INTRODUCTION
Humans have been conscious of the therapeutic potential of plants since ancient times. Almost every indigenous culture of humankind makes use of various medicinal plants for curing ailments. Ethnopharmacological knowledge is practiced amid the tribal population, but a great deal of this knowledge is pragmatic and lacks scientific validation. Since the advent of modern science, traditional medicine has made a significant come back. It has recognized that many important pharmaceuticals have been discovered from plants used by indigenous people.
Spathodea campanulata P. Beauv. is normally recognized as the African tulip tree. It has been planted for ecological, timber, firewood, fodder, life fence, and ornamental purposes (Fongod et al., 2014; Villanueva-Partida et al., 2019). The seeds are used as food and a poison is derived from the tough central part of the fruit to kill animals (CABI, 2021). The wood is used for firewood and for making drums (Simbo, 2010). Through a literature survey, it has been discovered that the ethnic community, dwelling at the foothills and forests of the Africa prescribed this plant for the treatment of malaria (Adebayo and Krettli, 2011; Komlaga et al., 2015; Osei-Djarbeng et al., 2015), diabetes (Tsabang et al., 2017), dysentery, asthma, stomach ache (Tugume and Nyakoojo, 2019a), fever (Emmanuel and Didier, 2012), and fungal and bacterial infections (Kamatenesi-Mugisha et al., 2008). There has been great interest in S. campanulata, as evidenced by the many works carried out in recent years (references therein). Hence, it is worth updating the association between the traditional uses and the current pharmacology of S. campanulata, as has been carried out for numerous plants. This review is intended to provide up-to-date information about the ethnopharmacological uses, photochemistry, pharmacological potential, and toxicity profile of this useful plant. It will provide dimensions to researchers who are willing to explore the plant further. It will enable traditional practitioners to promote the use a holistic approach of considering traditional and current medicine.
REVIEW METHODOLOGY
The literatures reviewed were collected using a systematic and comprehensive literature search on the medicinal plant S. campanulata. The articles were collected from various online search engines, viz. Google Scholar, PubMed, Science Direct, Core, and Semantic Scholar. Keyword combinations undertaken for the searches were Spathodea campanulata along with ethnomedicinal use or chemical constituent or pharmacology or bioactive compounds. Two chief criteria were employed for the collection of appropriate references for this review. For ethnomedicinal uses, all articles that were accessible with any relevant information on the ethnomedicinal and ethnoveterinary uses of S. campanulata were considered. Limitations to the journal of standard were not considered because the bulk of the publications are generally centered on the records of oral traditions. In observation to the claimed ethnomedicinal uses of S. campanulata, the papers that correlated with the disease description and validated by various biological studies were selected. Google Scholar provided about 6,470 search results for keyword S. campanulata, but around 80%–90% of the articles suggested were out of theme. Only about 10%–20% of the articles recommended were practical, out of which 182 references were used for this review. During the search, two compositions published earlier came to limelight (Boniface, 2017; Wagh and Butle, 2018). However, none of the reviews were comprehensive in nature. This review is proposed to make available the recent information on customary and local knowledge, classification of biologically important molecules, and pharmacological studies accomplished for validation of traditional use.
BOTANICAL DESCRIPTION AND VERNACULAR NAMES
The African tulip tree is a big erect tree with lustrous greenish pinnate leaves and wonderful reddish orange flowers. It can grow to up to 80 feet in suitable conditions. It has a solid trunk enclosed with a light gray bark. The leaves are usually opposite up to 10–15 cm long, elliptic to oblong, and are assembled at the branch’s tips. The leaves are compound, imparipinnate with seven to eight pairs of leaflets, and a 5–6 cm long petiole. Oval leaflets contain seven to eight major deep veins on each side. Horn-shaped silky buds emerge upside down at the end of the branch. The buds are filled with water and are frequently used by small children as “mini squirt-guns.” The blooms are big and cup-like with a brilliant reddish orange color. The fruits are 5–10 inches long, finger-like, 16 cm long, and points upward. Each fruit contains about 500 paper-like seeds. The tree blooms usually in the spring (CABI, 2021; Flowers of India, 2021; Sonibare and Osiyemi, 2012). The vernacular names of S. campanulata are mentioned in Table 1.
DISTRIBUTION
It is distributed worldwide, but most of them occur in the tropical and sub-tropical countries. It is native of Africa (i.e., in Angola, Burundi, Benin, Cameroon, Equatorial Guinea, Ghana, Gabon, Guinea, Nigeria, Liberia, Sierra Leone, Rwanda, Togo, and Zambia). It was introduced in Australia, Brazil, China, Costa Rica, Cuba, Egypt, French Guiana, French Polynesia, India, Indonesia, Kenya, Jamaica, Malaysia, Madagascar, Mexico, Papua New Guinea, Peru, Puerto Rico, Singapore, Saint Lucia, Sri Lanka, Spain, Thailand, United States (Hawaiian Is., Florida), Venezuela, and British Virgin Islands (IUCN, 2020). Spathodea campanulata has become an invasive species and has a threatened biodiversity in the Pacific islands. It has impacted the economy, cultural, and social welfare of pacific peoples (Brown and Daigneault, 2014; Labrada and Medina, 2009). It has invaded the discarded agricultural lands and jungle, and has become a dominant weed in Puerto Rico (Rivera and Aide, 1998). It is considered as a weed in the coffee orchards of Cuba (Herrera et al., 2002).
ETHNOMEDICINAL USE
A wide range of traditional uses are cited in the literature for S. campanulata. The stem bark of S. campanulata is mainly used in Africa to treat malaria (Iyamah and Idu, 2015). The leaves are used in India and Africa to treat skin disorders (Kumar and Dash, 2012; Shehu et al., 2018). The leaf is also used to treat epilepsy (Noumi and Fozi, 2003), liver disorder (Shiracko et al., 2016), asthma (Nwauzoma and Dappa, 2013), measles (Oladunmoye et al., 2011), and sore throat (Shiracko et al., 2016). The root is used for worm infections (Musinguzi et al., 2017), stomach ache (Musinguzi et al., 2017), dysentery (Tabuti et al., 2003), and hallucination (Ior et al., 2017). The flower is used as an antidote against animal poison (Santosh et al., 2019) and cataract (Tewari et al., 2019). Of the traditional uses cited, the most common conditions treated is malaria (11 articles), gastrointestinal tract (GIT) problem (9 articles), skin infections (7 articles), wound healing (6 articles), and kidney problem (4 articles). Traditional uses mostly employed the bark of the plant. The leaf and whole plant are also cited, but less frequently. The plant is used alone or in combination with other medicinal plants. Table 2 summarizes the various traditional uses of S. campanulata.
PHYTOCHEMICAL STUDIES
Exhaustive phytochemical investigations of various parts of S. campanulata lead to the isolation of many secondary metabolites such as iridoids (Fig. 1), triterpenoids (Fig. 2), sterol and cerebrosides (Fig. 3), and flavonoids (Figs. 4 and 5). Iridoids are the most important phytochemicals found in S. campanulata. Iridoids are a kind of monoterpenoids containing cyclopenta[c]pyranoid ring system. They are typically found as glycosides, which are associated with glucose molecules. In 1991, the first iridoid glycoside isolated from stem bark was verminoside (7) (Niyonzima et al., 1991). Later, five more iridoid glycosides (1–6) were isolated from the leaves (Gouda, 2009a). Specioside (8) has been detected in the flower of the plant (Elusiyan et al., 2011). Phytochemical analysis of leaves and stem bark (Ngouela et al., 1988, 1990, 1991) revealed the presence of different triterpenoids, viz. spathodic acid (9), 3β-acetoxyoleanolic acid (10), siaresinolic acid (11), 3β-acetoxy-12-hydroxyoleanan-28,13-olide, and oleanolic acid (16). Various other triterpenoids, such as ursolic acid (12), tomentosolic acid (13), 3β,20β-Dihydroxyurs-12-en-28-oic acid (15) (Amusan et al., 1996), α-amyrin (18) (Nazif, 2007), pomolic acid (19) (Mbosso et al., 2008), urs-12-en-27α, and 30 dioic acid 3-O-α-L-rhamnopyranosyl (1→2) α-L-arabinopyranoside (20) (Ilodigwe et al., 2010b), were identified from the bark and aerial parts. Several sterols, such as spathodol (21), β-sitosterol-3-O-β-D-glucopyranoside (22) (Ngouela et al., 1991), β-sitosterol-3-acetate (23) (Shehab et al., 2014), stigmasterol (24), cholesterol (25), and campesterol (26) (Nazif, 2007), have been detected from various parts of the plant. Regarding phenolic compounds, several researchers reported the presence of a range of flavonoids or anthocyanins (36–60) (Table 3) in the flower, stem bark, leaf, and aerial parts of the plant. Other groups of compounds found in S. campanulata include cinnamic acid derivatives (27–33), cerebrosides (34,35), carotenoids (61–71), monoterpenoids (68–74) and sesquiterpenoids (75–84), diterpenoids (abietatriene) (85), coumarins (86,87), aromatic acid, and their esters (88–95). Recently, a new cerebroside campanulatoside was isolated from the stem bark of S. campanulate (Magnibou et al., 2021). The phytochemicals possessing biological activities are listed in Table 4.
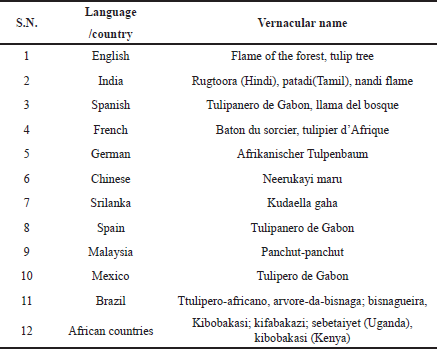 | Table 1. Vernacular names of S. campanulate. [Click here to view] |
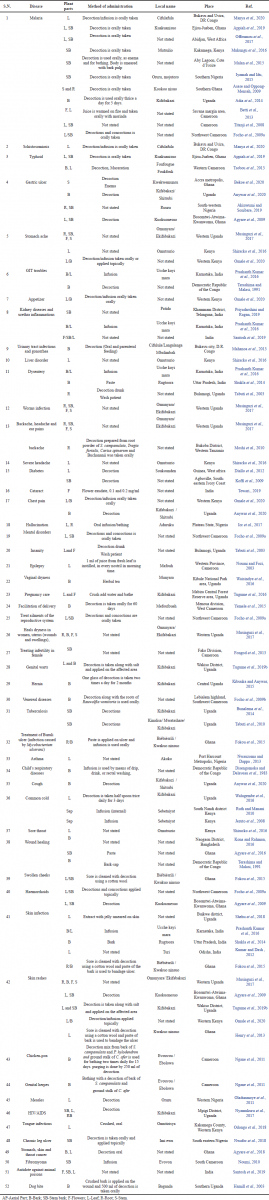 | Table 2. Ethnomedicinal uses of S. campanulate. [Click here to view] |
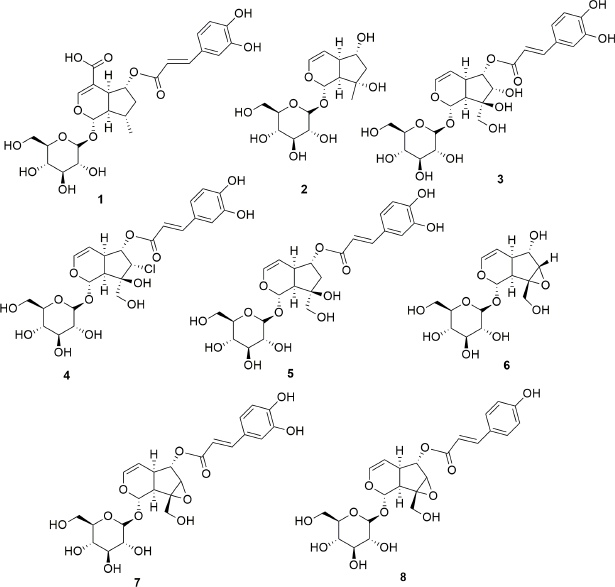 | Figure 1. Iridoids isolated from Spathodea campanulata. [Click here to view] |
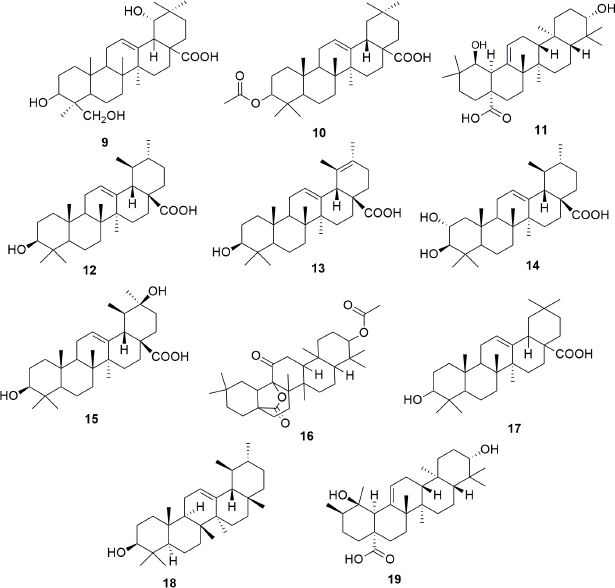 | Figure 2. Triterpenoids isolated from Spathodea campanulata. [Click here to view] |
PHARMACOLOGICAL ACTIVITY
Antimalarial activity
In observation of the significance of this plant in treating malaria by the traditional healer, systematic examinations have been carried for antimalarial action by Makinde and group. The schizontocidal activity of leaf extracts was evaluated against Plasmodium berghei in mice. The Extracts were tested in both early and established infections and proved to be more efficient in curing early infection. The aqueous leaf extract (ALE) exhibited highest antiplasmodial action at a concentration of 400 mg/kg/day and percentage of chemosuppression was 73.8%. The effective dose for 50% of the population was found to be around 100–400 mg/kg/day. Furthermore, the chromatographic fractions of the chloroform leaf extract (CLE) produced 61.0% chemosuppression at 40 mg/kg/day. The extracts were found to be less effective than chloroquine, which produced 99.3% suppression at the 20 mg/kg/day dose (9) (Makinde et al., 1987). The chloroform stem bark extract (CBE) and hexane stem bark extract (HBE) of S. campanulata were evaluated for blood schizontocidal activity by Rane and 4-day tests. Both extracts showed significant activities. The HBE suppressed 22%–80% of infections at the 50–400 mg/kg/day dose in the 4-day test with a mean survival time period of 18.0 and 13.0 days. The CBE also demonstrated significant activity at 100–400 mg/kg/day. Percentage suppression was in between 52% and 74%. The schizontocidal action of CBE was confirmed by measuring the mean survival time in mice, which was found to be 15.2 and 19.2 days. The CBE and HBE clearly demonstrated antimalarial action not only by suppressing parasitaemia but also by prolonging the lifetime of the mice (Makinde et al., 1988). The activity of the different fractions of CBE was evaluated by Fink and Kretschmar’s and Rane’s tests. Few of the chromatographic fractions exerted significant antimalarial action than the crude extracts. One of the fractions was mainly active at 40 mg/kg/day. 82% of the infection was suppressed with a mean survival time of 19.2 days (Makinde et al., 1990). The chemical entities revealed to be responsible for antimalarial activities were ursolic acid (6), tomentosolic acid (7), and 3β,20β-dihydroxyurs-12-en-28 oic acid (9). The isolated compounds suppressed parasitaemia in a dose-dependent manner and exhibited high mean survival times. Among the isolated compounds, ursolic acid (6) was highly active. At a dose of 60 mg/kg/day, it suppressed 97% of parasitaemia with a mean survival period of 25 days. The action was comparable to the chloroquine at a dose of 10 mg/kg/day. The triterpenoid ursolic acid was found to be non-toxic when fed to guinea pigs, rats, rabbits, and chickens at dose levels of 1,000–5,000 mg/kg/day body weight. It was also not toxic to humans at a dose of 20 mg/kg/day (Amusan et al., 1996). The in vitro antimalarial activity of ethanolic bark extract (EBE) and fractions was carried out by employing chloroquine-sensitive and resistant Plasmodium falciparum by schizont maturation inhibition assay. EBE (both 50% and 80%) did not show significant activity. Half-maximal inhibitory concentration value of 50% EBE was observed at 88.3 (chloroquine sensitive) and 108.2 μg/ml (chloroquine resistant) against P. falciparum strain. Accordingly, EBE (80%) exhibited IC50 values of 68.5 and 100 μg/ml (Dhanabalan et al., 2008). The ethyl acetate bark extract of S. campanulata displayed 28.9% inhibition of P. falciparum FcB1 at 10 μg/ml (Lacroix et al., 2011).
