INTRODUCTION
Periodontitis is a disease mediated by microorganisms that generates inflammation of the periodontal junction complex (Tonetti et al., 2018) and is one of the main causes of tooth loss and oral morbidity in the world (Jin et al., 2016). The onset and progression of periodontitis depend on dysbiotic ecological changes of the microbiome in response to nutrients from gingival inflammatory products and tissue decomposition that enrich some species and antibacterial mechanisms that attempt to contain microbial challenge within the gingival sulcus area by the spread of the bacterial biofilm along the root surface (Tonetti et al., 2018). The main pathogens related to the development of this disease are Aggregatibacter actinomycetemcomitans and the so-called “red complex”: Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia (Haffajee and Socransky, 2005; Holt and Ebersole, 2005). This disease and its relationship with antibiotics as adjuvant therapy has been of fundamental importance for the evolution of periodontology (McGowan et al., 2018). However, the evidence regarding its effectiveness when antibiotics are administered systemically is not consistent. Therefore, localized antibiotic therapy has been directed to be used in maintenance or supportive periodontal therapy (Feres et al., 2015). Periodontal therapy has evolved, and there is strong evidence about the clinical and microbiologic long-term results after the use of antibiotics as adjuvants for mechanical debridement therapy, especially in severe cases of periodontitis (Feres et al., 2015), as well as the evolutionary evasion of microorganisms in recent times against antibiotic therapy (Feres et al., 2015; Preus et al., 2013). The use of antimicrobials as adjuncts to periodontal mechanical therapy in patients with periodontitis has been controversial and is often reserved for cases of exacerbation of periodontal lesions or in patients who do not respond immediately to conventional mechanical therapy or in those who have the risk of systemic compromise. However, due to the recent interest in the treatment of periodontitis, not only for dental reasons but due to the association of periodontitis with systemic diseases, periodontal treatment has acquired greater importance and the recognition of microbial specificity in severe periodontitis supports the use of therapy with antibiotics (Slots, 2020).
Antibiotic resistance is a phenomenon that can be explained from different population conditions, such as (i) geographic location, (ii) access to the production of certain antibiotics, (iii) knowledge of clinicians and adhesion of patients to pharmacological therapies, and (iv) type of microorganisms specific to each population. This phenomenon is a serious problem at the therapeutic level within the health sciences throughout the world; however, the knowledge on the causes and strategies that will be taken to face the problem is recent and limited. There are few disciplines that have advanced in this subject from the medical sciences (McGowan et al., 2018).
Within dentistry as a discipline, periodontics is one the most advanced fields in the level of scientific evidence available for the analysis of resistance to antibiotics, which places it at a priority level to find trends in the behavior of antibiotics as adjuvant therapy and steps to be taken at a clinical, scientific, and public health level to try to minimize the phenomenon of antibiotic resistance in some regions of the world where the problem is still manageable (McGowan et al., 2018; Preus et al., 2013).
A bibliometric analysis allows a robust study of the bibliography from a conceptual, temporal, and network perspective that accounts for some evidence regarding the research questions that the scientific community is considering in a wide field of research (Ahmad et al., 2020; Bar-Ilan, 2008; Gómez-ríos and Ramírez-malule, 2019). However, a bibliometric analysis of resistance bacteria on periodontal disease is still scarce. The aim of this study was to analyze the relationship between periodontitis and antibiotic therapy and its evolution over time.
METHODS
Database selection
In this study, the Scopus database was used as a tool to retrieve the most relevant documents related to resistance bacteria on periodontal disease. Data collection was acquired from Scopus on May 25, 2020, and comprised records obtained from a systematic search of documents matching the search terms in the fields of article title, abstract, and keywords. The search was refined to article and review as the document type. Thus, the resulting search was as follows:
- Search equation: (periodontal disease OR periodontal diseases OR abscess, periodontal OR abscesses, periodontal OR adult periodontitis OR adult periodontitides OR aggressive periodontitis OR chronic periodontitis OR chronic periodontitides) AND (antimicrobial drug resistance OR antibiotic resistance, bacterial OR antibiotic resistance, microbial).
