INTRODUCTION
The immune system is a system responsible for the protection of the body against foreign matters entering, thereby preventing the body function from being disturbed. The functions of the immune system include improving human DNA, preventing infections from fungi, bacteria, viruses, and other organisms, and producing antibodies (immunoglobulin) to combat the assault of foreign bacteria and viruses on the body. The non-specific immune system is the first-line protection against foreign microorganisms or matters that come into the body (Balekar et al., 2014).
In general, diseases related to the immune system result from either deficient or excessive expression of the immune system. Deficiency in the immune system is called immunodeficiency. Such a condition makes the body vulnerable toward infectious disease attacks. It is, therefore, of utmost importance to preserve the body’s condition in order to maintain the immune system in the body. One effort that can be made for this purpose is to consume immunostimulants that are capable of promoting the immune system of the body (Balekar et al., 2014).
Some in vitro and in vivo tests may be conducted to examine the immunomodulatory activity. Phagocytosis is one of the methods frequently used to examine the immune response activity. It is a primary defensive mechanism against foreign substances that penetrate the body which is triggered by neutrophils and macrophages. The process of phagocytosis consists of sequential stages such as motility, adhesion to microorganisms, ingestion of microorganisms, degranulation, and intracellular killing of microorganisms (Uribe-Querol et al., 2017).
A previous study by Ulfah et al. (2013) has proven that the ethanol extract of the Hibiscus sabdariffa L. flower demonstrates immunomodulatory activity. The ethanol extract of the H. sabdariffa L. flower stimulates lymphocyte proliferation in Swiss Webster mice in vitro in the 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide assay at concentrations of 50, 100, 200, and 400 μg/ml. Another study by Fakaye (2008) tested the ethyl acetate fraction of H. sabdariffa L. which showed immunomodulatory activity at 100 mg/kg BW.
This study was conducted to further assay the immunomodulatory activity of H. sabdariffa L. flower. The aims of this study were to fractionate the ethyl acetate fraction of H. sabdariffa L. using vacuum liquid chromatography (VLC), to test the immunomodulatory activity of the H. sabdariffa L. fraction, and to identify the phytochemical compounds of the H. sabdariffa L. fraction using liquid chromatography-mass spectroscopy (LC-MS).
MATERIALS AND METHODS
Chemicals
The chemicals used in this study are as follows: 96% ethanol, ethyl acetate, n-hexane, chloroform, silica gel GF 254 (Merck), nutrient agar media, Aquadest, Stimuno, 1% BaCl2, 1% H2SO4, 0.9% NaCl, 4% Giemsa stain, methanol, and immersion oil. All chemicals were purchased from a standard local source.
Animals
Albino mice (20–30 g) were caged in a hygienic condition at the Pharmaceutical Biology Laboratory. They were caged at a standard temperature (25°C ± 5°C) in a 12:12 hour light and dark cycle, in which a standard pellet diet was applied to the mice. Animal testing was carried out with the approval of the Animal Ethics Committee with Letter No. 177a/UN29.20.1.2/PG/2021.
Plant materials and fractionation
The locally sourced H. sabdariffa L. flower was subjected to determination at the Department of Biology of Universitas Haluoleo. The flower was dried at room temperature and then pulverized and macerated with an ethanol solvent. The ethanol extract was freed from glycosides by the liquid–liquid extraction method using ethyl acetate and water. The ethyl acetate fraction was fractionated by the VLC method with hexane, a combination of hexane and ethyl acetate, ethyl acetate, and methanol solvents. From this fractionation, 20 fractions were obtained to be analyzed by node analysis using thin-layer chromatography (TLC) with a chloroform : methanol mobile phase of 1:9. Fractions with the same TLC profiles were merged as single fractions.