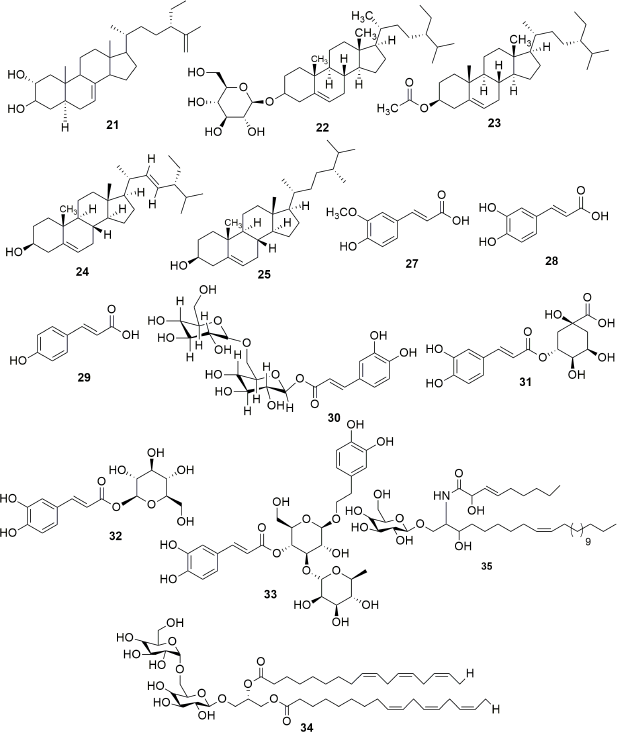 | Figure 3. Sterols, cinnamic acid derivatives and Cerebrosides isolated from Spathodea campanulata. [Click here to view] |
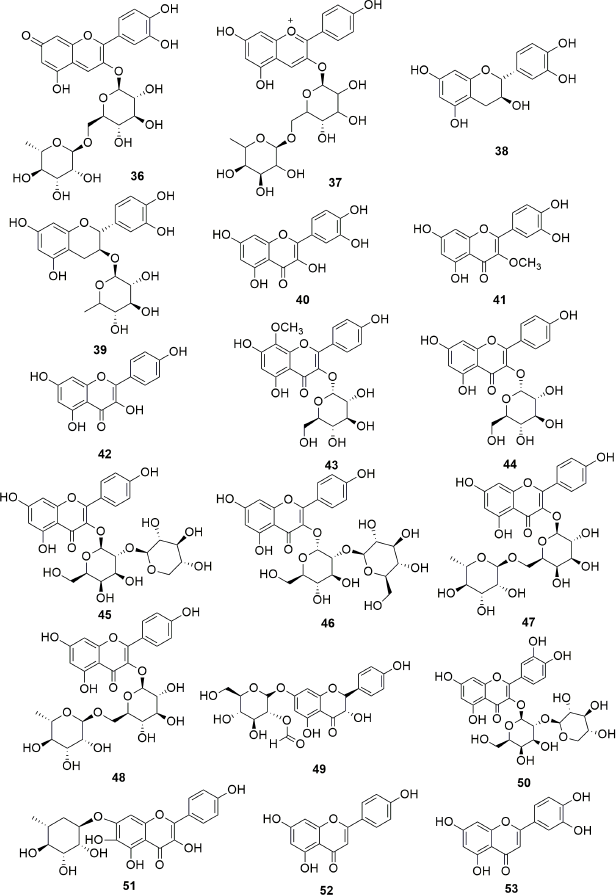 | Figure 4. Flavonoides isolated from Spathodea campanulata. [Click here to view] |
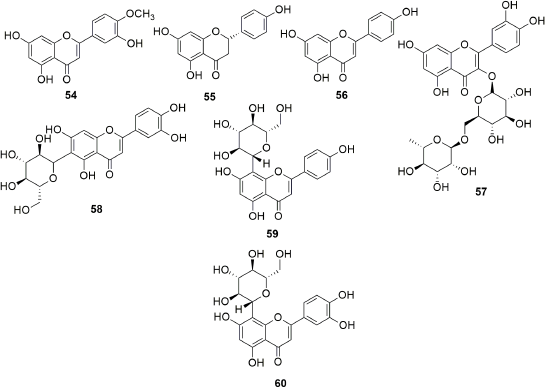 | Figure 5. Flavonoides isolated from Spathodea campanulata. [Click here to view] |
From the above-mentioned studies it may be concluded that most of the experiments were conducted by employing stem bark extracts. The chloroform bark extract showed better activity when compared to others. The toxicity reports indicated safety of the isolated ursolic acid. However, the high dose requirement of 60 mg/kg/day for pronounced activity compared to chloroquine (10 mg/kg/day) in the studies is a matter of concern. Furthermore, there is a scope to study the roots and seeds for analogous activities and isolation of compounds with convincingly superior biological activity.
Antidiabetic activity
The stem bark decoction (SBD) of S. campanulata (8 g bark powder/kg bw) was tested for antidiabetic effect in the streptozocin (STZ)-induced diabetic mice model. It showed a hypoglycemic effect but did not influence insulin levels (Niyonzima et al., 1990). SBD also decreased blood glucose amount during the oral glucose tolerance test (OGTT) in normal mice. The water and butanol fractions obtained from bark decoction also showed hypoglycemic activity but did not influence insulin levels in STZ diabetic mice (Niyonzima et al., 1993). Different fractions of the SBD separated by column chromatography were evaluated for their hypoglycemic effect. The most polar fraction composed mainly of polysaccharides, which considerably decreased the blood glucose levels after 30, 60, and 90 minutes of glucose load. However, few of the fractions showed a significant hyperglycemic effect toward the end of the experiment (Niyonzima et al., 1999). Aqueous methanolic stem bark extract significantly decreased blood glucose levels after 2 hour at a dose of 800 mg/kg in normoglycemic rats. In OGTT, the extract reduced glycemia (63%) at the 400 mg/kg dose. It also reduced glycemia (29%) in alloxan-induced diabetes mellitus rats with the 400 mg/kg dose in the acute phase. The multiple dose treatment with the extract led to the decrease in alloxan-induced hyperglycemia at the end of the 18th day (Tanayen et al., 2014). The methanolic extracts of the flowering branch and bark were screened for their antidiabetic activity in mice at a dose of 500 mg/kg bw. Blood insulin, glucose, cholesterol, and triacylglycerol levels and the concentration of insulin receptors in muscle tissue were estimated at the end of the treatment. The bark extract resulted in the significant reduction in blood glucose (44.5% decline) after 1 hour of treatment. A significant rise in insulin receptors level (28% increment) was also observed for bark extract. The study concluded that the bark extract modified the tissue expression of insulin receptors (Abdraboh and Ahmed, 2015). The solvent fractions of methanolic bark extract (MBE) were separately tested for antidiabetic activity using OGTT. All fractions demonstrated reductions in hyperglycemia. The residual aqueous fraction reduced hyperglycemia up to 74.7% at the 200 mg/kg dose (Kihdze et al., 2016). The ethanolic flower extract (EFE) of S. campanulata also exerted significant anti-hyperglycemic activity in alloxan-induced diabetic mice at a dose of 500 mg/kg. The activity was correlated to its phenolic components (Shehab et al., 2014).
From the above discussion, it can be concluded that aqueous and alcoholic extracts obtained from bark and flower of S. campanulata at high doses (400–800 mg/kg) showed significant anti-hyperglycemic activity. The antidiabetic activity was mainly attributed to the most polar fraction composed mainly of polysaccharides. The underlined mechanism of action for antidiabetic activity is due to expression of insulin receptor. With this information on the hypoglycemic prospective, there is scope by thoroughly studying and using different parts of the plant against a variety of models available for diabetes.
Wound healing activity
Traditionally, the stem bark of S. campanulata is considered as an effective remedy to heal wound. The researcher determined the wound healing activity of the MBE of S. campanulata in the experimental burn model in rats. The extract in ointment form (2%, 10%, and 49%) reduced the score damage at the burn site. The application of 49% extract changed the score damage from 5 to 0.2 after 15 days of experimental burn. It completely healed the burn on the 19th or 20th day (Sy et al., 2005). The activity was further validated in terms of its antimicrobial, antioxidant, and inhibition of nuclear factor kappa light chain enhancer of activated B cells (NF-KB) activity. It indicated antimicrobial activity against Trichophyton species. It diminished the peroxidation of bovine brain extract and showed the IC50 value of 0.24 μg/ml. It also manifested a notable antioxidant effect by protecting MRC-5 cells from H2O2-induced oxidation damage at 1–10 μg /ml. During antioxidant activity using liposomes, it exhibited an IC50 of 0.24 mg/ml. The lowest concentration of 1 μg/ml demonstrated better protection against oxidative damage. Nevertheless, when cells were treated with high concentrations of the extract (5 and 10 μg/ml), they indicated characteristic signs of cell damage probably due to cytotoxicity. On the other hand, the extracts did not show inhibition of NF-KB at 100 μg /ml (Mensah et al., 2006). The wound healing activity of MBE of S. campanulata was evaluated in excision wound model in Sprague Dawley rats. The extract was included into a cream (10% and 20% w/w) and put on the excision wounds. The wounds were further infected with Staphylococcus aureus. Treatment with the 20% w/w cream led to a rapid decrease in wound size. 95% of the uninfected wound was healed on the 20th day and complete healing was observed on the 24th day. In infected wounds, application of the 20% w/w cream led to 91% wound healing on the 24th day and complete wound closure on the 28th day (Ofori-Kwakye et al., 2011).
 | Table 3. Phytochemicals isolated from S. campanulate. [Click here to view] |
 | Table 4. Biological activity of phytochemicals isolated from S. campanulate. [Click here to view] |
The experimentations related to wound healing nature of the plant have been supportive in the conventional use of the plant as a wound healer. However, the plant extract was proved to be most effective at lower dose because higher dose leads to cell damage probably due to cytotoxicity. More detailed safety data pertaining to dose of crude extracts need to be generated.