- Timespan: 1948–2020; 688 articles and reviews were retrieved after removing duplicates.
Data export and analysis
The information retrieved from the Scopus in the search was (i) citation information, (ii) bibliographical information, and (iii) abstract and keywords. Retrieved data were exported from Scopus to Microsoft Excel. The software VOSviewer 1.6.13 was used for visualization and data analysis. Additionally, duplicated records were removed by using Mendeley software. In this study, the minimum number of occurrences of a keyword was set at five, and after removing the thesaurus terms, 52 of them met the threshold, which led to seven clusters.
Bibliometric indicators
In the current study, the following bibliometric indicators were evaluated:
- Volume and growth of publications related to resistance bacteria on periodontal disease.
- Subject areas related to resistance bacteria on periodontal disease.
- Co-occurrence keyword network visualization.
- Co-occurrence keyword overlay visualization.
- Most active countries and institutions.
RESULTS
A total of 688 documents related to resistance bacteria on periodontal disease were indexed in the Scopus database between 1953 and May 25, 2020. Figure 1 shows the evolution of the number of publications in this field. It is evident how the number of publications per year has been growing over time in this discipline, having peaks of ascent marked in the years 1990, 2008, and 2014, where historically there have been findings of great value for periodontics (Fig. 1).
Areas of knowledge related to publications about resistance to antimicrobials in periodontal diseases are (i) Medicine, (ii) Dentistry, and (iii) Immunology and Microbiology, and a large volume of articles are published in medicine journals (Fig. 2).
When analyzing the co-occurrence of keywords network, four well-defined clusters resulted, corresponding to infection (yellow), disease (blue), microbiology (red), and treatment (purple, red, and green) (Fig. 3a).
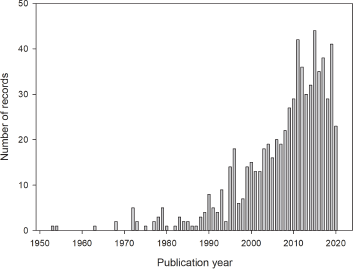 | Figure 1. Evolution of published studies related to antibiotic and periodontitis between 1948 and May 25, 2019. [Click here to view] |
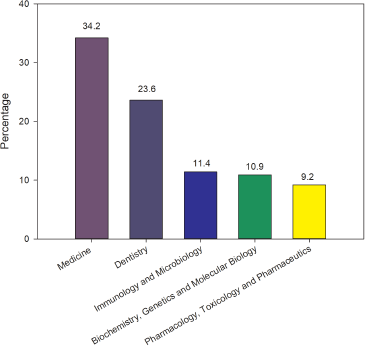 | Figure 2. Summary of publication grouped by areas on the field of resistance bacteria on periodontal disease studies between 1953 and May 25, 2020. [Click here to view] |
Regarding the time window of publications about bacterial resistance in periodontal diseases, it is observed how knowledge evolves over time and how the topics, in which researchers are concentrated in their research, change (Fig. 3b). From 2004 to more recent years such as 2015, it is noticed how at the beginning of the period they addressed issues related more to interventions, toward the middle of the period the research focuses more on the understanding of the disease and its associated factors. Afterwards (toward the end of the time window), it is seen how the different types of disease are analyzed and the most consistent therapeutic approaches with the different diagnoses were analyzed, also considering the endodontic-periodontal relationship between endodontic-periodontal lesions.
Table 1 shows the top 10 most presented keywords according to their frequency in the publications. Periodontal diagnosis in its variety of forms is the most frequent keyword, followed by words related to both the microbiota and the adjuvant antibiotic therapies.
Collaboration networks of countries with studies about resistance bacteria on periodontal disease are shown in Figure 4. The United States was the largest and most important node (Fig. 4a). Even though the United States was one of the countries that concentrated the studies in 2006, India, South Korea, Egypt, Saudi Arabia, and China were the leading countries between 2012 and 2014 (Fig. 4b). Besides, the United States, Germany, the United Kingdom, and Switzerland had the strongest collaboration network.