Phytochemical analysis
Hibiscus sabdariffa L. flower fractions were analyzed using LC-MS. LC-MS acted as an interfaced with a Quadrupole Time of Flight (Q-TOF) Mass Spectrometer fitted with an ESI source. A full-scan mode from m/z 500 to 1,200 was carried out. The solvents were methanol and water at 1:9. Solvents were delivered at a total flow rate of 0.3 ml/minute. Mobile phases consisted of 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B). Separation was carried out in 25 minutes under the following conditions: gradients starting at 95% A and 5% B in 8 minutes, to 60% A and 40% B in 3 minutes, to 100% B in 2 minutes, and to 95% A in 2 minutes. Components were identified by matching their mass spectra with those of the Wiley library database.
Immunomodulatory evaluation
The immunomodulatory activity testing involved 45 mice (Mus musculus). The experimental animals were assigned to nine treatment groups, with each group consisting of five mice (number of experimental animals per group based on Federer’s formula).
The divisions are as follows:
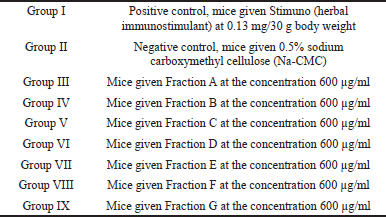 | [Click here to view] |
All the groups were given treatments with each sample orally every day for 1 week. On day 8, every mouse was intraperitoneally infected with a 0.5 ml (0.5 McFarland standard) Staphylococcus aureus bacterial suspension and left to stand for 1 hour. The mice were euthanized with ether to be dissected in their bellies using sterile surgical scissors and tweezers. The peritoneal liquid was extracted using a syringe. It was daubed on the object glass and fixated with methanol for 5 minutes. Then, it was stained with 4% Giemsa stain, left to stand for 20 minutes, and rinsed with flowing water. After the preparations were dried, they were then observed under a microscope using immersion oil at 10–100× and computed for macrophage phagocytosis (Fig. 1) (Parawansah et al., 2018).
The phagocytosis values were computed using the following formula (Parawansah et al., 2018):
Statistical analysis
Values are expressed as mean values ± standard error. One-way analysis of variance, followed by Tukey’s test was applied for the statistical evaluation of the results obtained in the tests. P-values < 0.05 were regarded as significant.
RESULTS AND DISCUSSION
Extraction and fractionation
The extraction of the H. sabdariffa L. flower yielded 42.5 g of thick extract (8.2% yield). The liquid–liquid extraction yielded 20.75 g of ethyl acetate extract. The fractionation of the ethyl acetate extract by the VLC method yielded 20 fractions. Of the 20 fractions from VLC fractionation with a chloroform : methanol mobile phase of 1:9, some shared the same TLC profiles, necessitating some merging. Seven fractions were derived as the final results: Fractions A (0.19 g), B (0.37 g), C (1.76 g), D (0.87 g), E (3.81 g), F (2.24 g), and G (1.99 g).
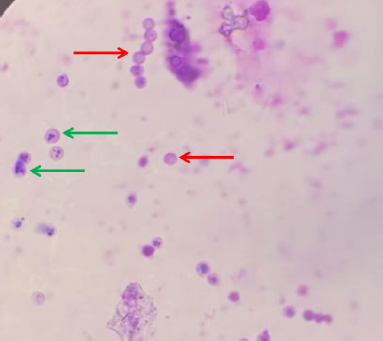 | Figure 1. Phagocytosis microscopic view (red arrows: inactivated phagocytes, green arrows: activated phagocytes). [Click here to view] |
Phytochemical analysis
The results of the phytochemical analysis of the H. sabdariffa L. fractions using LC-MS are presented in Table 1 and Figure 2.
Immunomodulatory evaluation
The results of the immunomodulatory evaluation can be seen in Table 2 and Figure 3. It was shown that all the treatments, except the treatment with Fraction E use, were significantly different from the negative control (Na-CMC). This shows that Stimuno and Fractions A, B, C, D, F, and G have immunomodulatory activity. The samples with Fractions A, B, F, and G were significantly different from the positive control (Stimuno), suggesting even better activity. Fraction A showed the best activity (95.83%).
DISCUSSION
This research aimed at examining the immunomodulatory activity of H. sabdariffa L. flower fractions with the phagocytosis method using mice (M. musculus) as experimental animals and at identifying the chemical compounds with the LC-MS method.
