INTRODUCTION
Globally, around 220 million peoples are affected with type-II diabetic disorder that might be doubled by 2030. Diabetic symptoms emerge with an increase in age, individual lifestyles, obesity due to food patterns, and socioeconomic pitfalls (Zhao et al., 2017). Nowadays, it has been proved that insulin resistance shows a key role in diabetes pathogenic conditions, and the lack of production of insulin from pancreatic β-cells causes a controlling effect on hyperglycemic disease development. The World Health Organization instigated the utilization of remedial plants as an alternative way of managing the diabetic condition (Baloyi et al., 2019). The control of diabetes needs a combined approach comprised of the initial involvement to prevent its occurrence and utilization of the integrated therapies to check associated complications and lipidemia in the delayed phases. Thus, an innovative natural hypoglycemic agent can influence the insulin sensation and has a preventive activity that effectively regulates insulin resistance syndrome. Hence, products of natural origin like triterpenoids and flavonoids were widely explored successfully in the drug development of antidiabetic agents (Chen et al., 2015).
The pentacyclic triterpenoid oleanolic acid (OA) is a plant-derived bioactive compound obtained from fruits and leaves of Oleaeuropaea L (Maczewsky et al., 2019). OA is biosynthesized by the process of cyclization of (3S)-2,3-oxidosqualene, and followed by the acetate/mevalonate pathway (Forestier et al., 2019) with triterpenes containing six groups of isoprene-moiety (Mahizan et al., 2019) and also acts as an aglycone of triterpenoid saponins attached with one or more sugar chains (Ludeña-Huaman et al., 2019). From investigations, it has been found that olive leaf is exceptionally rich in OA that is about 3.5% of the dry weight (Castellano et al., 2019). Besides, OA is an active constituent of various medicinal plants like ginseng (1%), papaya fruit, and the skin of an apple (Liu et al., 2019a). Based on pharmacological activities, OA has a significant role in the treatment of diabetic disorders (Zhang et al., 2018), neuroprotective effect in acute neurological disorder (Msibi et al., 2019), hypoglycemic, anti-inflammatory, cardio-protective (Sanchez-Rodriguez et al., 2018), antioxidant, lipid-lowering effect, antimicrobial (Jesus et al., 2015), hepatoprotective (Rohilla and Bhatt, 2018), and antiatherosclerotic activity along within the management of cancerous growth (Sen, 2020). Reports found that OA can produce hypoglycemic activity by modulating the insulin signaling process, carbohydrate metabolism in the skeletal muscle of streptozotocin (STZ)-induced experimental diabetic rats due to the direct interaction between the single protein molecule along with the β-phenolic -OH group present at C3 position and a -COOH group at C28 of a hydrophobic pentacyclic structure of OA (Figure 1), thus proved the versatility of OA through in vitro and in silico studies (Mukundwa et al., 2016). Hence, the main objective of this literature review is to outline the most remarkable concept about the molecular origin related to the antidiabetic activity of OA.
Role of OA in postprandial diabetes
In diabetic patients, postprandial glucose test indicates the concentration of glucose in plasma after assimilation of foods. Generally, digestion of carbohydrates occurs with the aid of the gastric enzymes (α-glucosidase and α-amylase) present across the intestinal membranes. Postprandial diabetes and the depletion of glycosylated proteins can be significantly controlled with inhibition of the gastric enzymes. Previous reports claimed that inhibition of α-glucosidase enzymes can be used as a novel effective therapeutic agent for diabetes by controlling blood sugars. For example, plant-derived triterpenoids saponins show stronger inhibitory potency with inhibitory concentration (IC50) = 23.1 μmol/l than standard acarbose with IC50 = 388.0 μmol/l; besides, terpenoids which isolated from Plectranthus madagascariensis and displayed potential α-glucosidase inhibitory activity with IC50 = 274.9 (Ding et al., 2018). In this context, OA triterpenoids exhibited potential α-glucosidase and α-amylase inhibitory activity with IC50 = 6.35 mg/ml in a reversible manner. The probable mechanism of α-glucosidase inhibition is due to conformational changes of structure of enzyme results decreases in the catalytic activity, suggest OA has glycemic control ability which could be used in the management of blood sugar in diabetic patients (Ding et al., 2018).
