INTRODUCTION
Transdermal drugs have high bioavailability on the site of action because of absence of the first-pass metabolism of oral drugs (Davies and Anderson, 1997). Importantly, transdermal drug deliveries of non-steroidal anti-inflamatory drugs (NSAIDs) were developed to decrease the side effects, associated with oral NSAIDs, on gastrointestinal, cardiovascular, and renal systems (Altman et al., 2015; Evans et al., 1995). Although some of the NSAIDs are absorbed into the systemic circulation from the dermal site of application, systemic exposure to drugs is decreased. Transdermal NSAID is commonly used to relieve pain and inflammation. Therefore, efficacy of therapy by transdermal drug is dependent on its ability to penetrate the skin and permeate to the target tissues. Different approaches were applied for their enhancer action, such as phospholipid micelles (Duan et al., 2015), liposomes (Cosco et al., 2015), niosomes (Tavano et al., 2013), polymers, lipotropic liquid crystals (Estracanholli et al., 2014), and surfactants (Som et al., 2012).
Vesicular systems such as liposomes and niosomes represent an important drug delivery system. The advantages of niosomes over liposomes include enhanced chemical and physical stability, lower cost, and ease of use of surfactants (Moghassemi and Hadjizadeh, 2014). Niosomes are classified as vesicles containing the bilayer of nonionic surfactants around the aqueous core (Sankhyan and Pawar, 2012; Onochie et al., 2013). The vesicular structure of niosomes presents an advantage with ability to load both hydrophilic and lipophilic drugs (Alomrani et al., 2015; Manca et al., 2014; Pando et al., 2015; Tavano et al., 2013). Niosomes is a very beneficial drug delivery system with several applications (Mahale et al., 2012; Moghassemi and Hadjizadeh, 2014). Niosomal vesicles were investigated to enhance the transdermal drug permeation for several active pharmaceutical ingredient (Auda et al., 2016; El-Ridy et al., 2017; Hagen and Baker, 2017; Ioele et al., 2015).
Diclofenac sodium (DiC) is an NSAID, which is administered by the oral, intravenous, intramuscular, and topical routes to relieve pain and inflammation in humans (Hagen and Baker, 2017). Oral dosage forms of DiC have been proven to be less effective and tolerated than transdermal DiC in the treatment of osteoarthritis (OA) (Lin et al., 2004; Mason et al., 2004; Rannou et al., 2016). Therefore, percutaneous dosage forms of DiC are recommended before the use of oral NSAIDs in certain international guidelines for OA of the knees or hands (Bruyère et al., 2014; Zhang et al., 2007). In a preference study, the amount of OA patients who choose to use a topical rather than oral NSAID was almost three times higher; particularly elderly were more concerned about side effects related to oral drugs (Carnes et al., 2008; Underwood et al., 2008).
The objective of the present study is to develop transdermal niosomal hydrogel of DiC to improve the skin permeation of drug. The current research work comprises an in vitro evaluation including preparation and characterization of DiC niosomes, as well as ex vivo and in vivo skin permeation studies.
MATERIALS AND METHODS
Materials
DiC sodium was purchased from Sinochem Ningbo (China); cholesterol was purchased from MP Biomedicals North America (US); Span 80 was purchased from Spectrum Chemical (US); ethanol 96% (v/v) was purchased from DG Chemical Company (Vietnam). Carboxymethylcellulose sodium (CMC sodium) was purchased from Sigma Aldrich (US); glycerin was purchased from Puyer Biopharma (China). Methanol for high performance liquid chromatography (HPLC) was purchased from J.T. Baker (US).
Animal
Adult male rats were used; each weighed between 200 and 250 g [the National Institute of Drug Quality Control (Vietnam)]. The rats were housed on a 12 hours light/dark cycle in a temperature of 25°C ± 2°C and relative humidity of 55% ± 15% in controlled room with commercial food and water ad libitum. The animals were allowed to acclimatize for at least 7 days before the start of the experiment. The protocol of the animal experiments was approved by the Animal Care and Use Committee of the Hanoi University of Pharmacy, Vietnam.