The activity examination was conducted by administering fractions, Stimuno (positive control), and Na-CMC (negative control) to the experimental animals for 7 days in a row orally to give the samples time to improve their nonspecific immune response. On day 8, each mouse was infected with a S. aureus bacterial suspension intraperitoneally at 0.5 ml. S. aureus was used as it is categorized as a Gram-positive bacterium. Gram-positive bacteria are able to bind Giemsa stain clearly, thereby making it easy to compute under the microscope. Moreover, containing no protein A, a protein that is antiphagocytic in nature, S. aureus is unable to escape from peritoneal macrophage phagocytosis (Fristiohady et al., 2019).
After being injected with bacterial suspension, all the treatment groups were left to stand for 1 hour prior to dissection. This was to know to which extent the macrophage bacterial activation ability was. The macrophages were able to withstand infection for the first period of about 1 hour before another immunity mechanism could be mobilized. Therefore, macrophage extraction was carried out approximately for 1 hour after bacterial induction. In this manner, to which extent the macrophages were able to deal with bacterial invasion could be identified (Wahyuni et al., 2019).
The peritoneal liquids obtained were then smeared thinly on preparate slides to be observed under a light microscope at 1,000× with immersion oil applied. Active macrophages were marked as growing macrophages in size and shape, with extremely varied pseudopodia protrusions. The phagosomes grew with increasingly wavy membranes, the lysosomes grew in quantity, the Golgi apparatuses enlarged, and the rough endoplasmic reticulums developed. On the other hand, the inactive macrophages were smaller in size and shape relative to the active macrophages (Rougerie et al., 2013).
The S. aureus bacterial infection involved in this research triggered infection in mice. During the infection process, T lymphocytes produce some lymphokines that will attract macrophages to places in need and activate them. Active macrophages release a number of important substances: enzymes, lysozymes, elastases, collagenases, complements, and cytokines. Cytokines that are secreted by macrophages include interleukin (IL)-1, IL-6, IL-8, IL-12, IL-15, and tumor necrosis factor (TNF-α). Under a normal condition, this process runs slowly, so the active macrophages that undergo phagocytosis resulting are small in quantity (Wahyuni et al., 2019).
The test results showed that the percentages of phagocytosis with Fractions A, B, C, D, F, and G suggested immunomodulatory activity levels that statistically were significant in their differences from the negative control (Na-CMC). This research also conducted a comparison with a positive control that used Stimuno. Stimuno is an immunomodulatory traditional medication preparation used in Indonesia. Fractions C and D did not show any difference in their activity from Stimuno. Meanwhile, Fractions A, B, F, and G did show better activity than Stimuno.
The results of the compound identification of the H. sabdariffa L. flower fractions show several compound components in the fractions. Some of the previous studies have reported these compounds in the H. sabdariffa L. flower: coumarin (Adeyemi et al., 2014), 3-hydroxy-7-methoxy baicalin (Alara et al., 2020), 5.7.2′,5′-tetrahydroxy-flavone (Da-Costa-Rocha et al., 2014), undecanoic acid (Jabeur et al., 2019), 9,12-octadecadienoic acid (Z,Z)-(2,2-dimethyl-1,3-dioxolan-4-yl)methyl ester (Hagr et al., 2020), salvianolic acid A (Amin et al., 2005), and kaempferol derivatives (Vargas-Álvarez et al., 2018).
Some compounds have been reported to exhibit activity as immunomodulators. Stefanova et al. (2007) have reported that coumarin treatment enhanced the macrophage migration activity in the presence and absence of lipopolysaccharide (LPS) and increased nitric oxide release. In vitro, coumarins induced IL-12 in murine macrophages and additively increased the LPS-induced IL-12 release. Orzechowska et al. (2014) reported that the Scutellariae radix extract, whose active compound is baicalin, was able to modulate the immune system by increasing the production of interferon-γ (IFN-γ) in peripheral blood leukocytes and reducing the production of TNF-α and IL-10 in bone marrow cells.
