INTRODUCTION
Curcumin (CUR) is a crystalline orange-yellow phytochemical that is isolated from Curcuma longa (Zingiberaceae) along with other two de-methoxy compounds which are desmethoxycurcumin and bisdemethoxycurcumin, and are classified as a functional food. Structurally, CUR is 1, 7- bis (4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione (Figure 1A) (Suchitra and Rajashree, 2016). Since its diverse molecular targets, it exhibits a wide variety of pharmacological activities including anti-cancer activity. CUR also possesses anti-inflammatory, chemo-sensitizing, radio-sensitizing, wound healing, antimicrobial, antiviral, antifungal, immunomodulatory, and antioxidant activities. CUR is relatively healthy and can be strongly tolerated well at high doses and has also been declared generally recognized as safe (GRAS) by the United States Food and Drug Administration (USFDA) (Moorthi and Kathiresan, 2012; Moorthi and Kathiresan, 2013; Moorthi et al., 2011, 2012; Sharma et al., 2005). PIP is a major alkaloid isolated from Piper nigrum L. (Piperaceae). Structurally, PIP is 1-[(2E,4E)-5-(1,3-Benzodioxol-5-yl)-1-oxo-2,4-pentadieneyl] piperidine (Figure 1B) (Maryadele, 2001). The most interesting point is that PIP increases the bioavailability of a number of therapeutic drugs, as well as phytochemicals, including CUR (Vipul et al., 2013). PIP has several reported pharmacological activities like central nervous system depressant, antipyretic, analgesic, hepatoprotective, antioxidant, and anti-inflammatory (Hamrapurkar et al., 2011). It is also listed by the USFDA as a GRAS molecule (Nurul et al., 2010).
The simultaneous estimate of CUR and PIP in their combined dosage form is not standard in any pharmacopoeia; therefore, no standard method of estimating CUR and PIP in their combined dosage forms is available (Kaushik et al., 2012). A literature survey reported that several methods are available for the estimation of CUR and PIP simultaneously in different formulations and in plasma. Namely, reverse phase high performance liquid chromatography (RP-HPLC) with photodiode array (PDA) detector (Moorthi et al., 2013), HPLC with electrospray tandem identification of the mass spectrometer in strong ionization mode (Xiu-Mei et al., 2012), RP-HPLC system with a fluorescence detector (Shaikh et al., 2009), RP-HPLC method using diode array detection system (Nagappan et al., 2009), RP-HPLC with ultraviolet (UV)/Visible Detector (Chahar and Mashru, 2016), ultra-fast liquid chromatography using a PDA (Shanmugam et al., 2014), and HPLC system UV-Visible detector(Prerana et al., 2009). Furthermore, it was found that only one method was developed by using UV Spectrophotometer, but it was for a mixture of CUR, PIP, and quercetin (Aneja et al., 2012). A UV-Visible spectroscopic approach was developed to estimate the dissolution profile of CUR, PIP, and their accurate measurement in formulated 0.5 Sodium lauryl sulfate (SLS) dissolution medium in pH 6.0 sodium phosphate buffer (Murti et al., 2019).The reported methods were not cost-effective due to the use of highly sophisticated instruments and detectors and costly solvents, such as tetrahydrofuran, trifluoro acetic acid, and some methods, were found to be less sensitive. The reported UV-Visible spectroscopic method was highlighting the estimation of CUR and PIP in the dissolution medium of the formulation. So in this research work, a quick, simplified, accurate, and sensitive simultaneous estimation approach (Vierordt’s and Q absorbance methods) was developed by using low-cost solvent methanol by using UV-Visible spectrophotometer which was highly sensitive to detect lower concentration. The developed approach was used for estimating the CUR and PIP in CUR+PIP dual drug-loaded nanostructured lipid carriers (NLCs) in which methanol was used for extraction of CUR and PIP. The use of methanol as an extracting solvent makes it more compatible with the developed method.
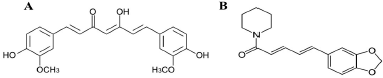 | Figure 1. Structure of (A) CUR and (B) PIP. [Click here to view] |
METHODS AND MATERIALS
Materials
CUR of purity 99.50% was obtained from VAV Life Science (Mumbai, India) as a gift sample. PIP of purity 97.00% was bought from Sigma-Aldrich (Mumbai, India). Analytical reagent grade methanol of purity 99.80% was bought from Rankem chemicals (Mumbai, India). Glyceryl distearate (Precirol ATO5), medium chain triglycerides (Labrafac Lipophile WL1349), and Stearoyl polyoxyl-32 glycerides (Gelucire 50/13) were gifted by Gattefosse (Mumbai, India). Polysorbate 80 (Tween 80) was gifted by BASF (Mumbai, India).
