INTRODUCTION
Diabetic neuropathy (DN) is well characterized by a range of symptoms and is a universal impediment of diabetes, which affects diabetic subjects across the globe (Berezin, 2016; Kaur et al., 2017; Singh et al., 2013, 2014). It has been reported that by 2030, the total number of patients affected by diabetes mellitus will rise to 350 million, which shows an alarming trend for the society. DN is a serious complication which results from persistent and chronic hyperglycemic conditions in vascular fluid (Kaur et al., 2017). The pathophysiology of DN is critical due to multi-factorial underlying causes. The fatal effects of chronic and long-lasting hyperglycemia are widely expected to play an essential role in the progression of DN (Kaur et al., 2017; Singh et al., 2013, 2014, 2015). DN may be shown in various forms, including sensory, focal/multifocal, and autonomous neuropathies. Patients often feel basic dazzling, tingling, or “electrical” discomfort. Moreover, sometimes the pain may get worse at night in patients (Barrett et al., 2017; Boulton et al., 2005) and the physical examination of patients revealed sensory loss to light touch, vibration, and temperature. There are several clinically approved drugs for treating neuropathy, but they provide only symptomatic relief.
The occurrence of symptoms and signs of peripheral nerve damage in diabetics has been identified as DN. About one-third of diabetic peripheral neuropathy patients and about 20% of all diabetic patients have painful signs, such as burning, tingling, and lancing. DPN begins at the toes and moves proximally gradually. When developed in the lower limbs, the sensory impairment is based on the traditional distribution pattern known as ’glove and stocking’ (Singh et al., 2013).
Chronic hyperglycemia is the principal inducer responsible for numerous cellular and biochemical changes during the progression of DN (Kishore et al., 2018; Singh et al., 2015). Hyperglycemia elicits plentiful biochemical pathways, viz. activation of the polyol pathway, mitogen-activated protein kinase pathway, formation of advanced glycation end products (AGEs) with subsequent activation of AGEs, and inducible nitric oxide receptor (Kishore et al., 2018; Oates, 2002; Singh et al., 2015; Smith, 2012; Toth et al., 2008). Excessive stimulation of the polyol pathway is deleterious, primarily by increasing the yield of cofactors such as reduced nicotinamide adenine dinucleotide phosphate and nicotinamide adenine dinucleotide (NAD+), which leads to a reduced level of glutathione as well as increased AGEs formation, activation of protein kinase-C (PKC), and diacylglycerol pathway (Adisakwattana et al., 2012; Singh et al., 2014). Reactive oxygen species (ROS) acts as an intermediary factor and is implicated in all pathways and further activates several downstream signaling pathways like mitogen-activated protein kinases and nuclear factor kappa B (Singh et al., 2015). Persistent activation of these signaling pathways further engages them in the oxidation of various cell components like proteins, lipids, and nucleic acids (Kaur et al., 2017; Singh et al., 2013, 2014, 2015).
There is continuous damage to the nerve fiber which augments pain, paraesthesia, and loss of sensation. Various mechanisms have been documented in favor of the development of painful DN, and these include oxidative stress, AGEs formation, increased activity of the polyol pathway, myoinositol exhaustion, decline in Na+/K+ATPase activity, scarcity of neurotrophin-3, and insulin-like growth factor, along with altered axonal transport and poly[ADP-ribose] polymerase overactivation (Kuwabara et al., 2008; Singh et al., 2015).
Although several drugs have been successfully investigated for diabetes neuropathy treatment in the past few decades, no drug has been identified as a gold standard for the treatment of DN due to its high side effects profile. Moreover, studies have shown that long-term use of herbal medicines appears to be effective in the management of DN. Curculigo orchioides (CO) which belongs to family Amaryllidaceae is widely used in traditional medicine with powerful remedies for cough treatment. The rhizomes of CO are reported to possess anti-diabetic, antioxidant, immunostimulant, aphrodisiac, and hepatoprotective activity (Thakur et al., 2012). CO is a rich source of active phytochemicals like flavonoids, polyphenols, and many more; till date, several reports have stated that as many as 46 active ingredients were isolated from CO and plenty of them show some extent of activity or produces synergistic effects in various diseases (Thakur et al., 2012). Thus, due to its richness in various phytoconstituents, different extracts of these plants are expected to act as a potential strategy to treat diabetes and its related complications. Therefore, the current experimental work aimed at exploring the neuroprotective potential of the hydroalcoholic extract of CO and the ethanolic extract of CO in streptozotocin–nicotinamide (STZ–NAD)-induced DN.