DISCUSSION
The initial publications about antibiotic resistance and periodontal disease emerged in the late 1970s and early 1980s when anaerobic culture and techniques of determination of antibiotic susceptibility allowed elucidating the relationship between microorganisms present in the subgingival plaque (Genco, 1981) and the onset and progression of periodontal disease were established (Feres et al., 2015; Haffajee, 1994; Socransky et al., 1998).
Around the year 1990, a first strong rise in publications related to antibiotic resistance of periodontopathic bacteria was presented, probably for two reasons: the publication of the first clinical consensus on periodontics follow-ups in the Workshop of the American Association of Periodontology (Periodontology, 1989) and in the following years the evidence of improvement in clinical markers such as clinical insertion levels as an indicator of success when nonsurgical root therapy was accompanied with systemic antibiotic therapy in patients with periodontitis (Aimetti et al., 2012; Guerrero et al., 2005; Preus et al., 2013; Zandbergen et al., 2013). Another peak of ascent in the publications was presented in 2008, and apparently, they are related to the evolution toward specific therapy with amoxicillin and metronidazole as drugs with greater effectiveness as adjuvants in periodontal therapy (Berglundh et al., 1998; Guerrero et al., 2005; Sgolastra et al., 2012; Winkel et al., 2001; Zandbergen et al., 2013). A final peak occurs around the year 2014 where the use of advanced molecular analysis techniques allowed the drastic increase in publications that enrich the knowledge mainly aimed at understanding more precise aspects of the pathogenesis of the disease, advances in support diagnosis techniques, and studies of the effectiveness of clinical treatments (McGowan et al., 2018).
The analysis of the different areas in which the articles related to resistance bacteria on periodontal disease are published confirms the link between oral and systemic health and the relevance of the concept of periodontal medicine coined by Offenbacher (1996). This led to a large number of publications about the medical implications of periodontal infection at the systemic level. This also prompted research on the indiscriminate use of antibiotics as adjuncts to periodontal mechanical therapy, and it has been widely discussed as a topic of interest that links the basic with the clinical sciences in periodontology (Mahuli et al., 2020).
The clusters of keywords network identified as infection, disease, microbiology and treatment show an idea of the perspectives from which the different problems surrounding this discipline and its clinical management are addressed and analyzed. Pathogenesis, diagnostics, prognosis, and treatment of periodontal diseases are topics addressed by the global workshops on periodontal diseases that are held regularly (Papapanou et al., 2018).
The high occurrence of the term P. gingivalis found (Table 1) was expected, since the mentioned bacteria have been reported as a principal member of the red complex, a microbial cluster implicated in the etiology of periodontal disease (Haffajee, 1994; Haffajee and Socransky, 2005). Additionally, metronidazole and amoxicillin appeared as the main adjunct therapy to prevent or reduce the pathology progression (Berglundh et al., 1998; McGowan et al., 2018). Periodontal diseases associated with bacteria such as P. gingivalis, A. actinomycetemcomitans, and Prevotella intermedia/nigrescens exhibited variable susceptibility to antimicrobials, finding more resistance strains to metronidazole and amoxicillin (Ardila et al., 2010; Jaramillo et al., 2005).
