INTRODUCTION
Microalgae contain lipids, carbohydrates, proteins, pigments, essential fatty acids, antioxidants, and vitamins (Mobin et al., 2019; Nethravathy et al., 2019; Safafar et al., 2015), where all bioactive compounds are potentially useful in pharmaceutical and nutraceutical applications (Mobin et al., 2019). In particular, antioxidant compounds have been exploited for use in human healthcare and therapeutic product formulation, and in this sense, natural microalgae antioxidants are considered a valuable resource compared to synthetic antioxidants obtained from chemical processes (Safafar et al., 2015; Smerilli et al., 2019; Tan et al., 2020). The synthetic antioxidants have raised many human health concerns and controversies; for instance, they trigger liver damage and carcinogenesis (Hamidi et al., 2020). Contrary to artificial antioxidants, microalgae are the ultimate choice to meet the incremental demands in the perspective of human population growth as they are fast growing with high biomass, aimed at complementing the antioxidant production from traditional plants (Barkia et al., 2019; Sansone and Brunet, 2019).
Microalgae are photosynthetic organisms that use light, carbon dioxide, and water to produce food in the form of biological macromolecules like proteins, lipids, and carbohydrates (Morales et al., 2019). Light and photoperiod affect the growth and yield of microalgae during cultivation (Ahmad et al., 2020; Zhang et al., 2019). In addition, the period of light and dark exposure until the saturation point at which the maximal photosynthetic rate is reached may control cellular contents (Darvehei et al., 2018; Sirisuk et al., 2018), including chlorophyll and antioxidants (Maroneze et al., 2016). Light promotes the synthesis of various bioactive compounds (Chandra et al., 2016; Mobin et al., 2019; Ye et al., 2017) in which they play a pivotal role in suppressing the development of reactive oxygen species (ROS) from molecular oxygen (O2) in chloroplasts, as well as mitochondria, apoplasts, and peroxisomes (Noshi et al., 2016), with superoxide anion radical (O2•−), singlet oxygen (1O2), hydroxyl radical (•OH), and hydrogen peroxide (H2O2) (Assunção et al., 2017; Khorobrykh et al., 2020). Constant illumination may cause an imbalance between ROS output and its scavengers (Khorobrykh et al., 2020). Excessive ROS development promotes oxidative damage to lipids and other molecules in cytosols or within chloroplasts, and in this regard, microalgae provide a wide array of antioxidants to combat oxidative stress (Mobin et al., 2019; SzymaÅ„ska et al., 2017). Antioxidants consist of enzymatic (superoxide dismutase, peroxidase, and catalase) and nonenzymatic groups (water-soluble vitamin C, lipid-soluble vitamin E, and quenchers such as β-carotene) (Assunção et al., 2017; Yu et al., 2017). Antioxidant production generally depends on the form and period of stress as well as the species of microalgae (SzymaÅ„ska et al., 2017; Yu et al., 2017).
Up until now, most of the research conducted with microalgae focused on biomass production (Che et al., 2019; Kato et al., 2019; Ren et al., 2020; Vendruscolo et al., 2019; Zhang et al., 2019) and determination of the cellular contents such as lipid (Che et al., 2019; Kato et al., 2019; Medved et al., 2020; Ren et al., 2020; Vendruscolo et al., 2019; Zhang et al., 2019), fatty-acid (Che et al., 2019; Kato et al., 2019; Vendruscolo et al., 2019), chlorophyll (Gonzalez-Camejo et al., 2019; Patel et al., 2019; Vendruscolo et al., 2019), protein (Medved et al., 2020; Vendruscolo et al., 2019; Zhang et al., 2019), and carbohydrate content (Chong et al., 2019; Kato et al., 2019; Medved et al., 2020) upon the variations in the light and dark exposure. The role of photoperiods in the development of antioxidants and their responses to microalgae, however, is poorly understood. The goal of this study was to determine the effect of the photoperiod on growth and antioxidant responses of three species of marine microalgae, Chlorella vulgaris, Isochrysis galbana (CB), and Tetraselmis chuii (CT), commonly found in tropical brackish and marine environments. This evaluation is important to extend the knowledge of optimizing the culture conditions of microalgae with high antioxidants producing capacity.
MATERIALS AND METHODS
Microalgae stock and inoculum culture
Chlorella vulgaris (UMT-M1), CB, and CT obtained from Institute of Marine Biotechnology, Universiti Malaysia Terengganu, Terengganu, Malaysia, were grown with F/2 medium containing NaNO3 (0.88 mM), NaH2PO4 (0.036 mM), Na2EDTA.H2O (0.012 mM), FeCl3.6H2O (0.012 mM), MnCl2.4H2O (0.91 µM), ZnSO4.7H2O (0.077 µM), CoCl2.6H2O (0.042 µM), Na2MoO4.2H2O (0.026 µM), CuSO4.5H2O (0.039 µM), thiamine-HCl (0.30 µM), biotin (8.84 nM), and cyanocobalamin (0.37 nM) (Guillard, 1975; Guillard and Ryther, 1962) at 30 ppt salinity and pH 8.0 ± 0.2. The cultures were incubated at 24°C ± 2°C under constant illumination (2,000 lux) using 6,500 K daylight white light emitting diode lamp. The aeration Hailea HAP-120 pump (Hailea Group Co., China) was supplied with an airflow rate of around 120 l/minutes of air under the pressure of 0.018 MPa, filtered through a 0.22 μm Minisart® Sartorius syringe filter to avoid contamination. The cultivation of stock culture was first introduced in 100 ml of the liquid medium in a 250 ml flask before upscaling to 500 ml took place. The inoculum cultures were upscaled to 450 ml of the liquid medium in 500 ml conical flasks and harvested at 1 × 106 cells/ml prior to use in experiments. Microalgae were tested for purity and density and subsequently inoculated into separate flasks for use in photoperiod experiments.