MATERIALS AND METHODS
Drugs, chemicals and reagents
STZ and NAD were purchased from Sigma-Aldrich, India, and Finar India Ltd., India, respectively. All reagents and chemicals used were of empirical grade.
Experimental animals
Inbred Wistar rats of either sex (age: 8–12 months; weight: 200–300 gm) were obtained from the animal house of Chitkara College of Pharmacy, Rajpura, Punjab, India. The rats were accommodated in groups of six in polypropylene cages filled with husk. The experimental protocol was evaluated and approved by the Institutional Animal Ethics Committee (IAEC) under registration number IAEC/CCP/18/PR-008 and the experimental work was executed in harmony with the guiding principles set by Committee for the Purpose of Control and Supervision of Experiments on Animals, Ministry of Environment and Forests, Government of India.
Collection and identification of plant
Rhizomes of C. orchioides were collected from the local market of Patiala and authenticated under Voucher no. 1623 from the Department of Botany, Sri-Venkateshwara University, Tirupati, Andhra Pradesh, India.
Preparation of extract
The rhizomes of the C. orchioides plant were dried under shade for a period of 15 days and then powdered. Approximately, 1 kg of powdered plant material was proceed using ethanol in the fraction of 1:2 (w/v) and then kept at room temperature for 15 hours. Furthermore, the suspension was filtered with Whatman filter (paper no. 4) and extracted with different solvents by increasing the order of polarity. Finally, the extracts were again filtered and then dried under vacuum. Crude extracts were dissolved in distilled water and used for further pharmacological study. The mass was then weighed and recorded and the percentage of yield was calculated. The weight of the dried crude extract obtained was approximately 1.5 g which was observed with the percentage yield of 15.5%.
Phytochemical screening
Phytochemical analysis of plant extracts was carried out as the method mentioned in Evans (2002), with a slight modification, for the identification of various phytoconstituents present in the ethanolic and hydroalcoholic extracts.
Induction of DN
DN was induced in rats by a single injection of the freshly prepared solution of STZ (65 mg/kg, i.p.) in citrate buffer, 15 minutes after the single injection of NAD (230 mg/kg, i.p.) (Kishore et al., 2018). After 72 hours of STZ injection, the fasting blood glucose (FBG) level was checked to confirm the development of diabetes. The inclusion criterion for rats in the study was an FBG level ≥ 250 mg/dl. Three different doses (i.e. 150, 300, and 600 mg/kg) of the CO ethanolic extracts (COEE) and CO hydroalcoholic extracts (COHAE) were selected based on previous reports of acute toxicity and pilot studies (Anandakirouchenanea et al., 2013; Madhavan et al., 2007). The symptoms of DN in rats typically develop after 7–9 weeks of STZ administration; so, keeping this in view, the treatment of CO extracts was started from the 60th day and continued up to the 90th day (Shaikh and Somani, 2010).
In-vitro antioxidant activity estimation
2, 2-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity
DPPH assay was performed for measuring the percentage of free radical scavenging activity of ethanolic and hydroalcoholic extracts of CO as per the method described by Shimada et al. (1992) and Kaur et al. (2016).
Hydrogen peroxide scavenging activity
The H2O2 scavenging action was performed for measuring the free radical scavenging activity of ethanolic and hydroalcoholic extracts of CO as per the method described by Ruch et al. (1989) and Kaur et al. (2016).
