INTRODUCTION
Efforts are being undertaken over the last few decades to develop efficient therapies for diseases such as diabetes, cancer, arthritis, cardiovascular diseases, etc. The medications available for treatment are costly and are associated with some side effects. Hence, it is prudent to search for options in herbal medicine. Herbal medicine or phytomedicine has been the oldest form of healthcare known to mankind. It is based on the usage of plant products as medication and it has been associated with different traditional medicine systems spread across the geo-cultural spheres in the world (David et al., 2015). The natural products have been used to isolate active constituents and some of these active leads have also been taken up for clinical trials (David et al., 2015; Fabricant and Farnsworth, 2001). Hence, in the past decade, there has been a resurgence of interest in the investigation of natural resources as a source of potential drug substance.
The Aloe species has been used for centuries for its health, beauty, medicinal, and skin care properties. Aloe barbadensis Miller commonly called as Aloe vera belongs to the family Liliaceae and is a cactus-like plant (Ahlawat and Khatkar, 2011). Aloes are perennial succulents or xerophytes which are around 60–100 cm tall; as such, they are adaptable to habitats with low or erratic water availability and are characterized by the capacity to store large volumes of water in their tissue. Aloes have in common green fleshy leaves covered by a thick cuticle or rind and an inner clear pulp. The concentration of Aloe latex in the rind depends on the leaf part, age, position of the leaf on the plant, leaf orientation, and season of collection. The latex in the plant is used as defense strategy to deter it from being consumed. The gel consists of about 99.5% water and the remaining 0.5–1% is solid material (Eshun and He, 2004). The dry weight solid material consists of a range of compounds, including water-soluble and fat-soluble vitamins, minerals, enzymes, mono (Femenia et al., 1999) and polysaccharides, lignin, proteins, polypeptides, phenolic compounds, and organic acids (Boudreau and Beland, 2006; Hamman, 2008).
The genus Aloe contains over 400 different species, among which A. barbadensis Miller (A. vera) is widely used compared to other species, like Aloe arborescens Mill, Aloe ferox Mill, for their therapeutic properties (Radha and Laxmipriya, 2015). The Aloe preparations have been used to treat frostbite, burns, radiation dermatitis, ulcers, psoriasis, wounds, skin infections, cancer, arthritis, and diabetes (Boudreau and Beland, 2006).
The phytochemistry of A. vera gel has reported the presence of more than 200 bioactive chemicals. The main constituents are monosaccharide, polysaccharides, amino acids, enzymes, chromones, anthraquinones (aloin and emodin), lignins, minerals, vitamins, salicylic acid, saponins, and sterols. The biological activities of several Aloe species have been reviewed by Reynolds and Dweck (1999) and their relationship between the components and biological effects have been reported by Choi and Chung (2003). Since then, further pharmacological activities of the components of A. vera have been reported. This review aims to provide updated information on bioactive components, their pharmacological properties, recent advances, and side effects of species of the genus Aloe.
METHOD
This review was prepared with the help of articles collected using databases such as Google Scholar, PubMed, Science Direct, and other peer reviewed journals. The Plant List (www.theplantlist.org), The International Plant Name Index (www.ipni.org), and Tropicos® (www.tropicos.org) were used for understanding the differences between the species from Aloe genus. ChemDraw Ultra 12 was used for drawing of the structures.
ETHNOMEDICAL USES OF THE GENUS ALOE
As mentioned earlier, the Aloe species are succulent, stem less xerophytes. The leaves are succulent and are usually greenish or greyish green in color. The flowers usually bloom in the months of October and January (Manvitha and Bidya, 2014). The flower grows around 90 cm, often being pendulous and consists of yellow tubular corolla around 2–3 cm (Babu et al., 2019). The Aloe plants are mostly used as decorative plants and are widely used in cosmetic industries. However, the Aloe species have a broad range of medicinal properties. Mostly, the gel portion of the plant is used for the treatment of diseases and disorders (Reynolds and Dweck, 1999). Aloe barbadensis Miller, commonly referred to as A. vera, is the most commonly used species of Aloe, followed by Aloe arborenscens, A. ferox, and Aloe chinensis. These are the species which are present in abundance geographically and also have therapeutic potential. The Indian Ayurveda medicine system uses the epidermis part of the A. vera for its anti-helminthic property and digestive system problems (Sandeep and Yadav, 2014). The Mexicans use A. vera for the treatment of burns and skin problems. Other medicinal properties of A. vera include treatment for rheumatoid arthritis, diabetes, cancer, and as an immunomodulatory agent (Cock, 2015).
The traditional use of A. arborescens involves the use as a topical agent to treat wounds and burns (Mabona and Van Vuuren, 2013). The Japanese use this species for the treatment of intestinal ailments and also as an antiseptic agent. Apart from that, it is also used as an immunomodulatory and chemo-preventive agent and it also has anti-diabetic and anti-inflammatory properties. Aloe chinensis is used for its antioxidant and immunomodulatory potential (Wu et al., 2006).
Aloe ferox is predominantly found in the African region, and according to folklore, it was used as a laxative and anti-purgative agent in the African medicine system. Scientific reports are available, which report the use of this species for treating wounds, psoriasis treatment, eczema, and melanoma. It has also been used for the treatment of infectious diseases such as syphilis and candida. The latest reports that have been published credit A. ferox for having anti-diabetic, anti-cancer, anti-microbial, and anti-inflammatory properties (Kambizi and Afolayan, 2008; Loots et al., 2011; Mwale and Masika, 2014).
Species native to the Arabian nations also have a history of therapeutic properties. Among these, Aloe perryi Baker has wider usage due to its medicinal properties. It has been used to treat skin and eye infections and intestinal disorders (Al-Fatimi et al., 2005). It is also used in treatment of malaria and has anti-bacterial property (Mothana et al., 2009). Other species belonging to this region are Aloe inermis, Aloe officinalis, and Aloe tomentosa, which have been known for their antioxidant and anti-microbial properties (Cock, 2015).
Apart from these species, other species of Aloe have narrow geographical distributions and in turn, their ethno-pharmacological benefits are also less. These include Aloe africana (used as a laxative), Aloe marlothii (used to treat microbial infections and respiratory disorders), and Aloe variegata (used as a laxative). More than 400 species of Aloes have been reported and most of these species have some or other medicinal benefits. Most of the work has been carried out using A. barbadensis Miller, Aloe arborescenes, and A. ferox (Cock, 2015). Table 1 lists out some of the species of A. vera that have been studied for its beneficial properties, while Figure 1 shows some of the important species of the genus Aloe.
BIOACTIVE CONSTITUENTS PRESENT IN THE GENUS ALOE
The gel of A. vera consists of around 200 active constituents such as saccharides, polyphenols, flavonoids, glycoproteins, minerals, vitamins, anthraquinones, lipids, amino acids, and enzymes (Hamman, 2008). In this section, we discuss the components of the gel and the epidermis of A. vera with some important chemical structures (Fig. 2).
