INTRODUCTION
Broad bean (Vicia faba L.) is an essential vegetable plant and is considered as a skim milk and meat substitute in diets due to its high protein and nutritional quality (Devi et al., 2011). Crop production is mainly affected by soil-borne pathogenic agents all over the world (Compant et al., 2005; Wu et al., 2015). Plant diseases as well as crops cause the loss of approximately 20% and even more (Kröber et al., 2014; Oerke, 2006) by plant pathogens worldwide depending on the type of the crop, the severity of the pathogen, the strategies of management, and environmental conditions.
Macrophomina phaseolina and Rhizoctonia solani are severe soil-borne plant pathogens associated with root rot and damping-off diseases of broad bean (Devi et al., 2011). The most essential tool to control plant phytopathogens is using chemical pesticides, but their tremendous overuse leads to serious environmental problems such as the development of pathogen resistance, decline in the number of nontarget beneficial microorganisms in the rhizosphere region, and negative human health effects (Dukare et al., 2020; Hesran et al., 2019).
Madhurankhi and Suresh (2020) stated that biofertilizers are the safest, most ecofriendly effective tool in comparison to chemical fungicides. They also enhance soil fertility as well as crop yield by 10%–40% (Bhardwaj et al., 2014). Furthermore, they have been found to aid in the long-term sustainability of the agricultural ecosystem. Biofertilizers include all the beneficial bacteria that can be either symbiotic or free-living bacteria in nature. They are together referred to as plant growth-promoting rhizobacteria (PGPR).
PGPR has been considered as one of the most promising tools to achieve the elimination and even prevent soil-borne disease and hence maximize crop yields (Aruna et al., 2020; Devi et al., 2011; Hass and Defago, 2005). It has been proved that PGPR has either indirect or direct effects on plants in order to promote their growth and to increase crop yields through several mechanisms (Devi et al., 2011), nitrogen fixing and phytohormones production (Cattelen et al., 1999), nitrogen availability stimulation, increase of phosphate solubilization and minerals in the soil (Richardson, 2003), and above all control or even prevention of plant pathogens’ activities (Babu et al., 2015; Glick, 1995; Glick et al., 1999; Hayat et al., 2010). Several research works studied the mechanisms which microorganisms may use to overcome the disease stress in plants including the production of hydrolytic enzymes (Maurhofer et al., 1994; Mostafa and Abd El Aty, 2013) and siderophores production and hydrogen cyanide (HCN) synthesis (Buysens et al., 1996; Naureen et al., 2017). Some PGPR was responsible for promoting the growth of several plants indirectly by a mechanism known as induced systemic resistance (ISR) (Abdelrahman et al., 2016; Jogaiah et al., 2010; Kloepper, et al., 2004; Mendis et al., 2018). Kröber et al. (2014) added efficient bacterial strains to colonize the plant root system, accelerate plant growth, and activate their defense system. The phenomenon of initiating the systemic resistance activities of the plant has been termed rhizobacteria-ISR (Arguelles-Arias et al., 2009; Kröber et al., 2014).
One of the most antagonistic PGPR is Bacillus sp. with many advantages such as easy isolation and culturing, wide distribution, antiadversity ability, and their ability to produce a wide range of antibiotics and enzymes with broad-spectrum antimicrobial activity (Arguelles-Arias et al., 2009; Chowdhury et al., 2015; Kröber et al., 2014; Tiago et al., 2004; Yuan et al., 2013). Bacillus licheniformis, Bacillus amyloliquefaciens, Bacillus pumilus, Bacillus megaterium, Bacillus subtilis, and so forth have been recorded as rhizosphere residents of a wide range of crops with plant growth-promoting properties (Abd El Aty and Zohair, 2020; Kloepper et al., 2004; Kumar et al., 2011). Previous studies of Kröber et al. (2014) documented B. amyloliquefaciens FZB42 as plant growth-promoting bacteria for their ability to promote plant growth as well as the ability to suppress different plant pathogens on cotton, cucumbers, lettuce, tobacco, and tomatoes (Chowdhury et al., 2015; Grosch et al., 1999; Guel et al., 2008; Idris et al., 2007; Koumoutsi et al., 2004; Wang et al., 2009; Yao et al., 2006).
The main goal of this work was to apply a novel rhizosphere bacterium with multiple plant growth-promoting traits and antifungal activity against phytopathogens for promoting the growth of V. faba, an important food legume in Egypt that can be developed as a commercial product.
MATERIALS AND METHODS
Bacterial strains
Five morphologically different rhizosphere bacterial strains isolated from outside the rhizosphere plant root tips of the species Salsola imbricata, collected from Tur Sinai in Egypt (Abd El Aty et al., 2018), were tested to select the most active strain with maximum antifungal activities against all tested phytopathogens. All rhizosphere strains were maintained on nutrient agar slants and subcultured periodically and preserved at 4°C.
In vitro antagonistic effects of isolated bacteria towards soil-borne plant pathogens
Different bacterial strains isolated by Abd El Aty et al. (2018) were screened against a panel of plant pathogenic fungal strains. The soil-borne plant pathogens Alternaria alternata NRC43, Fusarium solani NRC11, Fusarium oxysporum NRC23, and Sclerotium rolfsii NRC32 were supplied from the Culture Collection Unit at the Chemistry of Natural and Microbial Products Department, National Research Centre, Egypt (Abdel Wahab et al., 2018; Batrana et al., 2019). Petri dishes containing a Potato Dextrose Agar (PDA) (Difco) medium were inoculated with a 1 ml spore suspension containing about 1 × 106 spores of 7 days old fungal pathogens. After solidification of the agar medium, the tested bacterial colonies were inoculated individually in the center of each plate. Plates without bacteria were used as a control. The Petri dishes were incubated at a temperature of 28°C–30°C for 5 days. The mycelium growth inhibition and the diameter of bacterial colonies were measured and compared to the control without bacterial inoculation to select the most active bacterial strain (Zohair et al., 2018).
Bacillus amyloliquefaciens MH046937
The bacterial isolate identified as B. amyloliquefaciens MH046937 was used in the present study according to the antagonistic results obtained, and the plant growth-promoting tests were carried out only for that isolate.
Evaluation of B. amyloliquefaciens MH046937 for multiple plant growth-promoting traits
Indole acetic acid (IAA) production
Twenty-five milliliters of nutrient broth (NB) slants supplemented with 0.05% DL-tryptophan was inoculated with a 1 day old bacterial culture grown in a shaking incubator at 30°C at 200 rpm in the dark for 2 days (Ashour et al., 2016 a; Kumar et al., 2012a). The tested bacterial isolate was centrifuged in a refrigerated centrifuge at a range of 10,000–15,000 rpm for 10 minutes at 4°C. Indole-3-acetic acid [Indole-3-acetic acid (IAA)] was estimated in the supernatant by using the colorimetric assay test (Loper and Schroth, 1986). Half millilitre of bacterial supernatant was added and well mixed with 2 ml of Salkowski’s reagent and the produced pink color was measured after half an hour at 535 nm in a UV-visible spectrophotometer. The production of the pink color in the test tube was considered as an indication of IAA production which was previously indicated by Gordon and Weber (1951).
Production of ammonia
Bacillus amyloliquefaciens was investigated for the production of ammonia (NH3) as reported by Ashour et al. (2016a) and Cappuccino and Sherman (1992). A 1-day-old bacterial culture was inoculated in 5 ml peptone broth media then incubated at 30°C for 2 days in a shaking incubator. Then 0.25 ml of Nessler’s reagent was added to flasks and mixed well. The appearance of a faint yellow color to a dark brown color was considered as an indicator of ammonia production.
