INTRODUCTION
Fucoidan is a sulfated polysaccharide from brown algae and marine invertebrates (Klettner, 2016). It is a heteropolysaccharide that is mainly composed of fucose and sulfate esters. It also comprises other monosaccharides like galactose, glucose, mannose, rhamnose, arabinose, xylose, uronic acid, proteins, and acetyl groups (Wang et al., 2019). Based on its polysaccharide structure, fucoidan has immune stimulator potential. The main sugar component of fucoidan that has an immunomodulatory function is L-fucose. Because of that, it has been found to have various bioactivities, including antioxidant, anti-inflammatory, immunoregulatory (Sun et al., 2018; Zhang et al., 2015), antiviral, antitumor, antidiabetic, and antihepatic injury activities (Peng et al., 2019).
Fucoidan’s immunomodulatory activity is determined by several factors such as the type of extract, source algae, molecular weight, content, and other fucoidan components (Lee et al., 2019). The composition and structure of the fucoidan carbohydrate chain vary and are influenced by seaweed species as its source (Ale et al., 2011). Several studies have showed the ability of fucoidan extracted from several alga sources to induce the immunostimulant effect.
Teruya et al. (2010) reported that fucoidans isolated from Laminaria angustata var. Longissima stimulated the production of nitric oxide (NO), tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6) in RAW 264.7 cells. Jin and Yu (2015)also reported that fucoidan from Undaria pinnatifida induced IL-6, IL-8, and TNF-α production in human neutrophils via the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) signaling pathway. Other studies have shown that fucoidans with lower molecular weight have a more significant effect on inducing an immune response (Jang et al., 2014). Other researchers showed fucoidan’s ability to increase the immune response of Th1 and natural killers cells (Miyazaki et al., 2019). It involves several signaling pathways in macrophage activation; almost all of these signals are closely related to cytokine expression associated with inflammation and inducible nitric oxide synthase (iNOS). Several studies have shown that various polysaccharides can increase the transcription activity of iNOS by activating nuclear factor-kappa B, thus encouraging NO formation in macrophages (Kouakou et al., 2013; Park, 2014).
Brown seaweed Sargassum duplicatum are abundant in almost all Indonesian waters and can be obtained in a year. This study was conducted to evaluate the immunostimulatory activity of fucoidan extracts of S. duplicatum, expressed as macrophage NO production. The immunomodulatory improving effects of fucoidan compound agents were investigated using in vivo studies. The present research examined the effects of S. duplicatum fucoidan at high doses on hematological and serum biochemical to confirm the safety or ability of test animals to digest the fucoidan
MATERIALS AND METHODS
Materials
The brown seaweed S. duplicatum as the main raw material was collected from Binuangeun, Banten, Indonesia. The seaweed samples were collected by local fishermen from 0.5 to 10 m depth between July and August 2018.
Experimental animals and housing conditions
Male white Sprague Dawley rats were obtained from Laboratory of Animal Management Unit [Unit Pengelola Hewan Laboratorium (UPHL)], Faculty of Veterinary Medicine [Fakultas Kedokteran Hewan (FKH)], Bogor Agricultural University [Institut Pertanian Bogor (IPB)], Bogor, Indonesia, with a weight of 200–250 g. The feed contains water (13%), protein (19%), fat (21%), fiber (5%), ash (7%), calcium (0.9%), phosphorus (0.6%), and total energy of 3,000–3,100 kcal/g. Before testing the samples, the rats were acclimatized for 2 weeks to adapt to the animal enclosure environment. They were held in a 12-hour dark/light cycle at 22°C ± 3°C and permitted free access to standard laboratory diets [(purchased from National Food and Drug Agency, Indonesia (BPOM)] and tap water ad libitum during the experiments. Male and female rats were obtained from BPOM for experiments LD50 and granted by the Animal Ethical Committee of Pharmacy Faculty, University of Indonesia.
Extraction of fucoidan
The fucoidan extract was obtained from defatted S. duplicatum. The defatted algae were prepared using the modified method of Sinurat and Rosmawaty (2015). The fucoidan was extracted from the defatted algal powder by soaking in 0.01 M HCl of pH 4 at the ratio of 1 :10 (w/v) by stirring for 6 hours.