Photoperiod treatment
Microalgae inoculated from the stock cultures were grown under two photoperiod conditions; one was given continuous illumination (24:0 hours light/dark cycles) and the other 12 hours light (12:12 hours light/dark cycles), respectively. The scaled-up inoculum was culture and treated accordingly until the end of the exponential phase (8 days). The 12 hours light (12:12 hours light/dark cycles) was controlled with a 24-hour programmed electrical timer (MS1144, Eurosafe, Malaysia). Experiments were conducted in three batches of cultivation with three biological replications for each batch, followed by three technical replicates in individual biological replicate. The detailed experimental setup of continuous and 12 hours light was shown in Figure 1.
Determination of microalgae growth and biomass
Cell density
Cell density was estimated regularly for 13 days. Approximately 200 μl of the cultures was diluted with 800 μl of Lugol solution (White et al., 2014). 10 μl of the diluted solution was transferred to the Neubauer hemocytometer and the cells were counted using a compound microscope (Leica CME, Leica Microsystems GmbH, Germany) (Sahastrabuddhe, 2016). Cell densities were determined using the following formula:
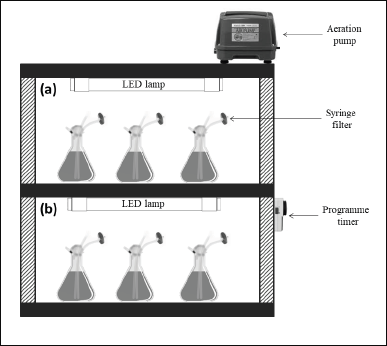 | Figure 1. Experimental setup of (a) 24:0 hours L/D and (b) 12:12 hours L/D treatments. [Click here to view] |
Wet and dry biomass determination
The wet and dry biomass of microalgae were determined on day 8 (at the end of the exponential and early stationary phase). 50 ml culture was centrifuged 10 minutes with an Allegra X-30R Centrifuge, Beckman Coulter, Inc., Krefeld, Germany at 1,000 × g. The supernatant was discarded and wet microalgae paste was washed twice with the same amount of distilled water. Microalgae paste was weighed with a balance (ME204, Mettler Toledo, Switzerland) and the wet biomass was recorded in g/l. The wet paste was dried in the oven at 70°C ± 2°C for 24 hours until a constant weight was obtained, with the latter reported as dry biomass (g/l) (Kong et al., 2011). The remaining microalgae culture was centrifuged at 1,000 × g for 10 minutes and microalgae cells were harvested for antioxidant assays.
Determination of antioxidative assays
Chlorophylls and carotenoids contents determination
The content of chlorophylls and carotenoids was determined as essentially defined by Torres et al. (2014). Approximately 0.05 g of fresh microalgae cells was homogenized for 10 minutes with 1.5 ml of absolute methanol using an ultrasonic bath (FisherbrandTM Elmasonic S 60H, Fisher Scientific, Schwerte, Germany) at 0oC–4oC. The homogenate was centrifuged (Microfuge 20R Centrifuge, Beckman Coulter, Inc., Krefeld, Germany) at 9,168 × g for 10 minutes at 4oC. 200 μl of supernatant was transferred to a 96-well plate, with 200 μl of absolute methanol used as control. The plate was shaken in a microplate reader for 10 seconds (VarioskanTM LUX, Thermo Fisher Scientific, Vantaa, Finland). The chlorophylls and carotenoids absorbance were measured at 470, 653, and 666 nm (Lichtenthaler and Wellburn, 1983). The chlorophylls and carotenoids contents were calculated using the following formula:
where A is the absorbance value; V is the total extract volume; FW is the microalgal fresh weight; and d is the light path length.
Ascorbic acid content determination
Ascorbic acid content was determined following the procedures by Norhayati et al. (2016) with minor modifications. 1 ml of 10% trichloroacetic acid was applied to 0.05 g fresh microalgae cells and then homogenized for 10 minutes using an ultrasonic bath (FisherbrandTM Elmasonic S 60H, Fisher Scientific, Germany) at 0oC–4oC. The homogenates were centrifuged (Microfuge 20R Centrifuge, Beckman Coulter, Inc., Krefeld, Germany) at 9,168 for 10 minutes ×g in cold condition (4oC). 30 μl of the supernatant was then diluted to a total volume of 200 μl with distilled water and subsequently transferred to a flat bottom 96-well plate with 200 μl of distilled water used as a control (blank). 20 μl of 10% of the Folin-Ciocalteu reagent was applied. The plate was incubated in a microplate reader (VarioskanTM LUX, Thermo Fisher Scientific, Vantaa, Finland) at 25oC for 10 minutes, where a blue color would be formed. The absorbance was measured at 760 nm (A760) and the ascorbic acid value was calculated on a standard ascorbic acid curve (y = 0.022 × −0.0015) constructed at a concentration of 0–5 μg/ml. The content of ascorbic acid was calculated using the following formula:
where A is the absorbance value; Vrm is the total reaction mixture volume in well; Veb is the total extraction buffer volume; d is the light path length; Ve is the total extract volume in well; FW is the microalgal fresh weight; and df is the dilution factor.
α-Tocopherol content determination
α-Tocopherol content was determined using methods adapted by Norhayati et al. (2016) with minor modifications. Approximately 0.05 g of fresh microalgae cells was homogenized with 1.5 ml of absolute acetone in cold (0oC–4oC) for 10 minutes. The mixture was extracted with 0.5 ml hexane and then homogenized with an ultrasonic bath (FisherbrandTM Elmasonic S 60H, Fisher Scientific, Germany) for 10 minutes at 0oC–4oC. Homogenates were then centrifuged (Microfuge 20R Centrifuge, Beckman Coulter, Inc., Germany) for 10 minutes at 9,168 × g in cold condition (4oC). After centrifugation, the upper layer of the supernatant was removed and the hexane extraction was repeated twice. 25 μl of hexane extract was transferred to the flat bottom of the 96-well plate, with 25 μl of absolute ethanol used as control. Then 20 μl of 0.1% 3-(2-pyridyl)-5,6-diphenyl-1,2,4 triazine and 20 μl of 0.1% ferric chloride were added to the layer. 85 μl of absolute ethanol was applied to the microplate reader (VarioskanTM, Thermo Fisher Scientific, Finland) for color production before the plate incubation at 25oC for 4 minutes. 10 μl of 0.2 M orthophosphoric acid was added to the mixture. The microplate was incubated at 25oC for another 10 minutes in the microplate reader prior to the 554 nm (A554) absorbance measurement. The α-tocopherol value was calculated based on the standard curve (y = 0.0045 × +0.0046) constructed at a concentration of 0–5 μg/ml. The content of α-tocopherol was measured using the following formula:
where A is the absorbance value; Vrm is the total reaction mixture volume in well; Veb is the total extraction buffer volume; d is the light path length; Ve is the total hexane extract volume in well; FW is the microalgal fresh weight; and df is the dilution factor.