Antibacterial and antifungal activity
The antibacterial activity of methanolic leaf extract (MLE) of S. campanulata was determined utilizing the disk diffusion method, against S. aureus and Escherichia coli. The extract does not show significant activity (Melendez and Capriles, 2006). Various concentrations (2.5–10 mg/ml) of the organic leaf extracts were evaluated for antibacterial activity against eight bacteria employing agar disk diffusion assay method. The extracts displayed significant activity on the tested bacterial strains in a dose-dependent manner. The Gram-negative bacterium, Klebsiella pneumoniae was discovered to be more vulnerable to petroleum ether leaf extract with an inhibition zone of 11 mm. However, the Gram-positive strains were found be least active (Dhanabalan et al., 2008). The antibacterial activity of seven isolated compounds from stem bark were assessed against Gram +ve and −ve bacteria using micro-broth dilution method. The tested compounds exerted significant antibacterial properties. Spathoside (35) hampered the growth of K. pneumoniae [minimum inhibitory concentration (MIC) = 6.25 mg/ml], Shigella flexneri (MIC = 12.5 mg/ml), and Bacillus subtilis (MIC = 25 mg/ml). However, it did not inhibit the growth of Shigella dysenteriae and E. coli (Mbosso et al., 2008). ALE was evaluated for in vitro antibacterial activity against 14 pathogenic bacteria by cup plate method and it was found to be inactive (Satish et al., 2008). Aqueous and alcoholic extracts of the aerial parts were subjected to screening for antibacterial activity against three Staphylococcus species: Staphylococcus epidermidis, S. aureus, and Neisseria subflava using disk and well diffusion methods. The alcoholic extract was found to be more effective than aqueous extracts (Parekh and Chanda, 2008). The ALE and MLE of S. campanulata were investigated by disk and well diffusion methods against bovine udder isolated bacteria. MLE showed 8.0 and 7.6 mm inhibition zones against S. aureus and Streptococcus agalactiae, respectively (Das et al., 2009). The antibacterial activity of petroleum ether, ethanol, methanol, and aqueous stem bark extracts (ABE) of S. campanulata were investigated in case of S. aureus, B. subtilis, Pseudomonas aeruginosa, and E. coli. The MBE showed very good antibacterial activity. The MIC of MBE was found to be B. subtilis (50–55 mg/ml), S. aureus (145–150 mg/ml), P. aeruginosa (60–65 mg/ml), E. coli (50–55 mg/ml), and Candida albicans (45–50 mg/ml) (Ofori-Kwakye et al., 2009). EFE and ethanolic leaf extract (ELE) were evaluated for antibacterial activity at 10 mg/ml by using disk diffusion method against E. coli, S. aureus, B. subtilis, K. pneumonia, Proteus vulgaris, Salmonella typhimurium, and Vibrio cholera. The EFE was found to be more active than ELE (Kowti et al., 2010). The antibacterial assay of aqueous and ethanol extracts of roots, leaves, stem, and flowers of S. campanulata were evaluated by agar disk and well diffusion methods. EFE of S. campanulata exerted significant zone of inhibition against E. coli (7.5 mm), S. aureus (7 mm) (Kumar, 2012). MLE and ALE of S. campanulata were evaluated against nine bacterial species and two fungal species. MLE at a 200 μg/ml concentration showed inhibition zone of 18 mm against S. pneumonia and 14 mm for S. aureus, respectively. The ALE demonstrated inhibition zone of 9 mm for S. aureus and 8 mm for E. coli (Vijayasanthi and Kannan, 2014). ELE of the plant showed activity against S. typhi (MIC = 1,024 μg/ml) in microdilution assay method (Roger et al., 2015). The methanolic flower extract (MFE) of S. campanulata showed strong activity against Enterococcus faecalis (MIC = 39.1) and K. pneumonia (MIC = 156.2 mg/ml) (Mbosso et al., 2016). 70% of the hydroethanolic extracts of S. campanulata root were screened against Mycobacterium ulcerans and Mycobacterium smegmatis using the Resazurin Microtiter Assay (REMA). The MIC were observed at 250 μg/ml and >250 μg/ml, respectively (Fokou et al., 2016). The antibacterial activity of petroleum ether, chloroform, ALE, and nanoparticle obtained were determined against Bacillus cereus and Actinomyces pseudofradea using well diffusion method. Both the silver nanoparticles and chloroform extract were very effective against A. pseudofradea showing an inhibition zone of 21.50 mm at a concentration of 100 mg/ml (Rai et al., 2017). MIC of MLE and methanol root extract (MRE) of S. campanulata was obtained for B. cereus, B. subtilis, Proteus mirabilis, S. typhi, and C. albicans. Good activity was observed against S. typhi at MIC value of 400 μg/ml. It also inhibited the growth of C. albicans at MIC of 400 μg/ml. Antibacterial activities were more pronounced than antifungal potentials (Moronkola et al., 2018). ALE of S. campanulata was evaluated for their possible antifungal activity against eight species of Aspergillus such as Aspergillus niger, Aspergillus ochraceus, Aspergillus flavipes, Aspergillus candidus, Aspergillus columnaris, Aspergillus flavus, Aspergillus fumigatus, and Aspergillus tamari isolated from paddy, sorghum, and maize seeds. The aqueous extract was found to be inactive against the tested strains (Satish et al., 2007). Dried residue obtained from watery fluid at floral base of S. campanulata exhibited antimicrobial potency at a concentration of 500 μg against different microorganisms (Killedar et al., 2011). EFE of S. campanulata displayed variable antibacterial activities with inhibition zones ranging from 16 to 25 mm in diameter against K. pneumonia, Streptococcus pyogenes, E. coli, and S. aureus at a concentration level of 100 μg/ml (Shehab et al., 2014). The in vitro antibacterial and antifungal activity of the fresh leaves extracts from S. campanulata was determined against bacterial species of Micrococcus luteus and Proteus vulagris and fungal strain of A. niger and C. albicans. The ALE exerted maximum inhibition with 17 mm against M. luteus, while the CLE showed an inhibition zone of 16 mm against C. albicans (Thampi and Kumar, 2015). 60% aqueous MBE of Saccharomyces campanulata was observed to be inactive against E. coli, S. cerevisiae, and Penicillium crustosum (Taniguchi et al., 1978). The methanolic extract from aerial parts were screened for possible antibacterial activity against six Enterobacteriaceae strains. It was found to be most active against Enterobacter aerogenes and K. pneumonia (Parekh and Chanda, 2007). ALE and ABE from 0.5 to 2.5 mg/ml were investigated in an agar diffusion test against Helicobacter pylori, using amoxicillin as positive control. The extracts did not affect the bacterial growth in any of the test concentrations (Agyare et al., 2009). The dichloromethane/methanol (1:1, v/v) crude bark extract and the fractions of were tested on H. pylori. The extract inhibited the growth of H. pylori by modulation of virulence factors and urease inhibition. One of the sub-fractions inhibited H. pylori urease in a heterologous bacterial model. Another sub-fraction had modulated the expression of one cytotoxin (CagA) and two adhesions (HopZ and BabA). Kaempferol (42) was identified as an active compound from the sub-fraction (Ngnameko et al., 2020).
The stem bark extract was found to be the most promising compared to other plant parts. Furthermore, the isolated compound, spathoside (35), was found to possess potent antibacterial activity in comparison to other isolated compounds. However, it was found to be less potent than the positive control ampicillin, which inhibited the growth of B. cereus and S. dysenteriae with MIC value of 0.4 mg/ml. Although activities have been reported for leaves and flowers against wide ranges of bacteria and fungi, the study results are not encouraging in terms of the concentration investigated. Bearing in mind the results of antimicrobial activities, supplementary studies are required in connection with the widespread use in treatment of the skin infections, wound, including even chronic leg ulcer.
Anthelmintic activity
The MLE of S. campanulata was evaluated for anthelmintic efficacy against earthworms Pheretima posthuma at 5, 15, and 20 mg/ml concentrations. The paralysis time and death time were considered to know anthelmintic potency. Significant activity was observed at a concentration of 20 mg/ml. The paralysis time and death time were observed at 4.23 and 10.32 minutes, respectively (Wagh et al., 2019). The EFE showed significant anthelmintic effect at 100 mg/ml concentration against non-parasitic earthworms, Allolobophora caliginosa (Shehab et al., 2014).
The investigation upon anthelminthic study is preliminary as the parasite examined in the studies was only earthworm. Experiments on other helminths of human significance should be considered like tapeworm, hookworms, pinworms, and flukes. It may be summarized that there is a plenty of scope in assessing the plant for anti-helminthic activity.
Anticancer activity
MFE of S. campanulata exhibited weak antiproliferative effect against cancer cell lines of lung, glioma, and melanoma with a mean IC50 value above 92 and 78 mg/ml, respectively (Mbosso et al., 2016). The antitumor activity of EFE of S. campanulata and its fractions was assessed in vitro against the two human cell lines MCF7 and hematocrit (HCT) 116 by the sulforhodamine B assay. EFE and its chloroform fraction demonstrated the IC50 values of 17.6 and 17.8 μg/ml against MCF7 cell line. Meanwhile, both hexane and n-butanol fractions showed identical IC50 (21.0 μg/ml) against the cell line (Shehab et al., 2014). Aqueous, methanol, ethanol, and CLE of S. campanulata were screened for their anticancer activity in MCF-7 cells. The CLE inhibited 67.98% proliferation on MCF-7 cells at 300 μg/ml (Dhanabalan et al., 2014). The cytotoxicity of the MRE was determined at different concentrations against drug-sensitive morphological variations in human leukemic lymphoblasts leukemia cells. IC50 was found to be more than 80 μg/ml (Kuete et al., 2016). The ELE showed very significant anticancer activity against Ehrlich-Lettre ascites carcinoma cell line (85%) in comparison to ethyl acetate and chloroform extracts (Sangeetha et al., 2016). The anticancer activity of MBE was examined against three human leukemic cell lines K562, U937, and HL-60. The extract exhibited IC50 values of 19.45, 20.5, and 20.14 μg/ml against the respective cell lines. Characteristic features of apoptosis such as chromatin condensation, cell shrinkage, and membrane blebbing were observed on the treated cells. The extract lead to the arrest of cell cycle in the sub-G1 and G1 phases. Activation of Caspase 9 and 3 and reduction in Caspase 8 was the underlying reason of apoptosis (Kumar et al., 2020a). The apoptosis activity of MBE of S. campanulata was investigated on Hepatoma G2 (HepG2) cells. The extract inhibited cell viability in a concentration-dependent way significantly. Various signs of apoptosis like nuclear fragmentation, chromatin condensation, and development of apoptotic bodies were observed in treated cells. Cell cycle arrest was detected in the G0/G1 phase (Kumar et al., 2020b). Recently, the compounds stigmasta-5,22-dien-3-ol, octadecenamide, and umbelliferone, isolated from chloroform extract of leaves of S. campanulate, were evaluated for their anticancer activity. The isolated compounds showed decreased cell viability in a dose-dependent manner against HL-60 cell lines. The IC50 values were found to be 44.12, 35.65, and 80.60 μg/ml, respectively. However, the activity was low compared to positive control Adriamycin (10.09 μg/ml) (Wagh et al., 2021).
Concluding the anticancer activity, it may be observed that the flower extract showed potent anticancer activity. The extracts should have been considered for isolation of active principle and in-vivo investigation against a range of cell lines should have been taken up. The selectivity toward cancer cells should also have been measured out during anticancer activity.
Anti-viral activity
Different fractions of the SBD were evaluated for the anti-human immunodeficiency virus (HIV) activity. The extracts were rather moderately active compared to azidothymidine (Niyonzima et al., 1999). MLE was tested against three viruses, viz. herpes simplex, virus targets sindbis, and polio, and was found to be inactive (Anani et al., 2000). MLE and ALE of S. campanulata were quantitatively evaluated for HIV-1 protease inhibitory effect by high performance liquid chromatography (HPLC). The %inhibition was found to be 42% and 31.1% at the concentration of 100 μg/ml (Takuya and Toru, 2009).
Analgesic and anti-inflammatory activity
The analgesic and anti-inflammatory activity of ELE of S. campanulata was determined using different pain models and carrageenan-induced acute inflammation in rats. 250–1,000 mg/kg of extract significantly increased the pain response times in hot-plate and tail flick pain models, and decreased acetic acid-induced writhing (AIW) in a dose-dependent manner. It also demonstrated significant reduction of inflammation induced by carrageenan (Ilodigwe and Akah, 2009). The analgesic effect of EFE of S. campanulata was investigated via AIW test, formalin test, and tail immersion experimental model. Pre-treatment with 100–500 mg/kg extract caused noteworthy dose-related analgesic effect. In tail immersion test, 500 mg/kg dose significantly reduced the pain response with percentage inhibition of 230%. The dose of 500 mg/kg inhibited 74.62% writhing induced by acetic acid when compared to control. In formalin-induced pain response, the dose of 500 mg/kg showed a significant percentage inhibition of 74.28% (Lamaeswari and Anuradha, 2013a). Anti-inflammation efficiency of 1-O-(E)-caffeoyl-β-gentiobiose (30) and (2S)-1,2-di-O-[(9Z,12Z,15Z)-octadeca-9,12,15-trienoyl]-3-O-[α-D-galctopyranosyl-(1?→6?)-O-β-D-galactopyranosyl] glycerol (34) isolated from MLE of S. campanulata produced 65.91% and 67.41% of membrane stability, respectively. It was concluded that S. campanulata might have exhibited its anti-inflammatory response by soothing the RBC membrane. During in silico studies, the compounds exhibited good affinity toward cyclooxygenase-II (Boniface et al., 2014). The ALE and MLE of S. campanulata were screened for anti-inflammatory activity in rats. The extract in the dose of 200 mg/kg bw demonstrated noteworthy inhibition of paw edema (0.431%) at the end of 180 minutes (Vijayasanthi et al., 2015).
Reliable analgesic activity of extracts obtained from leave and flowers were observed by both the methods. However, only alcoholic extracts were employed for the studies. The isolated compounds (30) and (34) showed almost equal anti-inflammatory activity as the standard anti-inflammatory agent diclofenac sodium, at a dose of 50 mg/ml. Toxicity studies demonstrated that the compounds were free of toxicity. The compounds further exhibited good affinity toward cyclooxygenase-II enzyme during in silico studies. It may be summarized that there is an ample of scope in developing drug candidates from the plant for anti-inflammatory activity.