Saccharides
Saccharides form the major component of the Aloe gel. Acemannan and mannose-6-phosphate are major constituents of the carbohydrates. Acetylated mannan, also called as acemannan, is considered to be one of the important bioactive polysaccharides present in the Aloe gel, which has been known for its therapeutic properties such as anti-diabetic and immunomodulation. It is made up of mannosyl residues that have been acetylated at C-2 and C-3 positions and the C-6 position consists of a side chain, which is usually galactose (Femenia et al., 1999). Other polysaccharides such as arabinan, arabinorhamnogalactan, galactan, galactogalacturan, glucogalactomannan, galactoglucoarabinomannan, and glucuronic acid containing polysaccharides have been reported to exist in the A. vera inner leaf gel part (Ni et al., 2004). Aloeride contains glucose (37.2%), galactose (23.9%), mannose (19.5%), and arabinose (10.3%). It is reported to have a molecular weight between 4 and 7 Million Dalton (Pugh et al., 2001). The saccharides are known to have wound healing properties, anti-diabetic properties, and anti-inflammatory properties (Cock, 2015).
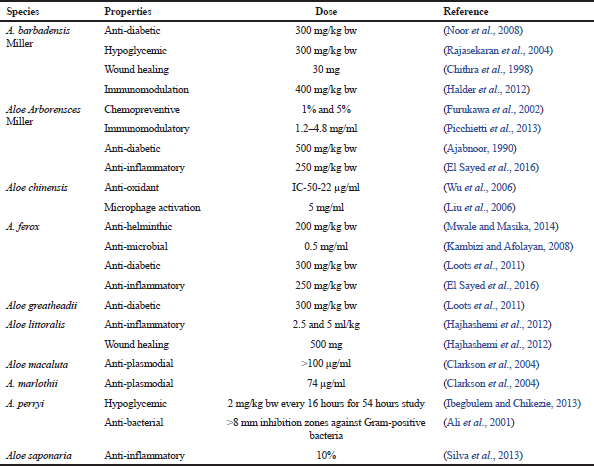 | Table 1. Medicinal properties of different species of the genus Aloe. [Click here to view] |
 | Figure 1. Some important species of the genus Aloe. [Click here to view] |
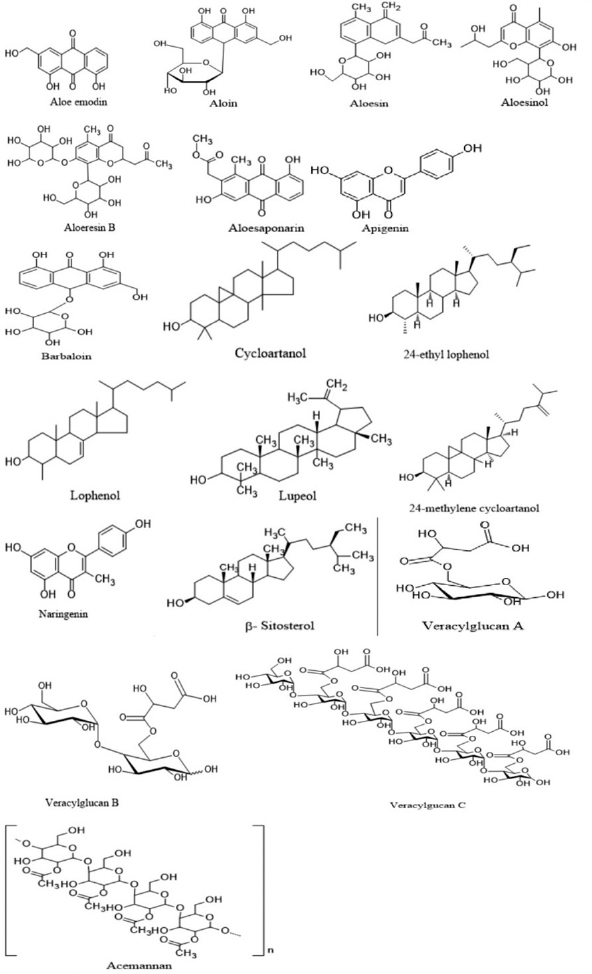 | Figure 2. Chemical structures of some of the bioactive constituents present in the genus Aloe. [Click here to view] |
Polyphenols
The polyphenols are known to exert their effect through their antioxidant properties. Apart from that, polyphenols from Aloe species have been reported to possess anti-diabetic potential, anti-inflammatory properties, and wound healing properties (Babu et al., 2019).
Coumarins, a class of polyphenols, are mostly found in the leaf of Aloe species and these are known to contribute to the bitter taste of Aloes. Feralolide is a coumarin which has been reported to be present in A. ferox (Speranza et al., 1993), while dihydroisocumarin is present in Aloe hildebrandtii (Veitch et al., 1994), which is known to stimulate the macrophages for fluid reabsorption in edema patients (Casley-Smith et al., 1993).
Flavonoids
The flavonoids that are present in the Aloe gel with antioxidant properties include naringenin, apigenin, myrcetin, quercitine, and cinnamic acid (Dagne et al., 2000), and are known to have inhibitory activity on platelets (Fuhrman and Aviram, 2001).
Sterols
Sterols are present in the leaf as well as the gel portion of the plant. Some of the important sterols which are known to be present in Aloe species are campesterol, β-sitosterol, lophenol, cycloaretonol, and lupeol (Dagne et al., 2000). These are said to have cell proliferative activities of endothelial cells. They are also reported to have a role in angiogenesis and hence are used in would healing process (Awad et al., 2007; Reynolds and Dweck, 1999).
Anthraquinones
Anthraquinones are usually present in the epidermis of the plant. Some of the anthraquinones present in the lead part are Aloin, Aloe emodin, aloesaponarin, chrysophanol, and isoxanthorin (Reynolds and Dweck, 1999). The concentration of aloin and Aloe emodin determines the antioxidant property. It is interesting to note that emodin acts as an antioxidant at high concentrations and at lower concentrations it acts as a prooxidant (Cock, 2015). Whereas aloin not only acts as a prooxidant at higher and lower levels, but it also acts as an antioxidant agent (Tian and Hua, 2005).
Anthrones
Anthrones are another set of compounds which are usually found in the leaf epidermis of the plant. These are the compounds which usually have purgative effects and are hence used as laxatives. Barbaloin is one of the first anthrones to be isolated from A. vera. Aloe ferox leaves are also reported to have higher amounts of anthrone (around 30%) (van Wyk et al., 1995). From Aloe marlotthi, anthrones such as homonataloin and nataloin have been extracted (Dagne et al., 2000). The anthrones are also known to have strong antioxidant properties. Yen et al. (2000) have suggested that the structures of anthrones determine their electrophilic nature and this enables them to have antioxidant potential. As mentioned earlier, the concentration of anthraquinones determines whether they can act as an antioxidant or a prooxidant.
Chromones
Chromones are mostly present in the leaf of the plant. Aloesin and Aloesinol are said to have anti-diabetic properties (Lee et al., 1997; Yimam et al., 2014). There are a number of isomeric forms of chromones present in the plant. These include aloeresin E, Aloerosin A, and Aloeresin F. Some of the chromones are present in a methylated form or are glycosylated at different positions (Dagne et al., 2000). These are usually said to possess antioxidant properties (Gomes et al., 2009); however, the levels of chromones would determine whether they would act as prooxidant or antioxidant agents (Maurya and Devasagayam, 2010).
Proteins/glycoproteins
In comparison to other constituents present in A. vera, not much has been reported on proteins or glycoproteins, although there have been literature reports which indicate the presence of amino acids in A. vera gel (Cock, 2015). Some lectins have also been isolated from A. vera. Most of the glycoproteins from A. vera are said to possess wound healing properties. Glycoproteins of different masses, such as 29KD, 5.5KD, 10KD, and 14KD, have been isolated from A. vera and their therapeutic potential, including anti-inflammatory, anti-diabetic, and immunomodulation, has been studied (Das et al., 2011; Siritapetawee et al., 2013; Yagi et al., 2009).