Reducing power assay
The reducing power assay is usually carried out to confirm the presence of reductions in the plant extract, which breaks the free radical string and thereby produces an antioxidant effect. The reducing power analysis was performed as per the scheme explained earlier (Kaur et al., 2016; Oyaizu et al., 1986).
Superoxide radical scavenging activity
The estimation of superoxide radical scavenging action was performed as per the procedure described by Sabu and Ramadasan (2002).
Experimental animals
Animals were randomly divided into nine different experimental groups (n = 6 in each group), where n is the number of animals in each group.
Group 1 Vehicle control (Saline + double distilled water)
Group 2 Diabetic control (STZ–NAD treated)
Group 3 Group 3 COEE 150 mg/kg + (STZ 65 mg/kg and NAD 230 mg/kg)
Group 4 COEE 300 mg/kg + (STZ 65 mg/kg and NAD 230 mg/kg)
Group 5 COEE 600 mg/kg + (STZ 65 mg/kg and NAD 230 mg/kg)
Group 6 COHAE 150 mg/kg + (STZ 65 mg/kg and NAD 230 mg/kg)
Group 7 COHAE 300 mg/kg + (STZ 65 mg/kg and NAD 230 mg/kg))
Group 8 COHAE 600 mg/kg + (STZ 65 mg/kg and NAD 230 mg/kg)
Group 9 Gabapentin 30 mg/kg; i.p. + (STZ 65 mg/kg and NAD 230 mg/kg)
CO extracts were evaluated for their effect on DN at different doses of 150, 300, and 600 mg/kg per oral (p.o.) and Gabapentin 30 mg/kg (i.p.). CO doses were chosen on the basis of prior acute toxicity trials demonstrating that CO extracts were practically non-toxic for oral use by rodents (Madhavan et al., 2007).
Assessment of body weight, food consumption, water intake, glucose level, and insulin level of DN rats
Body weight, food consumption (in terms of gm), and water intake (in terms of ml) of each animal was measured before administration of STZ. All three parameters were assessed routinely before the analysis was completed. After 3 days of STZ administration, glucose level was measured in order to observe the development of diabetes. In addition, FBG level was measured on 1st, 30th, 60th, and 90th day using commercially available enzymatic kits. Additionally, insulin level was determined on the 90th day using enzyme-linked immunosorbent assay kits.
Assessment of thermal hyperalgesia in DN rats
Tail immersion test
Hyperalgesia was indicated by a reduced reaction time for tail withdrawal. The test was performed as per the protocol described by Kaur et al. (2016) and Kishore et al. (2018). The reaction time was determined before and periodically after 30 minutes, 1, 2, and 3 hours of oral administration of the standard and test compound.
Hot-plate test
The latency was recorded before and after 30 minutes, 1, 2, and 3 hours following oral administration of the standard and test compound. The test was performed as per the protocol described by Inytska et al. (2006).
Assessment of mechanical hyperalgesia in DN rats
Randall-Selitto analgesiometer
Randall-Selitto analgesiometer (Ugo Basile, East Lyme, CT) was used to determine the hyperalgesia state expressed as grams. The experiments were repeated 5 times at 15–minutes intervals (Kishore et al., 2018)
Assessment of allodynia
Von Frey filaments (0.4–64 g) were used to assess the hind paw withdrawal threshold in experimental animals. The rats were placed in restraining cages with a mesh floor and the filaments were applied in an upright direction on the paw surface with adequate power to curve the string for a time period of 5 seconds. Pulling out of paw from the string was considered as an optimistic reaction (Morani and Bodhankar, 2008).
Motor nerve conduction velocity (MNCV) in DN rats
The MNCV was evaluated in the animals as per the procedure given by Greene et al. (1982).