The resulting extract was then filtered using a 350-mesh nylon screen, added to 4 M CaCl2, left at room temperature for 30 minutes, and refiltered using a 500-mesh nylon screen. After diluting with distilled water until the final CaCl2 concentration of 2 M, the filtrate was centrifuged at 15,344 g for 15 minutes, then increased with 3 M CaCl2, and centrifuged at a speed of 15,344 g for 15 minutes. The sample’s solubility was decreased by the addition of ethanol (1 : 2) and then left at room temperature overnight. The precipitated polysaccharides containing fucoidan were collected through centrifugation. The pellet formed was completely dried in spray drying. The fucoidan yield was estimated from the ratio of crude fucoidan weights to the defatted algal dry weight (Sinurat et al., 2016b). The whole process was carried out in triplicate, and the yield was mentioned as mean ± standard deviation (SD). All yields were collected and then analyzed and the yield was stated as mean ± SD.
Determination of chemical composition of crude fucoidan
The total polysaccharides content of fucoidan was analyzed according to the method described by Dubois et al. (1956) using phenol-H2SO4 reagent and L-fucose (Sigma) as the standard. The sulfate content was measured using K2SO4 (Merck) as the standard based on the BaCl2-gelatin method. Fucoidan sample was hydrolyzed (15 mg) in a solution of 3 M HCl for 17 hours at 100°C (Dodgson and Price, 1962). The content of uronic acid in fucoidan was analyzed by a carbazole–-sulfuric acid-borate method using D-glucuronic acid (Sigma) as standard (Bitter and Muir, 1962). All yields were calculated from the dried weight of fucoidan and converted into a percentage. Absorbance measurements were recorded in triplicate using an Ultrospec 2,100 UV/visible spectrophotometer (Thermo Scientific).
LD50 dose determination
LD50 is the amount of material which causes death to 50% of the population. LD50 of the extract was analyzed according to Indonesian Food and Drug Authority or Indonesian FDA (BPOM) guidelines no. 7/2014. The test item was fed orally to one rat at a dose of 6.941 mg/kg/ body weight (BW). Since the rat died within an hour after receiving the dose, a lower dose of 1 g/kg was tested in another group of three rats to find LD50.
After dosing, rats’ mortality was observed for up to 6 hours as a clinical sign of toxicity. Furthermore, it was recorded every day until 14 days when the animal was euthanized. This protocol was followed as per Committee for the Purpose of Control and Supervision of Experiments on Animals and approved by the Animal Ethical Committee of Pharmacy Faculty, University of Indonesia.
Feeding experiment
All animals were acclimatized for 2 weeks (14 days) before starting the experiment. Then, the experimental rats were divided into a randomized complete design with four treatments and seven replications. The experimental design pattern is presented in Table 1.
Hematology and serum biochemistry studies
After being acclimatized for 14 days, the rats had their blood drawn through vena coccygea to observe Red blood cell (RBC counts, hematocrit, and hemoglobin), white blood cells (WBC counts, neutrophils, lymphocytes, monocytes, basophils, and eosinophils), liver function [serum glutamic-pyruvic transaminase (SGPT) and glutamic-oxoloacetic transaminase (SGOT)], and kidney function (urea and creatinine). After blood sampling, the rats were treated according to the experimental design for 6 days.
 | Table 1. Experimental design for in vivo immunomodulatory test. [Click here to view] |
After 7 days, rats were injected intraperitoneally with 2 ml of Staphylococcus aureus bacterial suspension and left for 1 hour. Then, the rats were euthanized using a combination of ketamine and xylazine. Next, the stomachs of the rats waswere dissected using sterile tweezers and surgical scissors. Peritoneal fluid was taken using a micropipette. The peritoneal liquid was daubed on an object glass and fixed with methanol for 5 minutes, then stained with Giemsa staining, kept to stand for 20 minutes, and washed with running water. Then, the preparations were seen under a microscope to calculate the activity and capacity of macrophage phagocytosis. On day 8, we observed (RBC counts, hematocrit, and hemoglobin), (WBC counts, neutrophils, lymphocytes, monocytes, basophils, and eosinophils), liver function (SGPT and SGOT), and kidney function (urea and creatinine).
The parameters observed consisted of phagocytic capacity of macrophages, (RBC counts, hematocrit, and hemoglobin), and (WBC counts, neutrophils, lymphocytes, monocytes, basophils, and eosinophils). Hemocytometer was used as a tool for calculating the number of WBC using WBC fluid. The level in the leukocyte count was counted from a different type of WBC from the Giemsa-colored slides from each of the 30 oil fields immersed for microscopy purposes (Coles, 1974); liver function (SGPT and SGOT) and SGOT and SGPT enzymes were analyzed using the kit package (Pars Azmoon Co; Tehran, Iran) (Toghyani et al., 2011); and kidney function (ureum and urea creatinine) levels were analyzed using a photoelectric colorimeter.