Production of HCN
Production of HCN was estimated by streaking the B. amyloliquefaciens bacterial isolate on King’s B agar medium amended with glycine. The internal side of the Petri plate’s lid was covered with filter paper soaked in a picric acid solution (0.05% solution in 2% sodium carbonate). Then the plates were sealed air-tight with Parafilm stretch and incubated at 30°C ± 1°C for 2 days. The change of the filter paper color from deep yellow to reddish-brown indicated HCN production (Bakker and Schipperes, 1987; Geetha et al., 2014).
Production of siderophore
Siderophore productivity was estimated according to Sujatha and Ammani (2013). The tested bacterial isolates were streaked on the (King’s B) medium with and without the addition of (50 mg/l) FeCl3 then incubated at temperature 28°C ± 1°C for 2 days. The formation of fluorescent pigment was considered as evidence of siderophore production.
Phosphate solubilization
The phosphate solubilization qualitative test was carried out using Pikovskaya’s (PVK) medium containing (in g/l) glucose 10.0, Ca3 (PO4)2 5.0, (NH4)2SO4 0.5, KCl 0.2, MgSO4.7H2O 0.1, MnSO4 0.002, FeSO4 0.002, yeast extract 0.5, agar 16.0, and distilled water. The freshly prepared bacterial culture was streaked on PVK’s agar medium and incubated at a temperature of 30°C for 12 days. The formation of a clear zone surrounding the tested culture proved the phosphate solubilization ability (Kumar et al., 2012a).
Determination of the fungal cell-wall hydrolytic enzymes
Bacillus amyloliquefaciens was screened for chitinase and 1,3-β-glucanase enzymes production using an NB medium at different incubation periods (Hao et al., 2012). Fermentation was carried out in 250 ml conical flasks containing 50 ml of a sterile medium. For the cultivation process, a 24-hour-old culture was adjusted to ~1.0 at 600 nm, and each flask finally was inoculated with a 5 ml bacterial suspension. Flasks were incubated at 32°C, 180 rpm for 24, 48, and 72 hours. After incubation for different time intervals, the fermentation broth was centrifuged for chitinase and 1,3-β-glucanase activities determination.
Assay of chitinase
The chitinase assay was performed using the colorimetric method for the estimation of N-acetyl amino sugar using the modified dinitrosalicylic acid [Dinitrosalicylic acid (DNS)] method. The unit (U) of chitinase productivity was defined as the quantity of enzyme required to liberate 1.0 μmol/minute of N-acetyl glucosamine (Ashour et al., 2016b; Shehata et al., 2018).
Assay of 1,3-β-glucanase
Glucanase productivity was determined according to Burner (1964). The reaction mixture contained 250 μl of the freshly prepared crude enzyme solution and 250 μl of 1% laminarin (Sigma Chemical Co., St. Louis, MO) in a 0.2 M sodium acetate buffer at pH 5. The previous mixture was incubated with gentle shaking at 37°C for 30 minutes. The reaction was arrested by the addition of 1% dinitrosalicylate (DNS) and then boiled for another 10 minutes. One unit (U) of the glucanase productivity was estimated based on the quantity of enzyme that is needed to liberate 1 μmol/minute of glucose.
Extraction and detection of bioactive compounds with antifungal properties
The B. amyloliquefaciens fermentation medium was investigated for antifungal metabolites production.
Fermentation and extraction of metabolite compounds
For bacterial fermentation, 500 ml conical flasks containing 100 ml of sterile NB of total volume 500 ml were inoculated with a B. amyloliquefaciens suspension. The flasks were incubated for 7 days at 33°C and 180 rpm. Then the fermentation medium was centrifuged at 7,000 rpm for 15 minutes and the filtrate obtained was partitioned with ethyl acetate three successive times. The ethyl acetate layer was concentrated under a vacuum to obtain the ethyl acetate extract.
Fractionation, purification, and characterization of compounds
The crude ethyl acetate extract was separated on silica gel 60 with particle size 0.063–0.2 mm (70–230 mesh) column chromatography. N-Hexane and ethyl acetate with ratio (1,1) were applied to give 20 ml of three subfractions (A1–A3) and then ethyl acetate (100%) to give another three subfractions (A4–A6). Subfractions coded A3 and A4 were gathered and further purified by using YL9100 High-performance liquid chromatography (HPLC) equipped with a YL9112 isocratic pump and YL9120 UV/visible detector at λ = 254 and 280 nm. The used column was a normal phase (ZORBAX RX Silica 5 μm, 250 × 9.4 mm). The used solvent system was n-hexane, EtOAc with 2:1 ratio, and flow rate 4 ml/minutes. The detected eluted peaks at different retention times by the UV detector were recorded and the major compound 1 (4.0 mg) was purified. Structure elucidation of 1 was carried out using 1H and 13C NMR measured in CDCl3 using a Bruker 500 MHz.
In vitro antifungal activity
Nine fractions separated from the HPLC column were assayed for their antifungal properties using the agar diffusion method (Mohamed et al., 2017; El-serwy et al., 2015). Each fraction was loaded on a paper disc of blotting paper (5 mm in diameter) with a concentration of 100 μg/5 μl ethyl acetate/disc. The phytopathogen F. solani NRC11 was inoculated into plates containing a sterile PDA medium. The discs were placed on the surface of the inoculated plates and left for compounds diffusion for about 30 minutes at 4°C. Plates were incubated for 3 days at 28°C for the growth of pathogenic fungi. The diameters of the inhibition zone formed around the discs at three different points were measured in millimeters and the average values were recorded as mean ± SD by using the MS Excel program.
In vivo biocontrol estimation of root rot disease
The biocontrol activity of B. amyloliquefaciens against phytopathogens (F. oxysporum, F. solani, and S. rolfsii) was confirmed in the greenhouse experiment. The healthy V. faba seeds were cultivated in pots (each containing 400 g sterilized soil previously autoclaved at 121°C for 15 minutes). The infection technique was applied using 1-week-old soil-borne pathogenic fungi cultures on PDA. A 5-ml sterilized distilled water suspension was inoculated with a 5 mm diameter disc of soil-borne pathogenic fungi to prepare the inocula. To create an artificial infection, the prepared inocula were added to pots. The treatments were designed as mentioned in Table 1: T0: treatment of pots with pathogenic fungi without the addition of any control agents, T1: treatment of pots with the biocontrol agent B. amyloliquefaciens without infecting pots with pathogens, and T2: treatment of pots with both the biocontrol agent B. amyloliquefaciens and three different pathogenic fungi separately. The pots were kept under greenhouse conditions with a temperature of 25°C.
 | Table 1. Greenhouse experiment design. [Click here to view] |
Each treatment was carried out in triplicate. The tested vegetative growth parameters were estimated and recorded at the end of the experiment. The disease index (DI) was estimated on a scale from 0 to 4. The statistical design work was completely randomized according to the work of Harveson et al. (2014).
Statistical analysis
The recorded data were subjected to an analysis variation test and were analyzed by using the a computer based Statistical software packages (MSTATC) program. The differences of recorded means of the three replicates were compared applying the least significant differences (LSD) test at a 5% level of significance.