Statistical analysis
Statistical analysis was performed using Statistical Package for Social Science software version 20. The mean comparisons were evaluated using a one-way analysis of variance analysis. Multiple mean comparisons were determined by the Tukey test, while a comparison between 12:12 and 24:0 hours L/D cycles in each species was analyzed using a paired t-test sample. The differences were considered to be significant at p < 0.05. Bivariate (Pearson) correlations were conducted to evaluate the hypotheses of association between cell density, biomass (wet and dry), and antioxidative responses like chlorophyll a, chlorophyll b, carotenoids, ascorbic acid, and α-tocopherol.
RESULTS AND DISCUSSION
Effect of photoperiod on microalgae growth and biomass
Photoperiod affected the cell density and biomass of C. vulgaris (UMT-M1), CB, and CT during culture. The cell densities of UMT-M1, CB, and CT were higher in 24:0 hours L/D cycle than in 12:12 hours L/D cycle (Fig. 2), but only UMT-M1 and CT were significantly higher (p < 0.05) in 8 days of continuous light exposure (Table 1). The wet biomass obtained for UMT-M1, CB, and CT samples grown under constant light was higher (p < 0.05) compared to 12:12 hours L/D, observations consistent with Maroneze et al. (2016) analysis. It was discovered that, with constant illumination, Scenedesmus obliquus, a green microalga, achieved maximum cell density and biomass. Similar findings have been obtained with Nannochloropsis sp. when constant light was provided at 50 and 100 µmol m−2 s−1 (Wahidin et al., 2013) as well as N. salina and Phaeodactylum tricornutum at 250 µmol m−2 s−1 (Sirisuk et al., 2018). On the contrary, I. galbana reached optimum density when the culture was carried out with a 12:12 hours L/D cycle at 400 µmol m−2 s−1 (Che et al., 2019), suggesting that certain microalgae species do not need constant light to achieve maximum growth, as in the case of Neochloris conjuncta, N. terrestris, and N. texensis reported by KrzemiÅ„ska et al. (2014). Interestingly, this study showed that the dry biomass of UMT-M1 grown with these two photoperiod cycles was significantly different, but this condition was not observed in CB and CT, which showed that dry biomass is associated with cellular water content, which is dependent on microalgae organisms, cell size, and length of light exposure (Chioccioli et al., 2014; Khoeyi et al., 2012).
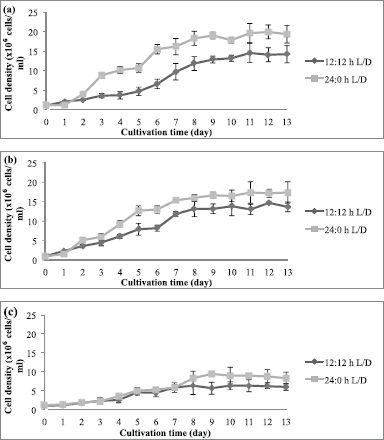 | Figure 2. Cell density of (a) UMT-M1, (b) CB, and (c) CT cultivated under 12:12 and 24:0 hours L/D cycles. Data were reported as mean ± standard deviation (n = 3). [Click here to view] |
Under the light regime, the cellular water content can be affected by the size of cellular components such as nucleus, cytoskeleton, chloroplasts, and mitochondria (Aratboni et al., 2019; Chioccioli et al., 2014), as well as by the accumulation of photosynthetic-related biomass compounds such as starch, glycerol, and protein (Khan et al., 2018; Xu et al., 2016). The period of light exposure influences the growth and biomass of microalgae as they are parallel to the rate of photosynthesis and the metabolism of microalgae (Matos et al., 2017). Increasing the exposure to light may lead to an increase in the reproduction rate before the intensity of the saturation point is reached. Individual organisms vary in terms of light requirements, life cycles, and reproduction patterns. The culture conditions, therefore, have a major impact on the growth and biomass of microalgae (Krzemińska et al., 2014).
Effect of photoperiod on antioxidative responses
Chlorophylls, carotenoids, ascorbic acid, and tocopherol are major antioxidative response compounds. All these components effectively detoxify excessive ROS production (Papalia et al., 2019). Light absorption by photosynthetic microalgae occurs in chlorophyll a as well as accessory chlorophyll b and carotenoids (Sirisuk et al., 2018). Figure 3 shows the content of chlorophyll a, chlorophyll b, chlorophyll a/b ratio, and carotenoids in UMT-M1, CB, and CT grown under different photoperiods. UMT-M1 contained the highest chlorophyll a (1.366 ± 0.057 mg/g FW), chlorophyll b (0.711 ± 0.038 mg/g FW), and carotenoids (0.264 ± 0.031 mg/g FW) but CB has the highest chlorophyll a/b ratio of three species. Interestingly, UMT-M1, CB, and CT chlorophyll a, chlorophyll b, and carotenoids were significantly induced (p < 0.05) in 24:0 L/D compared to 12:12 hours L/D cycle. Compared to other microalgae species such as Ankistrodesmus falcatus, chlorophyll a and carotenoids were higher in cultivation with 12:12 hours L:/D cycle, whereas chlorophyll b remains higher in culture with constant light (George et al., 2014). In another study, the chlorophyll a of S. obliquus grown with 12:12 hours L/D cycle was higher than 24:0 L/D cycle but chlorophyll b content remained constant in both photoperiod conditions (Vendruscolo et al., 2019). In addition, chlorophyll a/b ratio decreased in UMT-M1 but increased in CB while CT remained constant when continuous light was provided. These findings suggested that continuous illumination promotes chlorophyll a and b synthesis as well as carotenoid content in UMT-M1, CB, and CT, but the chlorophyll a/b ratio varies in these species.