MINERAL ELEMENTS AND OTHER CONSTITUENTS
Trace mineral elements, such as magnesium, zinc, copper, manganese, potassium, sodium, and iron, have been reported and have been studied for their therapeutic properties (Rajasekaran et al., 2005). Aloe vera also contains alkaloids, benzene derivatives, and furan derivatives (Cock, 2015).
These are some of the bioactive components which are present in the A. vera plants. It can be inferred that bioactive components such as saccharides, glycoproteins, polyphenols, sterols, and mineral elements are present in the gel. The leaf or the epidermis part usually consists of a large amount of anthraquinones, anthrones, and chromones. The therapeutic potential of A. vera depends not only on the amount of constituents present in the plant but also on the ratio of these chemicals and their nature. The interaction of these constituents with one others may also affect the medicinal benefit imparted by this plant. It is observed that even though more than 400 species are present in Aloe genus, most of the constituents (sterols, polyphenols, saccharides, proteins, glycoproteins, and minerals) have been reported either from A. vera, A. ferox, or A. arborescens. The therapeutic potential of these bioactive constituents, such as anti-diabetic, anti-oxidant, anti-inflammatory, anti-cancer, wound healing, and immunomodulation, have been discussed in the following sections with their pre-clinical/clinical studies and their possible mechanism of action.
THERAPEUTIC PROPERTIES OF THE GENUS ALOE BIOCONSTITUENTS
Role of Aloe gel and its constituents in the alleviation of diabetes
Most of the studies have focused on the effect of the Aloe gel for their pharmacological actions. According to the reports, A. vera extract has shown not only to reduce blood sugar levels (Noor et al., 2008; Rajasekaran et al., 2004), but has also shown a protective effect on organs like pancreas, liver, and small intestine in streptozotocin-induced diabetic rats (Noor et al., 2008; Noor et al., 2017). Clinical trials have been reported wherein the patients were administered 100 g of A. vera gel with 20 g of Psyllium seed husks (Agarwal, 1985) and another clinical trial reported on the anti-hyperglycemic and anti-hypercholesterolemia effects on type 2 diabetic patients (Huseini et al., 2012) and lowering of blood glucose levels in diabetic patients when administered A. vera juice (Choudhary et al., 2014; Radha and Laxmipriya, 2015). The literature available on the active constituents of A. vera for its anti-diabetic property has been summarized in Table 2.
Anti-hyperglycemic effect of Aloe polyphenols
The polyphenols are known to exert their effect through their antioxidant properties (Pandey and Rizvi, 2009). A polyphenol-rich A. vera extract (350 mg/kg) containing 181.7mg/g aloin and 3.6mg/g Aloe emodin when administered for a period of 4 weeks to insulin-resistant mice showed a decrease in blood glucose levels (Pérez et al., 2007). Aloesin and Aloesinol of A. ferox have shown to decrease blood sugar levels in mice with an increase in adiponectin levels in vitro and a decrease in plasma insulin levels in vivo (Yimam et al., 2014). Phytosterols (lophenol, 24-methyl lophenol, 24-ethyl lophenol, cycloartanol, and 24-methlyene cycloartanol) have shown to reduce blood sugar levels as well as triglyceride levels (Tanaka et al., 2006).
Anti-hyperlipidemia effect of Aloe polyphenols
Phytosterols (lophenol and cycloartanol) have shown to reduce the serum-free fatty acid and triglyceride levels in Zucker diabetic fatty rats. Reduction in levels of PPARÉ£/Liver X receptor α by A. vera gel can lead to proper functioning of insulin and these phytosterols may be one of the compounds acting via their inhibiting activity on PPARÉ£/Liver X receptor α, pro-inflammatory cytokines (Misawa et al., 2008). Abd-Alla et al. (2009) reported that Aloe hijazensis containing flavonols and flavones help in reducing blood sugar levels and triglyceride levels, thereby ameliorate the diabetic conditions (Abd-Alla et al., 2009).
Anti-hyperglycemic effect and anti-hypercholesterolemic effect of Aloe saccharides
Yagi et al. (2009) reported the hypoglycemic effect in diabetic patients with 1,000KD polysaccharide fraction along with glycoprotein verectin (29KD) (Yagi et al., 2009). Acemannan, a mucopolysaccharide enriched A. vera extract, when given to diabetic patients showed anti-hyperglycemic and anti-hypercholesterolemic activities (Huseini et al., 2012).
Hypoglycemic effect by Aloe Proteins/polypeptides
A glycoprotein named verectin (29KD), along with polysaccharide fraction of 1,000KD, has shown hypoglycemic effect in patients with type 2 diabetes (Yagi et al., 2009). Verectin indicated anti-oxidative, anti-thromboxane A2 synthase inhibition, and cyclooxygenase-2 inhibiting activities in vitro and these may be correlated with vasodilatation in diabetic patients (Yagi et al., 2003). The mechanism of action of these constituents in alleviating diabetes is shown in Figure 3.
Insulinotropic effect by Aloe minerals
Narayanan et al. (2007) reported that these minerals may play a direct or indirect role in insulin secretion in a synergistic way (Narayanan et al., 2007). Rajasekaran et al. (2005) reported that the presence of these minerals in A. vera extract plays a role in antidiabetic activity (Rajasekaran et al., 2005). Magnesium is one of the important compounds which usually takes part in the metabolism of carbohydrates and fats by improving glucose and insulin homeostasis (Rumawas et al., 2006). Zinc enhances the effectiveness of insulin acting as a cofactor. Narayanan et al. (2007) reported that the presence of zinc in A. vera can modulate the targets for insulin activity (Narayanan et al., 2007). Potassium takes part in releasing insulin from beta cells, calcium in stimulation of insulin in pancreatic islets, vanadium elicits glucose levels, copper is involved in insulin binding, and chromium is essential for normal carbohydrate metabolism (Rajasekaran et al., 2005).
In addition to antidiabetic potential, the Aloe constituents also exhibit other therapeutic properties, such as anti-inflammatory, anti-allergic, anti-bacterial, anti-cancer, angiogenic, antioxidant, immunomodulation, and wound healing. The details of the findings are discussed in the following sections and are summarized in Table 3.
Antioxidant and anti-cancer properties of Aloe constituents
APS-1, a polysaccharide from A. vera, was shown to have both anti-oxidant property and protective effect on heart tissue (Wu et al., 2006), which may be due to a higher content of rhaminose and arabinose in polysaccharide fraction (Kang et al., 2014). Aloe vera polyphenols have exhibited antioxidant and anti-cancer properties (Jeon et al., 2011; Naqvi et al., 2010) with Aloe emodin exhibiting anti-cancer potential against hepatoma cells. Aloin has shown to exhibit anti-cancer activity by inhibiting the tumor angiogenesis by inactivating the signal transduction and activators of transcription 3 pathway (Pan et al., 2013). Aloe polysaccharides were reported for antitumor activity against sarcoma 180 cells (Liu et al., 2006). Another polysaccharide G2E1DS2 was shown to activate macrophages and exhibit potent anti-tumor activity when injected into mice implanted with sarcoma cells. Polysaccharides also inhibit the activity of PMA-induced tyrosine kinase activity in human leukemic cells, leading to apoptosis of leukemic cells (Kim et al., 1999). A glycoprotein fraction and Aloesin was found to promote cell proliferation activity in human hepatoma SK-HEP1 cell lines, while a 53KD lectin was observed to have anti-proliferative activity against colon and lung cancer cell lines (Kaur et al., 2011). Figure 4a shows the mechanism of action of these constituents in cancer therapy.