RESULTS AND DISCUSSION
Evaluation of bacterial strains antagonistic effects
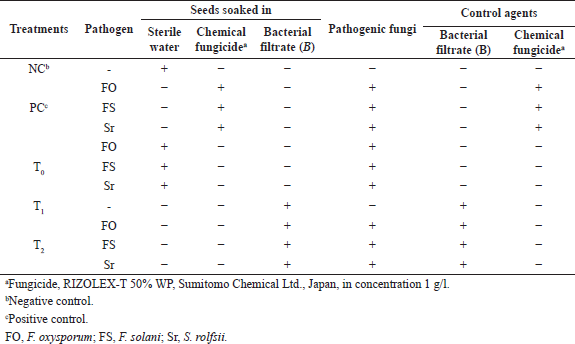
All bacterial strains isolated from the rhizosphere zone of S. imbricata in Tur Sinai, Egypt (Abd El Aty et al., 2018), were tested in vitro against four different pathogenic fungi: A. alternata, F. solani, F. oxysporum, and S. rolfsii. Out of all rhizosphere strains, the bacterial isolate identified as B. amyloliquefaciens was found to have broad-spectrum antifungal activities against all pathogens (Fig. 1). Antifungal effects depend on the production of cell-wall hydrolytic enzymes such as chitinases, a major enzyme group capable of degrading the polymeric chitin components of pathogenic fungal cell walls (Dubey et al., 2014; Kumar et al., 2012b). In this context, B. suly produced the chitinase enzyme that reduced the infection severity of Fusarium sp. under greenhouse conditions (Hariprasad et al., 2011).
Bacillus amyloliquefaciens showed high antagonistic activities against S. rolfsii (a) followed by A. alternata (b) and showed similar effects against the Fusarium sp. (c and d). Our results agree to some extent with Gowtham et al. (2018) who indicated that Pseudomonas aeruginosa has the maximum percentage of inhibition capability against Colletotrichum truncatum growth, followed by B. amyloliquefaciens and Burkholderia cepacia. On the other hand, Akinrinlola et al. (2018) exhibited limited antagonism of B. pumilus strains R174, R183, and R190 and B. megaterium R181 against F. graminearum, while the two Pythium spp. and R. solani were not affected.
Evaluation of B. amyloliquefaciens for plant growth-promoting properties
Physiological traits of B. amyloliquefaciens were estimated in vitro for the production of IAA, HCN, siderophores, and ammonia and capability for phosphate solubilization.
Production of IAA
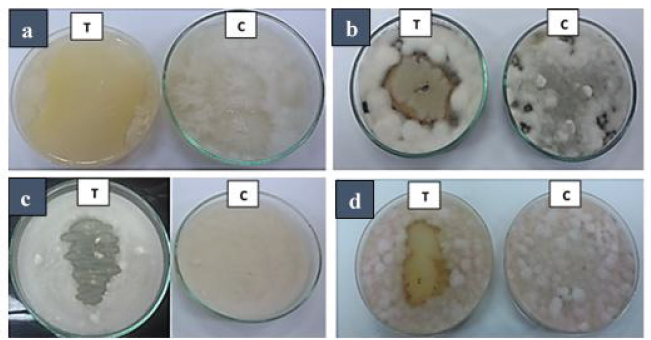
The PGPR secreting IAA through the interaction with the plant enhance and accelerate plant growth and help in the plant defense responses mechanism (Mohite, 2013; Tekalign et al., 2016). Our studies proved that B. amyloliquefaciens produced a substantial quantity of IAA equivalent to 32.9 ± 0.01 μg/ml (Fig. 2a). Idris et al. (2007) indicated that the Gram-positive bacterial strain B. amyloliquefaciens synthesized significant quantities of IAA, while mutants of B. amyloliquefaciens E101 (ΔtrpED) and E102 (ΔtrpAB) synthesized IAA in small quantities. The amount of IAA produced by B. amyloliquefaciens MH046937 was more than that reported by Asma et al. (2013) (7–14 μg/ml) due to the positive effect of tryptophan and higher than that recorded by Shao et al. (2015), 9.46 μg/ml, when L-tryptophan was added, while Chandra et al. (2018) proved that only three bacterial isolates out of the different isolates were IAA producers. Isolates CA2004 and CA1001 exhibit significant production capability of IAA (91.7 μg/ml) followed by 81.7 μg/ml and the isolate coded CA1001 produced 32 μg/ml of IAA which was similar to some extent to our results. Akinrinlola et al. (2018) showed the ability of Bacillus safensis and B. megaterium to synthesize IAA and none of the B. pumilus tested strains exhibited this activity.
 | Figure 1. Antagonistic effect of the rhizospheric B. amyloliquefaciens against (a) S. rolfsii, (b) A. alternata, (c) F. solani, and (d) F. oxysporum. T tested plates inoculated with bacteria. C, control plates without bacterial inoculation. All plates incubated at 28°C–30°C for about 5 days. [Click here to view] |
 | Figure 2. In vitro physiological traits of B. amyloliquefaciens MH046937 for the production of IAA (a), production of ammonia (b), production of HCN (c), and phosphate solubilization (d). [Click here to view] |
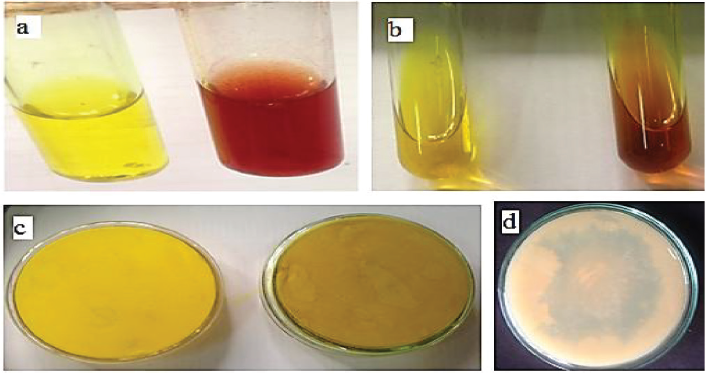
 | Figure 3. Agar diffusion assay of subfractions obtained from silica gel column chromatography (a) and nine fractions separated from HPLC column (b) against the phytopathogen F. solani. Each fraction was loaded with a concentration of 100 μg/5 μl ethyl acetate/disc. +ve, positive for inhibiting F. solani growth. −ve, negative for inhibiting F. solani growth. [Click here to view] |
Production of ammonia
Our results proved that B. amyloliquefaciens MH046937 produced a dark brown color with Nessler’s reagent indicating a considerable amount of ammonia production as shown in Figure 2b, and these results were found to agree with the Sushil et al. (2013) study which proved that B. amyloliquefaciens produced ammonia.
HCN and siderophore production
Bacillus amyloliquefaciens MH046937 was able to synthesize HCN at a moderate amount as shown in Figure 2c and this result goes along with Defago et al. (1990) who stated that B. amyloliquefaciens produced HCN at a moderate amount. Nadège et al. (2015) reported that over 75% of HCN producers were found to be Bacillus sp. strains isolated from the rhizosphere of rice.
Siderophore production was not recorded either in the presence or in the absence of FeCl3. These results were similar to some extent to Nadège et al.’s (2015) results, who showed siderophore synthesis without the addition of 1 μΜ FeSO4.7H2O to the culture flask. However, other Bacillus strains coded R181, R180, R232, and R190 were proved to be capable of siderophore synthesizing when cultivated on a Chrome Azurol S medium (CAS) agar medium (Akinrinlola et al., 2018).
Phosphate solubilization activity
In the work of Schwartz et al. (2013) and Khalid et al. (2004) the phosphate solubilization traits were connected to high differentiation, growth, and development efficacy. As shown in Figure 2d, the tested bacterial isolate had efficiency in phosphate solubilization and produced a large inhibition zone after 10 days. Akinrinlola et al.’s (2018) and Nadège et al.’s (2015) data declare that B. amyloliquefaciens and B. megaterium strains, respectively, exhibited phosphate solubilization activity on an (PVK’s) agar medium. These results were in excellent accordance with those recorded by other scientists (Fernandez et al., 2012; Riberio and Cardoso, 2012).
Results showed that B. amyloliquefaciens has many physiological traits like IAA, ammonia, and HCN production in addition to the ability to hydrolyze phosphate, which helps the bacterium prevent the plant pathogens activities and increase plant growth-promoting properties.