Antioxidant activity
EFE of S. campanulata showed significant antioxidant activity. The extract demonstrated good activity during the superoxide radicals scavenging (SRS) and nitric oxide (NO) assay with IC50 values of 246 and 175 μg/ml, respectively. Overall, antioxidant capacity was measured to be 50 and 500 μg/ml. 500 μg/ml extract showed 74% and 79% inhibition during NO and superoxide radical (SR) scavenging assay. Standard drug curcumin exhibited 79% inhibition at 15 μg concentration during NO scavenging activity and standard drug ascorbic acid displayed 89% inhibition at concentration of 100 μM in SRS assay (Hareesh et al., 2010a). EFE of S. campanulata exhibited a significant free radical scavenging activity toward the lipid hydroxyl radical (LHR), 2,2-diphenylpicrylhydrazyl (DPPH) radicals and lipid peroxidation (LP) inhibition, with IC50 values of 200, 225, and 201 μg/ml, respectively. Maximum inhibition of 84%, 68%, and 82% were observed at 500 μg/ml concentration during LHR, DPPH radical scavenging, and LP inhibition assay, respectively. Standard drug butylated hydroxyanisole displayed 85% inhibition at a concentration of 400 μM during LP inhibition assay and during DPPH radical inhibition assay, standard compound gallic acid showed 86% inhibition at 5 μg concentrations (Hareesh et al., 2010b). The DNA damage preventing capacity of ELE and EFE of S. campanulata are studied by agarose gel electrophoresis method. Both extracts prevented the DNA damage induced by H2O2 and t-Butyl hydroperoxide. It showed protection up to 95% at 50 μg concentration. Hence, it was experimentally proved that ethanol extract is very effective in preventing ROS-induced DNA damage (Kowti et al., 2011). The mechanism of antioxidant action was explored for S. campanulata bark and flower extracts. EFE and EBE exhibited antioxidant action on LP of liver microsome induced by Fe3+-ascorbic acid. The EFE was found to be five times less active than EBE. Flower extract, previously complexed with 20–100 μM concentrations of Fe3+, resulted in loss of antioxidant activity. However, previous incubation with Fe3+ did not lead to loss of antioxidant activity in bark extract. These experiments indicated an antioxidant mechanism independent of Fe3+ complex formation in case of bark extract. It was concluded that the antioxidant mechanisms of S. campanulata bark and flower extracts are divergent from each other, reflecting the extracts have diverse composition (Heim et al., 2012). Fresh leaves were analyzed for its antioxidant nature in terms of metal chelating ability, phosphomolybdenum assay, reducing power (RP) assay, and NO scavenging activity. Significant antioxidant activity justified the plant as a good source of antioxidant agents. However, the authors did not determine IC50 values for the assay carried out (Krishnaveni et al., 2013). Antioxidant activity of EFE of S. campanulata was determined by total antioxidant, NO scavenging activity, RP assay, and H2O2 scavenging activity. IC50 values observed for total antioxidant, NO scavenging activity, H2O2 scavenging activity and RP assay were 280, 150, 250, and 220 μg/ml, respectively. Similarly, the IC50 values for the standard drug ascorbic acid were observed at 250, 100, and 120 μg/ml, respectively. This clearly indicates that the flower extract has highly effective antioxidant properties (Lamaeswari and Anuradha, 2013b). Verminoside (7) and 1-O-(E)-caffeoyl-β-gentiobiose (30) isolated from the leaves of S. campanulata displayed outstanding antioxidant activity with concentration required to produce 50 % radical scavenging activity (RS50) value of 2.5 and 2.67 μg/ml in DPPH radical scavenging assay. The antioxidant activity was greater than that of standard ascorbic acid (RS50 = 3.25 mg/ml). The compounds act as a potential inhibitor of tyrosinase during in silico study, which is in line with the observed antioxidant activity (Boniface et al., 2015). In vitro antioxidant activities were shown by the petroleum ether, ethanol, methanol, and aqueous extracts in free radical (DPPH), hydroxyl scavenging activities and ferric-reducing antioxidant properties. During the in vivo antioxidant assay, improvement in enzymatic level of glutathione and catalase was observed, when mice infected with S. typhi were given ELE (Coolborn et al., 2015). Antioxidant activity of ALE was evaluated by H2O2 RP method. The aqueous extract showed 70.2% scavenging (Thampi and Kumar, 2015). 70% ELE of S. campanulata demonstrated high antioxidant effect in terms of radical scavenging activity against DPPH (84.67%), SR (72.69%), H2O2 (83.20%), hydroxyl radicals (70%), and phosphomolybdate RP (955 FAEA) in comparison to chloroform and ethyl acetate extracts. Ethanol extract also showed high level of total phenolic content (9.21 mg FAE/l), which is linked with the significant antioxidant activity (Sangeetha et al., 2016). Different fractions of MLE of S. campanulata were screened employing DPPH radical scavenging assay. They had considerable antioxidant potency at concentrations of 250–1,000 μg/ml. The highest antioxidant effect was observed for the hexane fraction with an IC50 of 178.46 μg/ml (Umenwa et al., 2017). The antioxidant activity and total phenolic content of leaves, flowers, and nectars of S. campanulata from different climatic regions and from different cities of Brazil were evaluated. In vitro antioxidant activity was carried out by ferric-reducing antioxidant power, oxygen radical absorbance capacity, and DPPH assay. The leaves and flowers of S. campanulata exerted significant antioxidant capacity and total phenols were independent of the city. The antioxidant activity and total phenolic content were high in flowers, leaves, and nectars, respectively (Santos et al., 2020).
It may be concluded that stem bark and flower and leaf extracts having iridoids, cinnamic acid, flavonoids, and phenolics are responsible for the antioxidant activity. The bark extract was found to be more potent than leaf extract. The underlined antioxidants not only acted as scavengers of free radicals but also prevented H2O2 and ROS-induced DNA damage. Hence, the plant can be strategically utilized to counteract the undesirable effects of oxidative stress. These natural antioxidants can be used as additives in food stuffs or to develop products such as nutraceuticals and/or functional foods to protect humans from various chronic diseases.
Cardioprotective activity
Cardioprotective effect of 70% EBE of S. campanulata was determined in Wister rats intoxicated with isoproterenol. Doses of 250 and 500 mg/kg were orally given for 14 days and cardioprotection was evaluated by estimating serum lactate dehydrogenase, aspartate amino transferase (AST), alanine amino transferase (ALT), creatin phosphokinase, cholesterol, high density lipoprotein, and low density lipoprotein. The observed results were further confirmed by analyzing levels of thiobarbituric acid reactive substance and reduced glutathione in heart homogenate. The prior administration of bark extract significantly prohibited the isoproterenol-induced cardiac alterations (Abubaker et al., 2012a).
Nephroprotective activity
Nephroprotective protective potential of 70% EBE of S. campanulata was evaluated on paracetamol-induced nephrotoxicity in rats. The extract was given for 7 days, at doses of 250 and 500 mg/kg po. Pre-treatment with the extract improved paracetamol-induced lipid peroxide formation and exhibited a decrease in serum marker enzymes. Ethanol extract also prohibited the reduction of tissue glutathione levels. Histopathological studies revealed a restoration of renal architecture (Abubaker et al., 2012b).
Hepatoprotective activity
The ABE of S. campanulata was investigated for hepatoprotective effect against acetaminophen-induced hepatic damage in mice. The mice were pretreated with 100, 300, and 625 mg/kg of the extract for 5 days prior to intoxication with acetaminophen. Hepatoprotective action was accessed by estimating ALT and AST levels in serum and total cytochrome P450, glutathione peroxidase, and superoxide dismutase levels in liver homogenate. Significant hepatoprotection was observed against liver damage as evident from decreased levels of marker enzymes and increased levels of total protein content in extract-treated groups. The extract reversed the decline in antioxidant enzymes; superoxide dismutase, and glutathione peroxidase levels. It also caused substantial inhibition of CYP450 enzymes responsible for activation of acetaminophen. The extract protected the liver by increasing antioxidant capacity and interfering with the bio-transformation of acetaminophen (Dadzeasah and Ansah, 2013). Hepatoprotective activity of ABE of S. campanulata was investigated in a carbon tetrachloride-induced hepatotoxicity model. Rats pre-treated with 625, 1,250, and 2,500 mg kg−1 dose for 3 days showed significant hepatoprotective activity as marked from reduced serum levels of ALT, AST, gamma glutamyl transferase, and bilirubin in extract-treated groups. Hepatoprotective potential was well correlated with histopathological findings and the antioxidant capacity. LP assayed showed that the extract also restored significantly increased level of thiobarbituric acid reactive substances to near normal in the carbon tetrachloride-treated rats. Administration of the extract (po) also considerably inhibited cytochrome P450 enzymes and thus interfered with CCl4 bioactivation and demonstrated hepatoprotective action (Ansah et al., 2013). The hepatoprotective potential effect of the stem and root bark extracts of S. campanulata was evaluated on dimethylnitrosamine (DEN)-induced hepatic impairment in albino rats. Treatment with root and stem bark extracts of S. companulata significantly relieved the changes in the loss of body weight and transaminase activity induced by DEN (Uchenna et al., 2021).
Larvicidal activity
The MLE of S. campanulata was found to be most active with LC50 values of 1.343, 1.607, 1.981, 2.165, and 2.432 against stage I, II, III, and IV of larvae and pupa, respectively (Aarthi and Murugan, 2010). The effectiveness of ALE of S. campanulata leaves was evaluated against the dengue vector Aedes aegypti. Extract tested at 0.1%, 0.2%, and 0.3% prolonged the larval and pupal period. The leaf extract showed EI50 and EI90 at 0.79% and 0.88% concentrations, respectively (Saranya et al., 2013a). Larvicidal and pupicidal activities and morphological deformities against Aedes aegypti were investigated for ALE of S. campanulata. LC10, LC50, and LC90 values at different time periods against I instar larvae were observed to be 1.42%, 4.0%, and 5.40% (24 hours); 0.47%, 0.96%, and 2.12% (48 hours); 0.28%, 1.14%, and 1.84% (72 hours); and 0.14%, 0.59%, and 1.12% (96 hours). Moreover, various morphological changes were observed at different stages of development (Saranya et al., 2013b).
The plant has great significance in designing of an efficient vector control strategy based on environmental benign alternative to synthetic larvicides due to its ability to kill various stages of the larvae.
Insecticidal activity
The insecticidal potential of S. campanulata nectar was assessed through mortality tests on Sitophilus zeamais. The pure nectar showed a control efficiency of 89% against the insect population (Franco et al., 2015). Insecticidal action of nectar from S. campanulata was tested against three different insects. viz. Helicoverpa zea, Euschistus heros, and Anticarsia gemmatalis. The activity was linked to pro-oxidant proteins present in the nectar. The gross nectars and the dialysate nectar exhibited a mortality of 60%, 35% against E. heros, respectively. The gross nectar offered the highest mortality (80%) against H. zea in comparison to dialysate nectar (55%) (Santos et al., 2017).
Natural products such as S. campanulata nectar could be considered as a replacement to chemical pesticides due to their lower perseverance in the environment. Therefore, S. campanulata nectar could be used to protect grains while in storage due to its ability to kill various insects and pests.
Anticonvulsant activity
The anticonvulsant activity of ELE was studied using picrotoxin, pentylenetetrazole (PTZ), and electroshock-induced mice models. Prior administration of S. campanulata extract (250–1,000 mg/kg po) protected the mice against PTZ and picrotoxin-induced convulsion. 100% protection was observed at the maximum dose of 1,000 mg/kg. Administration of the extract did not significantly affect centrally coordinated behaviors such as rota-rod performance, righting reflex, position sense, amphetamine-induced stereotypy, and phenobarbital sleep time in treated animals. The results also showed that S. campanulata extract is non-sedating and has no antipsychotic properties (Ilodigwe et al., 2010a). Anticonvulsant effect of the isolated glycoside, urs-12-en-27α, 30 dioic acid 3-0-α-Lrhamnopyranosyl (1→2) α-L-arabinopyranoside (20), from ELE of S. campanulata was determined using PTZ and electrically induced seizures. The consequence on phenobarbitone-induced sleeping time and rota-rod performance were also experimented. It exhibited significant eradication of seizures caused by PTZ and electro shock. Oral and intraperitoneal LD50 of 323.59 and 158 mg/kg were obtained during acute toxicity studies, respectively (Ilodigwe et al., 2010b).
From the experimental study, it can be concluded that ethanol leaf extract of S. campanulata exhibits significant anticonvulsant activity against PTZ and picrotoxin-induced convulsion in Swiss albino mice. The anticonvulsant activity was attributed to the glycoside (20) present. This study provides pharmacological evidence for the folk claim of this plant to treat mental disorder and insanity. Furthermore, there is scope to derive the possible mechanism involved. The roots and barks can be studied for analogous activities, as they are traditionally prescribed for convulsion.
Antidepressant activity
The methanolic flower extract of S. campanulata was evaluated for antidepressant activity in mice at doses of 200 and 400 mg/kg. It showed dose-dependent antidepressant activity in tail suspension test, force swim test, and lithium chloride-induced twitches test. The extract significantly decreased in immobility period. Furthermore, a significant reduction in head twitches and locomotor activity was observed after giving the extract. The HPLC/electrospray ionisation mass spectrometry study identified the existence of spathoside A (3) and spathoside B (4) in the extract. These two compounds showed good binding affinity for monoamine oxidase A enzyme (Bajaj et al., 2021).
Sedative and anxiolytic activities
The sedative and anxiolytic activities of methanol leaf extract were evaluated in different animal models, viz. open field and hole-cross test, elevated plus maze, light–dark box, and hole-board test. In both open field and hole-cross tests, the extract at different doses of 200, 400, and 600 mg/kg significantly reduced the number (squares and holes) crossed by mice. Moreover, the mice opted to stay more in open arms and light box instead of close arms and dark box in elevated plus maze and light-dark box tests, respectively. Furthermore, the hole-board test elevated the number of head dipping to a significant extent (Begum et al., 2020).
