INTRODUCTION
Acute and chronic wounds do not only cause pain but also significantly impose an economic burden (Guest et al., 2017; Järbrink et al., 2017). As a result, productivity, business returns, and tax revenue are greatly affected (Nussbaum et al., 2018; Sen et al., 2009). Given that the rate of accidents is very high in Indonesia, research into wound care has become a great priority (Jusuf et al., 2017; Karina et al., 2019; Tarawan et al., 2017).
Tissue repair in wound healing consists of three consecutive phases including inflammatory, proliferation, and maturation phases. Macrophages play an important role in all the phases of tissue repair (Shedoeva et al., 2019). Following activation, macrophages are polarized into two types, i.e., classically activated macrophages (M1) and alternatively activated macrophages (M2) (Yuliani et al., 2018). Inhibition of macrophage activity has been reported to be associated with prolonged wound healing time (Shedoeva et al., 2019). Similarly, as described among diabetic patients, persistent M1 macrophages in wound tissues and inhibition of M2 macrophage polarization in wound tissues make it difficult for chronic wounds to heal (Shah et al., 2017). Prolonged wound healing results in prolonged treatment and eventually increases the treatment cost. In view of this, a new breakthrough in wound care is required to produce effective, efficient, and inexpensive treatment options.
Medicinal plants for wound healing are not only cheap and affordable but also safe to use. These plants induce healing and regeneration of lost tissues by various mechanisms (Gao et al., 2015). According to the 2014 Horticultural Data, Curcuma longa, commonly known as turmeric rhizome, is the second highest biopharmaceutical plant in Indonesia (Jacob et al., 2007).
Furthermore, turmeric rhizome plants are widely used as an additive to food and also as a medicine for treating tissue inflammation problems (Chen et al., 2015). The turmeric rhizome contains curcumin, which modulates inflammatory responses through downregulation of cyclooxygenase-2 activity, lipoxygenase, and inducible nitric oxide synthase (iNOS) enzymes (Riquelme et al., 2013). In addition, in a macrophage cell line (Raw264.7), curcumin has been shown to induce polarization of M2 macrophages (Krzyszczyk et al., 2018).
Since curcumin in turmeric rhizome has been identified to modulate the inflammatory response and promote the polarization of M2 macrophages in vitro, investigating the effect of topical administration of turmeric rhizome extract gel on wound healing is warranted.
MATERIALS AND METHODS
Animal model
Thirty (30) Swiss Webster male mice, each weighing 22–26 g and about 8–10 weeks old, were obtained from the Faculty of Biology, Bandung Institute of Technology. Previously, these mice had been adapted for 1 week. The mice were divided into three groups and each group consisted of 10 mice. For wound induction, two circular skin excisions were performed on the back of each mouse using a biopsy punch with a diameter of 6 mm. After excision, the wound was cleaned with physiological NaCl, dried with sterile gauze, and covered with a transparent dressing; then, they were covered with dry gauze and plastered. Treatment of the wound was carried out on the 3rd day after the injury. In the positive control group, the open wounds were treated with tulle (polymyxin B sulfate + neomycin sulfate + bacitracin zinc). In the negative control group, the open wounds were given blank gel. In the last group, the open wounds of the mice were given turmeric (C. longa) rhizome extract gel. All mice were killed by cervical dislocation technique on the 6th day after the injury for excision and collection of the wounded skin tissue. For histopathology observation, the skin tissues collected were fixed for microscopic observation. One part of the skin tissue was taken and stored in liquid nitrogen immediately for Arginase-1 (Arg-1) and iNOS (M2 markers) gene expressions analysis using quantitative Polymerase Chain Reaction (qPCR).
Turmeric rhizome extract gel
Turmeric rhizome (C. longa)-based extract gel (3%) was provided by the Pharmacy Laboratory, Faculty of Pharmacy, Universitas Padjadjaran.
Histopathology (Collagen) observation
Collagenesis was observed by histopathology analysis of excised skin on day 6 after wound induction. Collagen was observed through Mason’s trichrome staining as zones of purple strands. In this study, collagen density was measured by taking an average of three collagen fibers that appeared intact on the two edges and center of the wound using a micrometer under a light microscope with a magnification of 10x and 40x objective lens. Scoring 0–4 indicates more solid collagen.