Biochemical assessment in sciatic nerve
After measurement of MNCV, the animals were sacrificed and the sciatic nerve was removed. The sciatic nerve was minced and homogenized in 10% (w/v) phosphate buffer saline and centrifuged at speed of 1,000 × g for 10 minutes at 4°C. The supernatant was separated out and stored in the refrigerator until the assessment of thiobarbituric acid reactive substances (TBARS) and antioxidant enzymes (Ohkawa et al., 1979). The reduced glutathione (GSH) level was estimated by using the method described by Ellman (1959). Superoxide dismutase (SOD) activity was measured by the method described by Misra and Fridovich (1972). The estimation of AGEs in the sciatic nerve homogenate was carried out as per the method described by Sensi et al. (1996) and Kishore et al. (2018).
Histopathological studies of the sciatic nerve of DN rats
The effects of COEE and COHAE on nerve tissues of DN rats were investigated by hematoxylin-eosin staining of transverse section of the sciatic nerve (X = 100) (Kishore et al., 2018).
Statistical analysis
Data were expressed as mean ± SEM. The data were analyzed using Sigma-stat software. For statistical analysis, one-way analysis of variance (ANOVA) was followed by Tukey’s post hoc multiple comparison test p < 0.05 was considered to be statistically significant.
RESULTS
Phytochemical screening
The phytochemical analysis of the plant extracts revealed the presence of alkaloids, flavonoids, phenols, steroids, saponins, carbohydrates, glycosides, fixed oils, proteins, and terpenoids.
In-vitro antioxidant activity
In-vitro antioxidant capacity of COEE and COHAE was assessed by DPPH assay, H2O2 scavenging activity, reducing power assay, and superoxide anion scavenging assay. Both the extracts showed a marked scavenging activity against DPPH radical. The effect of COEE and COHAE was compared with ascorbic acid, which was used as a positive control. The IC50 value of ascorbic acid was 2.29 µg/ml, whereas the IC50 values of COEE and COHAE were found to be 3.83 and 3.3 µg/ml, respectively. In addition, COEE and COHAE possess strong H2O2 scavenging activity. The IC50 values of COEE and COHAE were found to be 117.11 and 87.03 µg/ml, respectively, whereas the IC50 value of ascorbic acid was 59.53 µg/ml. Furthermore, in reducing power assay, EC50 (effective concentration) was found to be 70 and 75 µg/ml for COEE and COHAE, respectively. Superoxide anion is a powerful free radical which holds the capacity to produce direct cytotoxic damage on cell and DNA. A decrease in the absorbance with sample mixture points toward the scavenging of superoxide anion. In the present study, the IC50 of COEE and COHAE were observed to be 201.9 and 187 µg/ml, respectively (Table 1).
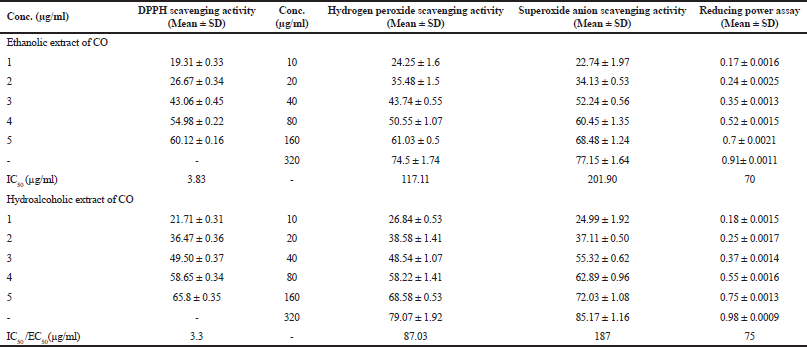 | Table 1. In vitro antioxidant activity of Curculigo orchioides ethanolic and hydroalcoholic extracts. [Click here to view] |
Effect of CO on body weight, feed and water intake, glucose level, and insulin level in DN-induced rats
Diabetic control rats exhibited a significant (p < 0.05) decline in body weight on days 60 and 90 after STZ administration. The administration of COEE and COHE at selected doses significantly increased body weight in a dose-dependent manner. Polydipsia (increase in water intake) and polyphagia (increase in food intake) were significantly elevated in diabetic control rats when compared to normal control rats. However, oral administrations of COEE and COHE (150, 300, and 600 mg/kg) and Gabapentin (30 mg/kg) for 30 days significantly (p < 0.05) attenuated the water intake and food intake as compared to diabetic rats (Table 2).