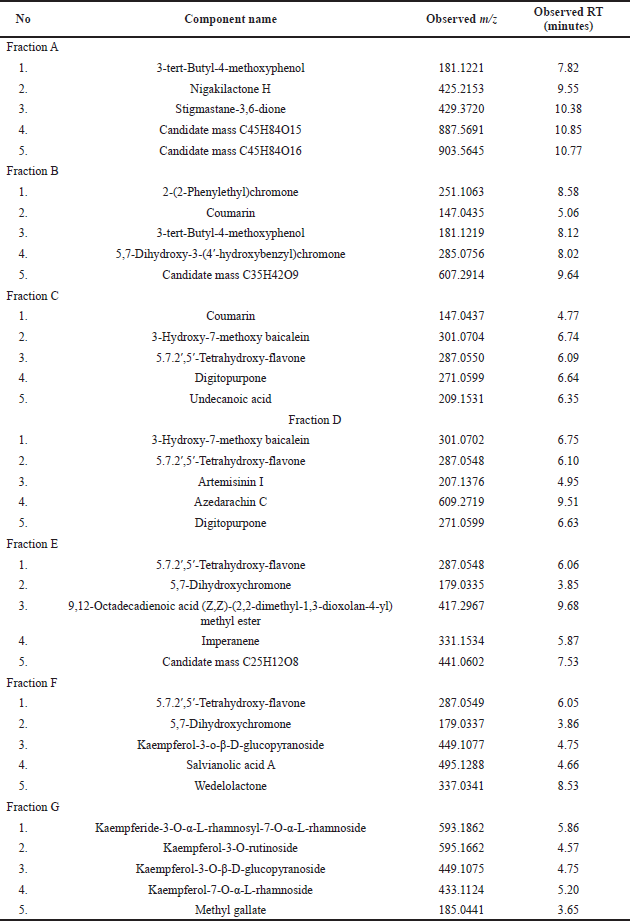 | Table 1. Results of LC-MS analysis of H. sabdariffa L. flower fractions. [Click here to view] |
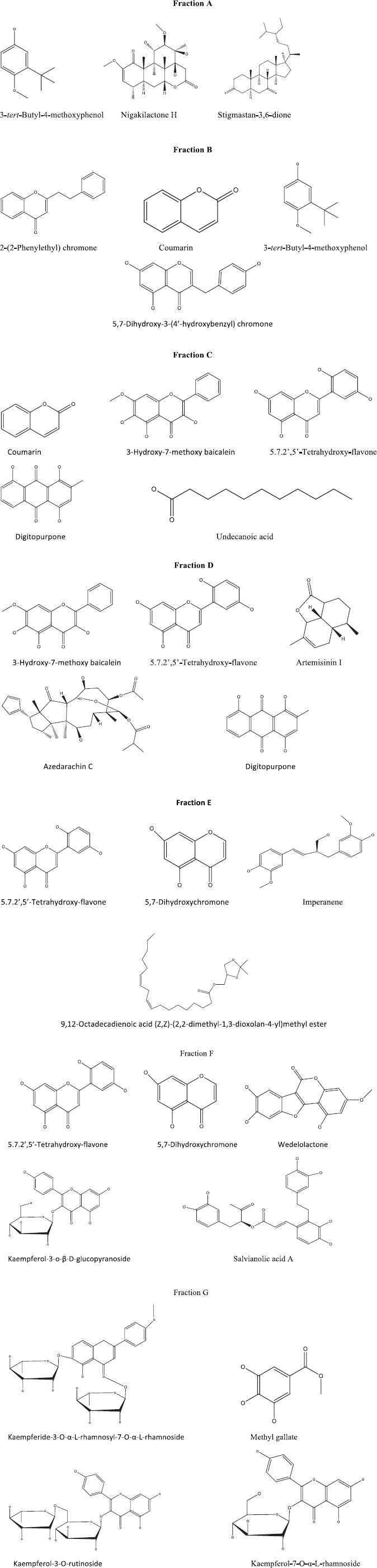 | Figure 2. Identified compounds of H. sabdariffa L. flower fractions. [Click here to view] |
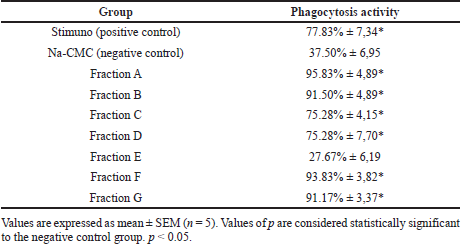 | Table 2. Immunomodulatory evaluation of H. sabdariffa L. flower fraction. [Click here to view] |
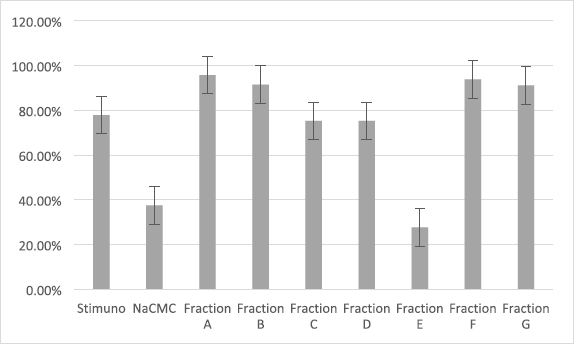 | Figure 3. Immunomodulatory evaluation of H. sabdariffa L. flower fraction. [Click here to view] |
Such fatty acid compounds as undecanoic acids have also been reported as immunomodulators, being able to affect both the innate and adaptive responses. Fatty acids are able to enhance neutrophil aggregation, neutrophil-endothelial cell attachment, and phagocytic and candidacidal capacities (Di Sotto et al., 2020). Other studies also reported that fatty acids are able to boost the production of anti-inflammatory cytokines like IL-4, in spite of a reduction of proinflammatory TNF-α (Kumar et al., 2019).
Hibiscus sabdariffa L. fractions contain many flavonoids such as 5.7.2’,5’-tetrahydroxy-flavone and kaempferol derivatives. Flavonoids have been widely known as immunomodulators. They have the potential of working against the lymphokines produced by T cells, thereby stimulating phagocytes to exhibit phagocytosis responses (Hosseinzade et al., 2019). Flavonoids have proven to be able to boost IL-2 and lymphocyte proliferation. Lymphocyte proliferation will influence CD4+ cells, which in turn will cause Th1 cells to be activated. Activated Th1 cells will influence specific macrophage-activating factors (SMAFs). SMAFs per se are