RNA extraction
The collected skin tissues were transferred into a homogenizer tube containing ceramic beads and 1 ml of Qiazol was added (Qiagen, Germany). Tissue lysis was carried out using Precellys homogenizer (6,000 rpm). The lysate was then transferred into 1.5 ml of microcentrifuge tube and 100 μl of chloroform was added gently. The tube was shaken for 10 seconds and incubated for 10 minutes at room temperature. The centrifugation was then carried out at 12,000 × g for 12 minutes at 4°C. After three phases were formed, 350 μl of the clear top layer consisting of the ribonucleic acid (RNA) was transferred into new tubes and washed with one volume of 70% ethanol. Afterward, RNA precipitate was transferred into a minicolumn tube, which consisted of RB column and 2 ml collection tube (Geneaid RNA Extraction kit, Taiwan) and centrifuged for 1 minutes with 14,000 × g at 4°C. The next step was performed according to the manufacturer’s (Geneaid, Taiwan) instructions. The extracted RNA was quantified using a spectrophotometer and the concentration of RNA was measured using absorbance at 260 and 280 nm.
Gene expression analysis
The Arg-1 and iNOS (M1 and M2 markers) gene expressions from wound skin tissues were analyzed using the quantitative real-time PCR method. At least 4.2 µg/ml from the total RNA was reverse-transcribed to be complementary deoxyribonucleic acid (cDNA) using ReverTra Ace® qPCR RT Master Mix (Toyobo, Japan), according to the manufacturer’s instruction. PCR was then carried out using Thunderbird® SYBR® qPCR Mix (Toyobo, Japan) kit, according to the manufacturer’s instruction. Real-time PCR was performed using Rotorgene Q cycler (Qiagen, Germany). The gene expressions assessed were Arginase-1 (Arg-1) as a marker for M2 macrophage and iNOS as M1’s marker. The gene expressions were analyzed using the following formula: 2-ΔΔCt, in which ΔΔCt = (Ct gene of interest−Ct reference gene) treatment group – (Ct gene of interest–Ct reference gene) control group.
The degree of gene expression was normalized with housekeeping gene β-actin as a reference gene and the result presented as a fold change to the control group. The primer sequences (mouse) are presented in Table 1. Quantitative real-time PCR was carried out at 95°C for 10 minutes, followed by at least 40 cycles at 95°C for 20 seconds and 60°C for 1 minutes.
Ethical statement
This study was approved by the Research Ethics Committee, Universitas Padjadjaran (705/UN6.C.10/PN/2017). All efforts were made to minimize the suffering of animals during the experimental procedures.
Statistical analysis
The Shapiro–Wilk test was used for the normality analysis and the Levene test was used for the homogeneity analysis. Analysis of the mean difference between the two groups based on the results of this normality test used independent t-test for variables with normal distribution or the analysis of variance (ANOVA) test, followed by Tukey’s multiple comparison test to compare more than two groups. Meanwhile, for variables with abnormal distribution, the Mann–Whitney U test was used for comparison between two groups. The analysis was performed with statistical software GraphPad Prism 8.
RESULTS
Histopathology
Representative pictures of microscopic skin tissue among positive control, negative control, and C. longa treatment groups are shown in Figure 1. The expression of collagen was highest in the C. longa treatment group compared to the positive (p < 0.0001) and negative control (p = 0.0418) groups. However, there was no statistical difference between the positive and negative control groups (Fig. 2).
Gene expression marker analysis for M1 and M2
Gene expression analysis on Arg-1 showed a significant increase in the C. longa treatment compared to the positive control (p = 0.0394) and negative control (p = 0.0313) (Fig. 3). Statistical analysis of the iNOS gene expression showed a decreasing trend in the C. longa treatment compared to the positive control and the negative control, but it was not significant (Fig. 4).