Method preparation of DiC niosomes
DiC niosomes were prepared by ethanol injection method. The solvent phase was prepared by dissolving of Span 80, cholesterol, and DiC in the 10 ml of ethanol 96% and then heated up to 65°C. Solvent phase was injected in the purified water at 65°C with magnet stirring. The mixture was evaporated under reduced pressureat 40°C using rotary evaporator R25 (Buchi, Germany) to remove ethanol to form niosomal suspension.
Drying. The suspension of DiC niosomes was dried in the vacuum oven at 40°C for 48 hours for differential scanning calorimetry (DSC) and Fourier transform infrared spectroscopy (FTIR) characterization. Dry samples less than 2% moisture were stored in the refrigerator at 2°C–8°C.
DiC niosomes characterization
Size analysis
Vesicle size and distribution of vesicle size were evaluated by dynamic light scattering method using Zetasizer ZS90 (Malvern, UK). Each sample was diluted with purified water until the count rate in the range of 200–300 kcps.The results of analysis givea mean vesicles size and a polydispersity index (PDI) and characterized the width parameter of size distribution.
Determination of DiC content in the niosomal suspension
Quantitative analysis of DiC in the niosomal suspension was determined by UV-visible spectrography at 276 nm, the maximum wavelength of DiC aqueous solution. Linearity was determined from six working standard solutions of DiC. The correlation R2 and the slope of standard curve were calculated.
Determination of entrapment efficiency
Free DiC was removed from niosomal suspension by filtration through Vivaspin 50 kDa molecular weight cut off (GE Healthcare, US). Accurately, 1 ml of DiC niosomal suspension was poured in the vivaspin and then was centrifuged at 5,000 rpm for 30 minutes. The filter solution at the bottom was collected to determine the free amount of DiC by UV-visible spectrography as described above. The total amount of DiC was determined in the niosomal suspension prior filtration. The entrapment efficiency (EE%) was calculated by the following equation:
where m is the total amount of DiC and m1 is the unencapsulated amount of DiC.
Morphology of DiC niosomes
Morphology of DiC niosomes was observed by Field Emission Scanning Electron Microscope. The sample was put on a plate and continued drying in vacuum oven at 40°C for 48 hours. The dried sample was coated with gold and observed in the field emission scanning electron microscopy (FESEM) S4800 (Hitachi, Japan).
Evaluation of DiC niosomal hydrogel
Preparation of DiC niosomal hydrogel and DiC hydrogel
DiC niosomal hydrogel 1% (w/v): CMC sodium was put in the suspension of DiC niosomes to form gel. Methylparaben was dissolved in the glycerin and then continued mixing with the gel.
DiC hydrogel 1% (w/v): CMC sodium was put in the water to form gel. Methylparaben and DiC were dissolved in the glycerin and then continued mixing with the gel.
Placebo hydrogel: CMC sodium was put in the water to form gel. Methylparaben was dissolved in the glycerin and then continued mixing with the gel.
Ex-vivo skin permeation study
Skin preparation. To evaluate the skin permeation of DiC niosomal hydrogel, formulations were subjected to ex vivo permeation study. Back skins were obtained from rats. A 2.0 cm × 2.0 cm patch of hair-removal skin was excised, continuing removing subcutaneous fat and connective tissue. To achieve higher reproducibility, the integrity of the excised skins was confirmed under an optical microscope before experiment to verify that there was no scratch or injury on the stratum corneum-epidermis layer. Then, the excised skins were stored at 2°C–8°C for later use (Butani et al., 2014; El-Ridy et al., 2017).
Ex-vivo permeation study. The ex vivo permeation study of DiC niosomal hydrogel was evaluated using hair-free back skin of the rat. Dynamic Franz diffusion cell was used for skin permeation study of DiC niosomal hydrogel; the effective diffusion area was 1.767 cm2. The phosphate buffer solution (7 ml) was used as a receptor in continuous stirring condition. The prepared skin was placed at the diffusion area between donor and receptor compartments. Experiments were carried out at 37°C to guarantee that the temperature of skin surface was 32°C ± 0.5°C. About 0.5 g of the experiment hydrogel (corresponding to 5 mg DiC) was applied on the epidermal side of the rat skin and 0.5 ml of the receptor medium was got after predetermined period of time at 1, 2, 3, 4, 5, 6, 7, and 8 hours for DiC determination by HPLC method.