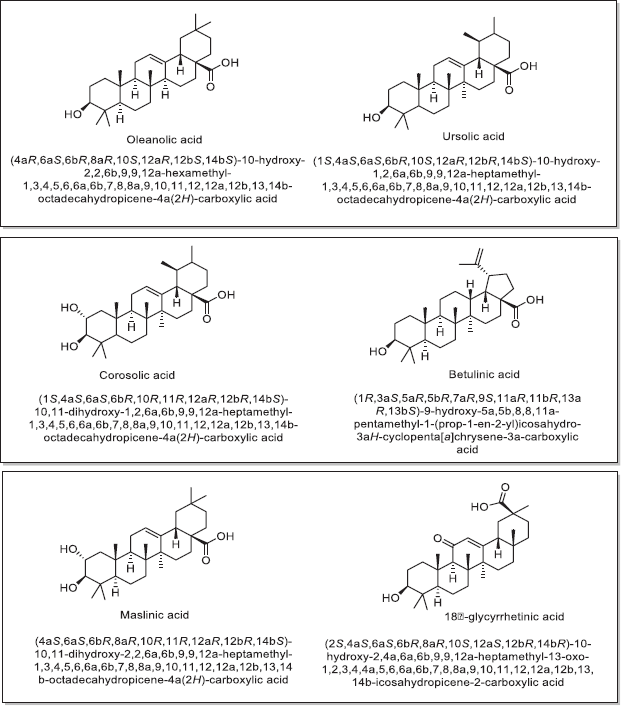 | Figure 1. Chemical structures and chemical name of associated natural triterpene analogs along with OA showing antidiabetic effects (Castellano et al., 2013 ). [Click here to view] |
Role of OA in pancreatic β-cell function
The report found that during diabetes mellitus, pancreatic β-cells become slowly degenerate and unable to liberate adequate insulin required for normal metabolic activities. From the experimental finding, it was found that OA has a significant role in β-cell function for secretion of insulin in a rat model. OA and standard tolbutamide were studied in insulin and content assay using INS-1 832/13 cell lines; results indicated OA increases in insulin secretion at stimulatory glucose level in isolated rats’ islets which is comparable with standard drugs. Further, molecular parameters like insulin protein and messenger ribonucleuc acid concentration also increased that suggests OA might contribute to potential anti-diabetic activities (Fig. 2). Clinical symptoms have appeared when there is more than 70% death of pancreatic β-cell which indicates that diabetes process occurs in a progressive manner and the probable mechanism involved in β-cell death is enhanced expression of apoptotic Fas, excess secretion of the pro-inflammatory cytokine, and more production of scavenging species such as nitric oxide in pancreatic cell (Rojas et al., 2018). As a natural analog, OA inhibits the cytokine synthesis occurred due to antigen-presenting cells and macrophages, reduces the efficiency of Interleukin (IL)-4, IL-7, and IL-2 generating T cells, γ-interferon and thus increases the durability of induced islet cells in STZ induced diabetic rats (Table 1). Similarly, the triterpenes like Asiatic acid and ursolic acid (UA) maintained the functionality of pancreatic β-cells and triggered the cell defense mechanism in STZ-induced diabetic rats (KalaycıoÄŸlu et al., 2018). Similarly, insulin production is an important aspect of type-II diabetes and it can achieve by decreasing the signaling activity of insulin. In this context, OA has proved its effectiveness at insulin receptor (IR) by down-regulating signaling mechanism.
