INTRODUCTION
The medicinal chemistry is an essential tool for development and discovery of new drug for prevention of cure and disease. The new drug design is very complex method for the discovery of new pharmacophore moiety (Jeyaprakash et al., 2009). The benzodiazepines are the class of compound having benzodiazepine type nucleus the only difference between then is of sulfur atom in place of the nitrogen atom in heterocyclic ring system (Jeyaprakash et al., 2009).
Epilepsy is the one of the oldest diseases which found in human being population according to the World Health Organization. The distinctive inclination to recurring seizures and is definite by one or more malicious seizures. Seizure may differ from the least period of time or muscle twitching to chronic and elongated convulsions. They may also diverge in regularity from several months to several days (Ameta et al., 2013).
World Health Organization, epilepsy is a result of cellular dysfunction in the brain region due to excessive firing of a hyper excitability of neurons, and leads to neuronal dysfunction (Pandeya et al., 2003).
1, 5-benzothiazepine nucleus containing drug molecules is used for the lead discovery because it acts against various target receptor site. 1, 5-benzothiazepine nucleus containing first molecule diltiazem that is used as clinical interest, then after clentiazem and clothiapine were used in CNS as well as cardiovascular disorders. Substituted 1, 5-benzothiazepine is an identical crucial agent in the field of new drug development and research (Raja et al., 2007).
1,5-benzothiazepine and its derivatives are significant class of compounds in organic as well as medicinal chemistry. 1,5-benzothiazepine nucleolus is an important pharmacophore ring system which contains various pharmacological biological activities (Ansari et al., 2008).
Literature survey revealed that various substitution at 3 and 5 positions yield a compound of clinical interest. In the past studies, the substitution has been done at various positions in the ring system. Our consideration is mainly at the position 3 and 5. At position number 3, the substitution is the done at the side chain and at position number 5, the H atom is replaced by different suitable substituent.
The newly synthesize molecules were identified by elemental and spectroscopic methods.
The newly synthesized molecules (6a–6j) were preliminary screen as anticonvulsant agent by MES model. Rota rod study was performed for evaluation of neurotoxicity of compounds. Hence, to ascertain and establish the safety for its use, acute toxicity studies as per OCED guidelines 420.
MATERIALS AND METHODS
The determination of melting point of synthesized compound was confirmed by open tube capillary technique. Chemical reaction was monitor through TLC and Rf value determine on silica gel G (E. Merck) plate using chloroform: methanol (9:1) as a mobile phase. Infrared was identified on PerkinElmer FTIR-8400S spectrometer (SHIMADZU, Japan) by pressed pellet technique. Proton NMR spectra were characterized by Bruker DRX-300 spectrophotometer in solvent DMSO-D6. Molecular mass was characterized by electro spray ionization (ESI)-MS (Schimadzu-2010 AT) under ESI technique. Microanalysis was determined by Carlo Erba EA 1108 elemental analyzer. Log P calculated by using octanol-phosphate buffer, and C log P value was identified by software Chem Draw Ultra 8.
All the solvents and reagents were obtained by Qualigens® Fine chemical.
Synthesis of substituted 1, 5-benzothiazepine (6a–6j)
2, 4-Pentadione and piperidine were dissolve in benzene, at the room temperature substituted benzaldehyde was mixed drop wise drop over 20 minutes. The chemical reaction mixture reflux for 2 hours with continuous stirring. Cool the reaction mixtures, organic layer was wash with aqueous cold 10% sodium carbonate, collect the product, and treat with o-aminothiophenol in methanol with stirring about 1 hour, collect the solid product, and washed with water and methanol.
Collected solid product was taken in methanol then acetic acid added until pH reaches 4 and stirred the reaction mixture for 12 hours. Collect the solid product, wash, and recrystallized by methanol. Schiff’s bases (6a–6j) were synthesized by reacting compound (5) with hydrazine hydrate for about 3 hours refluxing. The scheme is given in Figure 1.
Partition coefficient (log P) determination
Partition coefficient (log P) of synthesized compound determine by flask shake method by using octanol and phosphate buffer. The calculate log P (C log P) determine by using software chem. Draw ultra 8 version.