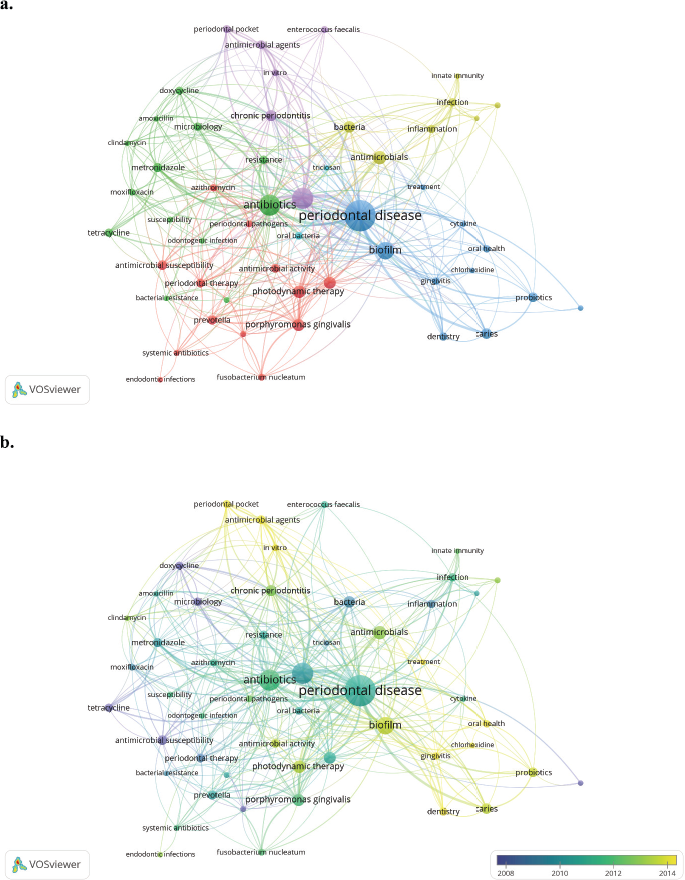 | Figure 3. Bibliometric network of resistance bacteria on periodontal disease studies between 1953 and May 25, 2020. (a) Research topic map. (b) Research topic map with time overlap. Minimum number of occurrences of a keyword is five. [Click here to view] |
Photodynamic therapy and probiotics are recently introduced as coadjuvant therapies for periodontitis. The first has not yielded conclusive results on its bactericidal effectiveness against periodontal pathogens such as P. gingivalis and A. actinomycetemcomitans (Peron et al., 2019). The use of probiotics containing lactobacilli or streptococci to model the periodontal microbiota in order to favor colonization by commensal bacteria has had positive effects on the control of the clinical parameters of periodontitis (Matsubara et al., 2016).
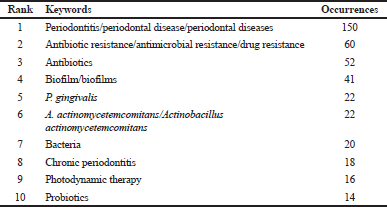 | Table 1. Top 10 author keywords of resistance bacteria on periodontal disease studies between 1953 and May 25, 2020. [Click here to view] |
Figure 4 shows an overview of the knowledge networks around the world in the investigation of periodontal disease. The United States is shown as a central object of research networks and publications on this subject. In South America, Brazil appears as the main contributor of research, but Chile and Colombia also appear as part of the network. There are also important research networks with several European countries such as Switzerland, Italy, Germany, and the United Kingdom. In terms of networks with the Asian continent, countries such as Japan, India, and China can be also observed with less participation.
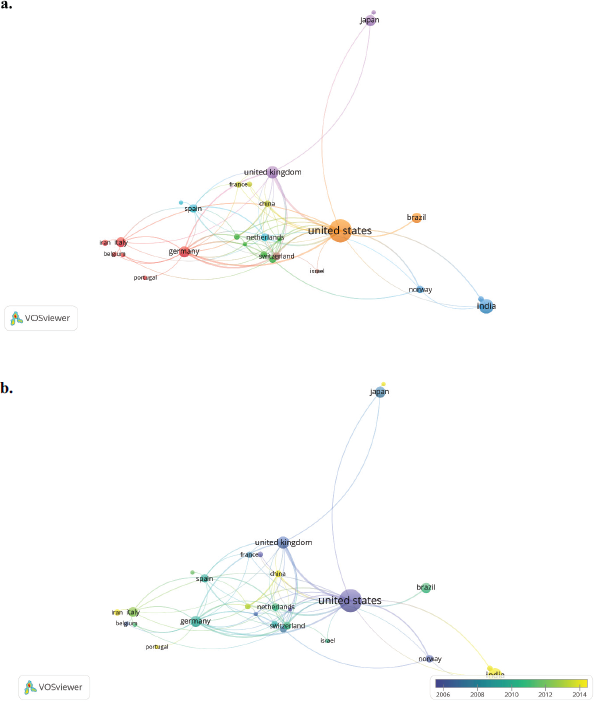 | Figure 4. Countries collaboration network of resistance bacteria on periodontal disease studies between 1953 and May 25, 2020. (a) Network visualization. (b) Overlay visualization. Countries contributing to a minimum number of both documents and citations of five. [Click here to view] |
CONCLUSION
Studies related to bacterial resistance to periodontal disease have been on the rise continuously from the 1970s to the present time. In this sense, the main areas of this topic were (i) Medicine, (ii) Dentistry, and (iii) Immunology and Microbiology, where the studies were focused mainly on the following clusters: infection, disease, microbiology, and treatment. The antibiotics mostly used (as adjunct therapy) to prevent or reduce periodontal disease were metronidazole and amoxicillin but P. gingivalis, A. actinomycetemcomitans, and P. intermedia/nigrescens exhibited resistance to these antibiotics probably due to their excessive use. Additionally, the countries that published the most studies on antimicrobial resistance in periodontics and had the strongest collaboration network were the United States, Germany, the United Kingdom, and Switzerland.