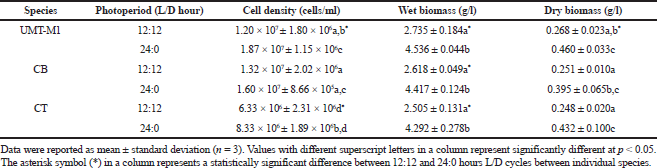 | Table 1. Effect of photoperiod cycle on the wet and dry biomass of UMT-M1, CB, and CT. [Click here to view] |
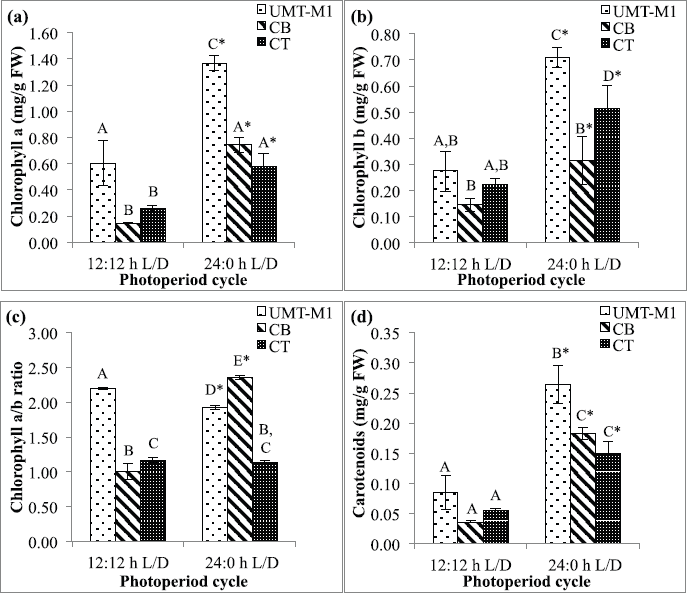 | Figure 3. Chlorophyll a (a), chlorophyll b (b), chlorophyll a/b ratio (c) and carotenoids (d) contents of UMT-M1, CB, and CT cultivated under 12:12 and 24:0 hours L/D cycles. Data were reported as mean of repetitions ± standard deviation (n = 3). Values with different capital letters (A, B, C, and D) were statistically significantly different at p < 0.05. The asterisk symbol (*) indicates that there was a significant difference between 12:12 and 24:0 hours L/D cycles between individual species. [Click here to view] |
On an applied note, the efficacy of microalgal chlorophyll a to capture light photons varies by pigment arrangement, cell structure, and chloroplast composition (Sirisuk et al., 2018). The elevation of chlorophyll a content as observed in this current study may be due to the increasing number of photosynthetic units during light harvesting, whereas the rise in chlorophyll b content may have been linked to the increment of photoprotection pigment during light exposure (Levasseur et al., 2018). The increase in the chlorophyll a/b ratio indicates a relatively significant role for chlorophyll a in photosynthesis, whereas the decrease in the chlorophyll a/b ratio suggests that chlorophyll b appears to be highly functional in photosynthesis, which is closely related to the shift in chlorophyll composition in the light-harvesting complex of thylakoid membranes in the reaction center of a photosystem (Beneragama and Goto, 2010; Negi et al., 2016; Perrine et al., 2012; Ramaraj et al, 2013).
Carotenoids exist in chloroplast membranes and play a crucial role in light harvesting as well as in photo protecting photosynthetic systems against the illumination tension (Ma et al., 2018; Smerilli et al., 2019). Increasing carotenoids in UMT-M1, CB, and CT under continuous light are therefore parallel to their involvement in alleviating and assisting the photosynthetic pigment-protein complex (Sirisuk et al., 2018). Carotenoids can quench peroxides and singlet oxygen thus inhibits the ROS formation (Smerilli et al., 2019). A balance between light energy harvesting in chloroplast and energy use during carbon fixation is needed to adapt light strain (Levasseur et al., 2018).
Besides chlorophyll and carotenoids, photoperiod also affects microalgae ascorbic acid accumulation. The ascorbic acid content of UMT-M1, CB, and CT vary considerably with exposure to the photoperiod conditions tested in this study (p < 0.05) as portrayed in Figure 4. Notably, the ascorbic acid content of UMT-M1, CB, and CT was higher in continuous light culture with an accumulation of 1.460 ± 0.167, 0.924 ± 0.044, and 1.264 ± 0.086 mg/g FW, respectively. Our findings showed that ascorbic acid production was enhanced under continuous illumination, with similar results in Desmonostoc salinum and S. quadricauda (De Alvarenga et al., 2020; Zahra et al., 2017). However, some microalgae species like Skeletonema marinoi do not show a shift in ascorbic acid content on crops with different light period (Smerilli et al., 2019).
Ascorbic acid (vitamin C), a water-soluble vitamin with antioxidant properties, can be used to scavenge ROS development under light stress (Galasso et al., 2019). De Alvarenga et al. (2020)stated that microalgae can produce 4–9 times ascorbic acid under continuous light. Ascorbic acid elevation in this study is possibly correlated with the 1O2 scavenging role as the photosynthesis process during light stress triggers scarce energy dissipation, thus intensifying 1O2 development (Sharma et al., 2012; Smirnoff, 2015). Besides, ascorbic acid also acts as a substrate for antioxidant enzymes such as peroxidase, •OH radical electron donor, and violaxanthin deepoxidase cofactor (Rezayian et al., 2019; Smirnoff, 2015; Smerilli et al., 2019). Moreover, the increase in ascorbic acid may be attributed to the suppression of H2O2 in the ascorbate-glutathione pathway, which serves as a hydrogen donor to turn H2O2 into water and monodehydroascorbate molecules (Rezayian et al., 2019; Zhang et al., 2020). Ascorbic acid production in microalgae under stressors is still poorly recognized (KováÄik et al., 2017).