In vivo skin permeation study
Experimental groups for determination of concentrations of DiC in rats. Before the initiation of the in vivo skin permeation study, rats were divided into three groups; each group contained eight rats. The animals were fed three times a day and had a free access to water. Before experiments, rats were inhaled with ether, and then hair of back thigh was removed, where rats were applied experiment hydrogels on. For the first group, rats were applied DiC niosomal N hydrogel 1%; rats of the second group were applied commercial prodrug of DiC sodium, Voltaren Emulgel 1%; rats in the third group were applied placebo hydrogel. DiC at dose 50 mg/kg was applied topically in rats of the first and second groups.
Thigh muscle and thigh skin were collected at the 1.5 hours after administration from four rats of each group. Blood was collected 1.5 hours after hydrogel application from four rats of each group. DiC concentrations in tissue and blood were quantified by HPLC in all rats of three groups.
Blood sampling. At the 1.5 hours moment after hydrogel application, the blood samples from the rats were collected retroorbitally. About 1 ml of blood was withdrawn with capillary and placed in the clean heparinized tube. Blood samples were centrifuged for 10 minutes at 4,000 rpm (Hanza, Korea) to receive the plasma samples, which were stored in a freezer at −20°C for later analysis.
Muscle/skin sampling. At the 1.5 hours moment after the application of hydrogels, rats were sacrificed with chloroform. Thigh muscle and skin were excised from the appropriate site using sterile surgical equipments. Skin was stripped and cleaned from subcutaneous fat. The excision of the tissue sample was weighed and stored at −20°C to be further homogenized for drug analysis.
Muscle sample homogenization
Muscle sample was thawed before the experiment and then was exactly weighed 1 g. The tissue was homogenized using Wise StirHS 30 E (Germany), following the addition of 0.9% sodium chloride solution to form 10 ml suspension. Later, the sample was sonicated for 10 minutes by Q 500 Sonicators (Qsonica, US) and then was centrifuged at 10,000 rpm by Hanil Supra R22 (Korea). DiC in the supernatant was extracted by a liquid-liquid extraction method using n-hexane as extract solvent.
Skin extraction. The cumulated amount of DiC in the rat skin was determined by cutting the striped skin into small pieces and put in a 5 ml vial, which contained absolute ethanol, with 5 minutes sonication for complete extraction of DiC. The validated extraction method showed that the recovered amount of DiC from the stripped skin was more than 90%.
Extraction of muscle samples. The tissue supernatant (500 µl) of each animal was added to each tube; 500 µl of phosphoric acid 1 M was added in each tube; and the sample was vortexed for 2 minutes; 500 µl of acetone was added, and the sample was vortexed for 2 minutes; 5 ml of n-hexane was added, extraction was carried out by homogenization (vortex) for 5 minutes, and then the tube was centrifuged at 5,500 rpm for 10 minutes to extract upper organic phase to another clean glass tube. The sample was dried by evaporation under nitrogen flow at 45°C; the dried residue was diluted with 1 ml of the mobile phase to form a sample solution, which was analyzed to determine the concentration of DiC by HPLC method.
Extraction of plasma samples. The plasma was extracted by the method like the extraction method of muscle samples. The plasma (500 µl) of each animal was added to each tube; 500 µl of phosphoric acid 1 M was added in each tube; the sample was vortexed for 2 minutes; 500 µl of acetone was added, and the sample was vortexed for 2 minutes; 5 ml of n-hexane was added, extraction was carried out by homogenization (vortex) for 5 minutes, and then the tube was centrifuged at 5,500 rpm for 10 minutes to extract upper organic phase to another clean glass tube. The sample was dried by evaporation under nitrogen flow at 45°C; the dried residue was diluted with 1 ml of the mobile phase to form sample solution, which was analyzed to determine the concentration of DiC by HPLC method.