Histopathological examination
Pathological changes were observed according to Raghuramulu et al. (2003). All the histopathological changes prior to seaweed water extract were observed according to Ganesan et al. (2019). All the rats divided into four groups, P1, P2, P3, and the control (K), were sacrificed 24 hours after their respective daily doses (Table 1). The liver and kidney were examined according to Ajibade et al. (2012).
Statistical analysis
All the tests were carried out independently in triplicate. Data were processed using the Statistical Package for the Social Sciences 19.0 software (IBM Corporation, Armonk, NY).
RESULTS AND DISCUSSION
Characterization extract fucoidan
Seaweed used as raw material for fucoidan extraction was brown seaweed S. duplicatum which grows at a depth of 0.5–10 m. Brown seaweed is attached to the substrate bottom of the water. The length of the brown seaweed thallus was around 30–100 cm. This process produced a fucoidan crude extract at amounts of 4.32% ± 0.40% (seaweed DW). The chemical compositions of fucoidan were 0.28% of uronic acid, 11.2% of sulfate, 8.25% of moisture content, and 60.5% of total polysaccharide.
Toxicity test LD50
Based on the results of the acute toxicity test using male mice and female mice using BPOM method No. 7 in 2014, the results showed that LD50 for female mice and male mice was the same at 6.941 g/kg BW. At this concentration, the mice died; an autopsy was conducted on the organs of the mice. The results showed that there are no differences in the size and shape of vital organs. These results of LD50 showed that fucoidan extract was declared nontoxic. From these results, there was no serious toxic effect on the male and female rats that were given fucoidan orally up to 6.941 g/kg BW. The highest dosage tested in this study was 4 g/kg BW and therefore may be safe for clinical use. A previous study showed that fucoidan derived from Laminaria japonica did not cause a significant toxicological effect on Wistar rats that were given 300 mg/kg BW/day for 6 months. Although there were prolonged clotting times at the administration of 900 and 1,500 mg/kg BW/day, it showed that fucoidan from L. japonica had no negative effect at 300 mg/kg BW/day (Ning et al., 2005). The same result also showed that fucoidan from Cladosiphon okamuranus was considered nontoxic in rats after oral administration for 3 months up to 600 mg/kg BW per day (Gideon and Rengasamy, 2008). Although there were slight differences between the experimental designs and settings in the studies, these results, including ours, suggested that the potential toxicity of fucoidan could be different from various sources and may require confirmation individually to maximize biological efficiency, while lowering any potentially harmful effects (Chung et al., 2010).
Hematology
Hematology parameters of rats before fucoidan treatment (in Table 2) show no significant differences in each hematological parameter value of rats’ blood in all groups before the treatment (p < 0.05). Hematology parameters of rats after fucoidan treatment are shown in Table 3. We added two new parameters, that isi.e., the number of macrophages and macrophage activity. From these parameters, we want to know the effect of fucoidan treatment on the rats’ blood.
Staphylococcus aureus is a pathogen bacteria responsible for the majority of human skin and soft tissue infections, including cellulitis, impetigo, folliculitis, ulcers infectious, and wounds. It can also cause invasive and life-threatening infections, such as pneumonia, meningitis, bacteremia, abscesses, endocarditis, osteomyelitis, and sepsis (Miller and Cho, 2011).
In this study, S. aureus has been shown to stimulate immune functions and this was indicated by increasing the levels of WBC. The WBC profiles showed that it increased after the administration of fucoidan. The increase in WBC might indicate the activation of the defense and immune system of the body and shows that there are edema and inflammation in the tissues (Mongi et al., 2011; Yousef et al., 2003). The percentage of neutrophils in the treated rats was higher than that in the untreated rats. Neutrophils, the major population of circulating leukocytes, played a vital role in the defense against bacterial and fungal infections. Mature neutrophils have the shortest half-life compared to other leukocytes when serving constitutive apoptosis (Jin and Yu, 2015). The rising number of macrophages and macrophage activity showed the influence of the fucoidan administration. The profiles also showed that there is an optimal concentration for fucoidan administration, that isi.e., the P2 group or the dose of 2 g/kg BW. A macrophage is one indicator of the increasing endurance of living things. The higher the number of macrophages, the stronger the body, being able to fight viruses or bacteria that enter the body.