Instrumentation
For spectra and absorbance imaging and calculation, a double beam UV-visible spectrophotometer (SHIMADZU UV-1800) (Mumbai, India) consisting of two matched quartz cells with 1 cm light direction and fitted with UV probe program (version 2.3) was used. In this project, an electronic analytical weighing balance (Shimadzu AU 220) (Mumbai, India) and a sonicator (Sonica, model 2200 MH) (Mumbai, India) were used.
Method development
Selection of the solvent
Various solvent mixtures were studied. The parameters used for the selection of solvent were the ease of processing the standards and samples, solubility, and stability of actives, cost, and applicability for the method. Both the actives showed good solubility profile in methanol which is inexpensive and thus it was chosen for the study.
Preparation of Standard stock solutions
Stock solutions containing standard CUR and PIP were independently prepared by dissolving 10 mg of CUR and 10 mg of PIP in 10 ml of methanol, respectively; the prepared solutions were sonicated for 10 min and the final volume of both solutions was rendered up to 100 ml with the same solvent to obtain stock solutions comprising 100 μg/ml of CUR and PIP each.
Determination of wavelength of maximum absorbance (λmax) and Iso-absorptive Point
The standard CUR and PIP stock solutions were diluted individually with methanol to provide a solution comprising 10 μg/ml CUR and 10 μg/ml PIP. Approximately 3.0 ml was collected and tested with the UV spectrophotometer from 200 to 800 nm. The wavelengths were selected in such a way that at each wavelength the absorptivity difference between the two actives was as large as possible. Considering this, the λmax of both actives were selected for the method.
Preparation of standard calibration curve of CUR and PIP
In both CUR and PIP, a calibration curve was plotted at a concentration range of 1–30 μg/ml. Accurately, measured working stock solutions (1, 2, 3, 4, 5, 6, and 7 μg/ml) for both CUR and PIP were prepared using methanol as a solvent in two different series of 10 ml volumetric flask. All the solutions were screened for absorbance at their respective λmax and iso-absorptive point. The calibration curves were developed by plotting the concentration against absorbance where every reading was mean of three determinations.
Sample preparation method
The sample solution in the ration of 2:1 (CUR:PIP) was prepared from standard stock solutions. Sample solution absorbance was measured simultaneously at 423, 342 nm, and also at 368.5 nm at its iso-absorptive point.
Preparation of CUR+PIP NLCs formulation
It was prepared by a modified hot melt emulsification/ultrasonication technique using lipids and surfactants at Vivekanand Education Society’s College of Pharmacy (Chembur, Mumbai). The formulation contains 3% w/v of glyceryl distearate (Precirol ATO5) as solid lipid, 2% w/v medium chain triglycerides (Labrafac Lipophile WL1349) as liquid lipid, and combination of 1.25% w/v of solid surfactant stearoyl polyoxyl-32 glycerides (Gelucire 50/13) with 1.25% w/v liquid surfactant Polysorbate 80 (Tween 80). The prepared nanoparticulate formulation contained 0.08% w/v of CUR and 0.04% w/v of PIP.
Method I (Vierordt’s simultaneous equation method)
The research is focused on the absorption of actives CUR and PIP at their wavelength maxima in this simultaneous equation system (Mohit et al., 2010). Two chosen wavelengths for the simultaneous calculations are 423 and 342 nm. The absorptivity measurement values for CUR are 1.377 (ax1), 0.270 (ax2), and for PIP are 0.004 (ayl), 1.257 (ay2) at 423 and 342 nm, respectively. These measurements are a mean of six estimations. This determines the concentration of actives and the absorbances at these wavelengths were substituted in equations (1) and (2).
where,
Cx and Cy are concentrations of CUR and PIP in μg/ml in sample solution, respectively.
A1 and A2 are the absorbance of sample solutions at 423 nm and 342 nm, respectively.
ax1 is the absorbance of CUR at 423 nm.
ax2 is the absorbance of CUR at 342 nm.
ay1 is the absorbance of PIP at 423 nm.
ay2 is the absorbance of PIP at 342 nm.
