol Lett, 2011; 32:487–90.
Otoom SA, Alkadhi KA. Epileptiform activity of veratridine model in rat brain slices: effects of antiepileptic drugs. Epilepsy Res, 2000; 38:161–70. CrossRef
Otoom SA, Handu SS, Wazir JF, James H, Sharma PR, Hasan ZA, Sequeira RP. Veratridine-induced wet dog shake behaviour and apoptosis in rat hippocampus. Basic Clin Pharmacol Toxicol, 2006; 98:423–6. CrossRef
Pradhan J, Panchawat S. Herbal Therapies for epilepsy: chemistry, biology and potential applications of selected plants and compounds. Chem Biol Interface, 2018; 8(4):205–24.
Ramazi S, Fahanik-Babaei J, Mohamadi-Zarch SM, Tashakori-Miyanroudi M, Nourabadi D, Nazari-Serenjeh M, Roghani M, Baluchnejadmojarad T. Neuroprotective and anticonvulsant effects of sinomenine in kainate rat model of temporal lobe epilepsy: involvement of oxidative stress, inflammation and pyroptosis. J Chem Neuroanat, 2020; 108:101800. CrossRef
Singh D, Goel RK. Anticonvulsant mechanism of saponins fraction from adventitious roots of Ficus religiosa: possible modulation of GABAergic, calcium and sodium channel functions. Revista Brasileira de Farmacognosia, 2016,26:579-85. CrossRef
Weuring WJ, Singh S, Volkers L, Rook MB, van ‘t Slot RH, Bosma M, Inserra M, Vetter I, Verhoeven-Duif NM, Braun KP, Rivara M. NaV1. 1 and NaV1. 6 selective compounds reduce the behavior phenotype and epileptiform activity in a novel zebrafish model for Dravet Syndrome. PloS One, 2020; 15(3):e0219106. CrossRef
Yadav D, Yadav SK, Jain G, Mazumder A, Khar RK. Protective effect of eugenol against electrical induced seizure model of epilepsy. Pharm Innov J, 2018.
Zhang C, Chen J, Zhao F, Chen R, Yu D, Cao Z. Iritectol G, a novel iridal-type triterpenoid from Iris tectorum displays anti-epileptic activity in vitro through inhibition of sodium channels. Fitoterapia, 2017;122:20–5. CrossRef
Zhang XY, Bi RY, Zhang P, Gan YH. Veratridine modifies the gating of human voltage-gated sodium channel Nav1. 7. Acta Pharmacol Sin, 2018; 39:1716–24. CrossRef