α-Tocopherol plays a crucial function in oxidative stress scavenger. A significant difference (p < 0.05) in α-tocopherol content was observed for UMT-M1 and CT in this study but not CB in 24:0 hours and 12:12 hours L/D photoperiod as shown in Figure 5. The α-tocopherol content of UMT-M1 and CT was higher in 24:0 hours L/D culture, with the accumulation of 12.534 ± 0.127 and 12.840 ± 0.546 mg/g FW, respectively. These findings were consistent with Durmaz (2007), where Nannochloropsis sp. accumulated high levels of α-tocopherol accumulated under continuous light exposure. Contrary to CB's outcome, I. galbana developed higher α-tocopherol in continuous light compared to 8:16 hours L/D period (Bandarra et al., 2003). Therefore, our findings suggest that continuous light stimulated α-tocopherol output in UMT-M1 and CT, but not in CB.
α-Tocopherol (vitamin E) is a lipophilic antioxidant in thylakoid membranes or plastid of microalgae (Papalia et al., 2019). The increase in α-tocopherol under continuous illumination can result from the defense of microalgae membrane lipids from photosynthesis-derived ROS, mainly in photosystem II (PSII) (Sharma et al., 2012). The protection mechanism uses scavenge 1O2 resonance energy transfer (Papalia et al., 2019; Smerilli et al., 2019). It is also a well-known chain-breaking molecule among the four tocopherol groups (α-, β-, and β-tocopherols) and is capable of suppressing 1O2, reducing O2•− and inhibiting lipid peroxidation by blocking ROS development involving inhibition of low-density lipoprotein oxidation (Galasso et al., 2019; Rezayian et al., 2019). Notwithstanding, α-tocopherol development in microalgae is rarely examined (Mudimu et al., 2017). Future studies should emphasize microalgal antioxidative responses under different environmental stressors to provide a practical understanding of cellular adaptation mechanisms and selection of the best stress-tolerant microalgae species for future pharmaceutical applications.
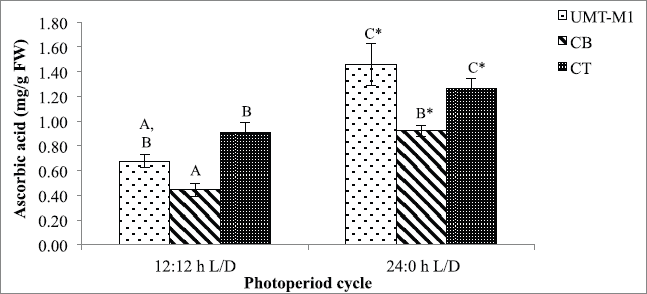 | Figure 4. Ascorbic acid contents of UMT-M1, CB, and CT cultivated under 12:12 and 24:0 hours L/D cycles. Data were reported as mean of repetitions ± standard deviation (n = 3). Values with different capital letters (A, B, C, and D) were statistically significantly different at p < 0.05. The asterisk symbol (*) indicates that there was a significant difference between 12:12 and 24:0 hours L/D cycles between individual species. [Click here to view] |
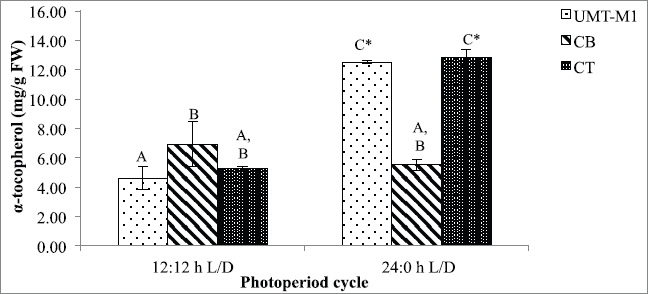 | Figure 5. α-Tocopherol contents of UMT-M1, CB, and CT cultivated under 12:12 and 24:0 hours L/D cycles. Data were reported as mean of repetitions ± standard deviation (n = 3). Values with different capital letters (A, B, C, and D) were statistically significantly different at p < 0.05. The asterisk symbol (*) indicates that there was a significant difference between 12:12 and 24:0 hours L/D cycles between individual species. [Click here to view] |
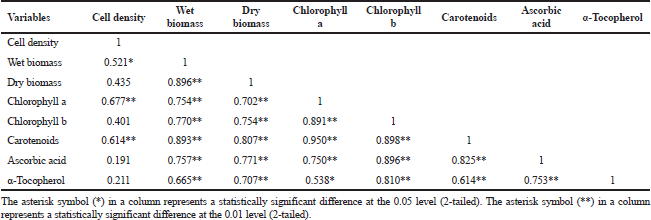 | Table 2. Pearson’s correlation coefficients of cell density, biomass, and antioxidative responses. [Click here to view] |
Correlation between cell density, biomass, and antioxidant responses
The relationship between cell density, biomass (wet and dry), and antioxidant responses (chlorophyll a, chlorophyll b, carotenoids, ascorbic acid, and α-tocopherol) with Pearson's correlation coefficient (R) was accessed as shown in Table 2.
Cell density showed a significant positive correlation of 0.521, 0.677, and 0.614, respectively, to wet biomass, chlorophyll a, and carotenoids. In comparison, there was no significant correlation between dry biomass cell density, chlorophyll b, ascorbic acid, and α-tocopherol. Due to cellular water content, microalgae cells are strongly affiliated, but the content varies by species, cell size, and light exposure (Chioccioli et al., 2014). Most unicellular microalgae have single or multiple chloroplasts per cell that contribute to the interactive division of microalgae cells and chloroplast (Sumiya, 2018), thus correlating with chlorophyll a and carotenoids output. Biomass (wet and dry) has a strong association with chlorophyll a and chlorophyll b, indicating that chlorophyll concentration serves as an indirect measurement of biomass concentration (Bauer et al., 2017).