By substituting the values of A1 and A2, the Cx and Cy can be calculated by solving equations (1) and (2).
Method II (absorbance ratio or Q-analysis method)
In the absorption ratio approach (Tushar et al., 2014), absorbances of both the actives are measured at two chosen wavelengths among which λ1 is the λmax of either drug among both drugs and λ2 is the wavelength of iso-absorptive point of both drugs. Here, λ1 is selected as 423 nm and from the overlain spectra wavelength (λ2) 368.5 nm (iso-absorption point) were selected for the formation of q absorbance equation [Eqs. (3) and (4)]. The absorbances at 423 and 368.5 nm for CUR were obtained and similarly for PIP absorbances are measured at 423 and 368.5 nm. The concentration of the individual actives was determined by using the following equations:
Where,
Cx and Cy are concentrations of CUR and PIP, respectively,
Qx = the ratio of absorbances of CUR at 423 and 368.5 nm.
Qy = the ratio of absorbances of PIP at 423 and 368.5 nm.
Qm = the ratio of absorbance of mixture at 423 and 368.5 nm.
A1 = the absorbance of mixture at iso-absorptive point.
ax = the absorbance value of CUR at iso-absorptive point.
ay =the absorbance value of PIP at iso-absorptive point.
Method validation
The methods mentioned have been validated using International Conference on Harmonization (ICH) parameters for the assay of the two active components of the mixture (ICH, 2005).
Linearity
Linearity was tested by using prepared standard solutions of actives at varying levels of concentration. Calibration curves were constructed using the standard solutions of 1–7 μg/ml and linear regression analysis was conducted.
Precision
The intraday and interday precision of the developed method was evaluated by determining the related response three times on the same day, and three separate concentrations (3:3, 5:5, 7:7 μg/ml) on two different days.
Accuracy (% recovery study)
The accuracy of the developed analytical method shows the closeness of agreement between the value recognized either as a traditional truth value or as an agreed reference value and the observed value. The recovery tests were carried out in triplicate by spiking samples previously studied with three separate concentrations of standards.
Limit of detection (LOD) and Limit of Quantitation (LOQ)
The LOD and the LOQ of the actives were obtained by calculating the signal-to-noise ratio (S/N) i.e., 3.3 for LOD and 10 for LOQ using the following equation as defined by the ICH guidelines.
Where, σ = standard deviation response and S = slope of the calibration curve.
Application of validated method for assay of CUR+PIP in NLCs formulation
In a volumetric flask, 1 ml formulation of CUR+PIP NLCs (equivalent to 0.80 mg of CUR and 0.40 mg of PIP) was combined with methanol up to 10 ml. This was subjected to 60 min of bath sonication for complete CUR and PIP extraction. The sonicated solution was mixed and was purified using a 0.45 μ Nylon syringe filter to get an 80 μg/ml of CUR and 40 μg/ml of PIP solution. The solution was further appropriately diluted and analysed UV-Visible spectrophotometer utilizing the simultaneous equations for the estimation of actives.
RESULTS AND DISCUSSION
Method development
Determination of wavelength of maximum absorbance (λmax) and Iso-absorptive Point
CUR exhibits maximum absorption at a wavelength (λmax) 423 nm, while PIP exhibits maximum absorption at a wavelength (λmax) 342 nm and from overlain spectra, it is evident that the iso-absorptive point is as 368.5 nm.