 | Table 1. Primer sequences used for quantitative real-time PCR. [Click here to view] |
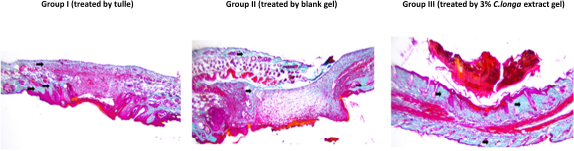 | Figure 1. Microscopic appearance of skin on the 6th day after injury; group I was positive control, group II was negative control, and group III was treated by 3% C. longa extract gel. The histopathological observation shows more collagen in the tissues that were treated with 3% C. longa extract gel. [Click here to view] |
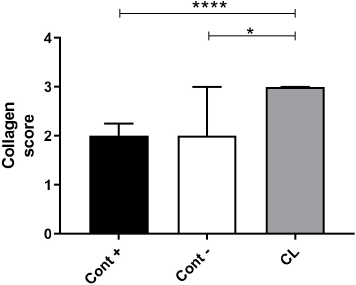 | Figure 2. Comparison graph of collagen density in positive controls (Cont +), negative controls (Cont −), and C. longa (CL). Data are presented as median ± interquartile range. Statistical analysis between two groups was tested using Mann–Whitney U test; *p < 0.05; ****p < 0.0001. [Click here to view] |
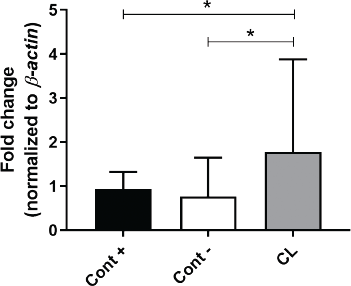 | Figure 3. Comparisons of Arginase-1 gene expression in positive controls (Cont +), negative controls (Cont −), and C. longa (CL). Graph is presented as median with interquartile range. Statistical analysis between two groups was tested using Mann–Whitney U test; *p < 0.05. [Click here to view] |
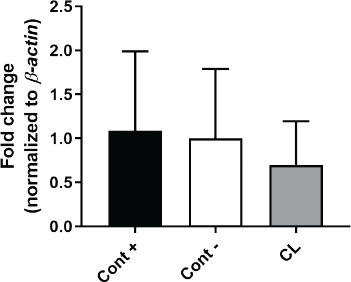 | Figure 4. Comparison of iNOS gene expression in positive controls (Cont +), negative controls (Cont −), and C. longa (CL). Graph is presented as mean with SD. Statistical analyses were tested using ANOVA, followed by Tukey’s multiple comparison test. [Click here to view] |
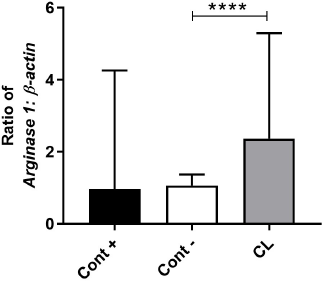 | Figure 5. Comparison of the ratio of Arginase-1 gene expression with iNOS in positive controls (Cont +), negative controls (Cont −), and C. longa groups. Graph is presented as median with interquartile range. Statistical analysis between two groups was tested using Mann–Whitney U test; ****p < 0.0001. [Click here to view] |
Comparison of Arg-1 and iNOS gene expression ratios (Fig. 5) showed a significantly higher ratio in C. longa compared to negative controls (p < 0.0001), but it was not significant when compared with positive controls (p = 0.0535).
DISCUSSION
In this study, we observed that C. longa has the ability to increase wound healing properties through the upregulation of Arg-1 gene in M2 polarized macrophages. The highest collagen fibers were formed in tissues that were treated with C. longa compared with the other groups. The higher amount of collagen fibers in mice of the C. longa-treated group could be due to the presence of beta-diketone and polyphenolic functional groups which act as strong antioxidants (Sha et al., 2019; Shedoeva et al., 2019). As a result, lipid peroxidation decreases but vascularity and collagen synthesis, as well as cross-linking of collagen significantly improve (Yuliani et al., 2018). Curcuma longa has anti-inflammatory properties and, therefore, topical application of C. longa gel extracts may have negative effects on the inflammatory phase in wound healing (Shah et al., 2017; Xue et al., 2018). To avoid this occurrence, the gel extract of C. longa was applied to the wounds of the mice on the 3rd day after injury. This study showed that Arg-1 significantly increased in the mice treated with C. longa compared to the positive controls and negative controls, and therefore, coupled with increased collagen density, wound healing was accelerated in the C. longa-treated group. These findings corroborate with several studies, wherein curcumin increased IL-4 and IL-13 proteins and messenger RNA (mRNA) expression which induced M2 polarization by upregulation of M2 markers (Arg-1, macrophage mannose receptor (MMR) gene, and Peroxisome proliferator-activated receptor gamma (PPAR-γ)) in Raw264.7 macrophages treated with C. longa (Gao et al., 2015; Jacob et al., 2007). According to other report, the inhibition of arginase activity significantly delayed wound healing time, and therefore, C. longa gel extracts can accelerate wound closure (Campbell et al., 2013). Generally, iNOS is induced by M1 macrophages and its expression is often low in human blood monocyte-derived macrophages (Ley, 2017; Riquelme et al., 2013). In this study, iNOS expression decreased in mice treated with C. longa compared with the negative controls and the positive controls. This may suggest that the local inflammatory processes and the resultant oxidative stress during injury may be reduced by topical application of C. longa gel extracts (Graf et al., 2017), thereby improving and hastening the healing of wounds (Choudhary et al., 2018). This finding concords with studies conducted by Ibrahim et al. (2018) and Mohanty et al. (2017), where C. longa gel extracts decreased the expression of iNOS and improved tissue repair, as well as the healing process.