 | Table 2. Hematology parameters of rats at before treated orally fucoidan extracts of S. duplicatum. [Click here to view] |
 | Table 3. Hematology parameters of rats at post treated orally fucoidan extracts of S. duplicatum. [Click here to view] |
In Table 3, there are no significant differences between each rat group in RBC, hematocrit, Hb, WBC, lymphocytes, monocytes, neutrophils, basophils, and eosinophils values (p < 0.05); however,, while there are significant differences between each rat group in the number of macrophages and macrophage activity values (p < 0.05). Both parameter values increased as the dose of fucoidan extract increased. The results show that the number of macrophages is higher in the P1 treatment group than that in the control group (P0). The same results are also shown in the P2 and P3 groups. However, the number of macrophages in the P2 group is higher than that in the P3 group. We assume that there is optimum fucoidan concentration that can increase the number of macrophages and macrophage activity values.
According to the hematology profile, the macrophage activity value in the P0 group shows 23.43 ± 4.58, whereas in the P1 group (dose 1 gr/kg BW), it shows an increase of almost two times. In the P2 group (dose 2 g/kg BW), the increase is three times higher than that in the control group, while the macrophage activity in the P3 group (dose 4 g/kg BW) decreases but remains above that of the control group. The same trend was also shown in the number of macrophage profiles.
Macrophages, innate immune cells, play a leading role in the elimination of hazardous foreign substances as well as cancer cells (Guiducci et al., 2005), infectious pathogens (Kar et al., 2011), and waste cells (Feng et al., 2015). In the human immune system, macrophages are the first cells that identify foreign bodies’ attack and rapidly become activated macrophages. The activated macrophages will lead to the innate immune responses and destroy the foreign bodies directly by phagocytosis and discharge of NO. Inducible iNOS produces NO and facilitates varied immune functions such as reducing the proliferation of pathogens and destroying pathogens (Aktan, 2004). iNOS is an important inflammatory gene whose transcription process is regulated by nuclear factor-kappa B (NF-κB). It is known that there are more than 400 stimuli that can activate NF-κB as a unit of pleiotropic transcription factors. NF-κB is an important transcription factor that regulates inflammatory response and the body’s immune system response. NF-κB generally plays a role in controlling immune and inflammatory responses, cell proliferation, and apoptosis and regulating the transcription of various proinflammatory genes. Fucoidan, as β-glucans, has the potential to stimulate innate immune cells and encourage protective Thl immune responses in tumor-bearing rats (Qi et al., 2015). The biological activities of a macromolecule such as polysaccharides are significantly affected by their structures such as monosaccharide composition, molecular weight, glycosidic linkage forms, and triple-helix structure. Preceding research showed that higher molecular weight polysaccharides (MW 57,500 Da) derived from Asparagus officinalis had significant antioxidant and antitumor activities than that the lower (MW 51,100 Da) (Zhao et al., 2012). Fucoidan extract used in this study has high molecular weight polysaccharide estimated above 75,000 Da (Sinurat et al., 2016a).
Serum biochemistry
Serum biochemistry parameters of rats before extract treatment wereare presented in Table 4 and serum biochemistry parameters of rats after extract treatment wereare presented in Table 5. There are no significant differences in each biochemical parameter value between the rat groups before the treatment (p < 0.05; Table 4). There are also no significant differences in each biochemical parameter value between rat groups after the treatment (p < 0.05; Table 5). However, the SGOT value increased in each rat group after the treatment.
(SGOT and SGPT enzymes are a precise indicator of serious liver cell impairment and pathological indicator of liver dysfunction. Severe damage to liver function will cause the release of SGOT and SGPT from the liver cells into the bloodstream; thus, any abnormal enhancement in their concentration could suggest liver failure (Toghyani et al., 2011). The urea and creatinine blood concentration can indicate the function of the kidney. In this study, the level of SGOT and SGPT of the treated rats’ group was not significantly different from the control group, and so was the level of urea and creatinine of the treated rat's’ group. It can be considered that the fucoidan administration up to 4 g/kg BW did not have an adverse effect on rat liver and kidney. Blood cell histopathological performance was significantly different because fucoidan effectively weakened the inflammatory cytokines. In this study, fucoidan post-treatment until a dose of 4 g/kg BW did not damage the liver and kidney. Meanwhile, fucoidan treatment was found to be not toxic for the liver and kidney.