multiple molecules, one of which is IFN-γ. IFN-γ will activate macrophages, leading them to undergo an increase in phagocytosis. This will result in the macrophages being capable of killing bacteria faster. Flavonoids also have a working mechanism by activating NK cells to stimulate IFN-γ production. IFN-γ is a main macrophage-activating cytokine, which will activate macrophages and trigger the elevation of phagocytosis activity. Activated macrophages and neutrophils will produce some proteolytic enzymes in phagolysosomes such as elastase and cathepsin G that function in destroying bacteria (Mendes et al., 2019; Sulistiani et al., 2015).
Steroid compounds have also been reported to act as immunomodulators. Heroor et al. (2020) reported that steroid compounds had higher activity in stimulating phagocytes when compared to alkaloids, flavonoids, and tannins. Steroid compounds are also known to induce a Th1 response in T-helper cells (Brüll et al., 2009).
The immunomodulatory activity of Fractions A, B, C, D, F, and G H. sabdariffa L. flower contains compounds that act as immunomodulators. Fraction A as the most active fraction contains 3-tert-butyl-4-methoxyphenol (phenol) and stigmastan-3,6-dione (steroid) compounds. The activity of Fraction A may be caused by the synergistic effect of the two compounds, especially steroid compounds which have high immunomodulatory activity.
Hibiscus sabdariffa L. flower fractions exhibit many compounds that have potential as immunomodulators. Immunomodulatory activity can result from the synergetic effect of the compounds. For further research, it is suggested to carry out active compounds isolation to determine the active compounds.