Aloe constituents with anti-inflammatory, anti-allergic, and anti-fungal properties
Aloin and aloesin were shown to exhibit anti-inflammatory activities by decreasing the levels of tumor necrosis factor-α (TNF-α) and plasma leukotriene B(4) (Park et al., 2011). Hutter et al. (1996) reported an anti-inflammatory compound called C-glucosylchromone which inhibits the cyclooxygenase pathway and reduces prostaglandin E2 production from arachidonic acid (Hutter et al., 1996). Veracylglucan B and Veracylglucan C have exhibited anti-inflammatory and cell proliferation activities (Esua and Rauwald, 2006). Alprogen, a glycoprotein, possessed anti-inflammatory activity (Ro et al., 2000). In another study carried out by Ro et al., 2000, alprogen was reported to have anti-allergic property by inhibiting the release of histamine in allergies through blocking the calcium influx (Ro et al., 2000). Anti-fungal activity against the Candida species and anti-inflammatory properties of a protein of 14KD from A. vera were reported by Das et al. (2011). The peptide/polypeptide fraction of A. vera has been reported to reduce inflammation through both in vitro and in vivo studies via reduction of inflammatory mediators (Babu and Noor, 2019). The anti-inflammatory action of these constituents is shown in Figure 4b.
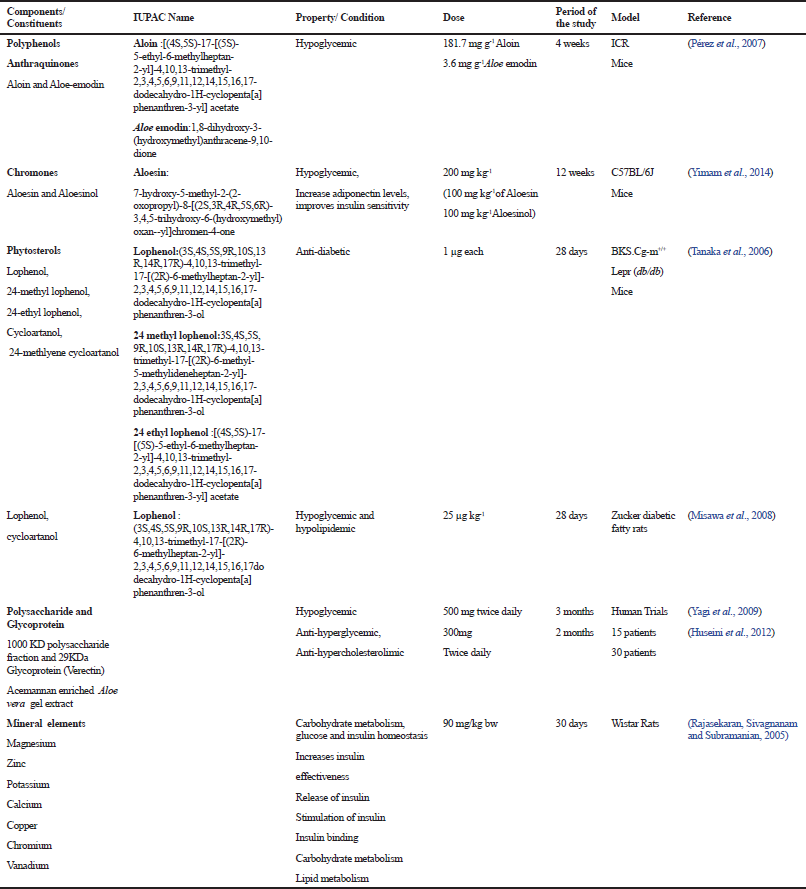 | Table 2. Role of different constituents of Aloe vera in diabetes mellitus [Click here to view] |
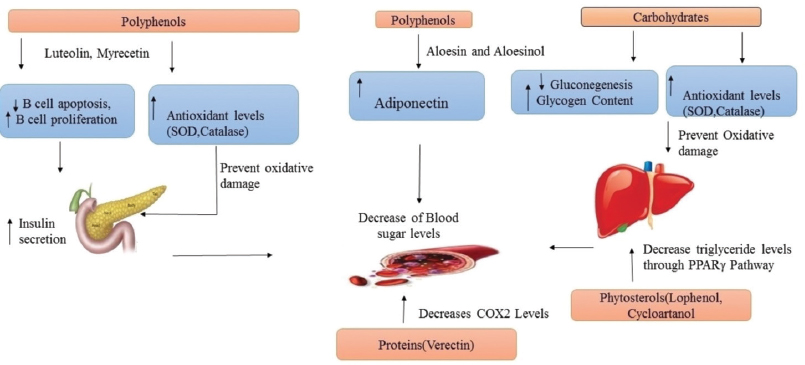 | Figure 3. Aloe constituents’ possible mechanism of action in diabetes. [Click here to view] |
Aloe constituents with anti-microbial properties
The polysaccharides have shown antibacterial activity by destroying the bacteria through generation of leukocytes (Pugh et al., 2001). Yates et al. (1992) demonstrated the anti-viral activity of acemannan in a pilot study of clinically symptomatic immunodeficiency virus-infected cat (Yates et al., 1992). Aloe emodin, purified from barbaloin, was also reported to inactivate a variety of viruses, including herpes simplex virus type I and type II, varicella-zoster, and the influenza virus (Jeon et al., 2011). The mechanism proposed for the anti-bacterial and anti-viral effects of Aloe emodin is the inhibition of nucleic acid biosynthesis, thereby inhibiting protein synthesis.
Aloe constituents with angiogenic property
β-Sitosterol possesses angiogenic activity and shows an increase in the formation of new blood vessels in a dose-dependent fashion (≥500 mg/kg) in gerbil brain cells by increasing the levels of vascular endothelial growth factor, the vascular endothelial growth factor receptor fetal liver kinase-1, and blood vessel matrix laminin, which has been damaged due to ischemia (Choi et al., 2002) .
Aloe constituents with immunomodulation and wound healing properties
Aloe polysaccharides between 400KD and 5KD molecules were shown to have immunomodulatory activity. Acemannan acts by activating and improving the macrophage action through an increased expression of cytokines IL-6 and TNF-α and the release of nitric oxide (Im et al., 2005; Kang et al., 2014). Lu et al. (2012) observed that A. vera polysaccharides mitigate ischemia and reperfusion injury in hemorrhagic rats (Lu et al., 2012).
The growth of endothelial, epithelial, and fibroblast cells plays a critical role in wound healing processes. Aloe polysaccharides increased the expression of matrix metalloprotease-3 and tissue inhibitor of metalloproteinases genes, thereby accelerating the process of wound healing (Tabandeh et al., 2014). A 5.5KD glycoprotein of A. vera was reported by Choi et al. (2001) for wound healing property by increasing the cell proliferation with elevated levels of epidermal growth factor receptor, fibronectin receptor, fibronectin, keratin 5/14, and keratin 1/10 (Choi et al., 2001). Figure 4c and d shows the immunomodulation action and wound healing action of these bioactive constituents, respectively.