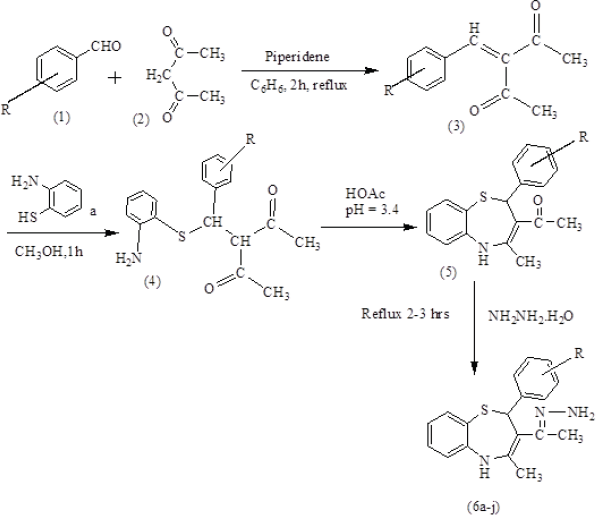 | Figure 1. Scheme for Synthetic route of the Schiff’s base (6a–6j). [Click here to view] |
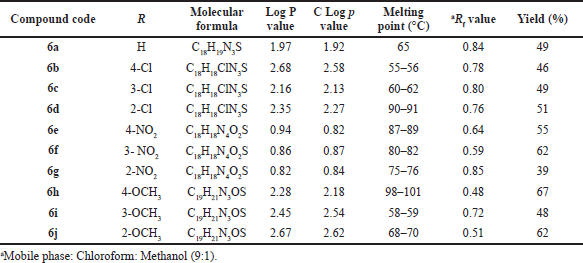 | Table 1. Physicochemical data of compound (6a–6j). [Click here to view] |
COMPOUND DETAIL
{1-[4-Methyl-2-phenyl-2, 5-dihydro-1,5-benzothiazepin-3-yl]-ethylidene}-hydrazine (6a)
Yield 49%; FTIR (KBr) ν, cm−1): 3,380 (N-Hstr), 3,080 (Ar. C-Hstr), 2,958 (Ali. C-Hstr), 1,685 (Ar. C—Cstr), 1,602 (C = Nstr), 1,554 (Ar. C-Cstr), 1,326 (Ar. C-Nstr), 703 (C-Sstr); 1H NMR, δ ppm: 1.210 (s, 3H, CH3), 2.300 (s, 3H, CH3), 4.207 (s, 2H, NH2, D2O exchange), 6.103 (s, 1H, Ar-H), 7.103–7.179 (t, 2H, Ar-H), 7.210–7.243 (d, 2H, Ar-H), 7.501–7.584 (m, 3H, Ar-H), 7.605–7.641 (d, 2H, Ar-H), 8.174 (s, 1H, N-H, D2O exchange); MS (m/z): 310 [M + 1]+; Elemental analysis: C, 68.92, H, 6.36, N, 12.31, S, 09.23%.
{1-[4-Chlorophenyl-4-methyl-2,5-dihydro-1,5-benzothiazepin-3-yl]-ethylidene}-hydrazine (6b)
Yield 46%; FTIR (KBr) ν, cm−1): 3,386 (N-Hstr), 3,089 (Ar. C-Hstr), 2,887 (Ali. C-Hstr), 1,686 (Ar. C-Cstr), 1,598 (C = Nstr), 1,531 (Ar. C-Cstr), 1,363 (Ar. C-Nstr), 1,064 (Ar. C-Clstr), 858 (C-H p-disub. benzene), 686 (C-Sstr); 1H NMR, δ ppm: 1.201 (s, 3H, CH3), 2.700 (s, 3H, CH3), 4.100 (s, 2H, NH2, D2O exchange), 5.951 (s, 1H, Ar-H), 7.203–7.244 (t, 2H, Ar-H), 7.410–7.425 (d, 2H, Ar-H), 7.501–7.527 (d, 2H, Ar-H), 7.854–7.895 (m, 3H, Ar-H), 8.901 (s, 1H, N-H, D2O exchange); MS (m/z): 344 [M + 1]+; 345 [M + 2]+; Elemental analysis: C, 64.83, H, 6.27, N, 12.41, S, 10.01, Cl, 11.05%.