Moreover, diabetic control rats showed a marked (p < 0.05) increase in serum glucose level, whereas a decrease in serum insulin level in diabetic rats as compared to normal control rats. The administration of COEE and COHE dose dependently (p < 0.05) reduced the serum glucose level in diabetic rats. Moreover, the administration of COEE and COHE also dose dependently (p < 0.05) increased the serum insulin level (Table 2).
Effect of CO on oxidative stress in the sciatic nerve of DN induced rats
The levels of TBARS and AGEs were significantly elevated, whereas the SOD and GSH levels were significantly (p < 0.05) decreased in the sciatic nerve supernatant of diabetic control rats in comparison to normal control rats. The administration of COEE and COHAE (150, 300, and 600 mg/kg) and Gabapentin (30 mg/kg) for 30 days significantly (p < 0.05) reduced the level of TBARS and AGEs, whereas it significantly (p < 0.05) improved the antioxidant enzyme levels (SOD and GSH) as compared to diabetic control rats (Table 3).
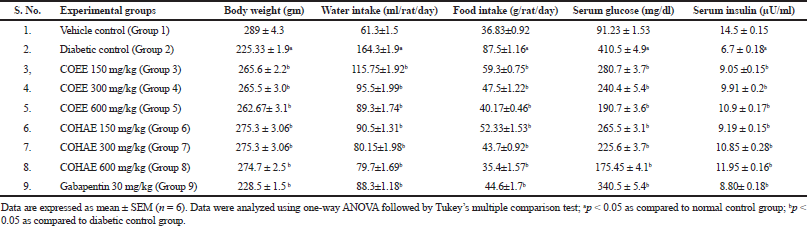 | Table 2. Effect of Curculigo orchioides ethanolic and hydroalcohlic extracts of on body weight, water intake, food intake, serum glucose, and serum insulin. [Click here to view] |
.png) | Table 3. Effect of Curculigo orchioides ethanolic and hydroalcohlic extracts on oxidative stress parameters in the sciatic nerve. [Click here to view] |
Effect of CO on anti-glycation activity in the sciatic nerve of DN-induced rats
A marked reduction in AGEs production (93.37%) was found against fructose-induced glycated bovine serum albumin. After incubation period of 3 weeks, the percentage reductions in AGEs by COEE and COHAE (50–500 μg/ml) were observed 75%–84% and 59%–97%, respectively (Fig. 1).
Effect of CO on mechanical hyperalgesia in DN-induced rats
Mechanical hyperalgesia was observed on the 90th day of study, with the help of Randall-Sellito and Von Fray filaments. Paw and tactile withdrawal thresholds were reduced in diabetic animals significantly (p < 0.05) when compared with normal control rats. Oral administration of ethanolic and hydroalcoholic CO extracts (150, 300, and 600 mg/kg) and Gabapentin (30 mg/kg, i.p.) for 30 days significantly (p < 0.05) augmented the paw withdrawal threshold as compared to diabetic control group (Fig. 2a,b).
Effect of CO on thermal hyperalgesia in DN-induced rats
Diabetic rats showed a marked decline in nociceptive pain threshold as compared to normal control group. Treatment with the COEE and COHAE (150, 300, and 600 mg/kg, p.o.) and Gabapentin (30 mg/kg, i.p.) for 30 days significantly (p < 0.05) and dose dependently increased the pain threshold in comparison to diabetic control group (Fig. 3a,b).
Effect of CO on change in MNCV in DN-induced rats
A significant decrease in MNCV was found in diabetic control rats when compared to normal control rats. Treatment with both extracts at selected doses (150, 300, and 600 mg/kg, p.o.) and Gabapentin (30 mg/kg, i.p.) for 30 days significantly (p < 0.05) improved the conduction velocity in motor nerve in dose-dependent manner when compared to diabetic control group (Fig. 4).