Carotenoids demonstrated a strong positive correlation with cell density, biomass, and other antioxidant responses. Among them, two major associations were observed between carotenoids and chlorophyll a (R = 0.980, p < 0.01), as well as carotenoids and chlorophyll b (R = 0.898, p < 0.01), which show a direct link between chlorophylls and carotenoids in light-harvesting reactions and their protective function against oxidative stress (Sirisuk et al., 2018). Furthermore, chlorophylls, carotenoids, ascorbic acid, and α-tocopherol have a clear association, and it can be expected that they have a strong synergistic contribution to scavenge photosynthetic-derived ROS, as previously stated by Papalia et al. (2019). The results of these Pearson’s correlation coefficients provide insight into the relationship between independent variables in this analysis, where most variables are highly interconnected, so it can be further manipulated to seek the best optimum growth conditions with high-value content.
CONCLUSION
Different microalgae species respond differently to photoperiod treatment. This study revealed continuous light exposure enhanced growth and antioxidative responses of C. vulgaris, I. galbana, and T. chuii. This study suggests that chlorophylls, carotenoids, ascorbic acid, and α-tocopherol have cooperative functions in quenching oxidative stress. Chlorella vulgaris, I. galbana, and T. chuii are good candidates for natural antioxidants to replace synthetic antioxidants in the industry, but more studies are needed to find ways to optimize the production of antioxidants from these microalgae.
ACKNOWLEDGMENTS
This project was funded by the Science and Technology Research Partnership for Sustainable Development (SATREPS), Japan Science and Technology Agency (JST), Japan International Cooperation Agency (JICA), and Ministry of Higher Education Malaysia (MOHE) under Vot No. 53221. Heartfelt thanks are due to the staff at the Institute of Marine Biotechnology (IMB), Centre of Research and Field Service (CRAFS), and Institute of Oceanography (INOS), Universiti Malaysia Terengganu, Terengganu, Malaysia, for their technical support.
AUTHORS’ CONTRIBUTIONS
YYS, HAZ, MEAW, and NY contributed to the conception and design of the work; NSY did the data acquisition, analysis, and data interpretation. NSY drafted the manuscript in consultation with NY; all authors discussed the results and critically reviewed the manuscript; and NY, YYS, MEAW, and HAZ were responsible for giving the final approval of the manuscript.
CONFLICT OF INTEREST
The authors declared that they do not have any conflicts of interest.
FUNDING
This work was funded by the Science and Technology Research Partnership for Sustainable Development (SATREPS), Japan Science and Technology Agency (JST), Japan International Cooperation Agency (JICA), and Ministry of Higher Education Malaysia (MOHE) under Vot No. 53221.
REFERENCES
Ahmad MT, Shariff M, Md. Yusoff F, Goh YM, Banerjee S. Applications of microalga Chlorella vulgaris in aquaculture. Rev Aquac, 2020; 12(1):328–46. CrossRef
Aratboni HA, Rafiei N, Garcia-Granados R, Alemzadeh A, Morones-Ramírez JR. Biomass and lipid induction strategies in microalgae for biofuel production and other applications. Microb Cell Fact, 2019; 18(1):178. CrossRef
Assunção MF, Amaral R, Martins CB, Ferreira JD, Ressurreição S, Santos SD, Varejão JMTB, Santos LM. Screening microalgae as potential sources of antioxidants. J Appl Phycol, 2017; 29(2):865–77. CrossRef
Bandarra NM, Pereira PA, Batista I, Vilela MH. Fatty acids, sterols and α-tocopherol in Isochrysis galbana. J Food Lipids, 2003; 10(1):25–34. CrossRef
Barkia I, Saari N, Manning SR. Microalgae for high-value products towards human health and nutrition. Mar Drugs, 2019; 17(5):304. CrossRef
Bauer LM, Costa JA, da Rosa AP, Santos LO. Growth stimulation and synthesis of lipids, pigments and antioxidants with magnetic fields in Chlorella kessleri cultivations. Bioresour Technol, 2017; 244:1425–32. CrossRef
Beneragama CK, Goto K. Chlorophyll a: b ratio increases under low-light in ‘shade-tolerant' Euglena gracilis. Trop Agric Res, 2010; 22(1):12–25. CrossRef
Chandra TS, Deepak RS, Kumar MM, Mukherji S, Chauhan VS, Sarada R, Mudliar SN. Evaluation of indigenous fresh water microalga Scenedesmus obtusus for feed and fuel applications: effect of carbon dioxide, light and nutrient sources on growth and biochemical characteristics. Bioresour Technol, 2016; 207:430–9. CrossRef
Che CA, Kim SH, Hong HJ, Kityo MK, Sunwoo IY, Jeong GT, Kim SK. Optimization of light intensity and photoperiod for Isochrysis galbana culture to improve the biomass and lipid production using 14-L photobioreactors with mixed light emitting diodes (LEDs) wavelength under two-phase culture system. Bioresour Technol, 2019; 285:121323. CrossRef
Chioccioli M, Hankamer B, Ross IL. Flow cytometry pulse width data enables rapid and sensitive estimation of biomass dry weight in the microalgae Chlamydomonas reinhardtii and Chlorella vulgaris. PLoS One, 2014; 9(5):e97269. CrossRef
Chong JF, Fadhullah W, Lim V, Lee CK. Two-stage cultivation of the marine microalga Chlorella salina for starch and carbohydrate production. Aquac Int, 2019; 27(5):1269–88. CrossRef
Darvehei P, Bahri PA, Moheimani NR. Model development for the growth of microalgae: a review. Renew Sustain Energy Rev, 2018; 97:233–58. CrossRef
De Alvarenga LV, Almeida AVM, de Castro NV, Oder JC, Esteves-Ferreira A, Nunes-Nesi A, Araújo WL, Vaz MGMV. Physiological responses to light intensity and photoperiod of the halotolerant cyanobacterium Desmonostoc salinum CCM-UFV059. Bioresour Technol Rep, 2020; 11:100443. CrossRef