Even though iNOS protein expression can hamper wound healing, it is a very essential enzyme that produces nitric oxide, a proinflammatory molecule that inactivates and destroys infectious microbes and as a result protects humans from diverse infectious diseases (Bogdan et al., 2000; Ellis et al., 2018). Since iNOS also helps the host, it is imperative to treat wounds with medications that balance the Arg-1 and iNOS ratio that will ensure that the benefits of both proteins are maintained but with decreased toxicity of iNOS. In this study, we revealed that the ratio of Arg-1 to iNOS was significantly higher in the C. longa treatment group than the negative controls; nevertheless, there was no substantial difference between C. longa treatment group and the positive controls. A high ratio of Arg-1 to iNOS activity improves the healing of wounds (Campbell et al., 2013), and by the findings of our study, it can be suggested that C. longa gel extracts will promote tissue repair and wound healing.
CONCLUSION
Based on this study, topical application of the turmeric rhizome (C. longa) gel extract accelerates the proliferation phase in the acute wound healing process through the induction of M2 macrophages and increasing collagen density in the wound tissue. However, further research is needed to explore more about the benefits of turmeric rhizome (C. longa) gel extract on the maturation/remodeling phase of the wound healing process.
ACKNOWLEDGMENTS
The authors thank the Directorate for Research and Community Service, Universitas Padjadjaran, for their financial support through Internal Research Grant for Fundamental Research.
CONFLICT OF INTEREST
The authors declare no conflict of interests.
FUNDING
This work was supported and funded by the Internal Research Grant for Fundamental Research of Universitas Padjadjaran (No. 855/UN6.3.1/PL/2017).
REFERENCES
Bogdan C, Röllinghoff M, Diefenbach A. The role of nitric oxide in innate immunity: no in innate immunity. Immunol Rev, 2000; 173(1):17–26. CrossRef
Campbell L, Saville CR, Murray PJ, Cruickshank SM, Hardman MJ. Local arginase 1 activity is required for cutaneous wound healing. J Invest Dermatol, 2013; 133(10):2461–70. CrossRef
Chen H, Shi R, Luo B, Yang X, Qiu L, Xiong J, Jiang M, Liu Y, Zhang Z, Wu Y. Macrophage peroxisome proliferator-activated receptor γ deficiency delays skin wound healing through impairing apoptotic cell clearance in mice. Cell Death Dis, 2015; 6(1):e1597. CrossRef
Choudhary V, Shivakumar HG. A review on curcumin: wound healing properties and biomarkers of wound healing. Int Res J Pharm, 2018; 9(9):1–5. CrossRef
Ellis S, Lin EJ, Tartar D. Immunology of wound healing. Curr Dermatol Rep, 2018; 7(4):350–8. CrossRef
Gao S, Zhou J, Liu N, Wang L, Gao Q, Wu Y, Zhao Q, Liu P, Wang S, Liu Y, Guo N, Shen Y, Wu Y, Yuan Z. Curcumin induces M2 macrophage polarization by secretion IL-4 and/or IL-13. J Mol Cell Cardiol. 2015; 85:131–9. CrossRef
Graf BL, Jaja-Chimedza AL, Raskin IL. Comparative anti-inflammatory effect of moringa-derived isothiocyanate versus curcumin in vitro and in vivo. FASEB J, 2017; 31(S1):972.22.