{1-[3-Chlorophenyl-4-methyl-2,5-dihydro-1,5-benzothiazepin-3-yl]-ethylidene}-hydrazine (6c)
Yield 49%; FTIR (KBr) ν, cm−1): 3,414 (N-Hstr), 3,093 (Ar. C-Hstr), 2,977 (Ali. C-Hstr), 1,618 (Ar. C-Cstr), 1,548 (C = Nstr), 1,461 (Ar. C-Cstr), 1,274 (Ar. C-Nstr), 1,074 (Ar. C-Clstr), 721 (C-H m-disub. benzene), 645 (C-Sstr); 1H NMR, δ ppm: 1.220 (s, 3H, CH3), 2.200 (s, 3H, CH3), 4.122 (s, 2H, NH2, D2O exchange), 6.167 (s, 1H, Ar-H), 7.123–7.169 (t, 2H, Ar-H), 7.363–7.367 (d, 1H, Ar-H), 7.500–7.534 (t, 1H, Ar-H), 7.705–7.721 (d, 2H, Ar-H), 7.902 (s, 1H, Ar-H), 7.910–8.032 (d, 1H, Ar-H), 9.140 (s, 1H, N-H, D2O exchange); MS (m/z): 344 [M + 1]+; 345 [M + 2]+; Elemental analysis: C, 62.82, H, 5.31, N, 13.83, S, 10.22, Cl, 10.21%.
{1-[2-Chlorophenyl-4-methyl-2,5-dihydro-1,5-benzothiazepin-3-yl]-ethylidene}-hydrazine (6d)
Yield 51%; FTIR (KBr) ν, cm−1): 3,365 (N-Hstr), 3,051 (Ar. C-Hstr), (Ali. C-Hstr), 1,677 (C = Nstr), 1,630 (Ar. C-Cstr), 1,419 (Ar. C-Cstr), 1,315 (Ar. C-Nstr), 1,091 (Ar. C-Clstr), 761 (C-H o-disub. benzene), 678 (C-Sstr); 1H NMR, δ ppm: 1.310 (s, 3H, CH3), 2.202 (s, 3H, CH3), 4.100 (s, 2H, NH2, D2O exchange), 4.402 (s, 1H, Ar-H), 7.043–7.103 (d, 2H, Ar-H), 7.216–7.243 (d, 1H, Ar-H), 7.401–7.435 (m, 3H, Ar-H), 7.509–7.524 (d, 2H, Ar-H), 9.162 (s, 1H, N-H, D2O exchange); MS (m/z): 344 [M + 1]+; 345 [M + 2]+; Elemental analysis: C, 62.45, H, 6.75, N, 11.39, S, 10.69, Cl, 11.03%.
{1-[4-Nitrophenyl-4-methyl--2,5-dihydro-1,5-benzothiazepin-3-yl]-ethylidene}-hydrazine (6e)
Yield 55%; FTIR (KBr) ν, cm−1): 3,320 (N-Hstr), 3,076 (Ar. C-Hstr), (Ali. C-Hstr), 1,722 (Ar. C-Cstr), 1,613 (C=Nstr), 1,510 (Ar. C-Cstr), 1,450 (N-O str.), 1,348 (Ar. C-Nstr), 847 (C-H p-disub. benzene), (C-Sstr); 1H NMR, δ ppm: 1.321 (s, 3H, CH3), 2.102 (s, 3H, CH3), 4.000 (s, 2H, NH2, D2O exchange), 5.410 (s, 1H, Ar-H), 7.053–7.109 (d, 2H, Ar-H), 7.210–7.235 (d, 2H, Ar-H), 7.421–7.439 (d, 2H, Ar-H), 7.654–7.690 (d, 2H, Ar-H), 9.102 (s, 1H, N-H, D2O exchange); MS (m/z): 355 [M + 1]+; Elemental analysis: C, 62.37, H, 6.62, N, 16.92, S, 08.39%.