Evaluation of B. amyloliquefaciens hydrolytic enzymes activities
The bacterial isolate was screened for chitinase and 1,3-β-glucanase enzymes activities at different incubation periods using the NB medium without any induction by substrate addition. The result showed that B. amyloliquefaciens was able to produce the chitinase enzyme (68 IUl−1) after 48 hours. In contrast, the tested bacterial isolate did not exhibit 1,3-β-glucanase enzyme activity. Other studies of Ashour et al. (2016a) and Wang et al. (2006) reported chitinase productivity in many Bacillus species such as B. diminuta KT277492, B. cereus KU058893, and B. megaterium using the NB medium, where other species were reported as β-glucanase producers, such as B. licheniformis (Chaari et al., 2012) and B. subtilis (Manjula and Podile, 2005; Narasimhan et al., 2013).
The ability of B. amyloliquefaciens to produce the hydrolytic enzyme chitinase, in addition to the previously mentioned physiological traits, increased its ability to overcome the disease stress in plants.
PGPR-based mechanisms employed in the biological control of phytopathogens include the production of cell-wall degrading chitinase enzyme (Husson et al., 2017), synthesis of antimicrobial metabolites (Couillerot et al., 2009), and HCN (Nandi et al., 2017), and siderophores (Shen et al., 2013) production is important for induction of ISR that causes a faster and stronger plant response to attacks by different pathogens (Olanrewaju et al., 2017).
Purification and characterization of B. amyloliquefaciens bioactive metabolites
The crude ethyl acetate extract of the tested bacterial isolate was first purified using silica gel column chromatography with different solvent systems. The antifungal activity of all obtained subfractions against F. solani was detected. Results indicated that subfractions A3 and A4 have the highest inhibition zone diameters (IZD) of 16 and 15 mm (Fig. 3a), respectively, that indicated the availability of bioactive antifungal metabolites. The A3 and A4 subfractions were combined and further purified using YL9100 HPLC, and the eluted peaks at various retention times were collected.
Nine fractions separated from the HPLC column at different retention times were assayed using the agar diffusion method. The fungal pathogen F. solani was used as an indicator to explore and determine the bioactive fractions for the identification of the major antifungal compound. Results in Figure 3b showed that fractions eight and nine were positive for inhibiting the fungal growth (IZD of 12 ± 0.71 mm). Fraction seven showed week antifungal activity (IZD of 8 ± 0.21 mm), where the other fractions were negative for inhibiting F. solani growth.
Eluted peaks 8 and 9 with good antifungal activities were subjected to structural elucidation analysis, and the major compound (1) was obtained. The structural analysis of the major compound (1) on the basis of the NMR measurements and EI mass spectroscopy was identified as 5-hydroxymethylfurfural (Fig. 4).
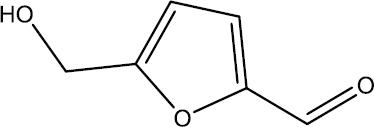 | Figure 4. Chemical structure of 5-hydroxymethylfurfural. [Click here to view] |
1H NMR (500 MHz, CDCl3) δ, 2.15 (br s, 1H), 4.74 (s, 2H), 6.52 (d, J = 3.6 Hz, 1H), 7.22 (d, J = 3.6 Hz, 1H), 9.61 (s, 1H), 13C NMR (125 MHz, CDCl3) δ, 57.7, 110.0, 122.6, 152.4, 160.4, 177.7, Low Rate- Electron Ionization (LR-EI) mass, 126 m/z.
Other studies of Rane Zab et al. (2012) stated that the ethyl acetate fraction of Punica granatum rind containing abundant 5-hydroxymethylfurfural showed antimicrobial activity by the agar diffusion method. Rigal and Gaset (1983) showed that the compound 2-furancarboxaldehyde,5-(hydroxymethyl) has antifungal and antibacterial activity and is applicable in many fields, including pesticides, cosmetics, and pharmaceuticals. Also, previous studies indicated that furfural could be used as a flavor and in many other products, such as herbicides, pesticides, fungicides, germicides, and insecticides, as mentioned by Morales (2008).
Plant growth-promoting and biocontrol properties of B. amyloliquefaciens
In greenhouse experiments, V. faba seeds treated with PGPR B. amyloliquefaciens were estimated for their positive effect on differentiation and vegetative growth parameters such as shoot dry and fresh weights and plant height, along with the number of leaves per plant. Our results showed that the bacterial treatment had significant stimuli on the height of infected plants with pathogens S. rolfsii, F. oxysporum, and F. solani at 34, 27, and 24 cm, respectively. The vegetative growth had been affected positively by the application of bacterial treatment.
The highest shoot dry and fresh weights were 0.4 and 4.2 g, respectively, in treated plants with the tested bacterial strain and infected with S. rolfsii compared with the positive control treatment.
The fresh and dry root weight in infected plants with F. solani or S. rolfsii and treated with the tested bacterial strain had been enhanced significantly in comparison with other treatments by 0.5 and 0.07 g, respectively.
The highest germination percentage was recorded with infected seeds with F. solani 86.7% and the DI was 0 grade significantly as the plants infected with F. oxysporum (Table 2). These results confirm the role of treatment with B. amyloliquefaciens as a biocontrol plant growth-promoting agent. In this context, Srivastava et al. (2016) recorded that B. amyloliquefaciens stimulated the production of rice plants under infection of pathogenic fungi Rhizoctonia.
Analysis of three different treatments indicated a significant enhancement in the plant height, the number of leaves, and stem thickness (28.33 cm, 3.66, and 0.53 mm, respectively) in the treatment with the tested bacterial strain compared with positive control (19.66 cm, 3, and 0.43 mm, respectively) as shown in Figure 5.
The treatment with B. amyloliquefaciens MH046937 (T2) compared with the control (PC) introduced a significant increase of germination percentage (60% and 40%, respectively) and reduced the disease incidence to be 0.44 and 1.66 for bacterial treatment and positive control, respectively. Our results agreed with Gowtham et al. (2018) whose work proved that out of eight PGPR isolates B. amyloliquefaciens provided the highest level of seed germination and also seedling vigor of chili seeds germination (84.8% and 1,423.8% respectively). Also, Hariprasad et al. (2011) stated that PGPR that had broad-spectrum antifungal and antibacterial activities was found to enhance the growth of many plants as well as the suppression of many phytopathogens of tomatoes by stimulating the plant’s defense mechanisms. The PGPR were also recorded to eliminate both bacterial and fungal infections effectively and enhance autoresistance in some plants (Chowdappa et al., 2013; Ramamoorthy et al., 2002). Other promising findings were observed by Yuan et al. (2013) who found the recorded rate of Fusarium infection causing wilt disease in banana plants cultivated in pots containing a bioorganic fertilizer was as high as 90% while it was only 28% in ones containing a bioorganic fertilizer inoculated with B. amyloliquefaciens NJN-6 which could be an indication of the role of B. amyloliquefaciens in suppressing the growth of the pathogens.