Guest JF, Vowden K, Vowden P. The health economic burden that acute and chronic wounds impose on an average clinical commissioning group/health board in the UK. J Wound Care, 2017; 26(6):292–303. CrossRef
Ibrahim NI, Wong SK, Mohamed IN, Mohamed N, Chin KY, Ima-Nirwana S, Shuid AN. Wound healing properties of selected natural products. Int J Environ Res Public Health, 2018; 15(11):1–23. CrossRef
Jacob A, Wu R, Zhou M, Wang P. Mechanism of the anti-inflammatory effect of curcumin: PPAR-γ activation. PPAR Res, 2007; 2007:1–5. CrossRef
Järbrink K, Ni G, Sönnergren H, Schmidtchen A, Pang C, Bajpai R, Car J. The humanistic and economic burden of chronic wounds: a protocol for a systematic review. Syst Rev, 2017; 6(1):15. CrossRef
Jusuf A, Nurprasetio IP, Prihutama A. Macro data analysis of traffic accidents in Indonesia. ITB J Public, 2017; 49(1):132–43. CrossRef
Karina, Samudra MF, Rosadi I, Afini I, Widyastuti T, Sobariah S, Remelia M, Puspitasari RL, Rosliana I, Tunggadewi TI. Combination of the stromal vascular fraction and platelet-rich plasma accelerates the wound healing process: pre-clinical study in a Sprague-Dawley rat model. Stem Cell Investig, 2019; 6:18. CrossRef
Krzyszczyk P, Schloss R, Palmer A, Berthiaume F. The role of macrophages in acute and chronic wound healing and interventions to promote pro-wound healing phenotypes. Front Physiol, 2018; 9:1–22. CrossRef
Ley K. M1 means Kill; M2 means heal. J Immunol, 2017; 199(7):2191–3. CrossRef
Mohanty C, Sahoo SK. Curcumin and its topical formulations for wound healing applications. Drug Discov Today, 2017;22(10):1582–92. CrossRef
Nussbaum SR, Carter MJ, Fife CE, DaVanzo J, Haught R, Nusgart M, Cartwright D. An economic evaluation of the impact, cost, and medicare policy implications of chronic nonhealing wounds. Value Health, 2018; 21(1):27–32. CrossRef
Riquelme P, Tomiuk S, Kammler A, Fändrich F, Schlitt HJ, Geissler EK, Hutchinson JA. IFN-γ-induced iNOS expression in mouse regulatory macrophages prolongs allograft survival in fully immunocompetent recipients. Mol Ther, 2013; 21(2):409–22. CrossRef
Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, Gottrup F, Gurtner GC, Longaker MT. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen, 2009; 17(6):763–71. CrossRef
Sha AM, Garib BT. Antibacterial effect of curcumin against clinically isolated porphyromonas gingivalis and connective tissue reactions to curcumin gel in the subcutaneous tissue of rats. BioMed Res Int, 2019; 2019:1–14. CrossRef
Shah A, Amini-Nik S. The role of phytochemicals in the inflammatory phase of wound healing. Int J Mol Sci, 2017; 18(5):1068. CrossRef
Shedoeva A, Leavesley D, Upton Z, Fan C. Wound healing and the use of medicinal plants. Evid Based Complement Alternat Med, 2019; 2019: 1–30. CrossRef
Tarawan VM, Mantilidewi KI, Dhini IM, Radhiyanti PT, Sutedja E. Coconut shell liquid smoke promotes burn wound healing. J Evid Based Complement Altern Med, 2017; 22(3):436–40. CrossRef
Xue Q, Yan Y, Zhang R, Xiong H. Regulation of iNOS on immune cells and its role in diseases. Int J Mol Sci, 2018; 19(12):1–13. CrossRef
Yuliani S, Mustofa, Partadiredja G. Turmeric (Curcuma longa L.) extract may prevent the deterioration of spatial memory and the deficit of estimated total number of hippocampal pyramidal cells of trimethyltin-exposed rats. Drug Chem Toxicol, 2018; 41(1):62–71. CrossRef