{1-[3-Nitrophenyl-4-methyl-2,5-dihydro-1,5-benzothiazepin-3-yl]-ethylidene}-hydrazine (6f)
Yield 62%; FTIR (KBr) ν, cm−1): (N-Hstr), 3,097 (Ar. C-Hstr), 2,913 (Ali. C-Hstr), 1,647 (Ar. C-Cstr), 1,612 (C=Nstr), 1,579 (Ar. C-Cstr), 1,483 (N-O str.), 1,337 (Ar. C-Nstr), 698 (C-H m-disub. benzene), 624 (C-Sstr); 1H NMR, δ ppm: 1.240 (s, 3H, CH3), 2.212 (s, 3H, CH3), 4.100 (s, 2H, NH2, D2O exchange), 6.107 (s, 1H, Ar-H), 7.103–7.189 (t, 2H, Ar-H), 7.303–7.342 (d, 1H, Ar-H), 7.510–7.574 (t, 1H, Ar-H), 7.705–7.731 (d, 2H, Ar-H), 7.907 (s, 1H, Ar-H), 7.915–8.035 (d, 1H, Ar-H), 9.240 (s, 1H, N-H, D2O exchange); MS (m/z): 355 [M + 1]+; Elemental analysis: C, 63.82, H, 6.18, N, 14.30, S, 10.72%.
{1-[2-Nitrophenyl-4-methyl-2,5-dihydro-1,5-benzothiazepin-3-yl]-ethylidene}-hydrazine (6g)
Yield 39%; FTIR (KBr) ν, cm−1): 3,370 (N-Hstr), 3,075 (Ar. C-Hstr), 2,925 (Ali. C-Hstr), 1,635 (Ar. C-Cstr), 1,623 (C=Nstr), 1,500 (Ar. C-Cstr), 1,421 (N-O str.), 1,325 (Ar. C-Nstr), 806 (C-H o-disub. benzene), 700 (C-Sstr); 1H NMR, δ ppm: 1.150 (s, 3H, CH3), 2.003 (s, 3H, CH3), 4.000 (s, 2H, NH2, D2O exchange), 5.502 (s, 1H, Ar-H), 7.215–7.269 (d, 2H, Ar-H), 7.304–7.325 (d, 1H, Ar-H), 7.610–7.630 (d, 2H, Ar-H), 7.904–7.923 (m, 3H, Ar-H), 9.213 (s, 1H, N-H, D2O exchange); MS (m/z): 355 [M + 1]+; Elemental analysis: C, 62.83, H, 6.26, N, 14.29, S, 9.71%.
{1-[4-Methoxyphenyl-4-methyl-2,5-dihydro-1,5-benzothiazepin-3-yl]-ethylidene}-hydrazine (6h)
Yield 67%; FTIR (KBr) ν, cm−1): 3,246 (N-Hstr), 3,114 (Ar. C-Hstr), 2,947 (Ali. C-Hstr), 1,608 (Ar. C-Cstr), 1,564 (C=Nstr), 1,500 (Ar. C-Cstr), 1,346 (Ar. C-Nstr), 1,064 (C-O-Cstr), 769 (C-H p-disub. benzene), 690 (C-Sstr); 1H NMR, δ ppm: 1.304 (s, 3H, CH3), 2.241 (s, 3H, CH3), 3.730 (s, 3H, OCH3), 4.000 (s, 2H, NH2, D2O exchange), 5.408 (s, 1H, Ar-H), 6.110–6.133 (d, 2H, Ar-H), 6.940–6.953 (d, 2H, Ar-H), 7.310–7.364 (t, 2H, Ar-H), 7.733–7.765 (d, 2H, Ar-H), 8.802 (s, 1H, N-H, D2O exchange); MS (m/z): 340 [M + 1]+; Elemental analysis: C, 66.54, H, 5.27, N, 11.03, S, 9.83%.
{1-[3-Methoxyphenyl-4-methyl-2,5-dihydro-1,5-benzothiazepin-3-yl]-ethylidene}-hydrazine (6i)
Yield 48%; FTIR (KBr) ν, cm−1): 3,386 (N-Hstr), 3,089 (Ar. C-Hstr), 2,970 (Ali. C-Hstr), 1,686 (Ar. C-Cstr), 1,598 (C=Nstr), 1,531 (Ar. C-Cstr), 1,363 (Ar. C-Nstr), 1,145 (C-O-Cstr), 730 (C-H m-disub. benzene), 686 (C-Sstr); 1H NMR, δ ppm: 1.264 (s, 3H, CH3), 2.320 (s, 3H, CH3), 3.734 (s, 3H, OCH3), 4.122 (s, 2H, NH2, D2O exchange), 5.601 (s, 1H, Ar-H), 6.080–6.093 (d,1H, Ar-H), 6.408 (s, 1H, Ar-H), 6.700–6.777 (t, 1H, Ar-H), 6.940–6.957 (d, 1H, Ar-H), 7.260–7.283 (t, 2H, Ar-H), 7.509–7.525 (d, 2H, Ar-H), 8.942 (s, 1H, N-H, D2O exchange); MS (m/z): 340 [M + 1]+; Elemental analysis: C, 66.72, H, 7.95, N, 13.83, S, 08.11%.