Inhibition of sickling
MFE was examined for in-vitro reverse sickling of erythrocytes at a low oxygen level according to the modified Dean and Schechter method. It displayed reversal activity of 89.6% at a concentration 4 mg/ml and inhibited polymerization of erythrocytes (Bamimore and Elujoba, 2018).
Antiulcer activity
Antiulcer activity of ELE of S. campanulata was determined against aspirin-induced gastric ulcer in rats. The extract at 200 and 400 mg/kg po decreased the ulcer index, ulcer number, gastric volume, pH, total acidity, and free acidity. It confirmed that S. campanulata extract has anti-ulcer activity due to secondary metabolites (Khatri et al., 2019).
Molluscicidal activity
Molluscicidal properties were studied against the bilharzia carrying snails Bulinus africanus. MBE was found to be strongly molluscicidal (Amusan et al., 1995). Screening of 70% of the ethyl alcohol leaf extract of S. campanulata showed molluscicidal activity against snails. The activity was attributed to the presence of tannins (Shams et al., 2012).
Aphrodisiac activity
Aphrodisiac activity of ABE of S. campanulata was determined in male rats. The rats were given 200, 400, and 800 mg/kg ABE for 8 days and the copulatory parameters were recorded. Bark extract increased the erectile function stimulation by increasing the number of erections, mount, and ejaculation frequency. These results proved the aphrodisiac properties of the trunk barks and justified the traditional use of this plant in curing erectile dysfunction (Clovis et al., 2019). To validate the traditional use of the bark as an aphrodisiac, the androgenic properties of the aqueous extract of S. campanulata trunk bark was evaluated in adult male rats. At the dose of 200 mg/kg, the extract significantly increased the penile nitrogen monoxide (136.36%) and the vesicular fructose (19.58%). Furthermore, there was an increase in the weight of certain androgen-dependent organs: the prostate (7.70%), the testes (11.20%), and penis (31.81%). These results validated the androgenic potential of S. campanulata and backing the use of S. campanulata trunk bark in the treatment of erectile dysfunction (Talla et al., 2021).
UV absorption ability
The flower extract of S. campanulata was screened for its cosmetic use. The extract absorbed UV radiation in the range of 200–400 nm and proved to possess UV protection ability (Patil et al., 2011). The flower extract prepared from distilled water and methanol (2:5) showed maximum absorbance at 200–240 nm, while good and moderate absorbances were noted at 240–325 nm and 310–340 nm, respectively (Patil et al., 2009).
CLINICAL STUDIES
One clinical study has been conducted to establish the effectiveness of S. campanulata for its use in malarial. Time Herbal Mixture® (THM), a Ghanaian herbal product composed of stem barks of S. campanulata, leaves of Solanum torvum, Vernonia amygdalina, and Bombax buonopozense, was evaluated to establish its safety and effectiveness in treatment of uncomplicated malaria. 40 patients diagnosed with uncomplicated malaria were given 60 ml of the formulation, three times daily for 6 days. The participants were followed-up for a period of 28 days. These patients comprised 15 males and 25 females, with a mean age of 42.29 years. 82.50% (33) of the participants were completely cured by day 7 (clearance of all parasites achieved). Partial clearance was observed in six patients and treatment failure in one. The product was found to have good safety profile as none of them reported any side effects. Kidney, liver, and blood profiles were also usual after the study (Tetteh et al., 2020).
TOXICITY STUDY
Acute toxicity of ELE was studied by administering doses in the range of 1,000–5,000 mg/kg. The subchronic study was carried out by giving 750–3,000 mg/kg of extract for 90 days. The LD50 of the extract was estimated to be 4,466.84 mg/kg. Deaths of animals were not observed during the study period but the rats had signs of weakness, sluggishness, anorexia, and increase in body weight. The extract significantly increased the serum liver enzymes, alkaline phosphatase (ALP), ALT, and AST. However, these changes were recovered after 28 days post-treatment. These outcomes suggest that the ELE of S. campanulata is safe in the treatment of various diseases (Ilodigwe et al., 2010c). Acute oral toxicity of the bark extract of S. campanulata was studied at a single oral dose of 2,000 mg/kg. Various parameters like body weight, behavior, general appearance, and mortality were calculated. It did not cause changes in general appearance and mortality and was found to be safe at a dose of 2,000 mg/kg (Palande, 2015). Toxicity profile of MBE was experimentally determined by both acute and sub-chronic toxicity studies in rats. Single oral dose of 5,000 mg/kg did not lead to any observable toxic effect or mortality. However, oral administration of the 800 mg/kg dose for 90 days resulted in an increase in bodyweight (increase in the stomach). Among the biochemical parameters accessed [Gamma-glutamyl transferase (GGT), AST, ALT, ALP, urea, and creatinine], only ALT level was increased at the 800 mg/kg dose. The levels of RBC, WBC, HCT, and red cell distribution width reduced significantly at the 800 mg/kg dose in the extract-treated groups. However, a noteworthy increase in mean corpuscular hemoglobin (MCH), mean corpuscular volume (MCV), and mean corpuscular hemoglobin concentration levels were observed at the 800 mg/kg dose in the treated animals. A histological analysis of the heart showed myocardial necrosis and hemorrhage at 400 and 800 mg/kg after 90 days administration. The acute use of extract was found to be safe. However, prolonged use at high doses affected liver enzyme (ALT) and the myocardial tissue (Tanayen et al., 2016). The cytotoxicity (250 μg/ml) of promising extracts was assayed on normal Chang liver cells by an (3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide) assay (Fokou et al., 2016).
CONCLUSION
Spathodea campanulata has been used for centuries for the treatment of malaria, diabetes, ulcers, wounds, and skin infection. The preliminary experimentations related to conditions like malaria, wounds, inflammations, convulsions, and diabetes have been supportive in bringing about the relationship between the pharmacological activity and types of the phytochemicals involved. However, the exact mechanism of its actions pertaining to its therapeutic potential is still uncertain. Malaria is reported to be traditionally treated by this plant, which is now justified by the modern findings of ursolic acid and tomentosolic acid. S. campanulata plant phytochemicals can be very useful as a first-aid treatment of malaria in the remote areas of Africa and Asia where modern medicine is not easily accessible. Additionally, the mechanism of action studies would further support the antimalarial activity. In addition to the above-mentioned observation, studies connected to wound healing activities are more distinct in crude extract. The antioxidant and antimicrobial activities advocate that the utilization of the plants in wound healing may be based on antiseptic and antioxidant effects of its constituents, which lend support to its folkloric use in the management of wounds. The insecticidal and larvicidal study is of great significance in designing an efficient vector control strategy based on environmental benign alternatives to synthetic insecticides and larvicides. Some of the validation studies like asthma, facilitation of delivery, treatment of reproductive system, hernia, and antidote against animal poisons have not been touched upon. The reviewed literature designates some gap in scientific studies that needs consideration as claimed in the traditional system of medicine. Further high-quality clinical studies are necessary to provide definitive clinical evidence of safety and efficacy.
ACKNOWLEDGMENT
The author expresses his thanks to Dr. Chandra Sekhar Patro, Principal, School of Pharmacy, Centurion University of Technology and Management, Rayagada, Odisha, for providing the opportunity to carry out this work.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Aarthi N, Murugan K. Larvicidal and smoke repellent activities of Spathodea campanulata P. Beauv. against the malarial vector Anopheles Stephensi Lis (Diptera: Culicidae). J Phytol, 2010; 2:61–9.
Abdraboh NR, Ahmed, N. Comparative study of biochemical changes in alloxan induced diabetic mice treated with extracts of Spathodea campanulata flowering branch and barks. Res J Med Plant, 2015; 9:395–405; doi:10.3923/rjmp.2015.395.405 CrossRef
Abubaker S, Shanmukha I, Jyoti TM, Gupt K. Cardioprotective effect of Spathodea campanulata bark on isoproterenol-induced myocardial infarction in rats. Asian Pacific J Trop Dis, 2012a; 2:S1–5; doi:10.1016/S2222-1808(12)60113-3 CrossRef
Abubaker S, Shanmukha I, Rajendra SV, Ramachandra Setty S. Protective effect of Spathodea campanulata bark against paracetamol-induced nephrotoxicity in rats. Int J Pharm Tech Res, 2012b; 4:398–403.
Adebayo JO, Krettli AU. Potential antimalarials from Nigerian plants: a review. J Ethnopharmacol, 2011; 133:289–302; doi:10.1016/j.jep.2010.11.024 CrossRef
Adia MM, Anywar G, Byamukama R, Kamatenesi-Mugisha M, Sekagya Y, Kakudidi EK, Kiremire BT. Medicinal plants used in malaria treatment by Prometra herbalists in Uganda. J Ethnopharmacol, 2014; 155:580–8; doi:10.1016/j.jep.2014.05.060 CrossRef
Agyare C, Asase A, Lechtenberg M, Niehues M, Deters A, Hensel A. An ethnopharmacological survey and in vitro confirmation of ethnopharmacological use of medicinal plants used for wound healing in Bosomtwi-Atwima-Kwanwoma area, Ghana. J Ethnopharmacol, 2009; 125:393–403; doi:10.1016/j.jep.2009.07.024 CrossRef
Agyare C, Boakye YD, Bekoe EO, Hensel A, Dapaah SO, Appiah T. Review: African medicinal plants with wound healing properties. J Ethnopharmacol, 2016; 177:85–100; doi:10.1016/j.jep.2015.11.008 CrossRef
Agyare C, Spiegler V, Asase A, Scholz M, Hempel G, Hensel, A. An ethnopharmacological survey of medicinal plants traditionally used for cancer treatment in the Ashanti region, Ghana. J Ethnopharmacol, 2018; 212:137–52; doi:10.1016/j.jep.2017.10.019 CrossRef
Akinwumi IA, Sonibare MA. Use of medicinal plants for the treatment of gastric ulcer in some parts of Southwestern Nigeria. Afr J Pharm Pharmacol, 2019; 13:223-35.
Amusan OOG, Msonthi JD, Makhubu LP. Molluscicidal activity of Spathodea campanulata Andrachne ovalis, Phytolacca dodecandra and Hypoxis rooperi. Fitoterapia, 1995; 66:113–6.
Amusan OOG, Adesogan EK, Makinde JM. Antimalarial active principles of Spathodea campanulata stem bark. Phyther Res, 1996; 10:692–3; doi:10.1002/(sici)1099-1573(199612)10:8<692::aid-ptr928>3.3.co;2-f CrossRef
Anani K, Hudson JB, De Souza C, Akpagana K, Tower GHN, Arnason JT, Gbeassor M. Investigation of medicinal plants of Togo for antiviral and antimicrobial activities. Pharm Biol, 2000; 38:40–5; doi:10.1076/1388-0209(200001)3811-bft040 CrossRef
Ansah C, Dadzeasah PE, Asiamah E. Aqueous stem bark extract of Spathodea campanulata (P. Beauv) modulates carbon tetrachloride induced hepatic damage in rats. Am J Pharmacol Toxicol, 2013; 8:39–50; doi:10.3844/ajptsp.2013.39.50 CrossRef
Anywar G, Kakudidi E, Byamukama R, Mukonzo J, Schubert A, Oryem-Origa, H. Indigenous traditional knowledge of medicinal plants used by herbalists in treating opportunistic infections among people living with HIV/AIDS in Uganda. J Ethnopharmacol, 2020; 246:112205; doi:10.1016/j.jep.2019.112205 CrossRef
Appiah K, Oppong C, Mardani H, Omari R, Kpabitey S, Amoatey C, Onwona-Agyeman S, Oikawa Y, Katsura K, Fujii Y. Medicinal plants used in the Ejisu-Juaben Municipality, Southern Ghana: an ethnobotanical study. Medicines, 2019; 6:1; doi:10.3390/medicines6010001 CrossRef
Asase A, Oppong-Mensah G. Traditional antimalarial phytotherapy remedies in herbal markets in southern Ghana. J Ethnopharmacol, 2009; 126:492–9; doi:10.1016/j.jep.2009.09.008 CrossRef
Bajaj J, Dwivedi J, Sahu R, Dave V, Verma K, Joshi S, Sati B, Sharma S, Seidel V, Mishra AP. Antidepressant activity of Spathodea campanulata in mice and predictive affinity of spatheosides towards type A monoamine oxidase. Cell Mol Biol, 2021; 67(1):1–8; doi:10.14715/cmb/2021.67.1.1 CrossRef
Bamimore V, Elujoba AA. Antisickling properties of three medicinal plants and their combinations. Int J Pharmacogn, 2018; 5:666–72.
Begum A, Biswas P, Shahed-Al-Mahmud M. Methanol extract of Spathodea campanulata P. (Beauv.) leaves demonstrate sedative and anxiolytic like actions on swiss albino mice. Clin Phytosci, 2020; 6(1):1–12; doi:10.1186/s40816-020-00182-z CrossRef
Bekoe EO, Agyare C, Boakye YD, Baiden BM, Asase A, Sarkodie J, Nettey H, Adu F, Otu PB, Agyarkwa B, Amoateng P, Asiedu-Gyekye I, Nyarko A. Ethnomedicinal survey and mutagenic studies of plants used in Accra metropolis, Ghana. J Ethnopharmacol, 2020; 248:112309; doi:10.1016/j.jep.2019.112309 CrossRef
Betti JL, Caspa R, Ambara J, Kourogue RL. Ethno-botanical study of plants used for treating malaria in a forest: savanna margin area, East region, Cameroon. Glob J Res Med Plants Indig Med, 2013; 2:692–708.