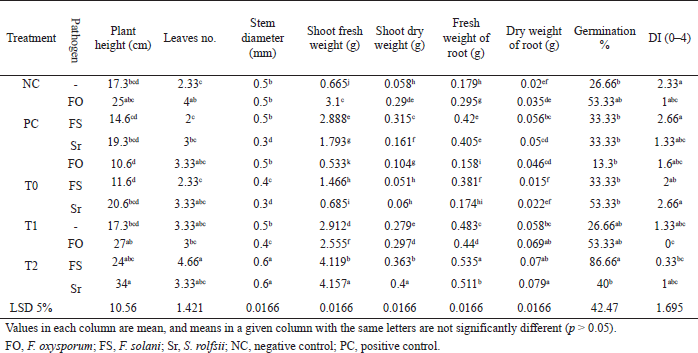 | Table 2. Effect of various treatments on biocontrol activities and plant growth-promoting properties. [Click here to view] |
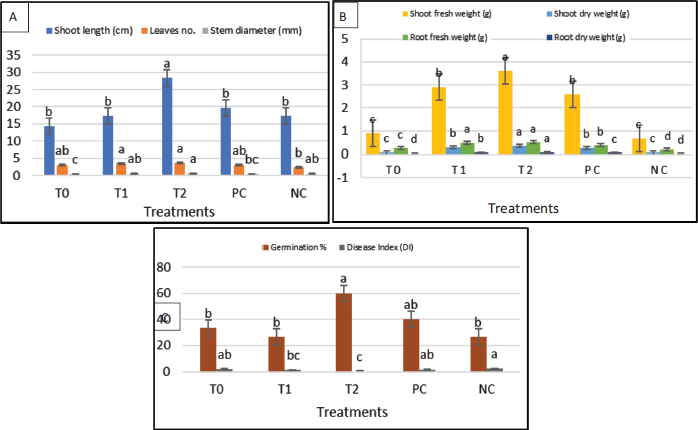 | Figure 5. Effect of various treatments on plant vegetative and root growth parameters (A and B) and biocontrol activities and germination percentage (C) of V. faba. [Click here to view] |
 | Figure 6. Greenhouse application of the PGPR (B. amyloliquefaciens MH046937) against phytopathogens. [Click here to view] |
The treatment with B. amyloliquefaciens MH046937 compared with the control introduced a significant stimulation of one or more growth factors. Bacterial treatment had considerable effects on shoot and root weights, compared to control. T2 had the highest shoot fresh weight at 3.6 g while the positive control had 2.59 g. In the same way, the treatment with bacteria had the highest significant effect on the root’s fresh weight at 0.495 g as shown in Figures 5 and 6. The same findings were reported by Gowtham et al. (2018) who found that plants treated with B. amyloliquefaciens provided the highest vegetative growth parameters of chili plants such as plant height at a value 18.32 cm, dry weight recorded as 1.5 g, shoot fresh weight reaching 3.5 g, and number of leaves at a value of 15.25 leaves per plant.
CONCLUSION
In conclusion, the B. amyloliquefaciens MH046937 rhizospheric strain showed antagonistic activity to several plant pathogenic fungi. It demonstrated that plant growth-promoting properties depending on the ability of IAA production, ammonia production, HCN production, and solubilization of phosphate to some extent are involved in V. faba growth promotion. Also, the results indicated that the tested bacterial isolate has the ability to produce the chitinase enzyme and bioactive metabolite identified as 5-hydroxymethylfurfural important in the suppression of plant pathogenic fungi. Results indicated that B. amyloliquefaciens MH046937 had broad-spectrum plant promotion activity for V. faba growth in greenhouse pot experiments. Its application as a biocontrol agent can significantly contribute to some extent to the damage that occurs due to the existence of soil-borne fungi.
ACKNOWLEDGMENTS
The authors sincerely acknowledge the Chemistry of Natural and Microbial Products Department as well as the National Research Centre of Egypt.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abd El Aty AA, Zohai, MM. Green-synthesis and optimization of an eco-friendly nanobiofungicide from Bacillus amyloliquefaciens MH046937 with antimicrobial potential against phytopathogens. Environ Nanotechnol Monit Manage, 2020; 14:100309; http:/doi.ord/10.1016/j.enmm.2020.100309 CrossRef
Abd El Aty AA, Shehata AN, Shaheen TI. Production and sequential optimization of Bacillus subtilis MF467279 pullulanase by statistical experimental designs and evaluation of its desizing efficiency. Biocatal Agric Biotechnol; 2018; 14:375–85. Available via https://www.sciencedirect.com/science/article/pii/S1878818118300021 CrossRef
Abdelrahman M, Abdel-Motaal F, El-Sayed M, Jogaiah S, Shigyo M, Ito SI, Tran LS. Dissection of Trichoderma longibrachiatum-induced defense in onion (Allium cepa L.) against Fusarium oxysporum F. sp. cepa by target metabolite profiling. Plant Sci, 2016; 246:128–38; http:/doi.ord/10.1016/j.plantsci.2016.02.008 CrossRef
Abdel Wahab WA, Abd El Aty AA, Mostafa FA. Improvement of catalytic, thermodynamics and antifungal activity of constitutive Trichoderma longibrachiatum KT693225 exochitinase by covalent coupling to oxidized polysaccharides. Int J Biol Macromol, 2018; 112:179–87. Available via https://pubmed.ncbi.nlm.nih.gov/29414729/
Akinrinlola RJ, Yuen GY, Drijber RA, Adesemoye AO. Evaluation of Bacillus strains for plant growth promotion and predictability of efficacy by in vitro physiological traits. Int J Microbiol; 2018:Article ID 5686874; http:/doi.ord/10.1155/2018/5686874
Arguelles-Arias A, Ongena M, Halimi B, Lara Y, Brans A, Joris B. Bacillus amyloliquefaciens GA1 as a source of potent antibiotics and other secondary metabolites for biocontrol of plant pathogens. Microb Cell Fact, 2009; 8:63; http:/doi.ord/10.1186/1475-2859-8-63 CrossRef
Aruna KA, Sam AM, Vinod SG. Isolation, screening and characterization of plant growth promoting rhizobacteria from rhizospheric soils of selected pulses. Biocatal Agric Biotechnol; 2020; 27:101685. CrossRef
Ashour WE, Hamed ER, El-Diwany AI, Swelim MA, Abd El Aty AA. Isolation and characterization of plant growth-promoting rhizobacteria from Trigonella foenum- graecum L rhizosphere and evaluation of their potential substances produced. Res J Pharm Biol Chem Sci, 2016a; 7(3):130–43; Available via https://www.rjpbcs.com/pdf/2016_7(3)/[18].pdf
Ashour WE, Abd El Aty AA, Hamed ER, Swelim MA, El-Diwany AI. Applications of Plackett–Burman and central composite design for the optimization of novel Brevundimonas diminuta KT277492 chitinase production, investigation of its antifungal activity. Brazil Arch Biol Technol, 2016b; 59:1–14. Available via https://www.scielo.br/scielo.php?script=sci_arttext&pid=S1516-89132016000100429 CrossRef
Asma AK, Noreddine KC, Laid D, Asma M, Mounia YA, Marc O, Philippe T. Biocontrol and plant growth promotion characterization of Bacillus species isolated from Calendula officinal is rhizosphere. Indian J Microbiol, 2013; 53:447–52. Available via https://pubmed.ncbi.nlm.nih.gov/24426149/ CrossRef
Babu AN, Jogaiah S, Ito S, Nagaraj AK, Tran LSP. Improvement of growth, fruit weight and early blight disease protection of tomato plants by rhizosphere bacteria is correlated with their beneficial traits and induced biosynthesis of antioxidant peroxidase and polyphenol oxidase. Plant Sci, 2015; 231:62–73; http:/doi.ord/10.1016/j.plantsci.2014.11.006
Bhardwaj D, Ansari MW, Sahoo RK, Tuteja N. Biofertilizers function as key player in sustainable agriculture by improving soil fertility, plant tolerance and crop productivity. Microb Cell Fact, 2014; 13:66. CrossRef
Bakker AW, Schipperes B. Microbial cyanide production in the rhizosphere in relation to potato yield reduction and Pseudomoas spp.—mediated plant growth stimulation. Soil Biol Biochem, 1987; 19:451–57. Available via https://www.sciencedirect.com/science/article/pii/003807178790037X CrossRef
Batrana RZ, Khedr MA, Abdel Latif NA, Abd El Aty AA Shehata AN. Synthesis, homology modeling, molecular docking, dynamics, and antifungal screening of new 4-hydroxycoumarin derivatives as potential chitinase inhibitors. J Mol Struct, 2019; 1180:260–71. Available via https://www.sciencedirect.com/science/article/pii/S0022286018314121 CrossRef
Burner RL. Determination of reducing sugar value 3,5-dinitrosalicylicacid method. Methods Carbohydr Chem, 1964; 4:67–71. Available via https://www.sciencedirect.com/book/9780127462080/general-methods
Buysens S, Heungens K, Poppe J, Hofte M. Involvement of pyochelin and pyoverdin in suppression of Pythium-induced damping-off of tomato by Pseudomonas aeruginosa 7NSK2. Appl Environ Microbiol, 1996; 62:865–71. Available via https://pubmed.ncbi.nlm.nih.gov/16535275/
Cappuccino JG, Sherman N. Biochemical activities of microorganisms. In: Microbiology, a laboratory manual. The Benjamin/Cummings Publishing Co. San Francisco, CA, 1992.