{1-[2-Methoxyphenyl-4-methyl-2,5-dihydro-1,5-benzothiazepin-3-yl]-ethylidene}-hydrazine (6j)
Yield 62%; FTIR (KBr) ν, cm−1): 3,290 (N-Hstr), 3,070 (Ar. C-Hstr), 2,937 (Ali. C-Hstr), 1,708 (Ar. C-Cstr), 1,610 (C=Nstr), 1,510 (Ar. C-Cstr), 1,348 (Ar. C-Nstr), 1,180 (C-O-Cstr), 744 (C-H o-disub. benzene), 692 (C-Sstr); 1H NMR, δ ppm: 1.208 (s, 3H, CH3), 2.203 (s, 3H, CH3), 3.734 (s, 3H, OCH3), 4.312 (s, 2H, NH2, D2O exchange), 6.280 (s, 1H, Ar-H), 6.503–6.548 (m, 3H, Ar-H), 6.957–7.060 (d, 1H, Ar-H), 7.269–7.299 (t, 2H, Ar-H), 7.305–7.359 (d, 2H, Ar-H), 8.350 (s, 1H, N-H, D2O exchange); MS (m/z): 340 [M + 1]+; Elemental analysis: C, 66.02, H, 7.32, N, 13.59, S, 08.96%.
PHARMACOLOGY
In this study, we take both sexes of albino mice 25–30 gm in my protocol. The animals were housed protocol standard condition at ambient temperature 25°C ± 2°C and tolerable free access to food and water except at time they brought out of cage. Experimental protocol was done as per institutional animal ethical committee of the Mahatma Gandhi Institute of Pharmacy, Lucknow (1957/PO/Re/S/17CPCSEA), who approved the protocol.
Anticonvulsant screening
Anticonvulsant screening was performed on laboratory experimental animal albino mice weight 20–25 g. All required experimental animal acclimatized to the experimental condition 1 day prior to initiation of biological activity. All experimental animals were fasted overnight. The test compounds mixed in PEG400 aq. Solution (30% v/v) and administered intraperitoneally to experimental animals for phase first pharmacological screening by maximum electroshock and neurotoxicity study.
Maximum electroshock (MES) test
Weight all the experimental animals, marked, and divided into three groups with six animals in each group named as control, standard, and test. At the first day of experiment, the control group was administered with 30% v/v PEG400 aq. Solution and to the standard group Phenytoin (30 mg/kg) in 30% v/v PEG400) was administered intraperitoneally and test compound administered intraperitoneally to test group at three different doses 30, 100, and 300 mg/kg in 30% v/v PEG400. Next day, the same procedure was followed for second and third group with others test compounds (Bhrigu et al., 2012; Garg, 2010; Siddiqui et al., 2015).
Handling the animal properly then corneal electrodes placed the upper eye lid and 150 mA current was given for 0.2 second time. We note down that the time spends at 0.5 and 4 hours after drug administration in different phases of seizures (a) tonic flexion, (b) tonic extensor, (c) clonic convulsion, (d) stupor, and (e) recovery or death was observed in each group of animals.
The above procedure repeated with control and standard group of the animals. We observed that the reduce time or abolish extensor phase after given electroshock and data presented in Tables 2 and 3 (Kulkarni, 2011).
Neurotoxicity studies
Motor impairment was screen in rats by using rotarod apparatus. Before the experiment start all the experimental animals trained to stay on accelerating rotarod at rotational speed up to 10 rpm. The test drug administered to animals then placed the animals on a knurled rotating rod. Neurotoxicity became visible by absence of rats to hold rod for 1 minute at least in every of three trials (Kulkarni, 2011).