CONCLUSION
Fractions A, B, C, D, F, and G of the H. sabdariffa L. flower showed immunomodulatory activity. Fraction A displayed the best activity among all the fractions. The results of the identification of the chemical compounds in the H. sabdariffa L. flower fractions showed that some of the compounds contained have potential as immunomodulators.
ACKNOWLEDGMENT
The authors are grateful to Universitas Mandala Waluya for the fund aid for this research completion.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
CONFLICT OF INTEREST
The authors confirm that this article’s content has no conflicts of interest.
ETHICAL APPROVALS
Animal testing was carried out with the approval of the Animal Ethics Committee with Letter No. 177a/UN29.20.1.2/ PG/2021.
LIST OF ABBREVIATIONS
TLC Thin-layer chromatography
VLC Vacuum liquid chromatography
Na-CMC Sodium carboxymethyl cellulose
LC-MS Liquid chromatography-mass spectroscopy
IL Interleukin
LPS Lipopolysaccharide
IFN Interferon
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Adeyemi DO, Ukwenya VO, Obuotor EM, Adewole SO. Anti-hepatotoxic activities of Hibiscus sabdariffa L. in animal model of streptozotocin diabetes-induced liver damage. BMC Complement Altern Med, 2014; 14(1):1–1. CrossRef
Alara OR, Abdurahman NH, Obanijesu EO, Alara JA, Abdul Mudalip SK. Extract-rich in flavonoids from Hibiscus sabdariffa calyces: optimizing microwave-assisted extraction method and characterization through LC-Q-TOF-MS analysis. J Food Process Eng, 2020; 43(2):e13339. CrossRef
Amin A, Hamza AA. Hepatoprotective effects of Hibiscus, Rosmarinus and salvia on azathioprine-induced toxicity in rats. Life Sci, 2005; 77(3):266–78. CrossRef
Balekar N, Bodhankar S, Mohan V, Thakurdesai PA. Modulatory activity of a polyphenolic fraction of Cinnamomum zeylanicum L. bark on multiple arms of immunity in normal and immunocompromised mice. J App Pharm Sci, 2014; 4(7):114–22.
Brüll F, Mensink R. Plant sterols: functional lipids in immune function and inflammation? Clin Lipidol, 2009; 4(3):355–65. CrossRef
Da-Costa-Rocha I, Bonnlaender B, Sievers H, Pischel I, Heinrich M. Hibiscus sabdariffa L.–A phytochemical and pharmacological review. Food Chem, 2014; 165:424–43. CrossRef
Di Sotto A, Vitalone A, Di Giacomo S. Plant-derived nutraceuticals and immune system modulation: an evidence-based overview. Vaccines, 2020; 8(3):468. CrossRef
Fakaye T. Toxicity and immunomodulatory activity of fractions of Hibiscus sabdariffa Linn (family Malvaceae) in animal models. Afr J Tradit Complement Altern Med, 2008; 5(4):394–8. CrossRef
Fristiohady A, Wahyuni W, Malik F, Leorita M, Yusuf MI, Febriansyah H, Sahidin S. Efek Imunomodulator Ekstrak Etanol Spons Xestospongia Sp. Terhadap Aktivitas Fagositosis Makrofag Pada Mencit Jantan Galur Balb/C. J Mandala Pharmacom Indones, 2019; 5(01):15–30. CrossRef
Hagr T, Adam I. Phytochemical analysis, antibacterial and antioxidant activities of essential Oil from Hibiscus sabdariffa (L) seeds, (Sudanese Karkadi). Prog Chem Biochem Res, 2020; 3(3):194–201.