HPLC method for determination of DiC concentration in biological tissue muscle. Quantitative analysis of DiC in the biological tissue was performed by high-performance liquid chromatography (HPLC-Agilent, US). The analytical column was C18 (150 × 4.6 mm, 5 µm) (Cosmosil, Japan);the operation was carried out at ambient temperature. The mobile phase was a mixture of methanol and phosphate buffer pH 2.5 in a ratio of 80:20 (v/v), the flow rate was 1.0 ml/minute, the detector was UV-visible at 254 nm, and the volume of injection was 50 µl. The sample was filtered through nylon membrane with pore size of 0.45 µm before injection to the column. Under the HPLC condition, the retention time of DiC was about 7.1 minutes. HPLC linearity was determined from six working standard solutions of DiC. HPLC method was validated by specificity, accuracy, precision, linearity, lower limit of detection, and lower limit of quantification.
Statistical analysis
The cumulative amount of DiC permeated (mg.cm−2) through excised rat skin was calculated by (Zhu et al., 2008)
where Cn (mg/ml) is the drug concentration of the solution in the receptor compartment at each sampling time (minimum n = 1; maximum n = 8), Ci is the drug concentration of the ith sample (minimum i = 1; maximum i = 7; i = n−1), and Vi (ml) and V0 (ml) are the volumes of the sample and the solution in the receptor compartment, respectively. S is the effective diffusion area (S = 1.767 cm2).
The permeation rate of DiC at a steady state (Jss, mg. cm−2.hours−1) through the rat skin was calculated from the slope of linear portion of the plots of Qt versus time (Butani et al., 2014; Das et al., 2017).
The results of all experiments were presented as an average ± SD. The data of all in vivo skin permeation experiments were analyzed by one-way analysis of variance with Tukey’s post hoc test. A p-value of < 0.05 was considered to be significant.
RESULTS AND DISCUSSIONS
Characterizations of DiC niosomes
Characterization of DiC niosomes is composed of Span 80 and cholesterol (chol) at molar ratios of 5:5 and 6:4 shown in Table 1.
As the results shown in Table 1, mean diameters of niosomal vesicles formulations M and N are smaller than 100 nm; they are distributed in the narrow range with PDI smaller than 0.2. FESEM image of DiC niosomes in Figure 1 shows that DiC niosomes are spherical; they have diameter in the range of 80–150 nm. Ethanol injection method was used to prepare niosomes loaded DiC to form small vesicles with diameter less than 100 nm, distributed in the narrow range with PDI smaller 0.2. Entrapment efficiency of niosomes of N formulation was higher than M formulation. But both N and M DiC niosomes had entrapment efficiency less than 50%, which is correlated with the solubility of drug in the aqueous phase. In some different researches, encapsulation of DiC increased by incorporation of positive-charge agents likely stearylamine to form lipophilic ion-pair between DiC and stearylamine. However, in this case, entrapment efficiency was not higher than 50% (Abdallah et al., 2013).
FTIR
Figure 2 presents the IR spectra of Span 80, cholesterol, sodium DiC, and niosomes of M and N formulations.
The DiC spectrum (Fig. 2E) exhibits board NH peaks at 3,440.39 cm−1. The Span 80 spectrum (Fig. 2A) presents peak at 3,438.46 cm−1 (O-H stretching) and 1,737.55 cm−1 (C=O stretching). The board peak at 3,438.46 cm−1 shows intermolecular hydrogen bond (Pretsch et al., 2009). The cholesterol exhibits board OH peak at 3,422 cm−1. The peak at 1,737.55 (C=O stretching) is changed in the niosomal samples, indicating their involvement in the formation of niosomal vesicles. This could be due to hydrogen bond between C=O of Span 80 and N-H and O-H groups of DiC and cholesterol, respectively.