ETHICAL APPROVAL
This article does not contain any studies with human participants or animals performed by any of the authors.
INFORMED CONSENT
For this type of study, formal consent is not required.
AUTHORS’ CONTRIBUTIONS
Natalia Aragón, Adriana Jaramillo-Echeverry, and Howard Ramírez-Malule contributed to conceptualization; Howard Ramírez-Malule contributed to methodology and supervision; Natalia Aragón and Adriana Jaramillo-Echeverry contributed to formal analysis and investigation, writing and preparation of the original draft, and writing, review, and editing.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
FUNDING
Funding information is not applicable. No funding was received.
REFERENCES
Ahmad P, Asif JA, Alam MK, Slots J. A bibliometric analysis of Periodontology 2000. Periodontol 2000, 2020; 82(1):286–97. CrossRef
Aimetti M, Romano F, Guzzi N, Carnevale G. Full-mouth disinfection and systemic antimicrobial therapy in generalized aggressive periodontitis: a randomized, placebo-controlled trial. J Clin Periodontol, 2012; 39(3):284–94. CrossRef
Ardila CM, Granada MI, Guzmán IC. Antibiotic resistance of subgingival species in chronic periodontitis patients, J Periodontal Res, 2010; 45(4):557–63. CrossRef
Bar-Ilan J. Informetrics at the beginning of the 21st century–a review. J Informetr, 2008; 2(1):1–52. CrossRef
Berglundh T, Krok L, Liljenberg B, Westfelt E, Serino G, Lindhe J. The use of metronidazole and amoxicillin in the treatment of advanced periodontal disease: a prospective, controlled clinical trial. J Clin Periodontol, 1998; 25(5):354–62. CrossRef
Feres M, Figueiredo LC, Soares GMS, Faveri M. Systemic antibiotics in the treatment of periodontitis. Periodontol 2000, 2015; 67(1):131–86. CrossRef
Genco RJ. Antibiotics in the treatment of human periodontal diseases. J Periodontol, 1981; 52(9):545–58. CrossRef
Gómez-ríos D, Ramírez-malule H. Bibliometric analysis of recent research on multidrug and antibiotics resistance (2017–2018). J Appl Pharm Sci, 2019; 9(5):112–16. CrossRef
Guerrero A, Griffiths GS, Nibali L, Suvan J, Moles DR, Laurell L, Tonetti MS, et al. Adjunctive benefits of systemic amoxicillin and metronidazole in non-surgical treatment of generalized aggressive periodontitis: a randomized placebo-controlled clinical trial. J Clin Periodontol, 2005; 32(10):1096–107. CrossRef
Haffajee AD. Microbial etiological agents of destructive periodontal diseases. Periodontol 2000, 1994; 5:78–111. CrossRef
Haffajee AD, Socransky SS. Microbiology of periodontal diseases: introduction. Periodontol 2000, 2005; 38(1):9–12. CrossRef
Holt SC, Ebersole JL. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: the ‘red complex’, a prototype polybacterial pathogenic consortium in periodontitis. Periodontol 2000, 2005; 38(1):72–122. CrossRef
Jaramillo A, Arce RM, Herrera D, Betancourth M, Botero JE, Contreras A. Clinical and microbiological characterization of periodontal abscesses. J Clin Periodontol, 2005; 32(12):1213–8. CrossRef
Jin LJ, Lamster IB, Greenspan JS, Pitts NB, Scully C, Warnakulasuriya S. Global burden of oral diseases: emerging concepts, management and interplay with systemic health. Oral Dis, 2016; 22(7):609–19. CrossRef