Acute oral toxicity studies
Most potent active compound was evaluated for acute oral toxicity in albino mice weight 20–25 g according to the OECD guidelines 420. The animals were randomly divided into two groups with six mice (both sex) in each. Group-I (Control) receive distal water orally. Group-II was administered 6c compound at single dose of 2,000 mg/kg, orally, respectively. Food and water was hold for about 2 hours after the administration of test drug. We notice that related to clinical sign and symptoms, for initially 24 hours, with special courtesy given starting 4 hours duration and daily subsequently 14 days after the test drug administration. Furthermore, factors like righting reflux, gripping, pupils, pain response, tremors, convulsion, skin color, corneal reflex, salivation, torch response, water intake, food intake, sleep, diarrhea, grooming, urination, alertness, lethargy, touch response, coma, and mortality were observed (Ali and Siddiqui, 2014; Jonsson et al., 2013; OECD 2000, 2000; Porwal et al., 2017) and presented in Table 4.
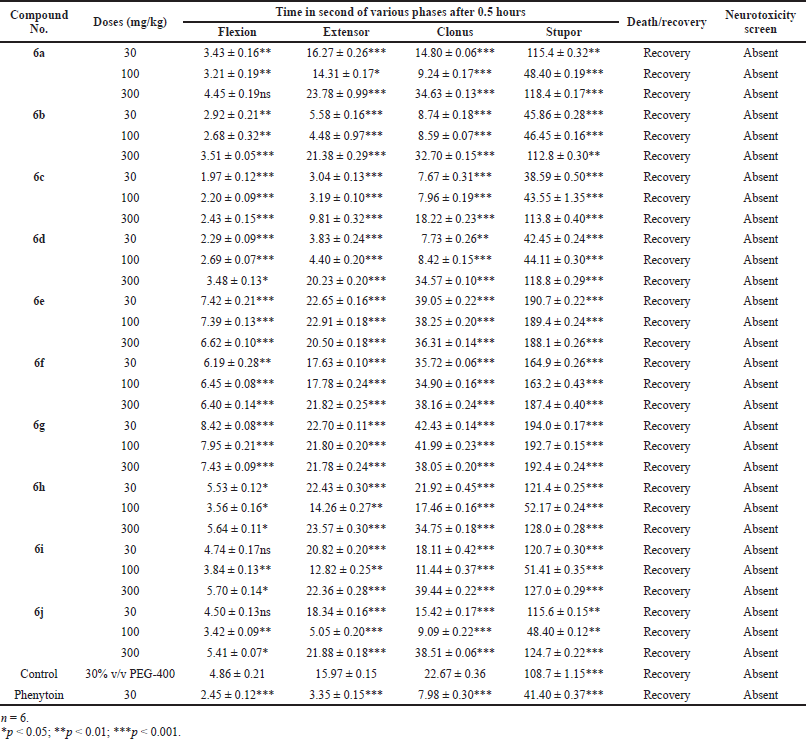 | Table 2. Anticonvulsant evaluation after 0.5 hours administration of synthesized compounds (6a–6j) using MES model. [Click here to view] |
Statistical analysis
All the statistical analysis were analyzed and collect the data by using graph pad prism 5 version software. All the values represented in the form of Mean ± SEM, and these all values analyzed by analysis of variance then multiple comparison test followed by Dunnett’s.
RESULTS AND DISCUSSION
Chemistry
All newly synthesized compounds (6a–6j) were identified by physicochemical parameters and related data described in Table 1. Finally, synthesized substituted Schiff’s bases identified by elemental and spectroscopic analytical methods.
Anticonvulsant activity
The newly synthesized substituted antiepileptic drug containing activity of Schiff’s bases carried out research under the antiepileptic drug development program. Classified active drug into three subsequent classes: class I drug active at 100 mg/kg or less, class II drug active more than 100 mg/kg, and class III drug inactive at 300 mg/kg body weight of the animal.