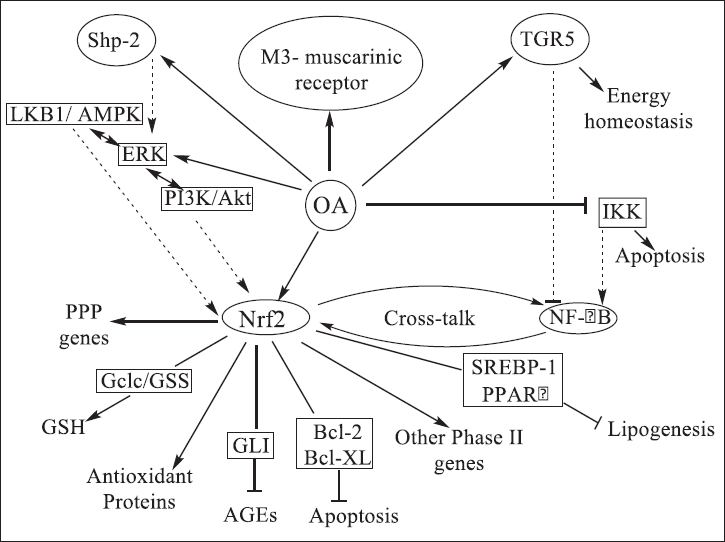 | Figure 2. The antidiabetic activity of OA proved by a secondary mechanism coupled with activation of the antioxidant protein Nrf2 where OA modulates the phase 2 response genes and stops Nf-kB through blockage of IκB kinase (IKK) (Castellano et al., 2013). [Click here to view] |
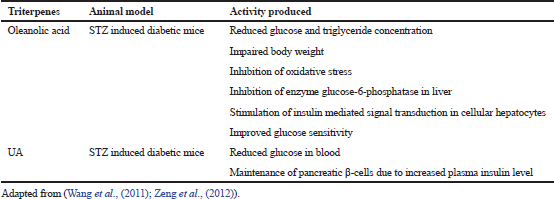 | Table 1. Triterpenes showed anti-diabetic effect in experimental animal groups. [Click here to view] |
Role of OA in M3 receptor
Muscarinic receptors (M3) associated with the pancreatic β-cell membrane help for the liberation of glucose-dependent insulin, which is triggered by acetylcholine (Jakubik and El-Fakahany, 2020). The report found that intraperitoneal administration of OA in Wistar rats results in decreases in fasting glycemia with subsequent action of enhancing the plasma insulin level in a parallel manner which suggests OA has acetylcholine enhancing effect at nerve endings. Experimental drugs like vesamicol and hemicholinium-3 (hemicholine) (Ravodina et al., 2019) decrease the acetylcholine synthesis by inhibiting the acetylcholine release and the choline reuptake simultaneously.
Role of OA in G protein-coupled bile acid receptor (TGR5)
TGR5 is a membrane receptor for bile acid which acts as a signaling molecule for various metabolic processes like alteration of blood glucose level and increase in energy expenditure; hence it can be used as a good candidate for the management of metabolic disorder including diabetic mellitus. Further, experimental data show an elevated level of bile acid in serum alters the insulin sensitivity through TGR5 signaling pathways. The signaling pathway of bile acid is involved with a profound systemic hormonal activity that enhances the synthesis of peptide-1 (Shapiro et al., 2018), glucagon by TGR5 intermediated pathway that triggers more insulin production with the renewal of β-cell. OA acts as an agonist for the TGR5 receptor at a half-maximal effective concentration of (EC50 = 1.42 mmol/l); similarly, UA performs the agonist action on TGR5 at a level of (EC50 = 1.43 mmol/l). The activation of the TGR5 mediated pathway enhances the formation of cyclic adenosine monophosphates, the enzyme deiodinase responsible for the activation of thyroid hormone (transform T4 into an active form of T3) (Shahid et al., 2020) and also induces oxidative phosphorylation in muscle. The C3-OH and C28-COOH functional groups present within OA play a vital role in activating the TGR5 receptor molecule. The triterpenes molecule can be bonded to TGR5 at three recognized binding sites: (a) a restricted H-bonding site allows the hydroxyl group to bind, (b) another polar region for carboxylate group binding, and (c) hydrophobic attachment for the skeleton of pentacyclic structure that allows adjustment of the polar moieties (Ladurner et al., 2017).
Protecting effect of OA over the pancreatic cell in physiological oxidative stress condition
The most usual stages of injury are the excessive accumulation of reactive oxygen species (ROS) in mitochondria that causes failure of the working capacity of pancreatic β-cell. The low concentration of enzymes required for the inactivation of hydrogen peroxide aggregation increases to a higher level, followed by the gradual formation of a hydroxyl radical. The cell mitochondria are damaged by the overproduction of ROS (Nita and Grzybowski, 2016). It is characterized by cross-linking of the peptides, peroxide formation in a phospholipid bilayer membrane, and increased destruction of DNA double-strand. But all these actions are stopped by the use of OA that gives protection to the pancreatic β-cells by firmly strengthening the cellular immune system.