Histological studies
To study the effect of CO on the sciatic nerve tissue of DN-induced rats
In vehicle control, microscopic investigations showed the normal structure and morphology of the nerve with intact cells and no change in myelin sheath was also observed in the control group. In DN control group, necrotic and degenerative changes were observed in nerve cells with severe edema and prominent degeneration of the myelin sheath. Moreover, prominent infiltration of inflammatory cells was also observed. Besides this, in the positive control group, no necrotic changes were observed in nerve cells, except mild edema. Moreover, few scattered inflammatory cells are also seen in the sciatic nerve. In the extracts treatment groups, COEE and COHAE extracts at doses of 600 mg/kg showed minor focal loss of myelin sheath and a few scattered inflammatory cells in the sciatic nerve (Fig. 5). About 600 mg/kg doses of C. orchioides extracts produced more significant changes in the structural architecture of the neuron as compared to STZ diabetic control rats.
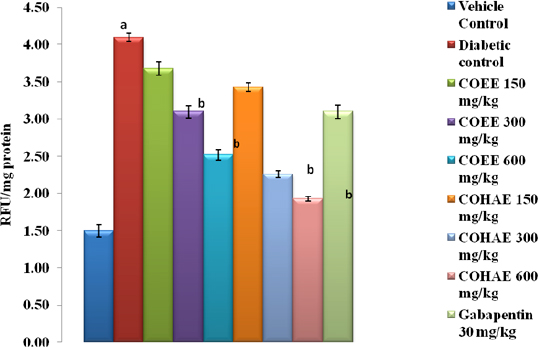 | Figure 1. Effect of ethanolic and hydroalcoholic extracts of Curculigo orchioides on AGEs in the sciatic nerve in DN rats. Data are expressed mean ± SEM; n = 6. Data were analyzed by using one-way ANOVA followed by Tukey’s multiple comparison test; ap < 0.05 as compared to vehicle control group; bp < 0.05 as compared to diabetic control group (on the 90th day). [Click here to view] |
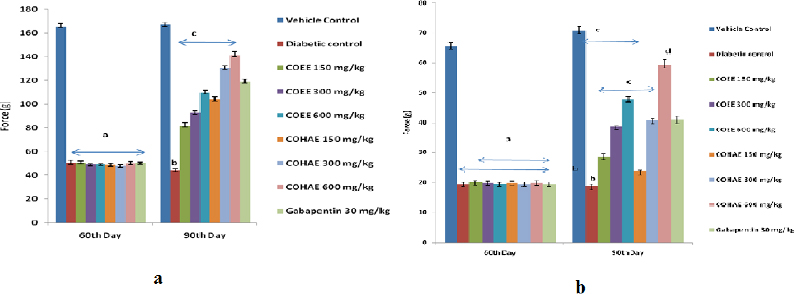 | Figure 2. (a) Effect of ethanolic and hydroalcoholic extracts of Curculigo orchioides on mechanical hyperalgesia in Randell-Selitto DN rats. Data are expressed mean ± SEM; n = 6. Data were analyzed by using one-way ANOVA followed by Tukey’s multiple comparison test; ap < 0.01 as compared to vehicle control group (on the 60th day); bp < 0.05 as compared to vehicle control group; cp < 0.05 as compared to diabetic control group (on the 90th day). (b) Effect of ethanolic and hydroalcoholic extracts of Curculigo orchioides on mechanical hyperalgesia in tactile allodynia in DN rats. Data are expressed mean ± SEM; n = 6. Data were analyzed by using one-way ANOVA followed by Tukey’s multiple comparison test; ap < 0.01 as compared to vehicle control group (on the 60th day); bp < 0.05 as compared to vehicle control group; cp < 0.05 as compared to diabetic control group (on the 90th day). [Click here to view] |
DISCUSSION
This study indicates that CO against experimentally induced peripheral neuropathy in diabetic rats has a neuroprotective capacity. Treatment with CO has shown improvement in mechanical and thermal hyperalgesia along with MNVC parameter as compared to DN control group. The pathophysiology of neuropathy is exemplified by a significant loss of nerve fibers (myelinated as well as non-myelinated), resulting in the augmentation of various signs and symptoms, for instance, excessive pain, loss of sensation, and paresthesia (Kishore et al., 2018; Morani and Bodhankar, 2008). The results of this study are supported by previous evidences which showed that morphologically, neuropathic pain is mainly characterized by degeneration, demyelination, and atrophy of axons of peripheral nerves (Singh et al., 2014). Studies have reported that excessive stimulation of several cellular signaling pathways, for instance, polyol pathways, free radical generation, amino acids glycation, as well as impaired PKC pathway, are principally accountable for loss of nerve fibers (Giacco and Brownlee, 2010; Morani et al., 2010; Singh et al., 2013, 2014; Xia et al., 1995).