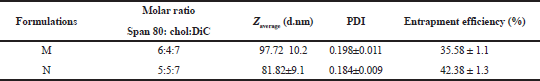 | Table 1. Particle size, distribution, and entrapment efficiency of DiC niosomes at different molar ratios of Span 80 and cholesterol (n = 4). [Click here to view] |
Differential scanning calorimetry
Figure 3 presents the DSC thermograms of DiC, cholesterol, and DiC niosomes of M and N formulations. DiC exhibits characteristic endotherm peaks at 89.58°C, indicating its crystal nature. The cholesterol displays an endotherm at 133.23°C corresponding to its melt temperature. The DiC and cholesterol had peak at 52.79°C, indicating the vaporing process of water. The DSC curve of DiC niosomes of M and N formulations showed that endothermic peaks of cholesterol and drug have disappeared, which indicated that the DiC and cholesterol dispersed in the niosomal membrane. The DSC results suggested that some weak hydrogen bonds or Vander Waals forces are formed between DiC, Span 80, and cholesterol (Jena et al., 2014; Ma et al., 2014; Singh et al., 2014, 2012; Yanyu et al., 2006).
Ex-vivo skin permeation study
M hydrogel 1%, N hydrogel 1%, DiC hydrogel 1%, and Voltaren Emulgel 1% (all hydrogels contained 1% DiC w/v) were used to evaluate ex-vivo skin permeation of drug. M, N, and DiC hydrogels 1% were prepared following formulations, shown in Table 2. All hydrogels were homogenized and had an elegant appearance. The cumulative amount of DiC, permeated through rat skin and permeation rate on steady state of the evaluated hydrogels, is given in Figure 4.
As illustrated in Figure 4A, cumulative amounts of DiC, released from the N and M niosomal hydrogels after 8 hours, were 0.74 and 0.44 mg/cm2, respectively, which were higher than those of Voltaren Emulgel and DiC hydrogel (0.15 and 0.02 mg/cm2, resp.). As reported in Figure 4B, the rates permeation (flux) of N and M hydrogels was higher than that of DiC hydrogel and Voltaren Emulgel. Specifically, the flux of DiC from N hydrogel (0.1017 mg.cm−2.hours−1) was about thirty times and five times that obtained with DiC hydrogel (0.0034 mg.cm−2. hours−1) and commercial formulation (0.0218 mg.cm−2.hours−1), respectively. These results have explained that the lower drug permeation observed from DiC hydrogel and commercial product in comparison with niosomal hydrogel proved the permeation enhancing effects of niosomal vesicles on drug delivery. Several excipients, such as surfactants, polyol, and alcohol in the Voltaren Emulgel®, play as percutaneous permeation enhancers. For this reason, the cumulative permeated DiC reached intermediate values, in comparison to the lower value of the DiC hydrogel prepared with only glycerol and CMC sodium. However, the presence of a raised amount of surfactant in the form of vesicular systems leads to the higher percutaneous penetration of DiC (Ioele et al., 2015). Enhancing skin permeation of drug from niosomal hydrogel was observed in the different studies (Ioele et al., 2015; Junyaprasert et al., 2012; Manca et al., 2014; Pando et al., 2015; Tavano et al., 2013).
 | Figure 1. FESEM of DiC niosomes of M formulation (A) and N formulation (B). [Click here to view] |
In vivo skin permeation study
Data in Table 3 shows that DiC from Voltaren Emulgel and N niosomal hydrogel could be depth permeated through skin and subcutaneous tissue and finally was observed in the blood after dermal administration. In Voltaren Emulgel, diethylammonium salt of the drug and several skin permeation enhancers, such as ethanol, polyalcohol, and surfactant, are used to penetrate the skin barrier. Several pieces of literature discussed that topical dosage form containing DiC overcomes through the skin and permeates to the muscle (Azevedo et al., 2013; Brun et al., 2016; Hagen and Baker, 2017).