Improved activity of the enzyme Shp-2 or protein tyrosine phosphatase-2
The major role of protein tyrosine phosphatase-2 (Shp-2) is promoting biosynthesis of insulin as well as cells and its signaling and activation through a receptor-operated mechanism. Besides, transcription of the insulin gene is also modulated by Shp-2 enzyme via extracellular signal-related kinase (ERK) with phosphotidylinositol-3-kinase/protein kinase B/FoxO1 transduction mechanisms, results in modulation activity of various insulin promoters like Ins1, Ins2, as well as regulation of the genetic expression of pancreatic and duodenal homeobox 1 (Pdx1). The effect of OA has been studied on the Shp-2 enzyme in STZ induced diabetic mice, and results indicate a low significant effect on phosphotyrosyl phosphatases like haematopoietic protein tyrosine phosphatase and Shp1 (Guo and Xu, 2020). However, in an experimental rat model, insulin biosynthesis is stimulated up to multiple folds by OA delivery at a dose of 30–50 μmol/l to β-cells of INS-1, its dose-dependent with selective activity at the transcriptional extent. OA also involves in the rising of the insulin protein level by ~25%, with a subsequent increase in proinsulin (Lyu et al., 2016). Alternatively, the triterpenoids like OA dropped the caspase-3 apoptotic cascade with increased mitochondrial Shp-2 enzyme activity suggesting OA has a significant role in controlling ROS.
Role of OA in IR activator
IR activators being the non-protein moiety can autophosphorylate themselves in insulin-unaffected cells, associated with the pathogenic condition of type II diabetes. The insulin moderated autophosphorylation of the IR is increased synergistically by activation of the natural analog of triterpenes like OA at a dose of 1 nmol/l in hamster ovarian cells. It has been found that β-subunit is the active site for binding OA with insulin rather than the insulin sites because the IR could not be activated at a lower or higher level within a range of 1–50 μg/ml in the lack of insulin, suggest it has a significant role in the management of diabetes.
Role of OA in protein kinase
The insulin response is regulated negatively in the living organism by the protein moieties like protein-tyrosine phosphatases 1B (PTP1B) and T-cell protein-tyrosine phosphatases (TCPTP). The PTP1B can be inhibited through a linear-mixed mechanism that depends on the kinetic constants Km and Vmax (Wang et al., 2015) with a natural product containing triterpenoids which could be the therapeutic target for the management of diabetes mellitus. The report found that triterpenes OA enhances insulin sensitivity as well as absorption of glucose (Ghosh et al., 2018) via inhibition of TCPTP and PTP1B in 3D QSAR pharmacophore models. The inhibitory effect of UA is more at a half-maximal inhibitory concentration of IC50 = 3.1 mmol/l. In comparison, OA has a half-maximal inhibitory concentration of IC50 = 3.4 mmol/l. The structural analysis determines that the triterpenes are attached to the enzyme at the secondary aryl phosphate group instead of binding at the catalytic region of the PTP1B molecule. Here the carboxylate group at the C28 position is bonded to the enzyme with an extended hydrogen bonding. Also, the triterpenes are connected to the protein molecule by Vanderwall's interaction. The above mechanism reveals that the natural analog of triterpenes like OA, UA possesses the additional activity for PTP1B inhibitors by modifying the carbon chain and specifying the phosphatases utilized in the insulin reaction (Shah et al., 2016). In addition to it, a group of synthetic derivatives of OA exhibited an inhibitory effect on PTP1B, associated with insulin resistance (Yang et al., 2020).
Similarly, serine/threonine specified protein kinase (PKB/Akt) which is known as phosphoinositide-3-kinase is responsible for glucose metabolism and glycogen synthesis, regulated by insulin-mediated pathways. Sangeetha et al. (2010) reported Akt can be activated in vascular smooth muscle as well as in 3T3L1 adipocytes with the help of phytoconstituents like OA and 3b-taraxerol. The protein kinase (Akt) and FoxO1 influence reduced activity of glucose-6-phosphatase and gluconeogenesis enzyme, which is related to the strong glucose-lowering effect of OA in diabetic mice (Liu et al., 2019b).