Cattelen AJ, Hartel PG, Fuhrmann JJ. Screening for plant growth promoting rhizobacteria to promote early soyabean growth. Soil Sci Soc Am J, 1999; 63:1670–80. CrossRef
Chaari F, Kamoun A, Bihri F, Bibech M, Ellouze-Ghrobeland R, Ellouz-Chaabouni S. Statistical optimization for the production of lichenase by a newly isolated Bacillus licheniformis UEB CF in solid state fermentation using pea pomace as a novel solid support. J Ind Crop Prod, 2012; 40:192–8. CrossRef
Chandra S, Askari K, Kumari M Optimization of indole acetic acid production by isolated bacteria from Stevia rebaudiana rhizosphere and its effects on plant growth. 2018; 16(2):581–6. Available via https://www.sciencedirect.com/science/article/pii/S1687157X18300829 CrossRef
Chowdappa P, Mohan Kumar SP, Jyothi Lakshmi M, Upreti KK. Growth stimulation and induction of systemic resistance in tomato against early and late blight by Bacillus subtilis OTPB1 or Trichoderma harzianum OTPB3. Biol Control, 2013; 65(1):109–17. CrossRef
Chowdhury SP, Anton H, Xue WG, Rainer B. Biocontrol mechanism by root-associated Bacillus amyloliquefaciens FZB42–areview. Microbiol 2015; 6:780; http:/doi.ord/10.3389/fmicb.2015.00780. CrossRef
Compant S, Duffy B, Nowak J, Clement C, Barka EA. Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl Environ Microbiol, 2005; 71:4951–9; http:/doi.ord/10.1128/AEM.71.9.4951-4959. CrossRef
Couillerot O, Prigent-Combaret C, Caballero-Mellado J, MoënneLoccoz Y. Pseudomonas fluorescens and closely-related fluorescent pseudomonads as biocontrol agents of soil-borne phytopathogens. Lett Appl Microbiol, 2009; 48:505–12; http:/doi.ord/10.1111/j.1472-765x.2009.02566.x CrossRef
Defago G, Berling CH, Burger U, Haas D, Kahr G, Keel C, Voisard C, Wurthner P, Wuthrich B. Suppression of black root of tobacco by a pseudomonas strain, potential application and mechanisms. In: Horby D, Cook RJ (ed.). 1990. Available via https://agris.fao.org/agris-search/search.do?recordID=GB9103166
Devi SI, Talukdar NC, Chandradev S, Jeyaram K, Rohinikumar M. Screening of rhizobacteria for their plant growth promotion ability and antagonism against damping off and root rot diseases of broad bean (Vicia faba L.). Indian J Microbiol, 2011; 51(1):14–21. CrossRef
Dubey RC, Khare S, Kumar P, Maheshwari DK. Combined effect of chemical fertilisers and rhizosphere-competent Bacillus subtilis BSK17 on yield of Cicer arietinum. Arch Phytopathol Plant Protect, 2014; 47:2305–18; http:/doi.ord/10.1080/03235408.2013.876744 CrossRef
Dukare AS, Singh RK, Jangra RK, Bhushan B. Non-fungicides-based promising technologies for managing post-production penicillium induced spoilage in horticultural commodities, a comprehensive review. Food Rev Int 2020; 1–41; http:/doi.ord/101080/8755912920201727497 CrossRef
El-serwy WS, Mohamed NA, El-serwy WS, Kassem EMM, Abd El Aty AA. Synthesis of new benzofuran derivatives and evaluation of their antimicrobial activities. Res J Pharm Biol Chem Sci, 2015; 6:213–24. Available via https://www.rjpbcs.com/pdf/2015_6(3)/[30].pdf
Fernandez L, Agaras B, Zalba P, Wall LG, Valverde C. Pseudomonas Spp. isolates with high phosphate-mobilizing potential and root colonization properties from agricultural bulk soils under no-till management. Biol Fertility Soils, 2012; 48:763–73. Available via https//link.springer.com/article/10.1007/s00374-012-0665-6 CrossRef
Geetha K, Venkatesham E, Hindumathi A, Bhadraiah B. Isolation, screening and characterization of plant growth promoting bacteria and their effect on Vigna radita (L.) R. Wilczek. Int J Curr Microbiol App Sci, 2014; 3(6):799–809. Available via https://www.ijcmas.com/vol-3-6/K.Geetha,%20et%20al.pdf
Glick BR. The enhancement of plant growth by free-living bacteria. Can J Microbiol, 1995; 41:109–17. Available via https://www.nrcresearchpress.com/doi/abs/10.1139/m95-015 CrossRef
Glick BR, Patten CL, Holguin G, Penrose D. Biochemical and genetic mechanisms used by plant growth promoting bacteria. World Sci, 1999. Available via https://www.worldscientific.com/worldscibooks/10.1142/p130
Gordon SA, Weber RP. Colorimetric estimation of indole acetic acid. Plant Physiol 1951; 26:192–5. Available via https://www.ncbi.nlm.nih.gov/pmc/articles/PMC437633/
Gowtham HG, Murali M, Singh SB, Lakshmeesha TR, Murthy KN. Plant growth promoting rhizobacteria- Bacillus amyloliquefaciens improves plant growth and induces resistance in chilli against anthracnose disease. Biol Control, 2018; 126:209–17; http:/doi.ord/10.1016/j.biocontrol.2018.05.022 CrossRef
Grosch R, Junge H, Krebs B, Bochow H. Use of Bacillus subtilis as a biocontrol agent. III. Influence of Bacillus subtilis on fungal root diseases and on yield in soilless culture. J Plant Dis Prot, 1999; 106:568–80.