Aloe constituents with anti-fibrinolytic and hemagglutination properties
A protease inhibitor protein of 11.8KD was reported to have anti-fibrinolytic potential (Siritapetawee et al., 2013). Aloctin I and Aloctin II have been reported with hemagglutination activity (Akev and Can, 1999), while 53KD lectin Mucin was shown to have hemeagglutinating activity and anti-proliferative activity against lung and colon cancer cell lines (Kaur et al., 2011).
 | Table 3. Pharmacological activities of Aloe vera constituents [Click here to view] |
RECENT ADVANCES ON EFFECTIVE UTILIZATION OF GENUS ALOE
Different formulations have been used to process A. vera as a commercial product. Depending on the requirement in product quality, various processing steps are followed by the industries. It is important to make sure that the constituents in A. vera do not lose their activity (Maan et al., 2018).
Research has been focused on controlled release of Aloe bioactive constituents. In case of wound healing, hydrogels and functional films have been developed for release of these constituents. The gel has been combined with biomaterials such as cellulose, alginate, and gelatin (Saibuatong and Phisalaphong, 2010; Silva et al., 2014). These formulations have prolonged drug release, better mechanical support, and prevention of body fluid loss. Attention has also been focused on tissue engineering, wherein growth factors and biomaterials have been employed for regeneration (Ikada, 2006). A nanofibrous scaffold comprising of gel and silk fibroin showed better synergistic effect in skin tissue regeneration (Suganya et al., 2014).
Micro and nanoparticles have also been used as a drug delivery system due to great stability of encapsulated drug. The microparticles consisting of A. vera, chitosan, and Vitamin E have been used for treating skin burns with better adhesive properties and prolonged release of the constituents (Pereira et al., 2014). Nanoparticles along with A. vera gel led to improved antiviral property along with high loading efficiency (Joshy et al., 2016). Co-encapsulation of A. vera along with curcumin has enhanced the antioxidant property of the phytoconstituents (Kitture et al., 2015). Liposomes of 200 nm with Mannose-6-phosphate have shown anti-inflammatory potential and are used for collagen synthesis and growth of skin cells (Conte et al., 2017; Takahashi et al., 2009). The use of liposomes, biomaterials, and micro and nano encapsulation studies on these constituents for their other therapeutic potential needs to be explored further.
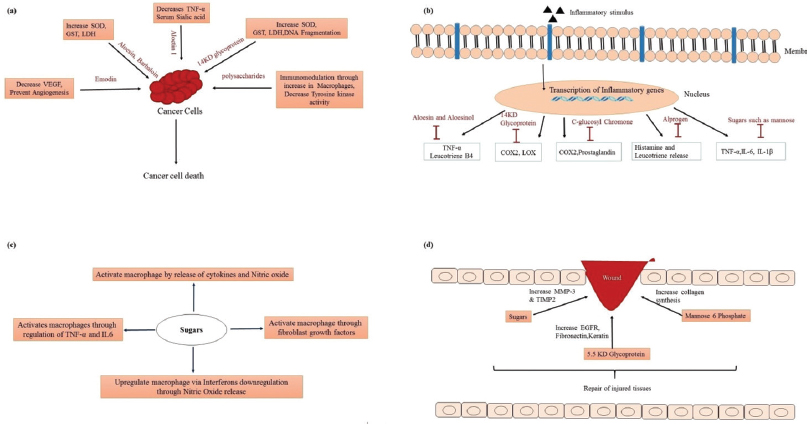 | Figure 4. Aloe constituents’ possible mechanism of action in (a) cancer, (b) inflammation, (c) immunomodulation, and (d) wound healing. [Click here to view] |
SIDE EFFECTS/TOXICOLOGICAL STUDIES OF THE GENUS ALOE
Most of the studies on A. vera and their constituents have been reported to be safe. But some side effects have also been mentioned. Application of A. vera to skin may cause redness, burning, or stinging sensations due to the presence of anthraquinones like aloin and barbaloin (Ferreira et al., 2007; Reider et al., 2005). It is also reported that prolonged use of Aloe latex or the Aloe epidermis region can increase the risk of arrhythmia (Ulbricht et al., 2008) due to its laxative property, leading to depletion of potassium. Based on our literature survey, most of the side effects reported are mainly because of the latex which has been poorly described (Kato et al., 2004).
Most of the works reported have used the Aloe plant or the gel portion alone. It can be inferred that the use of the entire plant or only the gel portion of Aloe can prevent these side effects. The presence of constituents, such as saccharides, proteins, and polyphenol minerals, in the gel may mask the effects of the Aloe latex which is usually rich in antharquinones such as aloin and barbaloin.
CONCLUSION
The genus Aloe consists of many species and the most prominent is the A. barbadensis Miller plant, commonly known as A. vera. There is no wonder in considering A. vera as the ‘Miracle plant’ as there is untapped potential in it for the management of therapeutic uses, and this review seeks to create more interest in screening of as many constituents as possible. Potential biological activity of constituents may be affected by the climate, season, harvesting, processing, storage conditions, and geographical origin which may bring about change in constituents, which may result in variations. Therefore, careful assessment of these constituents, their ecological and seasonal variation, metal contents, and toxicology studies need to be carried out to keep the toxic components, if any, at very low or permissible levels. The pharmacokinetic parameters, such as dose, its absorption and release and its metabolism also need to be studied. The action and the desired activity of these constituents, the time duration, and the therapeutic window need to be analyzed and validated through proper clinical trials. In addition to this, the route of delivery of the constituents needs to be studied. Studies regarding the usage, efficacy, safety of liposomes, polymers, bioadhesives, and nanoparticles to deliver these phytoconstituents need to be carried out. The bioactive constituent’s role in the clinical applications may help in development of new formulations, wherein these constituents may be used as an alternate source for treating various ailments. Therefore, it is considered that studies on the genus Aloe and its constituents should be expanded significantly to enable improvement in healthcare.
ACKNOWLEDGMENT
The authors thank the management of Vellore Institute of Technology,Vellore, for providing necessary facilities. The Indian Council of Medical Research (ICMR) is acknowledged for providing Senior Research Fellowship to Mrs Spoorthy N. Babu.
CONFLICT OF INTEREST
Authors declared that they do not have any conflicts of interest.
FUNDING
None.