Another most important kinase known as AMP-activated protein kinase (AMP-K), involved in metabolic pathways like glucose metabolism, synthesis, and oxidation of fatty acid in muscle and liver gluconeogenesis results in intracellular homeostasis. In pathological stress conditions especially in diabetes patients, AMP-K is stimulated which leads to impairment of adenosine triphosphate (ATP) to AMP ratio that excites the expression of Heme oxygenase-1 (HO-1), an Nrf2-regulated gene (Li et al., 2020). The triterpenes like OA, UA, and Betulinic acid activate AMP-K in hepatoma cells (Lewinska et al., 2017) via phosphorylation using the long-chain synthetic derivative of OA, 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid methyl ester (CDDO-Me). The upstream kinase ERK1/2 is controlled by CDDO-Me and methyl ester derivatives that enhance OA activity which could suggest the therapeutic role of OA in diabetes (To et al., 2017). The insulin response can be diminished by the protein kinase, i.e., the glycogen synthase kinase (GSK) 3β (To et al., 2017) in the absence of the catalyst. The triterpenes like OA, UA, and 3β-taraxerol hamper GSK-3β activity by undergoing phosphorylation reaction. The structural analysis has revealed that the triterpenes can be attached to the ATP binding position by forming hydrogen bonds (Perera et al., 2021). Further, impairment of the metabolic condition like apoptosis of β-cell and glycogen metabolism mediated by PKB-Akt pathway is generally occurred because of the interaction of AMPK-Nrf2 via phosphatidylinositol 3-kinase (PI3K). PI3K stimulation in β-cell is triggered by Shp-2 that is significantly activated by an activator OA. The remarkable generation of cytoplasmic Shp-2 by OA causes increased biosynthesis of insulin to a transcriptional extent, while the stimulation of mitochondrial Shp-2 protects the β-cell (Steensels et al., 2020). Alternatively, the Nfr2-antioxidant response element (ARE) cascade mechanism is activated by many anti-inflammatory mediators while the NF-kappa B (NF-kB) signaling is suppressed. The oxidative stress in hyperglycemia prompts destructive pathways like NF-kB creates ultimate cell death (Castellano et al., 2019). However, the process like oxidative stress and inflammatory response is significantly blocked by OA by stimulating the cytoprotective genes via Nrf2 activation and simultaneously stops NF-kB through blockage of IKK (Castellano et al., 2019). Therefore, the OA-mediated Nrf2 stimulation must be considered biologically in the sequence of ROS overproduction for maintaining the use of nicotinamide adenine dinucleotide phosphate (NADPH) to encourage the reduced glutathione concentration, to confirm the intracellular redox condition.
Role of OA in glycogen level
The diabetic condition has characterized a reduction in glycogenesis with a subsequent increase in glycogenolysis. Zhang et al. (2020a) reported liver glycogen was elevated gradually in STZ-induced diabetic mice by activating glucokinase as well as controlling glucose-6-phosphatase by the triterpenes like OA and UA. As glycogen phosphorylase inhibition is the rate-limiting step of glycogen degradation, hence, is a useful mechanism for curing diabetes. OA can suppress the effect in lung cancer A549 cells at a concentration of IC50 = 5.98 mmol/l (Zhang et al., 2020b) and IC50 = 14 mmol/l in the rabbit muscle consecutively. Enzyme inhibitions can occur by the alteration in the quaternary structure at C3 and C28 positions of the carbon skeleton of triterpenes as it binds at the AMP allosteric binding site.
Effect of OA in oxidative stress-mediated insulin resistance
The development of diabetic symptoms is characterized by the significant contribution of hyperlipidemia, advanced glycation end products (AGEs), hyperglycemia, and inflammatory cytokines. From the literature study, it has been revealed that overproduction of ROS and superoxide signifies the mechanism of the existence of injury (Fig. 4). Mitochondrial inhibition is one of the primary causes of Type 2 diabetes, which is characterized by the weakened oxidative mechanism resulting in the accumulation of ROS and superoxide (Jia et al., 2018). Thus, insulin resistance, β-cell dysfunction, and diabetic disorders are occurred by the excess production of superoxide radical components. Thus, insulin resistance, β-cell dysfunction, and diabetic disorders are occurred by the excess production of superoxide radical components which deregulate mitochondrial function by stimulating DNA fragmentation, crosslinking of the protein molecule, peroxidation of the membrane phospholipid bilayer, and finally promoting the stress mechanisms (Song et al., 2021). The triterpene analog OA protects the mitochondria from the formation of ROS and oxidative stress. So, it is used as an antidiabetic moiety in a better way. OA, obtained from natural origin, is found in many foods and medicinal plants that exist either in free form or combination with glycosides (Zhang et al., 2018).