Guel A, Kidoglu F, Tuzel Y, Tuzel IH. Effects of nutrition and Bacillus amyloliquefaciens on tomato (Solariumlycopersicum L.) growing in perlite. Span Agric J Res, 2008; 6:422–9; http:/doi.ord/10.5424/sjar/2008063-335 CrossRef
Hao Z, Cai Y, Liao X, Zhang X, Fang Z, Zhang D. Optimization of nutrition factors on chitinase production from a newly isolated Chitiolytic bacter meiyuanensis SYBC-H1. Braz J Microbiol, 2012; 43(1):177–86. CrossRef
Hariprasad P, Divakara ST, Niranjana SR. Isolation and characterization of chitinolytic rhizobacteria for the management of Fusarium wilt in tomato. Crop Prot, 2011; 30(12):1606–12; http:/doi.ord/10.1016/j.cropro.2011.02.032 CrossRef
Harveson RM, Nielsen KA, Eskridge KM. Utilizing a preplant soil test for predicting and estimating root rot severity in sugar beet in the central high plains of the United States. Plant Dis, 2014; 98:1248–52; http:/doi.ord/10.1094/PDIS-11-13-1186-RE CrossRef
Hass D, Defago G. Biological control of soil-borne pathogens by fluorescent pseudmonads. Nat Rev Microbiol, 2005; 3:307–19. CrossRef
Hayat R, Ali S, Amara U, Khalid R, Ahmed I. Soil beneficial bacteria and their role in plant growth promotion, a review. Ann Microbiol, 2010; 60:579–98. Available via https://www.academia.edu/333405/Soil_Beneficial_Bacteria_and_Their_Role_In_Plant_Growth_Promotion_a_Review CrossRef
Hesran S, Le RE, Wajnberg E, Beukeboom LW. Next generation biological control—an introduction. Entomol Exp Appl, 2019:579–83; http:/doi.ord/10.1111/eea.12805 CrossRef
Husson E, Hadad C, Huet G, Laclef S, Lesur D, Lambertyn V, Jamali A, Gottis S, Sarazin C, Van Nhien AN. The effect of room temperature ionic liquids on the selective biocatalytic hydrolysis of chitin via sequential or simultaneous strategies. Green Chem, 2017; 19:4122–31; http:/doi.ord/10.1039/c7gc01471f CrossRef
Idris EE, Iglesias DJ, Talon M, Borriss R. Tryptophan-dependent production of indole-3-acetic acid (IAA) affects level of plant growth promotion by Bacillus amyloliquefaciens FZB42. Mol Plant Microbe Interact, 2007; 20(6):619–26. Available via https://pubmed.ncbi.nlm.nih.gov/17555270/ CrossRef
Jogaiah S, Shivanna RK, Gnanaprakash PH, Hunthrike SS. Evaluation of plant growth-promoting rhizobacteria for their efficiency to promote growth and induce systemic resistance in pearl millet against downy mildew disease. Arch Phytopathol Plant Prot, 2010; 43:368–78; http:/doi.ord/10.1080/03235400701806377 CrossRef
Khalid A, Arshad M, Zahir ZA. Screening plant growth-promoting rhizobacteria for improving growth and yield of wheat. J Appl Microbiol, 2004; 96(3):473–80. Available via https://pubmed.ncbi.nlm.nih.gov/14962127/
Kloepper JW, Ryu CM, Zhang S. Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology, 2004; 94:1259–66. Available via https://pubmed.ncbi.nlm.nih.gov/18944464/ CrossRef
Koumoutsi A, Chen XH, Henne A, Liesegang H, Hitzeroth G, Franke P. Structural and functional characterization of gene clusters directing on ribosomal synthesis of bioactive cyclic lipopeptid esin Bacillus amyloliquefaciens strainFZB42. J Bacteriol, 2004; 186:1084–96; http:/doi.ord/10.1128/JB.186.4.1084-1096.2004 CrossRef
Kröber M, Wibberg D, Grosch R, Eikmeyer F, Verwaaijen B, Chowdhury SP, Hartmann A, Pühler A, Schlüter A. Effect of the strain Bacillus amyloliquefaciens FZB42 on the microbial community in the rhizosphere of lettuce under field conditions analyzed by whole metagenome sequencing. Front Microbiol, 2014; http:/doi.ord/10.3389/fmicb.2014.00252 CrossRef
Kumar A, Prakash A, Johri BN. Bacillus as PGPR in crop ecosystem in Bacteria. In: Maheshwari D (ed.). Bacteria in Agrobiology: Crop Ecosystems, Springer, Berlin, Heidelberg. Available via https,//link.springer.com/chapter/10.1007/978-3-642-18357-7_2
Kumar A, Amit K, Shikha D, Sandip P, Chandani P, Sushila N. Isolation, screening and characterization of bacteria from Rhizospheric soils for different plant growth promotion (PGP) activities, an in vitro study. Recent Res Sci Technol, 2012a; 4(1):01–5. Available via https://updatepublishing.com/journal/index.php/rrst/article/view/851
Kumar P, Dubey RC, Maheshwari DK. Bacillus strains isolated from rhizosphere showed plant growth promoting and antagonistic activity against phytopathogens. Microbiol Res 2012b; 167:493–9; http:/doi.ord/10.1016/j.micres. 2012.05.002 CrossRef
Loper JE, Schroth MN. Influence of bacterial sources of indole- 2-acetic acid on root elongation of sugar beet. Phytopathology, 1986; 76:386–9. Available via https://www.apsnet.org/publications/phytopathology/backissues/Documents/1986Articles/Phyto76n04_386.PDF CrossRef
Madhurankhi G, Suresh D. Isolation of a novel rhizobacteria having multiple plant growth promoting traits and antifungal activity against certain phytopathogens. Microbiol Res, 2020; 240:126516.
Manjula K, Podile AR. Production of fungal cell wall degrading enzymes by a biocontrol strain of Bacillus subtilis AF1. Indian J Exp Biol, 2005; 43:892–6. Available via https://pubmed.ncbi.nlm.nih.gov/16235723/
Maurhofer M, Keel C, Haas D, Défago G. Pyoluteorin production by Pseudomonas fluorescens strain CHA0 is involved in the suppression of Pythium damping-off of cress but not of cucumber. Eur J Plant Pathol, 1994; 100:221–32. Available via https://link.springer.com/article/10.1007/BF01876237 CrossRef
Mendis HC, Thomas VP, Schwientek P, Salamzade R, Chien J, Waidyarathne P, Kloepper J, La LD. Strain-specific quantification of root colonization by plant growth promoting rhizobacteria Bacillus firmus I-1582 and Bacillus amyloliquefaciens QST713 in non- sterile soil and field conditions. PLoS One, 2018; 13(2):e0193119; http:/doi.ord/10.1371/journal.pone.0193119 CrossRef
Mohamed TA, Hegazy MF, Abd El Aty AA, Ghabbour HA, Alsaid MS, Shahat AA, Paré PW. Antimicrobial sesquiterpene lactones from Artemisia sieberi. J Asian Nat Prod Res, 2017; 19(11):1093–101; http:/doi.ord/10.1080/10286020.2017.1302939 CrossRef
Mohite B. Isolation and characterization of indole acetic acid (IAA) producing bacteria from rhizospheric soil and its effect on plant growth. J Soil Sci Plant Nutr, 2013; 13(3). Available via https://scielo.conicyt.cl/scielo.php?script=sci_arttext&pid=S0718-95162013000300011 CrossRef
Morales FJ. Hydroxymethylfurfural (HMF) and related compounds. In: Stadler RH, Lineback DR (eds.). Process-Induced Food Toxicants, John Wiley & Sons, Inc., Hoboken, NJ, pp 135–74, 2008; http:/doi.ord/10.1002/9780470430101.ch2e CrossRef
Mostafa FA, Abd El Aty AA. Enzyme activities of the marine-derived fungus Alternaria alternata cultivated on selected agricultural wastes. J Appl Biol Sci, 2013; 7(1):39–46. Available via http://www.jabsonline.org/index.php/jabs/article/view/313
Nadège AA, Pacôme AN, Farid BM, Hafiz AS, Haziz S, Alphonse S, Honoré B, Adolphe A, Lamine BM. Characterization of potential plant growth promoting rhizobacteria isolated from maize (Zea mays L.) in Central and Northern Benin (West Africa). Appl Environ Soil Sci, 2015:Article ID 901656. Available via https://www.hindawi.com/journals/aess/2015/901656/