 | Table 4. Serum biochemical parameters at before treated orally fucoidan extract of S. duplicatum. [Click here to view] |
 | Table 5. Serum biochemistry parameters at post treated orally fucoidan extract of S. duplicatum. [Click here to view] |
 | Figure 1. Brown seaweed Sargassum duplicatum. [Click here to view] |
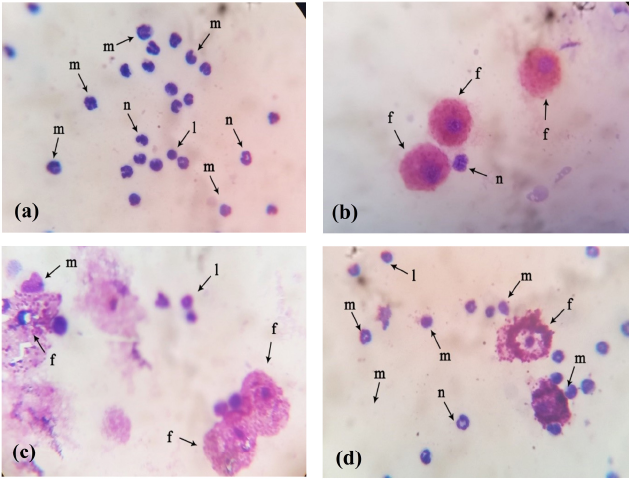 | Figure 2. Histopathology blood cell of experimental rats after feeding with fucoidan extract (a) Control group-standard diet, (b) Fucoidan extract administrated rats, (c) Fucoidan extract administrated rats, (d) Fucoidan extract administrated rats. [Click here to view] |
Histopathology
Histopathology results showed that there were visible blood clot stains in the rat blood cells of the fucoidan control group (Fig. 2). The stains appeared because of the S. aureus suspension administration to the experimental rats. On the contrary, the histopathology analysis appeared as blue and red stains that showed the effect of the fucoidan addition on the S. aureus infection in the remaining treatment groups, including Group 1 to Group 3. Such stains demonstrated the ability of fucoidan extract to destroy bacterial cells by phagocytosis so that bacterial cells are unable to damage other blood cells. Our result confirmed other results that showed the potential ability of fucoidan as antibacterial (Marudhupandi et al., 2013; Mohsin et al., 2016; Poveda-Castillo et al., 2018).
CONCLUSION
This study showed that fucoidan is nontoxic. In vivo immunostimulant test results also showed that the fucoidan administration starting from 1 g/kg BW can increase the immune system of rats almost two times higher than rats in the control group. However, we concluded that the optimum fucoidan administration is 2 g/kg BW based on the increased number of macrophages and macrophage activity.
AUTHORS’ CONTRIBUTIONS
ES designed the study and wrote the protocol as the main contributor. ES, EM, and SB interpreted the data, managed the analyses of the study. ES, EM and SB were the contributors to the analysis and interpretation of the data. All authors read and approved the final manuscript.
ACKNOWLEDGEMENTS
The authors acknowledge the research team at IPB Laboratory Management Unit (UPHL) Faculty of Veterinary Medicine, Bogor Agricultural University (FKH-IPB), Bogor, Indonesia for providing registered data. Contributors included: Dr. drh. Aulia, Dr. drh. Andriyanto, Msi and all staff laboratory.
CONFLICT OF INTEREST
We declare that we have no conflict of interest.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study protocol was approved by Animal Ethical Committee of Pharmacy Faculty, University of Indonesia.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Ajibade TO, Olayemi FO, Arowolo ROA. The haematological and biochemical effects of methanol extract of the seeds of moringa oleifera in rats. J Med Plants Res, 2012; 6(4):615–21. CrossRef
Aktan F. iNOS-mediated nitric oxide production and its regulation. Life Sci, 2004; 75(6):639–653. CrossRef
Ale, MT, Mikkelsen JD, Meyer AS. Important determinants for fucoidan bioactivity: a critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar Drugs, 2011; 9(10):2106–2130. CrossRef
Bitter T, Muir HM. A modified uronic acid carbazole reaction. Anal Biochem, 1962; 4(4):330–34. CrossRef
Chung HJ, Jeun J, Houng SJ, Jun HJ, Kweon DK, Lee SJ. Toxicological evaluation of fucoidan from Undaria pinnatifida in vitro and in vivo. Phytother Res, 2010; 24(7):1078–83. CrossRef
Coles EH. Veterinary clinical pathology. 2nd edition, WB Saunders, Philadelphia, PA, 1974.