In our study, Tmax (blood concentration) of N niosomal hydrogel and Voltaren Emulgel was observed at 1.5 hours after hydrogel application in rats (data not public). Therefore, at the moment of 1.5 hours after dermal administration of N niosomal hydrogel and Voltaren Emulgel, concentrations of DiC in the skin, muscle, and blood were shown in Table 3. The data show that drug concentration in the rat skin of the N hydrogel group was statistically higher by about three times than that in the Voltaren Emulgel group, p-value < 0.005. DiC concentration in the rat muscle of Voltaren Emulgel group was statistically lower by about 1.8 times than that of N niosomal hydrogel group, p-value < 0.005. Blood concentrations of drug in rats of Voltaren Emulgel and N niosomal hydrogel groups were not statistically different (21.24 µg/ml of Voltaren Emulgel group and 26.02 µg/ml of N niosomal group), p > 0.05.
 | Figure 2. FTIR spectra of Span 80 (A), cholesterol (B), DiC niosomes of M formulation (C), DiC niosomes of N formulation (D), and diclofenac sodium (E). [Click here to view] |
 | Figure 3. DSC thermograms of DiC (A), cholesterol (B), DiC niosomes of M formulation (C), and DiC niosomes of N formulation (D). [Click here to view] |
The data in Table 3 suggest that niosomal vesicles not only increase the permeation of DiC through the skin but also accumulate into the skin and they also carry a higher drug amount into the tissues (muscle) under the application site. However, the drug concentration of DiC in the blood was high because of good DiC solubility in water. The results of in vivo skin permeation study indicated that niosomal vesicles loaded with DiC had magnitude higher DiC concentration in the target tissue (muscle) than using the administration of commercial Voltaren Emulgel.
 | Table 2. Formulations of M, N, and DiC hydrogels 1% (w/v). [Click here to view] |
 | Figure 4. (A) Cumulative amount of DiC, permeated through rat skin; (B) permeation rate on steady state (Jss). [Click here to view] |
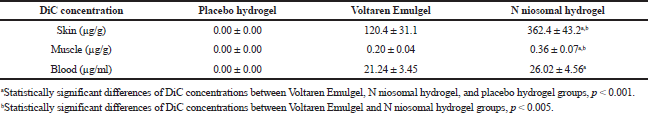 | Table 3. DiC concentration in the skin, muscle, and blood of rats (n = 4). [Click here to view] |
CONCLUSIONS
DiC niosomes, prepared by simple ethanol injection method, had spherical morphology and small diameter about 100 nm, distributed in a narrow range. Data of ex vivo and in vivo experiments suggested that DiC niosomal hydrogel not only improved the amount and rate transport of DiC through the skin but also increased drug concentration in the muscle in comparison to the commercial drug. These results strongly emphasized the potential application of DiC niosomes in transdermal drug delivery.
CONFLICT OF INTEREST
The authors report no conflicts of interest.
FUNDING
None.
REFERENCES
Abdallah M, Sammour O, EL-Ghamry H, Abu-Selem M. Preparation and in-vitro evaluation of diclofenac sodium niosomal formulations. Int J Pharm Sci Res, 2013; 5:1757–65.