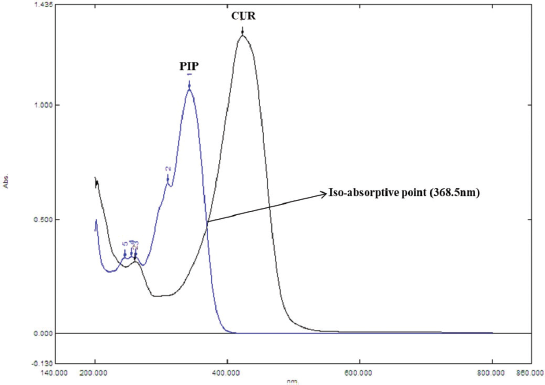 | Figure 2. UV overlay spectra of CUR and PIP. [Click here to view] |
Standard calibration curve of CUR and PIP
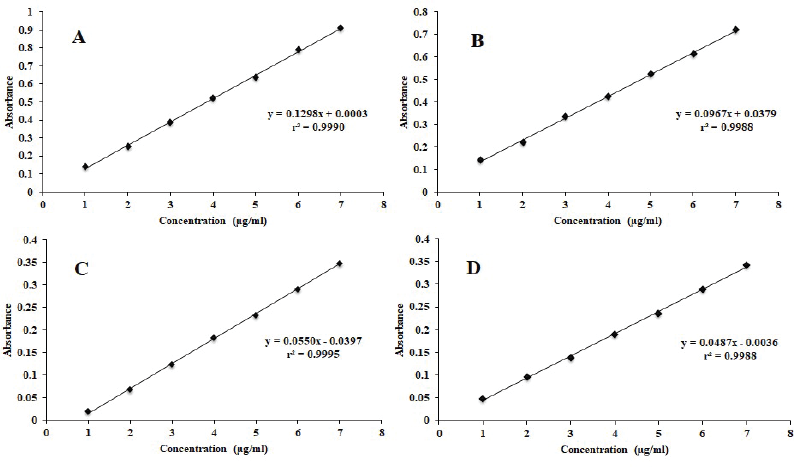 | Figure 3. Calibration curve of (A) CUR in methanol at wavelength (λmax) 423 nm, (B) PIP in methanol in methanol at wavelength (λmax) 342 nm, (C) CUR in methanol at iso-absorptive point 368.5 nm, (D) PIP in methanol at iso-absorptive point 368.5 nm. [Click here to view] |
Method validation
Linearity
The regression coefficients are reported in Table 1.
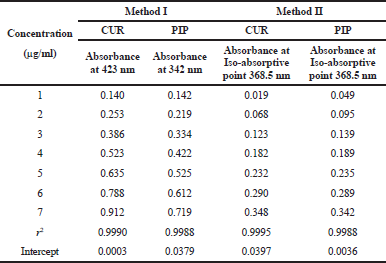 | Table 1. Linear regression analysis of calibration curves of CUR and PIP with both the methods. [Click here to view] |
Precision
The result was reported in terms of %relative standard deviation (RSD).
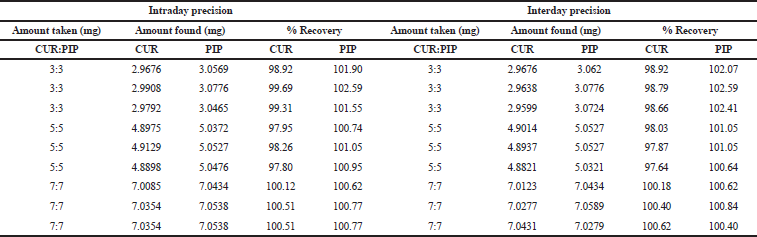 | Table 2. Data of precision and accuracy study for CUR and PIP for method I. [Click here to view] |
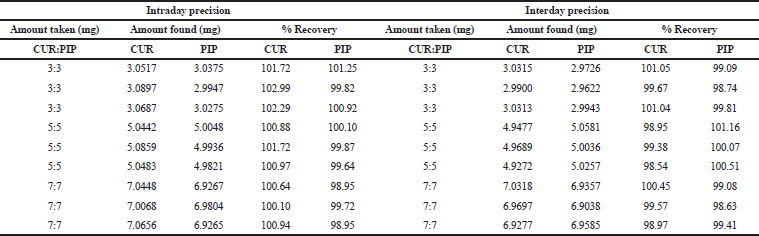 | Table 3. Data of precision and accuracy study for CUR and PIP for method II. [Click here to view] |
LOD and LOQ
 | Table 4. Results of LOD and LOQ. [Click here to view] |
Assay of CUR+PIP in NLCs formulation
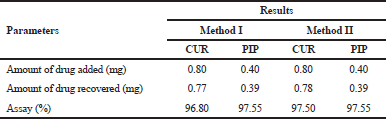 | Table 5. Assay results of CUR +PIP NLCs formulation. [Click here to view] |
The research was aimed at developing and validating a spectroscopic approach for estimating CUR and PIP actives in a nanoparticulate formulation system. The aim of the analytical method validation is to show the suitability and reliability of an analytical method for its intended function. Although many chromatographic methods are found in many publications for simultaneous estimation of CUR and PIP in various formulation systems. However, the reported approaches were having some drawbacks such as costly solvents, extensive sample preparation, which may involve multiple clean up steps, followed by extraction procedures and expensive analytical instruments which increases the sample analysis cost (Murti et al., 2019).