Boniface PK, Verma S, Shukla A, Khan F, Srivastava SK, Pal A. Membrane stabilisation: a possible anti-inflammatory mechanism for the extracts and compounds from Spathodea campanulata. Nat Prod Res, 2014; 28:2203–7; doi:10.1080/14786419.2014.930858 CrossRef
Boniface PK, Singh M, Verma S, Shukla A, Khan F, Srivastava SK, Pal A. RP-HPLC-DAD method for the identification of two potential antioxidant agents namely verminoside and 1-O-(E)-caffeoyl-β-gentiobiose from Spathodea campanulata leaves. Nat Prod Res, 2015; 29:676–80; doi:10.1080/14786419.2014.981538 CrossRef
Boniface PK. Advances on ethnomedicinal uses, phytochemistry, and pharmacology of Spathodea campanulata P. Beauv. EC Pharmacol Toxicol, 2017; 5:51–62.
Brown P, Daigneault A. Cost-benefit analysis of managing the invasive African tulip tree (Spathodea campanulata) in the Pacific. Environ Sci Policy, 2014; 39:65–76; doi:10.1016/j.envsci.2014.02.004 CrossRef
Bunalema L, Obakiro S, Tabuti JRS, Waako P. Knowledge on plants used traditionally in the treatment of tuberculosis in Uganda. J Ethnopharmacol, 2014; 151:999–1004; doi:10.1016/j.jep.2013.12.020 CrossRef
CABI. Spathodea campanulata. In: Invasive species compendium, CAB International, Wallingford, UK, 2021. Available via https://www.cabi.org/isc/datasheet/51139#tosummaryOfInvasiveness
Clovis T, Yaya I, Nga N, Didier DS, Emmanuel MM. Evaluation of aphrodisiac properties of the aqueous extract of the trunk barks of Spathodea campanulata P. Beauv. (Bignoniaceae) on albino rats (Rattus norvegicus). J Med Plants Res, 2019; 13:480–6; doi:10.5897/jmpr2019.6741 CrossRef
Coolborn AF, Bolatito B, Omolara AV, Adetuyi F. Phytochemical and antioxidant effect of Spathodea campanulata leaf extracts. Int J Biochem Res Rev, 2015; 7:148–59; doi:10.9734/ijbcrr/2015/16371 CrossRef
Dadzeasah PE, Ansah C. Spathodea campanulata extract attenuates acetaminophen-induced hepatic injury in mice. Int J Pharm Sci Drug Res, 2013; 5:158–64.
Das MP, Dhanabalan R, Doss A. In vitro antibacterial activity of two medicinal plants against bovine udder isolated bacterial pathogens from dairy herds. Ethnobot Leafl, 2009; 13:152–8.
Dhanabalan R, Doss A, Jagadeeswari M, Karthic R. Preliminary phytochemical screening and antimalarial studies of Spathodea campanulatum P. Beauv leaf extracts. Ethnobot Leafl, 2008; 12:811–9.
Dhanabalan R, Palaniswamy M, Devakumar J. In vitro cytotoxicity and anticancer activity of four folklore medicinal plants used among tribal communities of Western Ghats, Coimbatore, Tamil Nadu. Drug Invent Today, 2014; 6:751–9.
Dhanabalan R, Doss A, Balachandar S. In vitro phytochemical screening and antibacterial activity of organic leaf extracts of Spathodea campanulata P. Beauv against hospital isolated bacterial strains. Ethnobot. Leafl, 2008; 12:1022–8.
Diallo A, Traore MS, Keita SM, Balde MA, Keita A, Camara M, Van Miert S, Pieters L. Management of diabetes in Guinean traditional medicine: an ethnobotanical investigation in the coastal lowlands. J Ethnopharmacol, 2012; 144:353–61; doi:10.1016/j.jep.2012.09.020 CrossRef
Disengomoka I, Delaveau P. Medicinal plants used for child’s respiratory diseases in Zaire. Part I. J Ethnopharmacol, 1983; 8:257–63; doi:10.1016/0378-8741(83)90063-6 CrossRef
Eid HH, Shehab NG, El Zalabani SM. GC-MS profile and cytotoxicity of the hydrodistilled and extracted volatiles of the buds and flowers of Spathodea campanulata P. Beauv. J Biol Act Prod from Nat, 2014; 4:196–208; doi:10.1080/22311866.2014.936899 CrossRef
Elusiyan AC, Ani NC, Adewunmi CO, Olugbade TA. Distribution of iridiod glucosides and anti-oxidant compounds in Spathodea campanulata parts. African J Tradit Complement Altern Med, 2011; 8:27–33. CrossRef
Emmanuel M, Didier, D. Medicinal plant knowledge of ethnic groups in Douala town, Cameroon. Am J Food Nutr, 2011; 1:178–84. doi:10.5251/ajfn.2011.1.4.178.184 CrossRef
Flowers of India. African tulip tree. 2021. Available via http://www.flowersofindia.net/catalog/slides/African%20Tulip%20Tree.html
Focho DA, Newu MC, Anjah MG, Nwana FA, Ambo FB. Ethnobotanical survey of trees in Fundong, Northwest Region, Cameroon. J Ethnobiol Ethnomed, 2009a; 5:17; doi:10.1186/1746-4269-5-17 CrossRef
Focho DA, Ndam WT, Fonge BA. Medicinal plants of Aguambu - Bamumbu in the Lebialem highlands, southwest province of Cameroon. Afr J Pharm Pharmacol, 2009b; 3:1–13.
Fokou PVT, Nyarko AK, Appiah-Opong R, Tchokouaha Yamthe, LR, Addo P, Asante IK, Boyom FF. Ethnopharmacological reports on anti-Buruli ulcer medicinal plants in three West African countries. J Ethnopharmacol, 2015; 172:297–311; doi:10.1016/j.jep.2015.06.024 CrossRef
Fokou PVT, Kissi-Twum AA, Yeboah-Manu D, Appiah-Opong R, Addo P, Yamthe LRT, Mfopa AN, Boyom FF, Nyarko AK. In vitro activity of selected west African medicinal plants against Mycobacterium ulcerans disease. Molecules, 2016; 21:445; doi:10.3390/molecules21040445 CrossRef
Fongod A, Veranso M, Libalah M. Identification and use of plants in treating infertility in human females in Fako Division, Cameroon. Glob J Res Med Plants Indig Med, 2013; 2:724–37.
Fongod AGN, Ngoh LM, Veranso MC. Ethnobotany, indigenous knowledge and unconscious preservation of the environment: an evaluation of indigenous knowledge in South and Southwest Regions of Cameroon. Int J Biodivers Conserv, 2014; 6:85–99; doi:10.5897/ijbc2013.0637 CrossRef
Franco DP, Guerreiro JC, Ruiz MG, Da Silva RMG. Evaluation of insecticide potential of Spathodea campanulata (Bignoniaceae) nectar on Sitophilus Zeamais (Coleoptera: Curculionidae). Rev Colomb Entomol, 2015; 41:63–7.
Gormann R, Kaloga M, Kolodziej H. Novel flavonoids from leaf extracts of Markhamia acuminata and Spathodea campanulata. Planta Med, 2006; 72:35; doi:10.1055/s-2006-949835 CrossRef
Gouda YG. Iridoids from Spathodea campanulata P. Beauvais leaves. Nat Prod Commun, 2009a; 4:753–6. CrossRef
Gouda YG. Flavonoids and phenylpropanoids from Spathodea campanulata P. Beauvais leaves. Bull Pharm Sci, 2009b; 32:301–9; doi:10.21608/bfsa.2009.63500 CrossRef
Hamill FA, Apio S, Mubiru NK, Bukenya-Ziraba R, Mosango M, Maganyi OW, Soejarto DD. Traditional herbal drugs of Southern Uganda, II: literature analysis and antimicrobial assays. J Ethnopharmacol, 2003; 84:57–78; doi:10.1016/S0378-8741(02)00289-1 CrossRef
Hareesh AR, Harsha R, Ahmed MG, Satish Kumar BP, Thammanna Gowda SS, Joshi V, Chethan IA. Nitric oxide and superoxide radical scavenging activity of flower of Spathodea campanulata P. Beauv. Invent Rapid Ethnopharmacol, 2010a; 1:2–4.
Hareesh AR, Kowti R, Harsha R, Ahmed MG, Satish Kumar BP, Dinesha R, Mohammed IA, Thammanna Gowda SS. In vitro antioxidant and free radicals scavenging activity of flower of Spathodea campanulata P. Beauv. Int J Pharm Sci, 2010b; 2:508–14.
Heim S, Guarnier F, Ferreira D. Antioxidant activity of Spathodea campanulata (Bignoneaceae) extracts. Rev Bras Plants Med, 2012; 14:287–92. CrossRef
Henry SG, Francis A, Kofi A. Ethnobotanical survey of medicinal plants used for the treatment of diarrhoea and skin ulcer in the Brong Ahafo region of Ghana. J Med Plants Res, 2013; 7:3280–5; doi:10.5897/JMPR2013.5170
Herrera I, Brice L, Grillo R. Methodology for the artificial inoculation with Ceratocystis sp. for the control of Spathodea campanulata Beauv. Centro Agric, 2002; 29:56–8.
Ilodigwe EE, Akah PA, Nworu CS. Anticonvulsant activity of ethanol leaf extract of Spathodea campanulata P. Beauv (Bignoniaceae). J Med Food, 2010a; 13:827–33; doi:10.1089/jmf.2009.0144 CrossRef
Ilodigwe EE, Akah PA, Okoye TC, Omeje EO. Anticonvulsant effects of a glycoside isolated from the leaf of Spathodea campanulata P. Beauv. J Med Plants Res, 2010b; 4:1895–900; doi:10.5897/JMPR10.360
Ilodigwe EE, Akah PA, Nworu CS. Evaluation of the acute and subchronic toxicities of ethanol leaf extract of Spathodea campanulata P. Beauv. Int J Appl Res Nat Prod, 2010c; 3:17–21.
Ilodigwe EE, Akah PA. Spathodea Campanulata: An experimental evaluation of the analgesic and anti-inflammatory properties of a traditional remedy. Asian J Med Sci, 2009; 1:35–8.
Ior LD, Otimenyin SO, Okwori VA, Umar DM, Azila JJ. Ethnobotanical survey of plants used in the management of mental illnesses in some selected local government areas of Plateau State, Nigeria. J Pharmacogn Phyther, 2017; 9:146–56; doi:10.5897/jpp2017.0464 CrossRef
IUCN. The IUCN red list of threatened species version 2020-1. 2020. Available via https://www.iucnredlist.org/species/49196213/49196223
Iyamah PC, Idu M. Ethnomedicinal survey of plants used in the treatment of malaria in Southern Nigeria. J Ethnopharmacol, 2015; 173:287–302; doi:10.1016/j.jep.2015.07.008 CrossRef
Jeruto P, Lukhoba C, Ouma G, Otieno D, Mutai C. An ethnobotanical study of medicinal plants used by the Nandi people in Kenya. J Ethnopharmacol, 2008; 116:370–6; doi:10.1016/j.jep.2007.11.041 CrossRef
Kamatenesi-Mugisha M, Oryem-Origa H, Odyek O, Makawiti DW. Medicinal plants used in the treatment of fungal and bacterial infections in and around Queen Elizabeth Biosphere Reserve, western Uganda. Afr J Ecol, 2008; 46:90–7; doi:10.1111/j.1365-2028.2008.00935.x CrossRef
Khatri S, Goswami RB, Jain S. Phytochemical screening and evaluation of antiulcer activity of ethanolic extract of Spathodea campanulata leaves. J Drug Deliv Ther, 2019; 9:1012–5.
Kibuuka MS, Anywar G. Medicinal plant species used in the management of hernia by traditional medicine practitioners in central Uganda. Ethnobot Res Appl, 2015; 14:289–98; doi:10.17348/era.14.0.289-298 CrossRef
Kihdze TJ, Mayowa AA, Joseph O, Joseph Oc E, Ghaife TG, Bulus A, Van Aerschot A, Laekeman G, Ganafa AA. Phytochemical and antidiabetic evaluation of the methanolic stem bark extract of Spathodea campanulata (P. Beauv.) Bignoniaceae. Pharmacogn J, 2016; 8:243–9; doi:10.5530/pj.2016.3.12 CrossRef
Killedar SG, Kope KI, Sangle SB, Tamboli MS. Standardization and antimicrobial activity of watery fluid at floral base of Spathodea campanulata (Pal). Asian J Pharm Anal, 2011; 1:19–21.
Koffi N, Edouard KK, Kouassi K, Edouard KK, Kouassi K. Ethnobotanical study of plants used to treat diabetes, in traditional medicine, by Abbey and Krobou people of Agboville (Cote-d’Ivoire). Am J Sci Res, 2009; 4:45–58.
Komlaga G, Agyare C, Dickson RA, Mensah MLK, Annan K, Loiseau PM, Champy P. Medicinal plants and finished marketed herbal products used in the treatment of malaria in the Ashanti region, Ghana. J Ethnopharmacol, 2015; 172:333–46; doi:10.1016/j.jep.2015.06.041 CrossRef
Kona S, Rahman A. Inventory of medicinal plants at Mahadebpur Upazila of Naogaon district, Bangladesh. Appl Ecol Environ Sci, 2016; 4:75–83.
Kowti R, Harsha R, Ahmed MG, Hareesh AR, Thammanna Gowda SS, Dinesha R, Satish Kumar BP, Irfan Ali M. Antimicrobial activity of ethanol extract of leaf and flower of Spathodea campanulata P. Beauv. Res J Pharm Biol Chem Sci, 2010; 1:691–8.
Kowti R, Joshi V, Dabadi P, Thammanna GS, Satish BP, Dinesha R. Antioxydant activity of Spathodea campanulata in prevention of t BOOH and H2O2 induced DNA damage. Int J Curent Pharm Res, 2011; 3:87–9.
Krishnaveni M, Amsavalli L, Chandrasekar R, Madhaiyan P, Durairaj S. Antioxidant activity of plants at Govt. College of Engineering campus, Salem, Tamil Nadu, India. Int J Pharm Sci Rev Res, 2013; 21:160–3.