Recently, oxidative stress proved to be a vital mechanism in diabetes complications as well as insulin resistance. The superoxide radicals and hydroxyl groups are slowly detached with OA by a distinct mechanism than other antioxidants like butylated hydroxytoluene or LD-α-lipoic acid. The phenolic OH group present at the C3 position of OA is quite involved in this mechanism. The effect of glutathione peroxidase (GSHPx) and superoxide dismutase (SOD) enhances in liver cells on reaction with tert-butyl hydroperoxide (Matzinger et al., 2018). The triterpenes like OA and UA elevate the glutathione level and SOD activities based on the capability of reaction between PC12 cells and H2O2 or 1-methyl-4-phenylpyridinium (Castellano et al., 2019). Subsequently, followed by the impairment of lactate dehydrogenase release and the development of malondialdehyde. The myocardial ischemia-reperfusion injury (IRI) in the rat heart is characterized by the lack of blood supply or oxygen. The IRI can be protected by OA, as it can improve the glutathione-regulated mitochondrial antioxidant pathway (Sen, 2020). Likewise, in alloxan-induced diabetic rats, malondialdehyde concentration in the kidney and liver is decreased due to the enhanced activity of GSHPx and SOD that occurred by OA (Matzinger et al., 2018).
The OA activity is increased gradually to a great extent due to the stimulation of transcription factor (Nrf2) (Caltana et al., 2015) which acts as an essential mediator of endogenous antioxidants and phase II genes that causes restoration of defensive genetic transcription related to oxidative stresses occurred due to the attachment of AREs in the gene promoter sites. The transcription of a series of antioxidant enzymes like hemeoxygenase1 [HO-1] (Matzinger et al., 2018), catalase, SOD with glutamate-cysteine ligase, and glutathione synthase, genes associated with the biosynthesis of glutathione is improved by Nrf2. The genetic expression of various NADPH-generating genes like malic enzyme and other pentose phosphate pathway components are influenced by the antioxidant protein, Nrf2 (Strom et al., 2016). Besides, OA and UA activate the phosphorylation reaction of a group of protein Jun NH2-terminal kinase and ERK/mitogen-activated protein kinases (MAPK) (Lu et al., 2019) via oxidative stress mechanism. ERK is supposed to be a defensive pathway against peroxides and remarkably enhances mitochondrial membrane activity. But, from the literature study, it has been proved that the phosphorylation of proteins via MAPKs occurs indirectly as the direct phosphorylation inadequately controls antioxidant protein Nrf2 in vivo.
Effect of OA on inflammatory cytokine factors
The transcription factor NF-kB that is the nuclear factor influences most of the inflammatory cytokine mediators that produce an unfavorable effect on insulin signaling. Likewise, the inhibitory component IkB is phosphorylated by IKK that ultimately energizes the nuclear factor NF-kB with few endogenic and exogenic stimuli. Some natural triterpenes such as OA as well as UA stop the activation of nuclear factor NF-kB in the growth of silicosis (Peng et al., 2017) and IKK. So, the tumor necrosis factor mediated E-selectin expression is decreased in human endothelial cells, followed by the inhibition of the discharge of an endogenous chemical like IL-6 in monocytic cells (Mono-Mac-6 cells) and repression of endothelin-1 mechanism in Zucker diabetic fatty rats (Castellano et al., 2019). It has been proved that the inflammatory enzyme phospholipase A2 is invariably inhibited by OA at very low micromolar concentrations (Giresa et al., 2015). A combination of oxidized lipid components is produced like 4-hydroxynonenal (4-HNE) upon oxidation of membrane phospholipid bilayer of mitochondria via ROS (Xiao et al., 2017). The interaction of cytosolic Bcl-2/Bax protein is disturbed when 4-HNE causes apoptosis of pancreatic β-cell. The activity of IKK has stimulated because of the phosphorylation of Bcl-2 protein via kinase as well as the antioxidant protein Nfr2 checks the apoptosis of cells by regulating the transcription of Bcl-2 protein (Nguyen et al., 2019). Hence, OA, the triterpene, provides the capability to support the anti-inflammatory and antioxidant effects by rising PI3K/PKB/Akt pathway and Bcl-XL protein expression by controlling the activity of IKK. Simultaneously, OA stimulates the Nrf2 response that enhances the pancreatic β-cell activity as well as triggers the defense mechanism in hyperglycemic mice.