The CUR and PIP overlay spectra showed λmax at 423 nm and 342 nm, respectively, which are quite different from each other. In addition, an iso-absorptive value at 368.5 nm was found (Figure 2). Standard calibration curves for CUR and PIP were linear with correlation coefficients (r2) values of 0.9990 and 0.9988, respectively, at all the selected wavelengths. Standard calibration curves for CUR and PIP were linear with correlation coefficients (r2) values of 0.9995 and 0.9988, respectively, at all the iso-aborptive point (Figure 3). The procedure was replicated on the same day and it was observed that the %RSD was <0.4% for CUR and <0.59 for PIP, and <1.4% at iso-absorptive point, similarly the procedure was replicated on separate days and the %RSD was found to be <0.39% for CUR and <0.74% for PIP and <1.5% at iso-absorptive point (Table 2 and 3). The proposed method showed good accuracy by achieving a good percent recovery in the standard addition method. It ranged between 100.034% and 101.328% for CUR and 100.665% and 102.247% for PIP. The LOD was observed to be 0.092 μg/ml for CUR and 0.102 μg/ml for PIP. LOQ was observed to be 0.280 μg/ml and 0.311 μg/ml for CUR and PIP, respectively. The LOD of CUR and PIP at iso-absorptive point (368.5 nm) was observed to be 0.0683 times and 0.1024 μg/ml, respectively. The LOQ of CUR and PIP at iso-absorptive point (368.5 nm) was observed to be 0.207 and 0.310 μg/ml, respectively (Table 4). The developed method was successfully implemented for the assay of CUR+PIP in NLCs formulation. Assay of CUR and PIP was found to be 96.80% and 97.55%. The extracting solvent was methanol which helped in the complete extraction of CUR and PIP from the formulation. The results are summarized in Table 5. This proposed spectroscopic method confirms linearity, accuracy, and precision of the method. Although spectroscopic methods are not a selective option by analysts, the method is still helpful for the simultaneous estimation of two or more actives in various formulation systems. Despite the selectivity problem, the spectrophotometric method offers simplicity, rapidity, and reliability (Murti et al., 2019).
CONCLUSION
For the simultaneous estimation of CUR and PIP actives in the nanoparticulate formulation, the UV spectrophotometric Vierordt’s simultaneous equation (method I) and Q-absorption ratio (method II) were developed and validated. The suggested methods are simple, rapid, and validated in terms of linearity, accuracy, and precision. The methods can be effectively utilized for the routine estimation of actives CUR and PIP in pure and combined formulated systems.
LIST OF ABBREVIATIONS
UV - ultraviolet, CUR - Curcumin, PIP - Piperine, nm - Nanometer, cm - Centimeter, μg/ml - Microgram per milliliter, LOD - Limit of detection, LOQ - Limit of quantitation, US FDA - United States Food and Drug Administration, GRAS - Generally recognized as safe, RP-HPLC - Reverse Phase high performance liquid chromatography, PDA – Photodiode array, SLS – Sodium lauryl sulfate, NLCs - Nanostructured lipid carriers, ICH - International Conference on Harmonization, RSD - Relative standard deviation.
ACKNOWLEDGEMENT
The authors are thankful to the All India Council for Technical Education, New Delhi, for supporting this work (Ref. no: 8-159/RIFD/RPS/Policy-4/2013-14). The authors are thankful to VAV life sciences for providing gift sample of CUR. They are also thankful to Gattefosse, India, for providing the lipid samples for the development of the nanoparticulate system of CUR and PIP.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Aneja G, Dave U, Vadodaria K. Simultaneous estimation of piperine, quercetin, and curcumin in a mixture using U.V-visible spectrophotometer and method validation, Int J Ther Appl, 2012; 8:14–7.
Chahar MY, Mashru R. Development and validation of stability indicating RP-HPLC method for simultaneous estimation of curcumin and piperine in bulk mixture. World J Pharm Res, 2016; 5:1262–76.
Hamrapurkar PD, Kavita J, Sandip Z. Quantitative estimation of piperine in Piper nigrum and piper longum using high performance thin layer chromatography. J App Pharm Sci, 2011; 01:117–120.
ICH. Validation of analytical procedures: text and methodology. In International Conference on Harmonization (ICH), Q2(R1), IFPMA, Geneva, Switzerland, 2005.
Kaushik GK, Prakash MP, Hiral RT, Shital DF. Method development and validation of rifampicine and piperine in their combined dosage form. Int Bull Drug Res, 2012, 1:71–80.