Kuete V, Tchinda CF, Mambe FT, Beng VP, Efferth T. Cytotoxicity of methanol extracts of 10 Cameroonian medicinal plants towards multi-factorial drug-resistant cancer cell lines. BMC Complement Altern Med, 2016; 16:267. CrossRef
Kumar A. Evaluation of phytochemical screening & antibacterial activity of aqueous, ethanol extracts of medicinal plants against few common microorganisms. V Care Life Sci J, 2012; 2:17–28.
Kumar S, Dash D. Flora of Nandan Kanan sanctuary: medicinal plants with their role in health care. Int J Pharm Life Sci, 2012; 3:1631–42.
Kumar S, Gajbhiye RL, Besra SE. Apoptosis inducing activity of bark extract of Spathodea campanulata on human leukemia cell lines U937, K562 & HL60 cell lines via caspase cascade. Int J Pharm Sci Rev Res, 2020a; 64(1):114–22; doi:10.47583/ijpsrr.2020.v64i01.022 CrossRef
Kumar S, Gajbhiye RL, Besra SE. Apoptosis inducing potentiality of Spathodea campanulata bark against hepatocellular carcinoma: HEPG2 cells. World J Pharm Res, 2020b; 9(8)1578–94; doi:10.20959/wjpr20208-18129
Labrada R, Medina AD. The invasiveness of the african tulip tree, Spathodea campanulata beauv. Biodiversity, 2009; 10:79–82; doi:10.1080/14888386.2009.9712848 CrossRef
Lacroix D, Prado S, Kamoga D, Kasenene J, Namukobe J, Krief S, Dumontet V, Mouray E, Bodo B, Brunois F. Antiplasmodial and cytotoxic activities of medicinal plants traditionally used in the village of Kiohima, Uganda. J Ethnopharmacol, 2011; 133:850–5; doi:10.1016/j.jep.2010.11.013 CrossRef
Lamaeswari G, Anuradha R. Analgesic effects of the ethanolic extract of flowers of Spathodea campanulata studied in Albino mice. Int Res J Pharm Appl Sci, 2013a; 3:8–12.
Lamaeswari G, Anuradha R. In vitro antioxidant activity of ethanolic flower extract of Spathodea campanulata P. Beauv. Int J Biol Pharm allied Sci, 2013b; 2:2130–6.
Oladunmoye MK, Kehinde FY. Ethnobotanical survey of medicinal plants used in treating viral infections among Yoruba tribe of South Western Nigeria. African J Microbiol Res, 2011; 5:2991–3004; doi:10.5897/ajmr10.004 CrossRef
Magnibou L, Nyemb J, Magne C, Mbougnia J, Leutcha B, Henoumont C. Chemical constituents of Spathodea campanulata (Bignoniaceae), their antimicrobial and antioxidant activities. Nat Prod Chem Res, 2021; 9(4):1–7.
Mahanoa MFKAO, Bwirondea FM, Amanib AC, Mangambub JD, Nyakabwab DS, Wimbac LK, Tshibangud DST, Ngboluad KN, Kambalee JK, Mpianad PT. Ethnopharmacological survey of plants used against diabetes in Bukavu city (DR Congo). J Ethnobiol Tradit Med, 2013; 119:538–46.
Makinde JM, Amusan OOG, Adesogan EK. The antimalarial activity of Spathodea campanulata stem bark extract on Plasmodium berghei berghei in mice. Planta Med, 1988; 54:122–5; doi:10.1055/s-2006-962367 CrossRef
Makinde JM, Adesogan EK, Amusan OOG. The schizontocidal activity of Spathodea campanulata leaf extract on Plasmodium berghei berghei in mice. Phyther Res, 1987; 1:65–8; doi:10.1002/ptr.2650010205 CrossRef
Makindet JM, Amusan OOG, Adesogan EK. The antimalarial activity of chromatographic fractions of Spathodea campanulata stem bark extracts against Plasmodium berghei berghei in mice. Phyther Res, 1990; 4:53–6; doi:10.1002/ptr.2650040204 CrossRef
Malan DF, Neuba DFR, Kouakou KL. Medicinal plants and traditional healing practices in ehotile people, around the aby lagoon (eastern littoral of Côte d’Ivoire). J Ethnobiol Ethnomed, 2015; 11:21; doi:10.1186/s13002-015-0004-8 CrossRef
Manya MH, Keymeulen F, Ngezahayo J, Bakari AS, Kalonda ME, Kahumba BJ, Duez P, Stevigny C, Lumbu SJB. Antimalarial herbal remedies of Bukavu and Uvira areas in DR Congo: an ethnobotanical survey. J Ethnopharmacol, 2020; 249:112422; doi:10.1016/j.jep.2019.112422 CrossRef
Mbosso EJT, Ngouela S, Nguedia JCA, Penlap Bengs V, Rohmer M, Tsamo E. Spathoside, a cerebroside and other antibacterial constituents of the stem bark of Spathodea campanulata. Nat Prod Res, 2008; 22:296–304; doi:10.1080/14786410701766281 CrossRef
Mbosso Teinkela JE, Assob Nguedia JC, Meyer F, Vouffo Donfack E, Lenta Ndjakou B, Ngouela S, Tsamo E, Adiogo D, Guy Blaise Azebaze A, Wintjens R. In vitro antimicrobial and anti-proliferative activities of plant extracts from Spathodea campanulata, Ficus bubu, and Carica papaya. Pharm Biol, 2016; 54:1086–95; doi:10.3109/13880209.2015.1103273 CrossRef
Melendez PA, Capriles VA. Antibacterial properties of tropical plants from Puerto Rico. Phytomedicine, 2006; 13:272–6; doi:10.1016/j.phymed.2004.11.009 CrossRef
Mensah AY, Houghton PJ, Dickson RA, Fleischer TC, Heinrich M, Bremner P. In vitro evaluation of effects of two Ghanaian plants relevant to wound healing. Phyther Res, 2006; 20:941–4; doi:10.1002/ptr CrossRef
Moronkola DO, Olaoluwa OO, Oladosu IA, Aboaba SA, Aiyelaagbe OO. Phytochemical and antimicrobial activities of extracts from six medicinal plants utilized as antimalarials in ethno-medicine. Pharm Chem J, 2018; 5:52–61.
Moshi MJ, Otieno DF, Mbabazi PK, Weisheit A. Ethnomedicine of the Kagera region, north western Tanzania. Part 2: The medicinal plants used in Katoro Ward, Bukoba District. J Ethnobiol Ethnomed, 2010; 6:19; doi:10.1186/1746-4269-6-19 CrossRef
Mukungu N, Abuga K, Okalebo F, Ingwela R, Mwangi J. Medicinal plants used for management of malaria among the Luhya community of Kakamega East sub-County, Kenya. J Ethnopharmacol, 2016; 194:98–107; doi:10.1016/j.jep.2016.08.050 CrossRef
Musinguzi D, Tumushabe A, Sekabira K, Basamba TA, Byarugaba D. Medicinal plants use in and around Kalinzu central forest reserve, Western Uganda. J Med Plants Stud, 2017; 5:44–9.
Nazif NM. Phytochemical and antioxidant activity of Spathodea campanulata P. Beauvois. Growing in Egypt. Nat Prod Sci, 2007; 13:11–6.
Ngnameko CR, Marchetti L, Zambelli B, Quotadamo A, Roncarati D, Bertelli D, Njayou FN, Smith SI, Moundipa PF, Costi MP, Pellati F. New insights into bioactive compounds from the medicinal plant Spathodea campanulata P. Beauv. and their activity against Helicobacter pylori. Antibiotics, 2020; 9:258; doi:10.3390/antibiotics9050258 CrossRef
Ngane RAN, Mogtomo MLK, Tiabou AT, Nana HM, Chieffo PRM, Bounou ZM, Etame RME, Ndifor F, Biyiti L, Zollo PHA. Ethnobotanical survey of some Cameroonian plants used for treatment of viral diseases. African J Plant Sci, 2011; 5:15–21.
Ngouela S, Nyasse B, Tsamo E, Sondengam BL, Connolly JD. Spathodic acid: a triterpene acid from the stem bark of Spathodea campanulata. Phytochemistry, 1990; 29:3959–61; doi:10.1016/0031-9422(90)85376-Q CrossRef
Ngouela S, Tsamo E, Sondengam B. Extractives from Bignoniaceae: constituents of the stem bark of Spathodea campanulata. Planta Med, 1988; 54:476–6; doi:10.1055/s-2006-962516 CrossRef
Ngouela S, Tsamo E, Sondengam BL, Connolly JD. Spathodol, a new polyhydroxysterol from the leaves of Spathodea campanulata. J Nat Prod, 1991; 54:873–6; doi:10.1021/np50075a023 CrossRef
Niyonzima G, Laekeman G, Witvrouw M, Van Poel B, Pieters L, Paper D, De Clercq E, Franz G, Vlietinck AJ. Hypoglycemic, anticomplement and anti-HIV activities of Spathodea campanulata stem bark. Phytomedicine, 1999; 6:45–9; doi:10.1016/S0944-7113(99)80034-8 CrossRef
Niyonzima G, Laekeman GM, Scharpe S, Mets T, Vlietinck AJ. Hypoglycemic activity of Spathodea campanulata bark decoctions on streptozotocin-diabetic mice. Planta Med, 1990; 56:682; doi:10.1055/s-2006-961354 CrossRef
Niyonzima G, Pieters L, Balde AM, Claeys M, Laekeman GM, Vlietinck AJ. Isolation of 6-O-caffeoylcatalpol and some other compounds from Spathodea campanulata. Planta Med, 1991; 57:A85–6; doi:10.1055/s-2006-960359 CrossRef
Niyonzima G, Scharpe S, Van Beeck L, Vlietinck AJ, Laekeman GM, Mets T. Hypoglycaemic activity of Spathodea campanulata stem bark decoction in mice. Phyther Res, 1993; 7:64–7; doi:10.1002/ptr.2650070115 CrossRef
Noumi E, Fozi FL. Ethnomedical botany of epilepsy treatment in Fongo-Tongo village, Western Province, Cameroon. Pharm Biol, 2003; 41:330–9; doi:10.1076/phbi.41.5.330.15944 CrossRef
Noumi E. Treating fibromyoma with herbal medicines in South Cameroon. Indian J Tradit Knowl, 2010; 9:736–41.
Nwafor F, Tchimene M, Onyekere P, Nweze N, Orabueze C. Ethnobiological study of traditional medicine practices for the treatment of chronic leg ulcer in South eastern Nigeria. Indian J Tradit Knowl, 2018; 17:34–42.
Nwauzoma AB, Dappa MS. Ethnobotanical studies of Port Harcourt Metropolis, Nigeria. ISRN Bot, 2013; 2013:1–11; doi:10.1155/2013/829424 CrossRef
Nyamukuru A, Tabuti JRS, Lamorde M, Kato B, Sekagya Y, Aduma PR. Medicinal plants and traditional treatment practices used in the management of HIV/AIDS clients in Mpigi District, Uganda. J Herb Med, 2017; 7:51–8; doi:10.1016/j.hermed.2016.10.001 CrossRef
Odongo E, Mungai N, Mutai P, Karumi E, Mwangi J, Omale J. Ethnobotanical survey of medicinal plants used in Kakamega County, western Kenya. Appl Med Res, 2018; 4:22–40; doi:10.5455/amr.20180315095706 CrossRef
Offoumou M, Kipre G, Zirihi G, N’guessan K, Silue K, Bouable G, Djaman A, Grellier P. Ethnopharmacology study and phytochemical screening of twelve Ivorian antimalaria plants. Int J Recent Adv Multidiscip Res, 2017; 04:2441–3.
Ofori-Kwakye K, Kwapong AA, Adu F. Antimicrobial activity of extracts and topical products of the stem bark of Spathodea campanulata for wound healing. African J Tradit Complement Altern Med, 2009; 6:168–74; doi:10.4314/ajtcam.v6i2.57089 CrossRef
Ofori-Kwakye K, Kwapong AA, Bayor MT. Wound healing potential of methanol extract of Spathodea campanulata stem bark formulated into a topical preparation. African J Tradit Complement Altern Med, 2011; 8:218–23; doi:10.4314/ajtcam.v8i3.65280 CrossRef
Omale JM, Mutai P, Njogu P, Mukungu N, Mwangi J, Odongo E. Ethnobotanical survey of medicinal plants used in Emuhaya Subcounty, Vihiga County in Western Kenya. Appl Med Res, 2020; 7:6–25. CrossRef
Osei-Djarbeng S, Agyekum-Attobra E, Nkansah R, Solaga, D, Osei-Asante S, Owusu-Dapaah G. Medicinal plants constituting antimalarial herbal preparations in the ghanaian market. Br J Pharm Res, 2015; 5:153–62; doi:10.9734/bjpr/2015/14896 CrossRef
Palande N. Acute oral toxicity study of extract of Spathodea campanulata bark in wistar rats. J Pharm Sci Biosci Res, 2015; 5:613–6.
Parekh J, Chanda S. In vitro screening of antibacterial activity of aqueous and alcoholic extracts of various Indian plant species against selected pathogens from Enterobacteriaceae. Afr J Microbiol Res, 2007; 1:92–9.
Parekh J, Chanda SV. Antibacterial activity of aqueous and alcoholic extracts of 34 Indian medicinal plants against some Staphylococcus species. Turkish J Biol, 2008; 32:63–71.
Patil S, Patil V, Ghodke D, Kondawar M, Naikwade N, Magdum C. Formulation of gel and its UV protective study of some medicinal flowers. Asian J Pharm Anal, 2011; 1:34–5.
Patil V, Patil S, Kondawar M, Naikwade N, Magdum C. Study of methanolic extract of flower of Spathodea campanulata L. as an anti-solar. Int J Green Pharm, 2009; 3:248–9; doi:10.4103/0973-8258.56285 CrossRef
Pianaro A, Pinto JP, Ferreira DT, Ishikawa NK, Braz-Filho R. Iridoid glucoside and antifungal phenolic compounds from Spathodea campanulata roots. Semina, 2007; 28:251–6. CrossRef
Prashanth Kumar GM, Shiddamallayya N. Survey of wild medicinal plants of Hassan district, Karnataka. J Med Plants Stud, 2016; 4:91–102.