Effect of OA on polyol and AGEs
Diabetic condition is characterized by the significant rise in glucose flow by polyol mechanism. Glucose is reduced to sorbitol by the rate-limiting enzyme aldose reductase (AR) with the utilization of NADPH. NADPH has been involved in the transformation of GSH in turn extends the sensitivity to oxidative injury. Again, sorbitol is subsequently metabolized to fructose by the enzyme sorbitol dehydrogenase (SDH) (Aragno and Mastrocola, 2017). The formation of glycation end products is induced generally with an increased amount of sorbitol and fructose that ultimately cause the enhancement of stress-mediated signaling mechanisms. The analogs of triterpenes like OA as well as UA can prevent both the enzymes AR and SDH mostly in the kidney and liver of STZ-induced diabetic mice model (Sonowal and Ramana, 2019) along with increases in the concentration of glyoxalase-I and decreases in methylglyoxal concentration gradually result in controlling the AGEs production and preserving the mitochondrial redox condition. Also, OA can prevent the synthesis of pentosidine in vitro at a given dose. Pentosidine is the biomarker for AGEs and NÉ›-(carboxymethyl) lysine. At a given concentration, the efficiency of inhibition of OA is higher as compared to UA because, in alloxan-induced diabetic mice, the stages of plasma hemoglobin A1c, the CML and renal pentosidine, and glycated albumin are remarkably decreased by OA, which is comparatively more than that of UA.
Lipid-lowering effects of OA
In previous studies, it has been suggested that triterpene OA is capable of showing lipid-lowering and anti-atherosclerotic effects from the past nineties (Han-Qiong et al., 2018). The plasma concentrations of total cholesterol, low density lipoprotein and high density lipoprotein cholesterol, and triglyceride are decreased to a greater extent upon administration of OA and MA in Sprague-Dawley rats after feeding them with a high-fat diet. Subsequently, it is followed by the negative regulatory effect on the signaling of lipogenic genes acetyl-CoA carboxylase, the membrane-bound protein, sterol O-acyltransferase, stearoyl-CoA desaturase 2, and glycerol-3-phosphate acyltransferase. Similarly, the visceral fat in diabetic Swiss albino mice is decreased remarkably with the subsequent rise in the fat cells hormone leptin, in the fall of ghrelin, and plasma lipids occurred by OA. The lipid droplets and microvesicular steatosis produced by the diet are declined significantly by the utilization of OA that has been proved by the anatomical investigation of the liver (Gamede et al., 2019).
Role of OA in peroxisome proliferator-activated receptors (PPARs)
Generally, the process like lipid metabolism and glucose homeostasis are genetically regulated by the transcriptional regulators like PPARs (Mosana et al., 2020). From the study, it has been found that OA can control PPAR activity. As a PPAR-α protagonist, in an experimental like Zucker diabetic fatty rat OA stimulates cardiac lipid metabolism and can variate African green monkey kidney fibroblast (CV-1) cells from immortal keratinocytes HaCaT cell lines most effectively. Similarly, OA can prove the antidiabetic activity on diabetic KK-Ay mice by PPAR-γ receptor molecules stimulation (Medrano-Jiménez et al., 2019). Alternately, the down-regulation of the PPAR-γ receptor by OA results in the decreased accumulation of lipids as well as the amount of visfatin, the protein secreted by visceral fat in separated 3T3-L1cell lines in the adipose tissue. Accumulated evidence suggests that the triterpene analogs like glycosylated OA components isolated from Kalopanax pictus can stimulate three PPAR receptor subtypes (Zhang et al., 2020), as these PPAR activators control various metabolic disordered condition effectively by manipulating the insulin resistance, obesity, and atherogenic dyslipidemia successfully and simultaneously. The PPAR-γ plays a vital role in different physiological activities like apoptosis, angiogenesis as well as inflammation (Mirza et al., 2019).