Alomrani AH, Al-Agamy MH, Badran MM. In vitro skin penetration and antimycotic activity of itraconazole loaded niosomes: various non-ionic surfactants. J Drug Deliv Sci Technol, 2015; 28:37–45. CrossRef
Altman R, Bosch B, Brune K, Patrignani P, Young C. Advances in NSAID development: evolution of diclofenac products using pharmaceutical technology. Drugs, 2015; 8:859–77. CrossRef
Auda SH, Fathalla D, Fetih G, El-Badry M, Shakeel F. Niosomes as transdermal drug delivery system for celecoxib: in vitro and in vivo studies. Polym Bull, 2016; 5:1229–45. CrossRef
Azevedo MS, De La CÔrte FD, Brass KE, Dalmora SL, Machado FT, Pompermayer E, Santa’Ana LA. Bioavailability and tolerability of topical and oral diclofenac sodium administration in healthy ponies. J Equine Vet Sci, 2013; 1:22–6. CrossRef
Brun LV, Macolinets VI, Gubar SN. Pharmacokinetic study of diclophenac sodium in rat plasma by high-performance liquid chromatography. Ukraïns’kij BìofarmacevtiÄnij Žurnal, 2016; 45:62–70. CrossRef
Bruyère O, Cooper C, Pelletier JP, Branco J, Luisa Brandi M, Guillemin F, Reginster JY. An algorithm recommendation for the management of knee osteoarthritis in Europe and internationally: a report from a task force of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Semin Arthritis Rheum, 2014; 3:253–63. CrossRef
Butani D, Yewale C, Misra A. Amphotericin B topical microemulsion: formulation, characterization and evaluation. Colloids Surf B Biointerfaces, 2014; 116:351–8. CrossRef
Carnes D, Anwer Y, Underwood M, Harding G, Parsons S. Influences on older people’s decision making regarding choice of topical or oral NSAIDs for knee pain: qualitative study. BMJ, 2008; 7636:142–5. CrossRef
Cosco D, Paolino D, Maiuolo J, Marzio LD, Carafa M, Ventura C, Fresta M. Ultradeformable liposomes as multidrug carrier of resveratrol and 5-fluorouracil for their topical delivery. Int J Pharm, 2015; 1–2:1–10. CrossRef
Das B, Sen SO, Maji R, Nayak AK, Sen KK. Transferosomal gel for transdermal delivery of risperidone: formulation optimization and ex-vivo permeation. J Drug Deliv Sci Technol, 2017; 38:59–71. CrossRef
Davies NM, Anderson KE. Clinical pharmacokinetics of diclofenac. Clin Pharmacokinet, 1997; 3:184–213. CrossRef
Duan Y, Wang J, Yang X, Du H, Xi Y, Zhai G. Curcumin-loaded mixed micelles: preparation, optimization, physicochemical properties and cytotoxicity in vitro. Drug Deliv, 2015; 1:50–7. CrossRef
El-Ridy MS, Yehia SA, Mohsen AM, El-Awdan SA, Darwish AB. Formulation of niosomal gel for enhanced transdermal lornoxicam delivery: In-vitro and In-vivo evaluation. Cur Drug Deliv, 2017; 1:122–33. CrossRef
Estracanholli EA, Praça FSG, Cintra AB, Pierre MBR, Lara MG. Liquid crystalline systems for transdermal delivery of celecoxib: in vitro drug release and skin permeation studies. AAPS PharmSciTech, 2014; 6:1468–75. CrossRef
Evans JMM, McMahon AD, McGilchrist MM, White G, Murray FE, McDevitt DG, MacDonald, TM. Topical non-steroidal anti-inflammatory drugs and admission to hospital for upper gastrointestinal bleeding and perforation: a record linkage case-control study. BMJ, 1995; 6996:22–6. CrossRef
Hagen M, Baker M. Skin penetration and tissue permeation after topical administration of diclofenac. Curr Med Res Opin, 2017; 9:1623–34. CrossRef
Ioele G, Tavano L, De Luca M, Ragno G, Picci N, Muzzalupo R. Photostability and ex-vivo permeation studies on diclofenac in topical niosomal formulations. Int J Pharm, 2015; 1:490–7. CrossRef
Jena SK, Singh C, Dora CP, Suresh S. Development of tamoxifen-phospholipid complex: novel approach for improving solubility and bioavailability. Int J Pharm, 2014; 1–2:1–9. CrossRef
Junyaprasert VB, Singhsa P, Suksiriworapong J, Chantasart D. Physicochemical properties and skin permeation of Span 60/Tween 60 niosomes of ellagic acid. Int J Pharm, ;423, 2:303–11. CrossRef
Lin J, Zhang W, Jones A, Doherty M. Efficacy of topical non-steroidal anti-inflammatory drugs in the treatment of osteoarthritis: meta-analysis of randomised controlled trials. BMJ, 2004; 28:1–8. CrossRef
Ma H, Chen H, Sun L, Tong L, Zhang T. Improving permeability and oral absorption of mangiferin by phospholipid complexation. Fitoterapia, 2014; 93:54–61. CrossRef
Mahale NB, Thakkar PD, Mali RG, Walunj DR, Chaudhari SR. Niosomes: novel sustained release nonionic stable vesicular systems - an overview. Adv Colloid Interface Sci, 2012; 183–184:46–54. CrossRef
Manca ML, Manconi M, Nacher A, Carbone C, Valenti D, MacCioni AM, Fadda AM. Development of novel diolein-niosomes for cutaneous delivery of tretinoin: influence of formulation and in vitro assessment. Int J Pharm, 2014; 1–2:176–86. CrossRef
Marwa A, Omaima S, Hanaa EG, Mohammed AS. Preparation and in-vitro evaluation of diclofenac sodium niosomal formulations. Int J Pharm Sci Res, 2013; 5:1757–65.