Priyadarshini ES, Ragan A. Survey on folklore medicinal plants knowledge of inhabitants of Khammam District, Telangana, India. Ann Plant Sci, 2019; 8:3583–90.
Rai K, Pathan S, Dharamdasani L, Nair P, Bodhankar P. Synthesis of silver nanoparticle from Spathodea campanulata leaf extract and study of its antimicrobial and antioxidant activity. Int J Heal Sci Res, 2017; 7:155–64.
Rivera LW, Aide TM. Forest recovery in the Karst region of Puerto Rico. For Ecol Manage, 1998; 108:63–75; doi:10.1016/S0378-1127(97)00349-6 CrossRef
Roger T, Pierre-Marie M, Igor VK, Patrick VD. Phytochemical screening and antibacterial activity of medicinal plants used to treat typhoid fever in Bamboutos division, West Cameroon. J Appl Pharm Sci, 2015; 5:034–49; doi:10.7324/JAPS.2015.50606 CrossRef
Sangeetha S, Meenakshi S, Akshaya S, Vadivel V, Brindha, P. Evaluation of total phenolic content and antioxidant activity of different solvent extracts of leaf material of Spathodea campanulata P. Beauv. and investigation of their proliferation inhibition potential against EAC cell line. J Appl Pharm Sci, 2016; 6:121–7; doi:10.7324/JAPS.2016.60918 CrossRef
Santos VHM, Minatel IO, Lima GPP, Silva RMG, Chen CYO. Antioxidant capacity and phytochemical characterization of Spathodea campanulata growing in different climatic zones in Brazil. Biocatal Agric Biotechnol, 2020; 24:101536: doi:10.1016/j.bcab.2020.101536 CrossRef
Santos VHM, Minatel IO, Reco PC, Garcia A, Lima GPP, Silva RMG. Peptide composition, oxidative and insecticidal activities of nectar from flowers of Spathodea campanulata P. Beauv. Ind Crops Prod, 2017; 97:211–7; doi:10.1016/j.indcrop.2016.12.025 CrossRef
Santosh Kumar J, Krishna Chaitanya M, Semotiuk AJ, Krishna V. Indigenous knowledge of medicinal plants used by ethnic communities of South India. Ethnobot Res Appl, 2019; 18:1–112; doi:10.32859/era.18.4.1-112 CrossRef
Saranya M, Mohanraj RS, Dhanakkodi B. Adult emergence and prolong larval duration effects of Spathodea campanulata aqueous leaf extract against the dengue vector Aedes aegypti. Eur J Exp Biol, 2013a; 4:372–80.
Saranya M, Sundaram Mohanraj R, Dhanakkodi B. Larvicidal, pupicidal activities and morphological deformities of Spathodea campanulata aqueous leaf extract against the dengue vector Aedes aegypti. Pelagia Res Libr Eur J Exp Biol, 2013b; 3:205–13.
Satish S, Mohana DC, Raghavendra MP, Raveesha KA. Antifungal activity of some plant extracts against important seed borne pathogens of Aspergillus sp. J Agric Technol, 2007; 3:109–19.
Satish S, Raghavendra MP, Raveesha KA. Evaluation of the antibacterial potential of some plants against human pathogenic bacteria. Adv Biol Res (Rennes), 2008; 2:44–8.
Scogin R. Anthocyanins of the Bignoniaceae. Biochem Syst Ecol, 1980; 8:273–6; doi:10.1016/0305-1978(80)90058-7
Shams KA, Abou-Setta LM, Radwan HM, Nazif NM, Hassan RA, Soliman AMM. Molluscicidal activity and screening of forty Egyptian medicinal plants and determination of the active fractions. Asian J Chem, 2012; 24:3548–52.
Shehab NG, Eid HH, El Zalabani SM. Bioactivity and composition of the flowers of Spathodea campanulata P. Beauv. World J Pharm Res, 2014; 3:213–30.
Shehu MW, Bello I, Abdulkadir N, Shehu A, Jamil SE, Waziri SA. Utilization of medicinal plants used in the management of HIV/AIDS opportunistic infections in Njeru sub-county, buikwe district, Uganda. MOJ Bioequiv Availab, 2018; 5:66–72.
Shiracko N, Owuor BO, Gakuubi MM, Wanzala W. A survey of ethnobotany of the AbaWanga people in Kakamega County, western province of Kenya. Indian J Tradit Knowl, 2016; 15:93–102.
Shukla AN, Srivastava S, Rawat AKS. A survey of traditional medicinal plants of Uttar Pradesh (India) – used in treatment of infectious diseases. Nat Sci, 2014; 11:24–36.
Simbo DJ. An ethnobotanical survey of medicinal plants in Babungo, Northwest Region, Cameroon. J Ethnobiol Ethnomed, 2010; 6:8; doi:10.1186/1746-4269-6-8
Sonibare MA, Osiyemi OA. Morphological and anatomical studies of two medicinal plants: Harrisonia abyssinica Oliv. (Simaroubaceae) and Spathodea campanulata P. Beauv. (Bignoniaceae) and their systematic significance. J Chem Pharm Res, 2012; 4:800–7.
Subramanian SS, Nagarajan S, Sulochana N. Flavonoids of eight Bignoniaceous plants. Phytochemistry, 1972; 11:1499; doi:10.1016/S0031-9422(00)90115-8
Subramanian SS, Sulochana N, Nagarajan S. Caffeic acid from leaves of Spathodea campanulata. Curr Sci, 1973; 42:403.
Sy GY, Nongonierma RB, Ngewou PW, Mengata DE, Dieye AM, Cisse A, Faye B. Healing activity of methanolic extract of the barks of Spathodea campanulata Beauv (Bignoniaceae) in rat experimental burn model. Dakar Med, 2005; 50:77–81.
Tabuti JRS, Lye KA, Dhillion SS. Traditional herbal drugs of Bulamogi, Uganda: plants, use and administration. J Ethnopharmacol, 2003; 88:19–44; doi:10.1016/S0378-8741(03)00161-2
Tabuti JRS, Kukunda CB, Waako PJ. Medicinal plants used by traditional medicine practitioners in the treatment of tuberculosis and related ailments in Uganda. J Ethnopharmacol, 2010; 127:130–6; doi:10.1016/j.jep.2009.09.035
Takuya K, Toru O. Screening of medicinal plants in Egypt for anti-human immunodeficiency virus type-1 (HIV-1) activity. Jasmr, 2009; 4:1–8.
Talla C, Nga N, Ngoupayo J, Mpondo EM. Evaluation of the androgenic properties of the aqueous extract of the trunk bark of Spathodea campanulata P. Beauv. (Bignoniaceae) in male rats. Health Sci Dis, 2021; 22(3):60–8.
Tanayen JK, Ajayi AM, Ezeonwumelu JOC, Oloro J, Tanayen GG, Adzu B, Agaba AG. Antidiabetic properties of an aqueous-methanolic stem bark extract of Spathodea campanulata (Bignoniaceae) P. Beauv. Br J Pharmacol Toxicol, 2014; 5:163–8; doi:10.19026/bjpt.5.5182
Tanayen JK, Ezeonwumelu JOC, Ajayi AM, Oloro J, Tanayen GG, Adzu B, Van Aerschot A, Laekeman G, Agaba AG. Evaluation of the acute and subacute chronic toxicities of the methanolic stem bark extract of Spathodea campanulata (P. Beauv.) Bignoniaceae. Br J Pharmacol Toxicol, 2016; 7:9–19.
Taniguchi M, Chapya A, Kubo I, Nakanishi K. Screening of east african plants for antimicrobial activity. I. Chem Pharm Bull, 1978; 26:2910–3; doi:10.1248/cpb.26.2910
Terashima H, Malasi NM. Ethnobotany of the Lega in the tropical rain forest of eastern Zaire: part one, Zone de Mwenga. Afr Study Monogr Suppl, 1991; 15:1–61.
Tetteh A, Thomford K, Mensah M, Boadu K, Thomford A, Amposah I, Amofa G, Turkson B, Agyemang M, Owusu-Ansah EJ. Ghanaian herbal medicines for malaria: an evaluation of the clinical safety and effectiveness of “Time Herbal Mixture” in uncomplicated malaria. Pharmacognosy Res, 2020; 12:71; doi:10.4103/pr.pr_23_19
Tewari D, Samoila O, Gocan D, Mocan A, Moldovan C, Devkota HP, Atanasov AG, Zengin G, Echeverria J, Vodnar D, Szabo B, Crisan G. Medicinal plants and natural products used in cataract management. Front Pharmacol, 2019; 10:1–22; doi:10.3389/fphar.2019.00466
Thampi N, Kumar SN. Evaluation of antimicrobial and free radical scavenging potentials of three valuable medicinal plants-Hyptis suaveolens, Spathodea campanulata and Shorea robusta. J Che Pharm Res, 2015; 7:609–15.
Titanji VPK, Zofou D, Ngemenya MN. The antimalarial potential of medicinal plants used for the treatment of malaria in Cameroonian folk medicine. African J Tradit Complement Altern Med, 2008; 5:302–21.
Tsabang N, Fongnzossie E, Keumeze V, Jiofack R, Njamen D, Sonwa DJ, Nguelefack BT. Ethnomedical and ethnopharmacological study of plants used by indigenous people of Cameroon for the treatments of diabetes and its signs, symptoms and complications. J Mol Biomark Diagn, 2017; 8:1–5.
Tsobou R, Mapongmetsem PM, Van Damme P. Medicinal plants used against typhoid fever in Bamboutos division, western Cameroon. Ethnobot Res Appl, 2013; 11:163–74; doi:10.17348/era.11.0.163-174
Tugume P, Kakudidi EK, Buyinza M, Namaalwa J, Kamatenesi M, Mucunguzi P, Kalema J. Ethnobotanical survey of medicinal plant species used by communities around Mabira Central Forest Reserve, Uganda. J Ethnobiol Ethnomed, 2016; 12:5; doi:10.1186/s13002-015-0077-4
Tugume P, Nyakoojo C. Ethno-pharmacological survey of herbal remedies used in the treatment of paediatric diseases in Buhunga parish, Rukungiri District, Uganda. BMC Complement Altern Med, 2019a; 19:353; doi:10.1186/s12906-019-2763-6
Tugume P, Nambejja C, Nyakoojo C, Kamatenesi-Mugisha M. Medicinal plant species used in the treatment of skin diseases in Katabi Subcounty, Wakiso District, Uganda. Ethnobot Res Appl, 2019b; 18:1–17; doi:10.32859/era.18.20.1-17
Uchenna M, Nwozo S, Mohammed B, Garba R, Mohammed Y. Spathodea companulata (African tulip tree) stem and root barks extracts ameliorated n-nitroso diethylamine induced hepatic impairment in male rats. BIOMED Nat Appl Sci, 2021; 1(1):57–65.
Umenwa CE, Ojah EO, Moronkola DO, Adesanwo JK. Phytochemical and antioxidant assessments of three fractions from methanol extract of Spathodea campanulata Beauv. leaves. J Complement Altern Med Res, 2017; 3:1–10.
Vijayasanthi M, Doss A, Kannan KV. Anti-inflammatory activity of Spathodea campanulata P. Beauv. leaves against carrageenan induced paw edema. Elixir Bio Tech, 2015; 78:29450–2.
Vijayasanthi M, Kannan V. Antimicrobial activities of Delonix elata (Bojer ex Hook.) Raf. and Spathodea campanulata P. Beauv. African J Microbiol Res, 2014; 8:697–701; doi:10.5897/ajmr2013.6192
Villanueva-Partida CR, Casanova-Lugo F, Gonzalez-Valdivia NA, Villanueva-Lopez G, Oros-Ortega I, Cetzal-Ix W, Basu SK. Traditional uses of dispersed trees in the pastures of the mountainous region of Tabasco, Mexico. Agrofor Syst, 2019; 93:383–94; doi:10.1007/s10457-017-0125-2
Wagh A, Butle S, Telang P. In vitro anthelmintic efficacy of Spathodea campanulata P. Beauv. (Bignoniaceae) against Pheretima posthuma. Asian J Pharmacogn, 2019; 3:32–8.
Wagh A, Butle S, Raut D. Isolation, identification, and cytotoxicity evaluation of phytochemicals from chloroform extract of Spathodea campanulata. Future J Pharm Sci, 2021; 7(1):1–8; doi:10.1186/s43094-021-00205-7
Wagh AS, Butle SR. Plant profile, phytochemistry and pharmacology of Spathodea campanulata P. Beauvais (African Tulip Tree): a review. Int J Pharm Pharm Sci, 2018; 10:1; doi:10.22159/ijpps.2018v10i5.24096
Waisindye N, Kamatenesi M, Anywar G, Kazibwe F. An ethnobotanical documentation of medicinal plants used by local communities around Kibale National Park, a case of Kanyawara, Kanyansowera and Ibura Villages. Int J Adv Inf Sci Technol, 2016; 5:21–36.
Walugembe J, Iramiot JS, Katuura E. Indigenous knowledge and antibacterial activity of selected herbs used locally to treat common cold in Central Uganda. J Med Plants Res, 2016; 10:520–8; doi:10.5897/jmpr2016.6181
Yemele MD, Telefo PB, Lienou LL, Tagne SR, Fodouop CSP, Goka CS, Lemfack MC, Moundipa FP. Ethnobotanical survey of medicinal plants used for pregnant womens health conditions in Menoua division-West Cameroon. J Ethnopharmacol, 2015; 160:14–31; doi:10.1016/j.jep.2014.11.017