Mahuli SA, Zorair AM, Jafer MA, Sultan A, Sarode G, Baeshen HA, Raj AT, Sarode S, Patil S. Antibiotics for periodontal infections: biological and clinical perspectives. J Contemp Dent Pract, 2020; 21(4):372–6. CrossRef
Matsubara VH, Bandara HMHN, Ishikawa KH, Mayer MPA, Samaranayake LP. The role of probiotic bacteria in managing periodontal disease: a systematic review. Expert Rev Anti Infect Ther, 2016; 14(7):643–55. CrossRef
McGowan K, McGowan T, Ivanovski S. Optimal dose and duration of amoxicillin-plus-metronidazole as an adjunct to non-surgical periodontal therapy: a systematic review and meta-analysis of randomized, placebo-controlled trials. J Clin Periodontol, 2018; 45(1):56–67. CrossRef
Offenbacher S. Periodontal diseases: pathogenesis. Ann Periodontol, 1996; 1(1):821–78. CrossRef
Papapanou PN, Sanz M, Buduneli N, Dietrich T, Feres M, Fine DH, Flemmig TF, Garcia R, Giannobile WV, Graziani F, Greenwell H, Herrera D, Kao RT, Kebschull M, Kinane DF, Kirkwood KL, Kocher T, Kornman KS, Kumar PS, Loos BG, Machtei E, Meng H, Mombelli A, Needleman I, Offenbacher S, Seymour GJ, Teles R, Tonetti MS. Periodontitis: consensus report of workgroup 2 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J Periodontol. 2018; 89:S173–82. CrossRef
Periodontology AA. Proceedings of the world workshop in clinical periodontics. American Academy of Periodontology, Chicago, IL, 1989.
Peron D, Bergamo A, Prates R, Vieira SS, de Tarso Camillo de Carvalho P, Serra AJ. Photodynamic antimicrobial chemotherapy has an overt killing effect on periodontal pathogens? A systematic review of experimental studies. Lasers Med Sci, 2019; 34(8):1527–34. CrossRef
Preus HR, Gunleiksrud TM, Sandvik L, Gjermo P, Baelum V. A randomized, double-masked clinical trial comparing four periodontitis treatment strategies: 1-year clinical results. J Periodontol, 2013; 84(8):1075–86. CrossRef
Sgolastra F, Gatto R, Petrucci A, Monaco A. Effectiveness of systemic amoxicillin/metronidazole as adjunctive therapy to scaling and root planing in the treatment of chronic periodontitis: a systematic review and meta-analysis. J Periodontol, 2012; 83(10):1257–69. CrossRef
Slots J. Primer on etiology and treatment of progressive/severe periodontitis: a systemic health perspective. Periodontol 2000, 2020; 83(1):272–6. CrossRef
Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent Jr RL. Microbial complexes in subgingival plaque. J Clin Periodontol, 1998; 25(2):134–44. CrossRef
Tonetti MS, Greenwell H, Kornman KS. Staging and grading of periodontitis: framework and proposal of a new classification and case definition. J Periodontol, 2018; 89:S159–72. CrossRef
Winkel EG, Van Winkelhoff AJ, Timmerman MF, Van der Velden U, Van der Weijden GA. Amoxicillin plus metronidazole in the treatment of adult periodontitis patients: a double-blind placebo-controlled study. J Clin Periodontol, 2001; 28(4):296–305. CrossRef
Zandbergen D, Slot DE, Cobb CM, Van Der Weijden FA. The clinical effect of scaling and root planing and the concomitant administration of systemic amoxicillin and metronidazole: a systematic review. J Periodontol, 2013; 84(3):332–51. doi:10.1902/jop.2012.120040 CrossRef