Durmaz Y. Vitamin E (α-tocopherol) production by the marine microalgae Nannochloropsis oculata (Eustigmatophyceae) in nitrogen limitation. Aquaculture, 2007; 272(1–4):717–22. CrossRef
Galasso C, Gentile A, Orefice I, Ianora A, Bruno A, Noonan DM, Sansone C, Albini A, Brunet C. Microalgal derivatives as potential nutraceutical and food supplements for human health: a focus on cancer prevention and interception. Nutrients, 2019; 11(6):1226. CrossRef
George B, Pancha I, Desai C, Chokshi K, Paliwal C, Ghosh T, Mishra S. Effects of different media composition, light intensity and photoperiod on morphology and physiology of freshwater microalgae Ankistrodesmus falcatus–a potential strain for bio-fuel production. Bioresour Technol, 2014; 171:367–74. CrossRef
Gonzalez-Camejo J, Viruela A, Ruano MV, Barat R, Seco A, Ferrer J. Effect of light intensity, light duration and photoperiods in the performance of an outdoor photobioreactor for urban wastewater treatment. Algal Res, 2019; 40:101511. CrossRef
Guillard RR, Smith, M.L. and Chanley, M.H., Eds. Culture of phytoplankton for feeding marine invertebrates. In Culture of marine invertebrate animals. Springer, Boston, MA, pp 29–60, 1975. CrossRef
Guillard RRL, Ryther JH. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt and Detonula confervacea Cleve. Can J Microbiol, 1962; 8(2):229–39. CrossRef
Hamidi M, Kozani PS, Kozani PS, Pierre G, Michaud P, Delattre C. Marine bacteria versus microalgae: who is the best for biotechnological production of bioactive compounds with antioxidant properties and other biological applications? Mar Drugs, 2020; 18(1):28. CrossRef
Kato Y, Fujihara Y, Vavricka CJ, Chang JS, Hasunuma T, Kondo A. Light/dark cycling causes delayed lipid accumulation and increased photoperiod-based biomass yield by altering metabolic flux in oleaginous Chlamydomonas sp. Biotechnol Biofuels, 2019; 12(1):1–11. CrossRef
Khan MI, Shin JH, Kim JD. The promising future of microalgae: current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb Cell Fact, 2018; 17(1):36. CrossRef
Khoeyi ZA, Seyfabadi J, Ramezanpour Z. Effect of light intensity and photoperiod on biomass and fatty acid composition of the microalgae, Chlorella vulgaris. Aquac Int, 2012; 20(1):41–9. CrossRef
Khorobrykh S, Havurinne V, Mattila H, Tyystjärvi E. Oxygen and ROS in photosynthesis. Plants, 2020; 9(1):91. CrossRef
Kong W, Son H, Cao Y, Yang H, Hua S, Xia C. The characteristics of biomass production, lipid accumulation and chlorophyll biosynthesis of Chlorella vulgaris under mixotrophic cultivation. Afr J Biotechnol, 2011; 10(55):11620–30.
KováÄik J, Klejdus B, Babula P, Hedbavny J. Ascorbic acid affects short-term response of Scenedesmus quadricauda to cadmium excess. Algal Res, 2017; 24:354–9. CrossRef
KrzemiÅ„ska I, Pawlik-SkowroÅ„ska B, TrzciÅ„ska M, Tys J. Influence of photoperiods on the growth rate and biomass productivity of green microalgae. Bioprocess Biosyst Eng, 2014; 37(4):735–41. CrossRef
Levasseur W, Taidi B, Lacombe R, Perre P, Pozzobon V. Impact of seconds to minutes photoperiods on Chlorella vulgaris growth rate and chlorophyll a and b content. Algal Res, 2018; 36:10–6. CrossRef
Lichtenthaler HK, Wellburn AR. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans, 1983; 11:591–2. CrossRef
Ma R, Thomas-Hall SR, Chua ET, Eltanahy E, Netzel ME, Netzel G, Lu Y, Schenk PM. LED power efficiency of biomass, fatty acid, and carotenoid production in Nannochloropsis microalgae. Bioresour Technol, 2018; 252:118–26. CrossRef
Maroneze MM, Siqueira SF, Vendruscolo RG, Wagner R, de Menezes CR, Zepka LQ, Jacob-Lopes E. The role of photoperiods on photobioreactors–A potential strategy to reduce costs. Bioresour Technol, 2016; 219:493–9. CrossRef
Matos ÂP, Cavanholi MG, Moecke EHS, Sant'Anna ES. Effects of different photoperiod and trophic conditions on biomass, protein and lipid production by the marine alga Nannochloropsis gaditana at optimal concentration of desalination concentrate. Bioresour Technol, 2017; 224:490–7. CrossRef
Medved VO, Gorbunova ZN, Vitovetska TV. Peculiarities of accumulation of proteins, carbohydrates and lipids in the cells of green algae under different light conditions and photoperiod. Hydrobiol J, 2020; 56(3):97–104. CrossRef
Mobin SM, Chowdhury H, Alam F. Commercially important bioproducts from microalgae and their current applications–a review. Energy Procedia, 2019; 160:752–60. CrossRef
Morales M, Hélias A, Bernard O. Optimal integration of microalgae production with photovoltaic panels: environmental impacts and energy balance. Biotechnol Biofuels, 2019; 12(1):239. CrossRef
Mudimu O, Koopmann IK, Rybalka N, Friedl T, Schulz R, Bilger W. Screening of microalgae and cyanobacteria strains for α-tocopherol content at different growth phases and the influence of nitrate reduction on α-tocopherol production. J Appl Phycol, 2017; 29(6):2867–75. CrossRef
Negi S, Barry AN, Friedland N, Sudasinghe N, Subramanian S, Pieris S, Holguin FO, Dungan B, Schaub T, Sayre R. Impact of nitrogen limitation on biomass, photosynthesis, and lipid accumulation in Chlorella sorokiniana. J Appl Phycol, 2016; 28(2):803–12. CrossRef
Nethravathy MU, Mehar JG, Mudliar SN, Shekh AY. Recent advances in microalgal bioactives for food, feed, and healthcare products: commercial potential, market space, and sustainability. Compr Rev Food Sci Food Saf, 2019; 18(6):1882–97. CrossRef
Norhayati Y, Afzan AW, Jannah SSN, Nurul Wahidah MR. Antioxidative responses of Cocos nucifera against infestation by the Red Palm Weevil (RPW), Rhynchophorus ferrugineus, a new invasive coconut pest in Malaysia. Sains Malaysiana, 2016; 45(7):1035–40.