REFERENCES
Abd-Alla HI, Shaaban M, Shaaban KA, Abu-Gabal NS, Shalaby NMM, Laatsch H. New bioactive compounds from Aloe hijazensis. Nat Prod Res, 2009; 23(11):1035–49. CrossRef
Agarwal OP. Prevention of atheromatous heart disease. Angiology, 1985; 36(8):485–92. CrossRef
Ahlawat KS, Khatkar BS. Processing, food applications and safety of Aloe vera products: a review. J Food Sci Technol, 2011; 48(5):525–33. CrossRef
Ajabnoor MA. Effect of aloes on blood glucose levels in normal and alloxan diabetic mice. J Ethnopharmacol, 1990; 28(2):215–20. CrossRef
Akev N, Can A. Separation and some properties of Aloe vera L. leaf pulp lectins. Phytother Res, 1999; 13(6):489–93. CrossRef
Al-Fatimi M, Friedrich, Jenett-Siems K. Cytotoxicity of plants used in traditional medicine in Yemen. Fitoterapia, 2005; 76(3–4):355–8. CrossRef
Ali NAA, Jülich WD, Kusnick C, Lindequist U. Screening of Yemeni medicinal plants for antibacterial and cytotoxic activities. J Ethnopharmacol, 2001; 74(2):173–9. CrossRef
Awad AB, Chinnam M, Fink CS, Bradford PG. beta-Sitosterol activates Fas signaling in human breast cancer cells. Phytomedicine, 2007; 14(11):747–54. CrossRef
Babu S, Noor A. Aloe barbadensis Miller peptide/polypeptide fraction alleviates inflammation through inhibition of proinflammatory cytokines and mediators in vitro and in rats with Freund’s adjuvant-induced hind paw edema. Asian Pac J Trop Biomed, 2019; 9(12):524–30. CrossRef
Babu SN, Govindarajan S, Vijayalakshmi MA, Noor A. Evaluation of In vitro anti-diabetic and anti-oxidant activities and preliminary phytochemical screening of gel, epidermis and flower extract of Aloe vera. Res J Pharm Technol, 2019; 12(4):1761–8. CrossRef
Boudreau MD, Beland F. An evaluation of the biological and toxicological properties of Aloe barbadensis (miller), Aloe vera. J Environ Sci Health C, 2006; 24(1):103–54. CrossRef
Casley-Smith JR, Wang CT, Casley-Smith JR. Zi-hai C. Treatment of filarial lymphoedema and elephantiasis with 5,6-benzo-alpha-pyrone (coumarin). BMJ, 1993; 307(6911):1037–41. CrossRef
Chithra P, Sajithlal GB, Chandrakasan G. Influence of Aloe vera on the healing of dermal wounds in diabetic rats. J Ethnopharmacol, 1998; 59(3):195–01. CrossRef
Choi S, Chung MH. A review on the relationship between Aloe vera components and their biologic effects. Semin Integr Med, 2003; 1(1):53–62. CrossRef
Choi S, Kim KW, Choi JS, Han ST, Park YI, Lee SK, Kim JS, Chung MH.. Angiogenic activity of beta-sitosterol in the ischaemia/reperfusion-damaged brain of Mongolian gerbil. Planta Med, 2002; 68(4):330–5. CrossRef
Choi SW, Son BW, Son YS, Park YI Lee SK, Chung MH. The wound-healing effect of a glycoprotein fraction isolated from Aloe vera. Br J Dermatol, 2001; 145(4):535–45. CrossRef
Choudhary M, Kochhar A, Sangha J. Hypoglycemic and hypolipidemic effect of Aloe vera L. in non-insulin dependent diabetics. J Food Sci Technol, 2014; 51(1):90–6. CrossRef
Clarkson C, Maharaj VJ, Crouch NR, Grace OM, Pillay P, Matsabisa MG, Bhagwandin N, Smith PJ, Folb PI. In vitro antiplasmodial activity of medicinal plants native to or naturalised in South Africa. J Ethnopharmacol, 2004; 92(2–3):177–91. CrossRef
Cock IE. The genus Aloe: phytochemistry and therapeutic uses including treatments for gastrointestinal conditions and chronic inflammation. Prog Drug Res, 2015; 70:179–235. CrossRef
Conte R, Marturano V, Peluso G, Calarco A, Cerruti P. Recent advances in nanoparticle-mediated delivery of anti-inflammatory phytocompounds. Int J Mol Sci, 2017; 18(4): E709. CrossRef
Dagne E, Bisrat D, Viljoen A,Van Wyk BE. Chemistry of Aloe species. Curr Org Chem, 2000; 4(10):1055–78. CrossRef
Das S, Mishra B, Gill K, Ashraf MS, Singh AK, Sinha M, Sharma S, Xes I, Dalal K, Singh TP, Dey S. Isolation and characterization of novel protein with anti-fungal and anti-inflammatory properties from Aloe vera leaf gel. Int J Biol Macromol, 2011; 48(1): 38–43. CrossRef
David B, Wolfender JL, Dias DA. The pharmaceutical industry and natural products: historical status and new trends. Phytochem Rev, 2015; 14(2):299–315. CrossRef
El Sayed AM, Ezzat SM, El Naggar MM, El Hawary SS. In vivo diabetic wound healing effect and HPLC–DAD–ESI–MS/MS profiling of the methanol extracts of eight Aloe species. Braz J Pharmacogn, 2016; 26(3):352–62. CrossRef
Eshun K, He Q. Aloe vera: a valuable ingredient for the food, pharmaceutical and cosmetic industries–a review. Crit Rev Food Sci Nutr, 2004; 44(2):91–6. CrossRef
Esua MF, Rauwald JW. Novel bioactive maloyl glucans from Aloe vera gel: isolation, structure elucidation and in vitro bioassays. Carbohydr Res, 2006; 341(3):355–64. CrossRef
Fabricant DS, Farnsworth NR. The value of plants used in traditional medicine for drug discovery. Environ Health Perspect, 2001; 109(Suppl. 1):69–75. CrossRef
Femenia A, Sánchez ES, Simal S, Rosselló C. Compositional features of polysaccharides from Aloe vera (Aloe barbadensis Miller) plant tissues. Carbohydr Polym, 1999; 39(2):109–17. CrossRef
Ferreira M, Teixeira M, Silva E, Selores M. Allergic contact dermatitis to Aloe vera. Contact Dermatitis, 2007; 57(4):278–9. CrossRef
Fuhrman B, Aviram M. Flavonoids protect LDL from oxidation and attenuate atherosclerosis. Curr Opin Lipidol, 2001; 12(1):41–8. CrossRef
Furukawa F, Nishikawa A, Chihara T, Shimpo K, Beppu H, Kuzuya H, Lee IS, Hirose M. Chemopreventive effects of Aloe arborescens on N-nitrosobis(2-oxopropyl)amine-induced pancreatic carcinogenesis in hamsters. Cancer Lett, 2002; 178(2):117–22. CrossRef
Gomes A, Neuwirth O, Freitas M, Couto D, Ribeiro D, Figueiredo AGPR, Silva AMS, Seixas RSGR, Pinto DCG, Tomé AC, Cavaleiro JaS, Fernandes E, Lima JLFC. Synthesis and antioxidant properties of new chromone derivatives. Bioorg Med Chem, 2009; 17(20):7218–26. CrossRef
Hajhashemi V, Ghannadi A, Heidari AH. Anti-inflammatory and wound healing activities of Aloe littoralis in rats. Res Pharm Sci, 2012; 7(2):73–8.
Halder S, Mehta AK, Mediratta PK. Augmented humoral immune response and decreased cellmediated immunity by Aloe vera in rats. Inflammopharmacology, 2012; 20(6):343–6. CrossRef
Hamman JH. Composition and applications of Aloe vera leaf gel. Molecules, 2008; 13(8):1599–616. CrossRef
Huseini H, Kianbakht S, Hajiaghaee R, Dabaghian FH. Anti-hyperglycemic and anti-hypercholesterolemic effects of Aloe vera leaf gel in hyperlipidemic type 2 diabetic patients: a randomized double-blind placebo-controlled clinical trial. Planta Med, 2012; 78(4):311–6. CrossRef
Hutter J, Salman M, Stavinoha WB, Satsangi N, Williams RF, Streeper RT, Weintraub ST. Antiinflammatory C-glucosyl chromone from Aloe barbadensis. J Nat Prod, 1996; 59(5):541–3. CrossRef
Ibegbulem CO, Chikezie P. Hypoglycemic properties of ethanolic extracts of Gongronema latifolium, Aloe perryi, Viscum album and Allium sativum administered to alloxan-induced diabetic albino rats (Rattus norvegicus). Pharmacogn Commun, 2013; 3(2):12–6. CrossRef
Ikada Y. Challenges in tissue engineering. J R Soc Interface, 2006; 3(10):589–601. CrossRef
Im SA, Oh ST, Song S, Kim MR, Kim DS, Woo SS, Jo TH, Park YI, Lee CK. Identification of optimal molecular size of modified Aloe polysaccharides with maximum immunomodulatory activity. Int Immunopharmacol, 2005; 5(2):271–9. CrossRef
Jeon W, Jeon YK, Nam MJ. Apoptosis by aloe-emodin is mediated through down-regulation of calpain-2 and ubiquitin-protein ligase E3A in human hepatoma Huh-7 cells. Cell Biol Int, 2011; 36(2):163–7. CrossRef
Joshy KS, Sharma CP, Kalarikkal N, Sandeep K, Thomas S, Pothen LA. Evaluation of in-vitro cytotoxicity and cellular uptake efficiency of zidovudine-loaded solid lipid nanoparticles modified with Aloe vera in glioma cells. Mater Sci Eng C, 2016; 66(9):40–50. CrossRef
Kambizi L, Afolayan AJ. Extracts from Aloe ferox and Withania somnifera inhibit Candida albicans and Neisseria gonorrhoea. Afr J Biotechnol, 2008; 7(1):012–5.