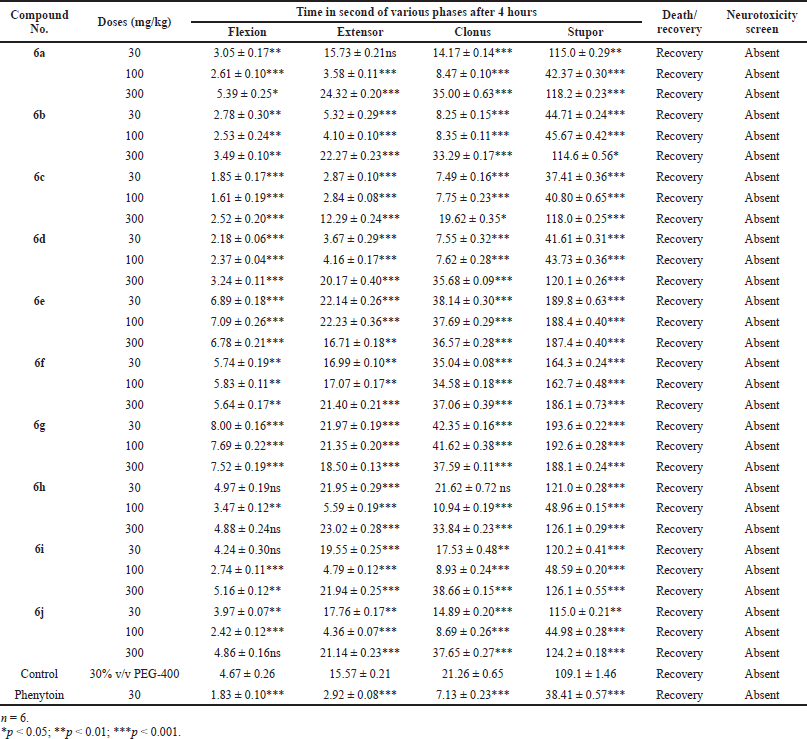 | Table 3. Anticonvulsant evaluation after 4 hours administration of synthesized compounds (6a–6j) using MES model. [Click here to view] |
Mostly compounds exhibit potent activity at 30 and 100 mg/kg dose without neurotoxin in nature except compounds 6e and 6g. Most of the compounds produced neurotoxicity at a maximum dose 300 mg/kg. Limited dose range (30–100 mg/kg) for activity showed the concept of therapeutic window phenomena. Compound 6c was found to be most potent compound having large range of dose for activity and produces less neurotoxicity at 300 mg/kg. Compounds 6e, 6f, and 6g (nitro substituted) showed poor or less activity due to low log P.
From the above results, it was found that the chloro derivatives exhibit better activity and the o-substituted derivative was found to possess more significant activity, alteration at p-position affect the activity. All the nitro substituted compounds showed poor activity due to low CNS penetration. The introductions of the chloro group in ring enhance the anticonvulsant activity. Graphical data given in Figures 2 and 3.
Acute oral toxicity studies
Compound 6c with a dose of 2,000 mg/kg delivered no harmful impact on the social reactions of the treated rodents (dosed once) and watched for 14 days. All the animals show neither any poisonous nor deadly impact. My current investigation shows that the administration of test compound up to 2,000 mg/kg did not any indication of harmfulness toxicity or mortality in animals at the time of experimental period.
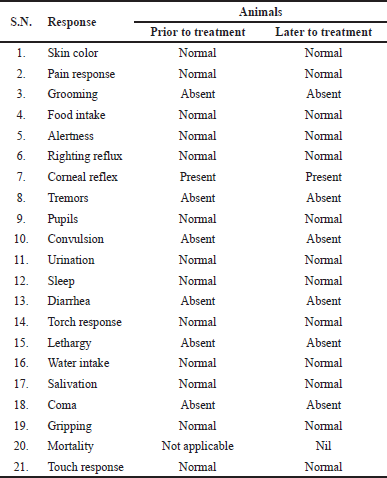 | Table 4. Effect of compound 6c for acute toxicity study. [Click here to view] |
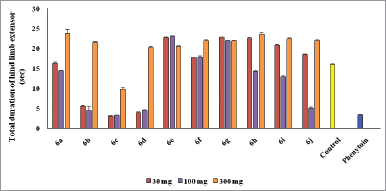 | Figure 2. Graphical representation of anticonvulsant evaluation after 0.5 hours administration of synthesized compounds (6a–6j) using MES model. [Click here to view] |
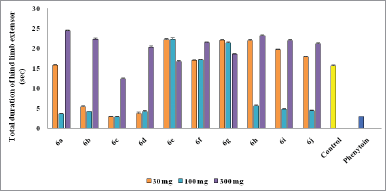 | Figure 3. Graphical representation of anticonvulsant evaluation after 4 hours administration of synthesized compounds (6a–6j) using MES model. [Click here to view] |
CONCLUSION
In conclusion, a series of substituted Schiff’s bases synthesized successful and every compound screen for anticonvulsant activity by utilizing MES model. The most potent compound 6c was found as a primary class of anticonvulsants that have shown practically identical anticonvulsant action with uniquely lower neurotoxicity. The non-toxic nature of compound 6c was determined by acute toxicity study according to the OECD 420 guidelines. Total 14 days observation normal behavior of animals that suggest the safety and innocuous nature of newly synthesized potent compound up to dose 2,000 mg/kg. Compound 6c found as a lead molecule for further modification and improvement in field of antiepileptic drug development.