Maryadele JO. The Mercek index: an encyclopedia of chemicals, drugs and biological. Whitehouse Station, NJ: The Royal Society of Chemistry.
Mohit R, Abhinav A, Ashish KJ, Narendra KL, Anil KK, Agrawal GP. Development of simultaneous spectrophotometric method of mesalazine and prednisolone in same dosage form. Int J Appl Pharm, 2010; 2:8–11.
Moorthi C, Kathiresan K. Curcumine piperine/curcumine quercetin/curcumine silibinin dual drug loaded nanoparticulate combination therapy: a novel approach to target and treat multidrug resistant cancers. J Med Hypotheses Ideas, 2013; 7:15–20. CrossRef
Moorthi C, Kathiresan K. Nanotoxicology: toxicity of engineered nanoparticles and approaches to produce safer nanotherapeutics. Int J Pharm Sci, 2012; 2:117–24.
Moorthi C, Kiran K, Manavalan R, Kathiresan K. Preparation and characterization of curcumin-piperine dual drug loaded nanoparticles. Asian Pac J Trop Biomed, 2012; 2:841–8. CrossRef
Moorthi C, Manavalan R, Kathiresan K. Nanotherapeutics to overcome conventional cancer chemotherapy limitations. J Pharm Pharm Sci, 2011; 14:67–77. CrossRef
Moorthi C, Senthil K, Mohan S, Kiran K, Kathiresan K. Application of validated RPeHPLCePDA method for the simultaneous estimation of curcumin and piperine in Eudragit E 100 nanoparticles. J Pharm Res, 2013; 7:224–9. CrossRef
Murti YB, Hartini YS, Hinrichs WLJ, Frijlink HW, Setyaningsih D. UV-Vis spectroscopy to enable determination of the dissolution behavior of solid dispersions containing curcumin as well as piperine. J Young Pharm, 2019; 11:26–30. CrossRef
Nagappan KV, Meyyanathan SN, Raja RB, Kannan E. A liquid chromatography method for the simultaneous determination of curcumin and piperine in food products using diode array detection. Asian J Res Chem, 2009; 2:115–8.
Nurul H, Halimaton H, Lee SL. Piperine loaded silica aerogel and silica xerogel as NANO-enabled drug delivery system. World Appl Sci J, 2010; 9: 06–16.
Prerana S, Virendra KD, Mohant S, Mishra SK, Rajeev J, Edwards G. Development and validation of a reversed phase HPLC method for simultaneous determination of curcumin and piperine in human plasma for application in clinical pharmacological studies, J Liq Chromatogr Relat Technol, 2009; 32:2961–74. CrossRef
Shaikh J, Ankola DD, Beniwal V, Singha D, Ravi Kumar MNV. Nanoparticle encapsulation improves oral bioavailability of curcumin by at least 9-fold when compared to curcumin administered with piperine as absorption enhancer. Eur J Pharm Sci, 2009; 37:223–30. CrossRef
Shanmugam R, Gowthamarajan K, Priyanka DL, Madhuri K, Lokesh M. Elango K. Development and validation of simultaneous estimation method for curcumin and piperine by RP-UFLC. Pak J Pharm Sci, 2014; 27:901–6.
Sharma RA, Gescher AJ, Steward WP. Curcumin: the story so far. Eur J Cancer, 2005; 41:1955–68. CrossRef
Suchitra P, Rajashree H. A new stability-indicating RP-HPLC method for determination of curcumin: an application to the nanoparticulate formulation. Int J Pharm Pharm Sci, 2016; 8:149–55. CrossRef
Tushar KK, Darshil BS, Dilip GM. Development and validation of q-absorbance ratio spectrophotometric method for simultaneous estimation of cilnidipine and metoprolol succinate in bulk and combined dosage form. Int J Pharm Pharm Sci, 2014; 6:401–7.
Vipul U, Neeru S, Himanshu MJ, Amreesh M, Manoj M, Singh BP, Sanjeev T. Development and validation of rapid RPHPLC method for estimation of piperine in Piper nigrum L.. Int J Herb Med, 2013; 1:6–9.
Xiu-Mei W, Qi-Zhi Z, Jian Y, Rong-Hua Z, Jun Z, Li-Jing C, Wen-Xing P. Validated HPLC–MS/MS method for simultaneous determination of curcumin and piperine in human plasma. Trop J Pharm Res, 2012; 11:621–9. CrossRef