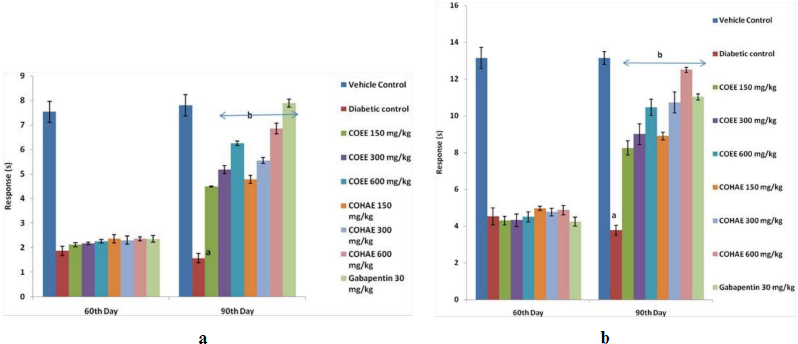 | Figure 3. (a) Effect of ethanolic and hydroalcoholic extracts of Curculigo orchioides on pain threshold in hot plate in DN rats. Data are expressed mean ± SEM; n = 6. Data were analyzed by using one-way ANOVA followed by Tukey’s multiple comparison test; ap < 0.05 as compared to vehicle control group; bp < 0.05 as compared to diabetic control group (on the 90th day). (b) Effect of ethanolic and hydroalcoholic extracts of Curculigo orchioides on pain threshold in tail immersion assays in DN rats. Data are expressed mean ± SEM; n = 6. Data were analyzed by using one-way ANOVA followed by Tukey’s multiple comparison test; ap < 0.05 as compared to vehicle control group; bp < 0.05 as compared to diabetic control group (on the 90th day). [Click here to view] |
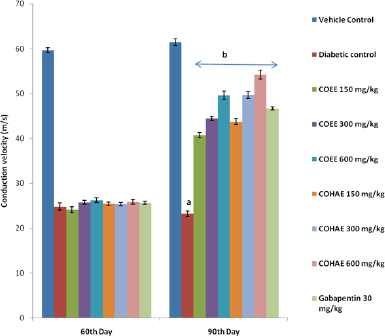 | Figure 4. Effect of ethanolic and hydroalcoholic extracts of Curculigo orchioides on motor nerve conduction velocity (MNCV) in DN rats. Data are expressed mean ± SEM; n = 6. Data were analyzed by using one-way ANOVA, followed by Tukey’s multiple comparison test; aP<0.05 as compared to vehicle control group; bP<0.05 as compared to diabetic control group (on the 90th day). [Click here to view] |
The pathophysiology of DN is well documented to engage numerous multifaceted factors, viz. constant hyperglycemia, free radical generation, oxidative stress, mitochondrial dysfunction, and dyslipidemia (Kumar et al., 2007). Numerous preclinical and clinical data stand in favor of the notion that the generation of ROS adversely affects diabetic subjects and the commencement of diabetic-related symptoms is usually or closely coupled with increased oxidative stress (Singh et al., 2015).