Mason L, Moore RA, Edwards JE, Derry S, McQuay HJ. Topical NSAIDs for chronic musculoskeletal pain: systematic review and meta-analysis. BMC Musculoskeletal Disord, 2004; 5:1–8. CrossRef
Moghassemi S, Hadjizadeh A. Nano-niosomes as nanoscale drug delivery systems: an illustrated review. J Control Release, 2014; 1:22–36. CrossRef
Onochie ITO, Nwakile CD, Umeyor CE, Uronachi EM, Usonwa UE, Attama AA, Esimone OO. Formulation and evaluation of niosomes of Benzyl penicillin. J Appl Pharm Sci, 2013; 12:066–71.
Pando D, Matos M, Gutiérrez G, Pazos C. Formulation of resveratrol entrapped niosomes for topical use. Colloids Surf B Biointerfaces, 2015; 128:398–40. CrossRef
Pretsch E, Bühlmann P, Badertscher M. Structure determination of organic compounds. 3rd edition, Springer. New York, NY, 2009.
Rannou F, Pelletier JP, Martel-Pelletier J. Efficacy and safety of topical NSAIDs in the management of osteoarthritis: evidence from real-life setting trials and surveys. Semin Arthritis Rheum, 2016; 4:18–21. CrossRef
Sankhyan A, Pawar P. Recent trends in niosome as vesicular drug delivery system. J Appl Pharm Sci, 2012; 6:20–32.
Singh C, Bhatt TD, Gill MS, Suresh S. Novel rifampicin-phospholipid complex for tubercular therapy: synthesis, physicochemical characterization and in-vivo evaluation. Int J Pharm, 2014; 1–2:220–7. CrossRef
Singh D, Rawat SMM, Semalty A, Semalty M. Quercetin-phospholipid complex: an amorphous pharmaceutical system in herbal drug delivery. Curr Drug Discov Technol, 2012; 9:17–24. CrossRef
Som I, Bhatia K, Yasir M. Status of surfactants as penetration enhancers in transdermal drug delivery. J Pharm Bioallied Sci, 2012; 1:2–9. CrossRef
Tavano L, Gentile L, Oliviero RC, Muzzalupo R. Novel gelniosomes formulations as multicomponent systems for transdermal drug delivery. Colloids Surf B: Biointerfaces, 2013; 110:281–8. CrossRef
Underwood M, Ashby D, Cross P, Hennessy E, Letley L, Martin J, Whyte K. Advice to use topical or oral ibuprofen for chronic knee pain in older people: randomised controlled trial and patient preference study. BMJ, 2008; 7636:138–42. CrossRef
Yanyu X, Yunmei S, Zhipeng C, Qineng P. The preparation of silybin–phospholipid complex and the study on its pharmacokinetics in rats. Int J Pharm, 2006; 1:77–82. CrossRef
Zhang W, Doherty M, Leeb BF, Alekseeva L, Arden NK, Bijlsma JW, Zimmermann-Górska I. EULAR evidence based recommendations for the management of hand osteoarthritis: report of a Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis, 2007; 3:377–88. CrossRef
Zhu W, Yu A, Wang W, Dong R, Wu J, Zhai G. Formulation design of microemulsion for dermal delivery of penciclovir. Int J Pharm, 2008; 360:184–90. CrossRef