Dodgson KS, Price RG. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem J, 1962; 84:106–10. CrossRef
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugar and related subtances. Anal Chem, 1956; 28(3):350–56. CrossRef
Feng M, Chen JY, Weissman-Tsukamoto R, Volkmer JP, Ho PY, McKenna KM, Cheshier S, Zhang M, Guo N, Gip P, Mitra SS, Weissman IL. Macrophages eat cancer cells using their own calreticulin as a guide: roles of TLR and Btk. Proc Natl Acad Sci U S A 2015; 112(7):2145–50. CrossRef
Ganesan AR, Subramani K, Balasubramanian B, Liud WC, Arasue MV, Al-Dhabie NA, Duraipandiyan Vu D. Evaluation of in vivo sub-chronic and heavy metal toxicity of under-exploited seaweeds for food application. J King Saud Univ Sci, 2019; 40:0–7.
Gideon PT, Rengasamy R. Toxicological evaluation of fucoidan from Cladosiphon okamuranus. J Med food, 2008; 11(4):638–42. CrossRef
Guiducci C, Vicari AP, Sangaletti S, Trinchieri G, Colombo MP. Redirecting in vivo elicited tumor infiltrating macrophages and dendritic cells towards tumor rejection. Cancer Res, 2005; 65(8):3437–47. CrossRef
Jang JY, Moon SY, Joo HG. Differential effects of fucoidans with low and high molecular weight on the viability and function of spleen cells. Food Chem Toxicol, 2014; 68:234–38. CrossRef
Jin JO, Yu Q. Fucoidan delays apoptosis and induces pro-inflammatory cytokine production in human neutrophils. Int J Biol Macromol, 2015; 73(1):65–71. CrossRef
Kar S, Gunjan S, Das PK. Fucoidan cures infection with both antimony-susceptible and-resistant strains of leishmania donovani through Th1 response and macrophage-derived oxidants. J Antimicrob Chemother, 2011; 66(3):618–25. CrossRef
Klettner A. Fucoidan as a potential therapeutic for major blinding diseases-a hypothesis. Mar Drugs, 2016; 14(31):1–13. CrossRef
Kouakou K, Schepetkin IA, Yapi A, Kirpotina LN, Jutila MA, Quinn MT. Immunomodulatory activity of polysaccharides isolated from alchornea cordifolia. J Ethnopharmacol, 2013;146(1):232–42. CrossRef
Lee HH, Cho YJ, Yu D, Chung D, Kim GH, Kang H, Cho H. Undaria Pinnatifida ucoidan-rich extract induces both innate and adaptive immune responses. Nat Prod Commun, 2019;14(8):1–8. CrossRef
Marudhupandi T, Thipparamalai TAK. Antibacterial effect of fucoidan from Sargassum Wightii against the chosen human bacterial pathogens. Int Curr Pharm J, 2013; 2(10):156–58. CrossRef
Miller LS, John SC. Immunity against Staphylococcus aureus cutaneous infections. Nat Rev Immunol, 2011; 11(8):505–18. CrossRef
Miyazaki Y, Yuri I, Juneha B, Hayato N. Biochemical and biophysical research communications the cooperative induction of macrophage activation by fucoidan derived from Cladosiphon okamuranus and b-glucan derived from saccharomyces cerevisiae. Biochem Biophys Res Commun, 2019; 516(1):245–50. CrossRef
Mohsin SRM, Sumayya A, Kurup GM. Bifunctional effect of fucoidan from padina tetrastromatica against human pathogenic microbes and free radicals. J Med Herbs Ethnomed, 2016; 2(2):1–10.