Kang MC, Kim SY, Kim YT, Kim E, Lee SH, Ko SC, Wijesinghe WAJP, Samarakoon KW, Kim YS, Cho JH, Jang HS, Jeon YJ. In vitro and in vivo antioxidant activities of polysaccharide purified from Aloe vera (Aloe barbadensis) gel. Carbohydr Polym, 2014; 99(1):365–71. CrossRef
Kato Y, Hou K, Saga Y, Yamaguchi S, Yachiku S, Kawakami N. Ammonium acid urate stone due to laxative abuse: a case report. Hinyokika Kiyo, 2004; 50(11):799–803.
Kaur M, Singh J, Kamboj SS, Saxena K. Purification and characterization of a lectin from leaf pulp of Aloe vera (L.) BURM. F. J Pharm Res, 2011; 4(7):2441–6.
Kim HS, Kacew S, Lee BM. In vitro chemopreventive effects of plant polysaccharides (Aloe barbadensis miller, Lentinus edodes, Ganoderma lucidum and coriolus versicolor). Carcinogenesis, 1999; 20(8):1637–40. CrossRef
Kitture R, Ghosh S, More PA, Date K, Gaware S, Datar S, Chopade BA, Kale SN. Curcumin-loaded, self-assembled Aloe vera template for superior antioxidant activity and trans-membrane drug release. J Nanosci Nanotechnol, 2015; 15(6):4039–45. CrossRef
Lee KY, Park JH, Lee YJ, Lee SK, Chung MH, Park YI, Kim KW, Lee YJ, Lee SK. Aloesin up-regulates cyclin E/CDK2 kinase activity via inducing the protein levels of cyclin E, CDK2, and CDC25A in SK-HEP-1 cells. IUBMB Life, 1997; 41(2):285–92. CrossRef
Liu C, Leung MYK, Koon JCM, Zhu LF, Hui YZ, Yu B, Fung KP. Macrophage activation by polysaccharide biological response modifier isolated from Aloe vera L. var. chinensis (Haw.) Berg. Int Immunopharmacol, 2006; 6(11):1634–41. CrossRef
Loots DT, Pieters M, Islam MS, Botes L. Antidiabetic effects of Aloe ferox and Aloe greatheadii var. Davyana leaf gel extracts in a low-Dose streptozotocin diabetes rat model. S Afr J Sci, 2011; 107(7–8):46–51. CrossRef
Lu ZQ, Deng YJ, Lu JX. Effect of aloe polysaccharide on caspase-3 expression following cerebral ischemia and reperfusion injury in rats. Mol Med Rep, 2012; 6(2):371–4. CrossRef
Maan AA, Nazir A, Khan MKI, Ahmad T, Zia R, Murid M, Abrar M. The therapeutic properties and applications of Aloe vera: a review. J Herb Med, 2018; 12(6):1–10. CrossRef
Mabona U, Van Vuuren SF. Southern African medicinal plants used to treat skin diseases. S Afr J Bot, 2013; 87:175–93. CrossRef
Manvitha K, Bidya B. Aloe vera: a wonder plant its history, cultivation and medicinal uses. J Pharmacogn Phytochem, 2014; 2(25):85–8.
Maurya DK, Devasagayam TPA. Antioxidant and prooxidant nature of hydroxycinnamic acid derivatives ferulic and caffeic acids. Food Chem Toxicol, 2010; 48(12):3369–73. CrossRef
Misawa E, Tanaka M, Nomaguchi K, Yamada M, Toida T, Takase M, Iwatsuki K, Kawada T. Administration of phytosterols isolated from Aloe vera gel reduce visceral fat mass and improve hyperglycemia in Zucker diabetic fatty (ZDF) rats. Obes Res Clin Pract, 2008; 2(4):239–45. CrossRef
Mothana RA, Lindequist U, Gruenert R, Bednarski PJ. Studies of the in vitro anticancer, antimicrobial and antioxidant potentials of selected Yemeni medicinal plants from the Island Soqotra. BMC Complement Altern Med, 2009; 9:7; doi:10.1186/1472-6882-9-7 CrossRef
Mwale M, Masika PJ. In vivo anthelmintic efficacy of Aloe ferox, agave sisalana, and gunnera perpensa in village chickens naturally infected with Heterakis gallinarum. Trop Anim Health Prod, 2014; 47(1):131–8. CrossRef
Naqvi S, Ullah MF, Hadi SM. DNA degradation by aqueous extract of Aloe vera in the presence of copper ions. Indian J Biochem Biophys, 2010; 47(3):161–5.
Narayanan V, Gnanavel I, Campus G, Care L, Remedies P. Study on the analysis of trace elements in Aloe vera and its biological importance. J Appl Sci Res, 2007; 3(14):1476–8.
Ni Y, Turner D, Yates KM,Tizard I. Isolation and characterization of structural components of Aloe vera L. leaf pulp. Int Immunopharmacol, 2004; 4(14)1745–55. CrossRef
Noor A, Gunasekaran S, Soosai Manickam A, Vijayalakshmi MA. Antidiabetic activity of Aloe vera and histology of organs in streptozotocin-induced diabetic rats. Curr Sci, 2008; 94(8):1070–6.