Nandi M, Selin C, Brawerman G, Fernando WGD, de Kievit T. Hydrogen cyanide, which contributes to Pseudomonas chlororaphis strain PA23 biocontrol, is upregulated in the presence of glycine. Biol Control, 2017; 108:47–54; http:/doi.ord/10.1016/j.biocontrol.2017.02.008 CrossRef
Narasimhan A, Bist D, Suresh S, Shivakumar S. Optimization of mycolytic enzymes (chitinase, β-1,3-glucanase and cellulose) production by Bacillus subtilis, a potential biocontrol agent using one-factor approach. J Sci Ind Res, 2013; 72:172–8. Available via http://nopr.niscair.res.in/handle/123456789/16056
Naureen Z, Rehman NU, Hussain H, Hussain J, Gilani SA, Al Housni SK, Mabood F, Khan AL, Farooq S, Abbas G, Harrasi AA. Exploring the potentials of Lysinibacillus sphaericus ZA9 for plant growth promotion and biocontrol activities against phytopathogenic fungi. Front Microbiol, 2017; 8:1477. Available via https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5563071/
Oerke EC. Crop losses to pests. J. Agric Sci, 2006; 144:31–43; http:/doi.ord/10.1017/S0021859605005708 CrossRef
Olanrewaju OS, Glick BR, Babalola OO. Mechanisms of action of plant growth promoting bacteria. World J Microbiol Biotechnol 2017; 33:197. CrossRef
Ramamoorthy V, Raguchander T, Samiyappan R. Induction of defense-related proteins in tomato roots treated with Pseudomonas fluorescens Pf1 and Fusarium oxysporum F. sp. lycopersici. Plant Soil, 2002; 239:55–68. CrossRef
Rane Z, Anish-Kumar P, Bhaskar A. Phytochemical evaluation by GC-MS and in vitro antioxidant activity of Punica granatum fruit rind extract. J Chem Pharm Res, 2012; 4(6):2869–73. Available via https://www.jocpr.com/abstract/phytochemical-evaluation-by-gcms-and-in-vitro-antioxidant-activity-of-punica-granatum-fruit-rind-extract-1471.html
Riberio CM, Cardoso EJBN. Isolation and characterization of root- associated growth promoting bactertia in Brazil pine (Araucaria Angustifolia). Microbiol Res, 2012; 167:69–78. Available via https://pubmed.ncbi.nlm.nih.gov/21596540/
Richardson AE. Making microorganisms mobilize soil phosphorus. In: First virtual international meeting on microbial phosphate solubilization. 2003. Available via http://webcd.usal.es/web/psm/abstracts/ Richardson2. htm. pp 1–5
Rigal L, Gaset A. Direct preparation of 5-hydroxymethyl-2-furancarboxaldehyde from polyholosides, a chemical valorisation of the Jerusalem artichoke (Helianthus tuberosus L.). Biomass, 1983; 3151–63. Available via https://agris.fao.org/agris-search/search.do?recordID=US201302611561 CrossRef
Schwartz AR, Ortiz I, Maymon M, Herbold CW, Fujishige NA, Vijanderan JA, Villella W, Hanamoto K, Diener A, Sanders ER, DeMason DA. Bacillus simplex—a little known PGPB with anti-fungal activity—alters pea legume root architecture and nodule morphology when coinoculated with Rhizobium leguminosarum bv. viciae. Agronomy, 2013; 3(4):595–620. Available via https://www.mdpi.com/2073-4395/3/4/595
Shao J, Zhihui X, Nan Z, Qirong S, Ruifu Z. Contribution of indole-3-acetic acid in the plant growth promotion by the rhizospheric strain Bacillus amyloliquefaciens SQR9. Biol Fertility Soils, 2015; 51:321–30. Available via https://link.springer.com/article/10.1007/s00374-014-0978-8?shared-article-renderer CrossRef
Shehata AN, Abd El Aty AA, Darwish DA, Abdel Wahab WA, Mostafa FA. Purification, physicochemical and thermodynamic studies of antifungal chitinase with production of bioactive chitosan-oligosaccharide from newly isolated Aspergillus griseoaurantiacus KX010988. Int J Biol Macromol, 2018; 107:990–9. CrossRef
Shen X, Hu H, Peng H, Wang W, Zhang X. Comparative genomic analysis of four representative plant growth-promoting rhizobacteria in Pseudomonas. BMC Genomics, 2013; 14:271; http:/doi.ord/10.1186/1471-2164-14-271 CrossRef
Srivastava S, Bist V, Srivastava S, Singh PC, Trivedi PK, Asif MH, Chauhan PS, Nautiyal CS. Unraveling aspects of Bacillus amyloliquefaciens mediated enhanced production of rice under biotic stress of Rhizoctonia solani. Front Plant Sci 2016; 7:587. Available via https://pubmed.ncbi.nlm.nih.gov/27200058/ CrossRef
Sujatha N, Ammani K. Siderphore production by the isolates of fluorescent pseudomonads. IJGRR, 2013; 5(20):1–7. Available via https://www.ijcrr.com/uploads/1052_pdf.pdf
Sushil KS, Aketi R, Bhavdish NJ. Isolation and characterization of plant growth promoting Bacillus amyloliquefaciens strain sks_bnj_1 and its influence on rhizosphere soil properties and nutrition of soybean (Glycine max L. Merrill). J Virol Microbiol, 2013:Article ID 446006; http:/doi.ord/10.5171/2013.446006
Tekalign K, Vasudev RT, Parth T. Bacillus species (BT42) isolated from Coffea Arabica L. rhizosphere antagonizes Colletotrichum gloeosporioides and Fusarium oxysporum and also exhibits multiple plant growth promoting activity. BMC Microbiol, 2016; 16:277. Available via https://pubmed.ncbi.nlm.nih.gov/27863465/ CrossRef
Tiago I, Teixeira I, Silva S, Chung P, Verissimo A, Manaia CM. Metabolic and genetic diversity of mesophilic and thermophilic bacteria isolated from composted municipal sludge on poly- epsilon- caprolactones. Curr Microbiol, 2004; 49:407–14; http:/doi.ord/10.1007/s00284-004-4353-0. CrossRef
Wang SL, Lin TY, Yen YH, Liao HF, Chen YJ. Bioconversion of shellfish chitin wastes for the production of Bacillus subtilis W-118 chitinase. Carbohydr Res, 2006; 341:2507–15. Available via https://pubmed.ncbi.nlm.nih.gov/16920090/ CrossRef
Wang S, Wu H, Qiao J, Ma L, Liu J, Xia Y. Molecular mechanism of plant growth promotion and induced systemic resistance to tobacco mosaic virus by Bacillus spp. J Microbiol Biotechnol, 2009; 19:1250–8; http:/doi.ord/10.4014/jmb.0901.008 CrossRef
Wu L, Hui-Jun W, Junqing Q, Xuewen G, Rainer B. Novel routes for improving biocontrol activity of Bacillus based bioinoculants. Front Microbiol, 2015; 6:1395; http:/doi.ord/10.2289/fmicb.2015.01395 CrossRef
Yao AV, Bochow H, Karimov S, Boturov U, Sanginboy S, Sharipov K. Effect of FZB24 Bacillus subtilis as abiofertilizer on cotton yields in field tests. Arch Phytopathol Plant Prot, 2006; http:/doi.ord/10.1080/03235400600655347 CrossRef
Yuan J, Ruan Y, Wang B, Zhang J, Waseem R, Huang Q, Shen Q. Plant growth-promoting rhizobacteria strain Bacillus amyloliquefaciens NJN-6-enriched bio-organic fertilizer suppressed Fusarium wilt and promoted the growth of banana plants. J Agric Food Chem, 2013; 61(16):3774–80. Available via https://pubmed.ncbi.nlm.nih.gov/23541032/
Zohair MM, El-Beih AA, Sadik MW, Hamed ER, Sedik MZ. Promising biocontrol agents isolated from medicinal plants rhizosphere against root-rot fungi. Biocatal Agric Biotechnol, 2018; 15:11–18. Available via https://www.sciencedirect.com/science/article/pii/S1878818118301889 CrossRef