Mongi, S, Mahfoud M, Boumendjel A, Jamoussi K, Abdelfattah EF. Protective effects of vitamin C against haematological and biochemical toxicity induced by deltamethrin in male wistar rats. Ecotoxicol Environ Saf, 2011; 74(6):1765–69. CrossRef
Ning L, Zhang Q, Song J. Toxicological evaluation of fucoidan extracted from Laminaria Japonica in wistar rats. Food Chem Toxicol, 2005; 43:421–26. CrossRef
Park HJ. Immune stimulatory activity of BRP-4, an acidic polysaccharide from an edible plant, Basella Rubra L. Asian Pac J Trop Med, 2014; 7(11):849–53. CrossRef
Peng Y, Yuefan S, Wang Q, Hu Y, Yunhai H, Dandan R, Wu L, Liu S, Cong H, Zhou H. In vitro and in vivo immunomodulatory effects of fucoidan compound agents. Int J Biol Macromol, 2019; 127:48–56. CrossRef
Poveda-Castillo GDC, Dolores R, Martínez A, Pérez MP. Bioactivity of fucoidan as an antimicrobial agent in a new functional beverage. Beverages, 2018; 4(3):64. CrossRef
Qi C, Yihua C, Lacey G, Chuanlin D, Bing L, Goetz K, Keqing Q, John V, Shinobu S, Yoichiro I, John R. Y, Jun Y. Differential pathways regulating innate and adaptive antitumor immune responses by particulate and soluble yeast-derived β-glucans. Blood J Am Soc Hematol, 2015; 117(25):6825–37. CrossRef
Raghuramulu N, Madhavan Nair K, Kalyanasundaram S, National Institute of Nutrition (India). A manual of laboratory techniques. National Institute of Nutrition, Hyderabad, India, pp 56–8, 2003.
Ren Y, Guiqing Z, Lijun Y, Lingrong W, Chao L, Xiong F, Lin Z. Structural characterization and macrophage immunomodulatory activity of a polysaccharide isolated from Gracilaria Lemaneiformis. J Funct Foods, 2017; 33:286–96. CrossRef
Sinurat E, Rosmawaty P. Evaluation of fucoidan bioactivity as anti-gastric ulcers in mice. Procedia Environ Sci Ictcred, 2015; 25:407–11. CrossRef
Sinurat E, Saepudin E, Rosmawaty P, Hudiyono S. Characterization of fucoidan extracted from Sargassum polycystum different habitats. 5th International Conference on Earth Science and Environmental Engineering (ICESEE-16), 4(1), pp 2–5, 2016a.
Sinurat E, Saepudin E, Rosmawaty P, Hudiyono S. Immunostimulatory activity of brown seaweed-derived fucoidans at different molecular weights and purity levels towards white spot syndrome virus (WSSV) in shrimp Litopenaeus Vannamei. J Appl Pharm Sci, 2016b; 6(10):082–91. CrossRef
Sun Y, Gong G, Guo Y, Wang Z, Song S, Zhu B, Zhao L, Jiang J. Purification, structural features and immunostimulatory activity of novel polysaccharides from Caulerpa Lentillifera. Int J Biol Macromol, 2018; 108:314–23. CrossRef
Teruya T, Shinji T, Yukihiro T, Masakuni T. Fucoidan isolated from Laminaria angustata Var. Longissima induced macrophage activation. Biosci Biotechnol Biochem, 2010; 74(9):1960–62. CrossRef
Toghyani M, Majid T, Abbasali G. Evaluation of cinnamon and garlic as antibiotic growth promoter substitutions on performance, immune responses, serum biochemical and haematological parameters in broiler chicks. Livest Sci, 2011; 138(1–3):167–73. CrossRef
Wang Y, Xing M, Qi C, Ji Q, Liang H, Song S. Biological activities of fucoidan and the factors mediating its therapeutic effects: a review of recent studies. Mar Drugs, 2019; 17(3):1–18. CrossRef
Yin M, Zhang Y, Li H. Advances in research on immunoregulation of macrophages by plant polysaccharides. Frontiers in immunology, 2019; 10(145): 1-19. CrossRef
Yousef MI, Abdallah GA, Kamel KI. Effect of ascorbic acid and vitamin E supplementation on semen quality and biochemical parameters of male rabbits. Anim Reprod Sci, 2003; 76(1–2):99–111. CrossRef
Zhang W, Tatsuya O, Qing Y, Jun OJ. Fucoidan from Macrocystis Pyrifera has powerful immune-modulatory effects compared to three other fucoidans. Mar Drugs, 2015; 13(3):1084–104. CrossRef
Zhao Q, Bingxian X, Jun Y, Fangchun Z, Jie X, Lingyun Y. In vitro antioxidant and antitumor activities of polysaccharides extracted from Asparagus officinalis. Carbohydr Polym, 2012; 87(1):392–96. CrossRef