Heroor S, Beknal A, Mahurkar N, Hiremath S, Inamdar S. Sterols possess immunomodulatory property: activity guided isolation and in vitro screening of phytoconstituents of Pongamia glabra and Ficus glomerata. Chin Herb Med, 2020; 12(3):310–5. CrossRef
Hosseinzade A, Sadeghi O, Naghdipour Biregani A, Soukhtehzari S, Brandt GS, Esmaillzadeh A. Immunomodulatory effects of flavonoids: possible induction of T CD4+ regulatory cells through suppression of mTOR pathway signaling activity. Front Immunol, 2019; 10:51. CrossRef
Jabeur I, Pereira E, Caleja C, Calhelha RC, Sokovi? M, Catarino L, Barros L, Ferreira IC. Exploring the chemical and bioactive properties of Hibiscus sabdariffa L. calyces from Guinea-Bissau (West Africa). Food Funct, 2019; 10(4):2234–43. CrossRef
Kumar NG, Contaifer D, Madurantakam P, Carbone S, Price ET, Van Tassell B, Brophy DF, Wijesinghe DS. Dietary bioactive fatty acids as modulators of immune function: implications on human health. Nutrients, 2019; 11(12):2974. CrossRef
Mendes LF, Gaspar VM, Conde TA, Mano JF, Duarte IF. Flavonoid-mediated immunomodulation of human macrophages involves key metabolites and metabolic pathways. Sci Rep, 2019; 9(1):14906. CrossRef
Orzechowska B, Chaber R, Wi?niewska A, Pajtasz-Piasecka E, Jatczak B, Siemieniec I, Gulanowski B, Chybicka A, B?ach-Olszewska Z. Baicalin from the extract of Scutellaria baicalensis affects the innate immunity and apoptosis in leukocytes of children with acute lymphocytic leukemia. Int Immunopharmacol, 2014; 23(2):558–67. CrossRef
Parawansah P, Nurtamin T, Mulyawati SA, Nuralifah N, Misnaeni WO. Immunomodulatory effect of Momordica charantia L. fruit ethanol extract on phagocytic activity and capacity of mice peritoneal macrophages. Indon Biomed J, 2018; 10(2):144–7. CrossRef
Rougerie P, Miskolci V, Cox D. Generation of membrane structures during phagocytosis and chemotaxis of macrophages: role and regulation of the actin cytoskeleton. Immunol Rev, 2013; 256(1):222–39. CrossRef
Stefanova TH, Nikolova NJ, Toshkova RA, Neychev HO. Antitumor and immunomodulatory effect of coumarin and 7-hydroxycoumarin against Sarcoma 180 in mice. J Exp Ther Oncol, 2007; 6(2):107–15. CrossRef
Sulistiani RP, Rahayuningsih HM. Pengaruh Ekstrak Lompong Mentah (Colocasia esculenta L Schoot) terhadap Aktivitas Fagositosis dan Kadar NO (Nitrit Oksida) Mencit Balb/C Sebelum dan Sesudah Terinfeksi Listeria monocytogenes. J Nutr Coll, 2015; 4(4):409–15. CrossRef
Ulfah M, Ulfah M, Sasmito E. Uji Aktivitas Imunostimulator Fraksi Etil Asetat Ekstrak Etanol Kelopak Bunga Rosella (Hibiscus Sabdariffa L.) Terhadap Proliferasi Sel Limfosit Mencit Galur Swiss Secara in vitro Beserta Identifikasi Kandungan Senyawa Kimianya. J Ilmu Farmasi dan Farmasi Klinik, 2013; 10(1):23–30.
Uribe-Querol E, Rosales C. Control of phagocytosis by microbial pathogens. Front Immunol, 2017; 8:1368. CrossRef
Vargas-Álvarez D, Chino-Patricio P, Damián-Nava A, Palemón-Alberto F, Hernández-Castro E, Silva-González M. Quercetin, kaempferol and apigenin in roselle (Hibiscus sabdariffa L.). Int J Adv Res Biol Sci, 2018; 5(1):62–6.
Wahyuni W, Yusuf MI, Malik F, Lubis AF, Indalifiany A, Sahidin I. Efek Imunomodulator Ekstrak Etanol Spons Melophlus sarasinorum Terhadap Aktivitas Fagositosis Sel Makrofag Pada Mencit Jantan Balb/C. J Farmasi Galenika, 2019; 5(2):147–57. CrossRef