ACKNOWLEDGMENTS
One of the authors are grateful thankful to the Department of Pharmaceutical Chemistry, Mahatma Gandhi Institute of Pharmacy, Lucknow (Uttar Pradesh, India) for providing facilities for this research work. The authors are also thankful to Central Drug Research Institute, Lucknow and IIT, New Delhi (India) for providing spectral data and elemental analysis of the newly synthesized compounds.
ETHICAL APPROVALS
Experimental protocol was done as per institutional animal ethical committee of the Mahatma Gandhi Institute of Pharmacy, Lucknow (1957/PO/Re/S/17CPCSEA), approved the protocol.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Ali R, Siddiqui N. Preliminary anticonvulsant and toxicity screening of substituted benzylidenehydrazinyl-N-(6-substituted benzo[d]thiazol-2-yl)propanamides. Sci World J, 2014; 2014:1–10. CrossRef
Ameta KL, Rathore NS, Kumar B. Synthesis and preliminary evaluation of novel 1,5-benzothiazepine derivatives as anti-lung cancer agents. Int J Pharm, 2013; 3(2):328–33.
Ansari FL, Iftikhar F, Ihsan-Ul-Haq, Mirza B, Baseer M, Rashid U. Solid phase synthesis and biological evaluation of parallel library of 2, 3-dihydro-1,5-benzothiazepene. Bioorg Med Chem, 2008; 16(16):7691–7. CrossRef
Bhrigu B, Siddiqui N, Pathak D, Alam Md S. Anticonvulsant evaluation of some newer benzimidazole derivatives: design and synthesis. Acta Pol Pharm-Drug Res, 2012; 69(1):53–62.
Garg N. Synthesis and evaluation of some new substituted benzothiazepine and benzoxazepine derivatives as anticonvulsant agents. Eur J Med Chem, 2010; 45(12):1529–35. CrossRef
Jeyaprakash RS, Tiwari M, Hashif K, Srinivasan KK. Synthesis and evaluation of antimicrobial activity of some 2-substituted benzimidazole. Pharmacologyonline, 2009; 3:737–42.
Jonsson M, Jestoi M, Nathanail AV, Kokkonen UM, Koivisto MAP, Karhunen P, Peltonen K. Application of OECD guideline 423 in assessing the acute oral toxicity of moniliformin. Food Chem Toxicol, 2013, 53:27–32. CrossRef
Kulkarni SK. Hand book of experimental pharmacology. Vallabh Prakashan, Delhi, India, 2011.
OECD. Guidance document on acute oral toxicity. Environmental health and safety monograph series on testing and assessment No 24. OECD, Paris, France, 2000a.
OECD. Guidance document on the recognition, assessment and use of clinical signs as humane endpoints for experimental animals used in safety evaluation environmental health and safety monograph series on testing and assessment No 19. OECD, Paris, France, 2000b.
Pandeya SN, Kohali S, Siddique N, Stables JP. Synthesis and anticonvulsant activities of 4-n-substituted arylsemicarbazones. Pol J Pharmacol, 2003; 55:565–71.
Porwal M, Khan NA, Maheshwari KK. Evaluation of acute and subacute oral toxicity induced by ethanolic extract of Marsdenia tenacissima leaves in experimental rats. Sci Pharm, 2017; 85(29):1–11. CrossRef
Raja AS, Pandeya SN, Panda SS, Stables JP. Synthesis and anticonvulsant evaluation of semicarbazones of acetophenone mannich bases. Pharm Chem J, 2007; 41(6):302–7. CrossRef
Siddiqui N, Singh PK, Kumar N, Pathak D. Synthesis and evaluation of 1-(2-(Substituted benzylidenehydrazinyl)acetyl)-3-(hydroxyimino)indolin-2-one as potential anticonvulsants. Der Pharmacia Sinica, 2015; 6(2):30–40.