Noshi M, Mori D, Tanabe N, Maruta T, Shigeoka S. Arabidopsis clade IV TGA transcription factors, TGA10 and TGA9, are involved in ROS-mediated responses to bacterial PAMP flg22. Plant Sci, 2016; 252:12–21. CrossRef
Papalia T, Sidari R, Panuccio MR. Impact of different storage methods on bioactive compounds in Arthrospira platensis biomass. Molecules, 2019; 24(15):2810. CrossRef
Patel AK, Joun JM, Hong ME, Sim SJ. Effect of light conditions on mixotrophic cultivation of green microalgae. Bioresour Technol, 2019; 282:245–53. CrossRef
Perrine Z, Negi S, Sayre RT. Optimization of photosynthetic light energy utilization by microalgae. Algal Res, 2012; 1(2):134–42. CrossRef
Ramaraj R, Tsai DD, Chen PH. Chlorophyll is not accurate measurement for algal biomass. Chiang Mai J Sci, 2013; 40(4):547–55.
Ren HY, Dai YQ, Kong F, Xing D, Zhao L, Ren NQ, Ma J, Liu BF. Enhanced microalgal growth and lipid accumulation by addition of different nanoparticles under xenon lamp illumination. Bioresour Technol, 2020; 297:122409. CrossRef
Rezayian M, Niknam V, Ebrahimzadeh H. Oxidative damage and antioxidative system in algae. Toxicol Rep, 2019; 6:1309–13. CrossRef
Safafar H, Van Wagenen J, Møller P, Jacobsen C. Carotenoids, phenolic compounds and tocopherols contribute to the antioxidative properties of some microalgae species grown on industrial wastewater. Mar Drugs, 2015; 13(12):7339–56. CrossRef
Sahastrabuddhe AP. Counting of RBC and WBC using image processing: a review. Int J Res Eng Technol, 2016; 5(05):356–60. CrossRef
Sansone C, Brunet C. Promises and challenges of microalgal antioxidant production. Antioxidants, 2019; 8(7):199. CrossRef
Sharma P, Jha AB, Dubey RS, Pessarakli M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot, 2012; 2012:1–26. CrossRef
Sirisuk P, Ra CH, Jeong GT, Kim SK. Effects of wavelength mixing ratio and photoperiod on microalgal biomass and lipid production in a two-phase culture system using LED illumination. Bioresour Technol, 2018; 253:175–81. CrossRef
Smerilli A, Balzano S, Maselli M, Blasio M, Orefice I, Galasso C, Sansone C, Brunet C. Antioxidant and photoprotection networking in the coastal diatom Skeletonema marinoi. Antioxidants, 2019; 8(6):154. CrossRef
Smirnoff N. The evolution of vitamin C biosynthetic pathways in plants and algae. Free Radic Biol Med, 2015; 86:S10. CrossRef
Sumiya N. Mechanism of coordination between cell and chloroplast division in unicellular algae. Plant Morphol, 2018; 30(1):83–9. CrossRef
SzymaÅ„ska R, Åšlesak I, Orzechowska A, Kruk, J. Physiological and biochemical responses to high light and temperature stress in plants. Environ Exp Bot, 2017; 139:165–77. CrossRef
Tan JS, Lee SY, Chew KW, Lam MK, Lim JW, Ho SH, Show PL. A review on microalgae cultivation and harvesting, and their biomass extraction processing using ionic liquids. Bioengineered, 2020; 11(1):116–29. CrossRef
Torres PB, Chow F, Furlan CM, Mandelli F, Mercadante A, Santos DYACD. Standardization of a protocol to extract and analyze chlorophyll a and carotenoids in Gracilaria tenuistipitata Var. Liui. Zhang and Xia (Rhodophyta). Braz J Oceanogr, 2014; 62(1):57–63. CrossRef
Vendruscolo RG, Fagundes MB, Maroneze MM, do Nascimento TC, de Menezes CR, Barin JS, Zepka LQ, Jacob-Lopez E, Wagner R. Scenedesmus obliquus metabolomics: effect of photoperiods and cell growth phases. Bioprocess Biosyst Eng, 2019; 42(5):727–39. CrossRef
Wahidin S, Idris A, Shaleh SRM. The influence of light intensity and photoperiod on the growth and lipid content of microalgae Nannochloropsis sp. Bioresour Technol, 2013; 129:7–11. CrossRef
White LH, Martin DW, Witt KK, Vogt F. Impacts of nutrient competition on microalgae biomass production. J Chemom, 2014; 28(5):448–61. CrossRef
Xu Y, Ibrahim IM, Harvey PJ. The influence of photoperiod and light intensity on the growth and photosynthesis of Dunaliella salina (chlorophyta) CCAP 19/30. Plant Physiol Biochem, 2016; 106:305–15. CrossRef
Ye S, Shao Q, Xu M, Li S, Wu M, Tan X, Su L. Effects of light quality on morphology, enzyme activities, and bioactive compound contents in Anoectochilus roxburghii. Front Plant Sci, 2017; 8:857. CrossRef
Yu W, Liu Y, Song L, Jacobs DF, Du X, Ying Y, Shao Q, Wu J. Effect of differential light quality on morphology, photosynthesis, and antioxidant enzyme activity in Camptotheca acuminata seedlings. J Plant Growth Regul, 2017; 36(1):148–60. CrossRef
Zahra SSA, Omidvar F, Mahdi K. Combine effects of temperature and photoperiod on the ascorbic acid of green algae Scenedesmus quadricauda. J Fish Sci Technol, 2017; 6(3):61–73.
Zhang S, He Y, Sen B, Wang G. Reactive oxygen species and their applications toward enhanced lipid accumulation in oleaginous microorganisms. Bioresour Technol, 2020; 307:123234. CrossRef
Zhang X, Yuan H, Guan L, Wang X, Wang Y, Jiang Z, Cao L, Zhang X. Influence of photoperiods on microalgae biofilm: photosynthetic performance, biomass yield, and cellular composition. Energies, 2019; 12(19):3724. CrossRef