Noor A, Gunasekaran S, Vijayalakshmi MA. Improvement of insulin secretion and pancreatic β-cell function in streptozotocin-induced diabetic rats treated with Aloe vera extract. Pharmacognosy Res, 2017; 9(5):99–104. CrossRef
Pan Q, Pan H, Lou H, Xu Y, Tian L. Inhibition of the angiogenesis and growth of Aloin in human colorectal cancer in vitro and in vivo. Cancer Cell Int, 2013; 13(1):69–78. CrossRef
Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev, 2009; 2(5):270–8. CrossRef
Park MY, Kwon HJ, Sung MK. Dietary aloin, aloesin, or aloe-gel exerts anti-inflammatory activity in a rat colitis model. Life Sci, 2011; 88(11–12):486–92. CrossRef
Pereira GG, Santos-Oliveira R, Albernaz MS, Canema D, Weismüller G, Barros EB, Magalhães L, Lima-Ribeiro MHM, Pohlmann AR, Guterres SS. Microparticles of Aloe vera/vitamin E/chitosan: microscopic, a nuclear imaging and an in vivo test analysis for burn treatment. Eur J Pharm Biopharm, 2014; 86(2):292–300. CrossRef
Pérez YY, Jiménez-Ferrer E, Zamilpa A, Hernández-Valencia M, Alarcón-Aguilar FJ, Tortoriello J, Román-Ramos R. Effect of a polyphenol-rich extract from Aloe vera gel on experimentally induced insulin resistance in mice. Am J Chin Med, 2007; 35(6):1037–46. CrossRef
Picchietti S, Bernini C, Belardinelli MC, Ovidi E, Taddei AR, Guerra L, Abelli L, Fausto AM. Immune modulatory effects of Aloe arborescens extract on the piscine SAF-1 cell line. Fish Shellfish Immunol, 2013; 34(5):1335–44. CrossRef
Pugh N, Ross SA, ElSohly MA, Pasco DS. Characterization of aloeride, a new high-molecular-weight polysaccharide from Aloe vera with potent immunostimulatory activity. J Agric Food Chem, 2001; 49(2):1030–4. CrossRef
Radha MH, Laxmipriya NP. Evaluation of biological properties and clinical effectiveness of Aloe vera: a systematic review. J Tradit Complement Med, 2015; 5(1):21–6. CrossRef
Rajasekaran S, Sivagnanam K, Ravi K, Subramanian S. Hypoglycemic effect of Aloe vera gel on streptozotocin-induced diabetes in experimental rats. J Med Food, 2004; 7(1):61–6. CrossRef
Rajasekaran S, Sivagnanam K, Subramanian S. Mineral contents of Aloe vera leaf gel and their role on streptozotocin-induced diabetic rats. Biol Trace Elem Res, 2005; 108(1–3):185–95. CrossRef
Reider N, Issa A, Hawranek T, Schuster C, Aberer W, Kofler H, Fritsch P, Hausen BM. Absence of contact sensitization to Aloe vera (L.) Burm. f. Contact Dermatitis, 2005; 53(6):332–4. CrossRef
Reynolds T, Dweck C. Aloe vera leaf gel: a review update. J Ethnopharmacol, 1999; 68(1–3):3–37. CrossRef
Ro JY, Lee BC, Kim JY, Chung YJ, Chung MH, Lee SK, Jo TH, Kim KH, Park YI. Inhibitory mechanism of aloe single component (alprogen) on mediator release in guinea pig lung mast cells activated with specific antigen-antibody reactions. J Pharmacol Exp Ther, 2000; 292(1):114–21.
Rumawas ME, McKeown NM, Rogers G, Meigs JB, Wilson PWF, Jacques PF. Magnesium intake is related to improved insulin homeostasis in the framingham offspring cohort. J Am Coll Nutr, 2006; 25(6):486–92. CrossRef
Saibuatong O, Phisalaphong M. Novo Aloe vera-bacterial cellulose composite film from biosynthesis. Carbohydr Polym, 2010; 79(2):455–60. CrossRef
Sandeep K, Yadav JP.Ethnobotanical and pharmacological properties of Aloe vera: a review. J Med Plants Res, 2014; 8(48):1387–98.
Silva MA, Trevisan G, Klafke JZ, Rossato MF, Walker CIB, Oliveira SM, Silva CR, Boligon AA, Flores FC, Silva CB, Athayde ML, Ferreira J. Antinociceptive and anti-inflammatory effects of Aloe saponaria haw on thermal injury in rats. J Ethnopharmacol, 2013; 146(1):393–401. CrossRef
Silva SS, Oliveira MB, Mano JF, Reis RL. Bio-inspired Aloe vera sponges for biomedical applications. Carbohydr Polym, 2014; 2(4):264–70. CrossRef
Siritapetawee J, Sojikul P, Soontaranon S, Limphirat W, Thammasirirak S. A protein from Aloe vera that inhibits the cleavage of human fibrin(ogen) by plasmin. Appl Biochem Biotechnol, 2013; 170(8):2034–45. CrossRef
Speranza G, Manitto P, Cassara P, Monti D. Feralolide, a dihydroisocoumarin from cape aloe. Phytochemistry, 1993; 33(1):175–8. CrossRef
Suganya S, Venugopal J, Ramakrishna S, Lakshmi BS, Dev VRG. Naturally derived biofunctional nanofibrous scaffold for skin tissue regeneration. Int J Biol Macromol, 2014; 68(7):135–43. CrossRef
Tabandeh MR, Oryan A, Mohammadalipour A. Polysaccharides of Aloe vera induce MMP-3 and TIMP-2 gene expression during the skin wound repair of rat. Int J Biol Macromol, 2014; 65:424–30. CrossRef
Takahashi M, Kitamoto D, Asikin Y, Takara K, Wada K. Liposomes encapsulating Aloe vera leaf gel extract significantly enhance proliferation and collagen synthesis in human skin cell lines. J Oleo Sci, 2009; 58(12):643–50. CrossRef
Tanaka M, Misawa E, Ito Y, Habara N, Nomaguchi K, Yamada M, Toida T, Hayasawa H, Takase M, Inagaki M, Higuchi R. Identification of five phytosterols from Aloe vera gel as anti-diabetic compounds. Biol Pharm Bull, 2006; 29(7):1418–22. CrossRef
Tian B, Hua Y. Concentration-dependence of prooxidant and antioxidant effects of aloin and aloe-emodin on DNA. Food Chem, 2005; 91(3):413–8. CrossRef
Ulbricht C, Armstrong J, Basch E, Basch S, Bent S, Dacey C, Dalton S, Foppa I, Giese N, Hammerness P, Kirkwood C, Sollars D, Tanguay-Colucci S, Weissner W. An evidence-based systematic review of Aloe vera by the natural standard research collaboration. J Herb Pharmacother, 2008; 7(3–4):279–323. CrossRef
van Wyk BE, van Rheede van Oudtshoorn MC, Smith GF. Geographical variation in the major compounds of Aloe ferox leaf exudate. Planta Med, 1995; 61(3):250–3. CrossRef
Veitch NC, Simmonds MSJ, Blaney WM, Reynolds T. A dihydroisocoumarin glucoside from Aloe hildebrandtii. Phytochemistry, 1994; 35(5):1163–6. CrossRef
Wu JH, Xu C, Shan CY, Tan RX. Antioxidant properties and PC12 cell protective effects of APS-1, a polysaccharide from Aloe vera var. chinensis. Life Sci, 2006; 78(6):622–30. CrossRef
Yagi A, Hegazy S, Kabbash A, Wahab EAE. Possible hypoglycemic effect of Aloe vera L. high molecular weight fractions on type 2 diabetic patients. Saudi Pharm J, 2009; 17(3):209–15. CrossRef
Yagi A, Kabash A, Mizuno K, Moustafa SM, Khalifa TI, Tsuji H. Radical scavenging glycoprotein inhibiting cyclooxygenase-2 and thromboxane A2 synthase from Aloe vera gel. Planta Med, 2003; 69(3):269–71. CrossRef
Yates KM, Rosenberg LJ, Harris CK, Bronsta DC, King GK, Biehle GA, Walker B, Ford CR, Hall JE, Tizard IR. Pilot study of the effect of acemannan in cats infected with feline immunodeficiency virus. Vet Immunol Immunopathol, 1992;35(1–2):177–89. CrossRef
Yen GC, Duh P Der, Chuang DY. Antioxidant activity of anthraquinones and anthrone. Food Chem, 2000; 70(4):437–41. CrossRef
Yimam M, Zhao J, Corneliusen B, Pantier M, Brownell L, Jia Q. Blood glucose lowering activity of aloe based composition, UP780, in alloxan induced insulin dependent mouse diabetes model. Diabetol Metab Syndr, 2014;6(1):61–79. CrossRef