The disease begins from a series of alterations in microvascular, vascular, and neuronal compartments. In addition, AGEs and hexosamine pathways are documented to be directly engaged in altering the redox status of the cell through ROS and reduction of obligatory elements of glutathione reprocessing (Kaur et al., 2017; Singh et al., 2015). The results of the current studies are in accordance with previous studies’ results, which showed that STZ administration in rats produces severe cytotoxic action, resulting in oxidative stress and further leading to progress of diabetes and associated impediments (Kishore et al., 2018). In the current study, initiation of DN via STZ–NAD administration produced significant elevations in TBARS and AGEs.
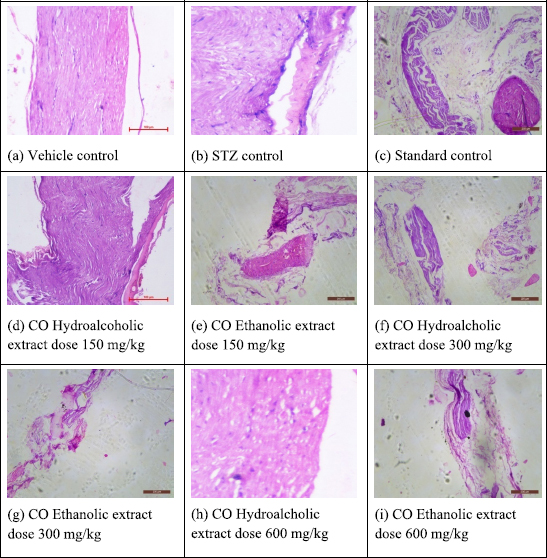 | Figure 5. Effect of ethanolic and hydroalcohlic extracts of Curculigo orchioides on the sciatic nerve in DN rats. [Click here to view] |
Oxidative stress-induced formation of AGEs may result from the interaction between carbohydrate molecule and free amino group of proteins (Stadtman et al., 2003). AGEs formation has been widely implicated in DN via the formation of various cytokines and growth factors, thereby producing serious diabetic complications (Vlassara et al., 2002). The current work reveals that both the extracts COEE and COHAE ameliorated STZ-induced oxidative harm in the sciatic nerve via escalating the antioxidant enzymes level and by decreasing the levels of TBARS and AGEs, thereby supporting the potential effect of plants in attenuating hyperglycemia-induced chronic oxidative stress and increasing the antioxidant level. Hyperglycemia is solely accountable for dwindling the level of antioxidant enzymes (SOD, catalase, and GSH) (Kumar et al., 2007; Palsamy et al., 2010; Singh et al., 2013, 2014). The probable explanation for excessive oxidative stress during diabetes may be due to glucose auto-oxidation, altered redox status, and diminished antioxidant levels (GSH and vitamin E) (Kumar et al., 2007; Palsamy et al., 2010).
A variety of functional deformities, like dwindling in nerve conduction velocity (of sensory and motor neurons) along with allodynia and hyperalgesia, have been reported to build up very early after the induction of hyperglycemia (Kishore et al., 2018). In the present study, signs of DN are well evidenced by mechanical and thermal hyperalgesia, allodynia, increased pain perception, and decreased MNCV. Previous studies have reported that thermal hyperalgesia emerges progressively because of chronic hyperglycemia-induced damage on small myelinated and unmyelinated fibers and ultimately cause a marked C-fiber loss (Lauria and Lombard, 2012; Singh et al., 2015). In the current study, the treatment of diabetic rats with COEE and COHAE effectively attenuated the sign of DN. Chronic administration of COEE and COHAE for 30 days produced a marked improvement in the level of aforementioned parameters.
It is extensively reported that plants rich in phytochemical constituents show potential antioxidant and anti-diabetic activities (Pradayna et al., 2012; Singh et al., 2013, 2014). Earlier study reported that phytochemical screening of rhizomes of CO revealed the presence of flavonoids, alkaloids, glycosides, saponins, phytosterols, curculigoside, glycosides, 2,6-dimethoxyl benzoic acid, curculigines, curculigol, and curculigo saponins (Wu et al., 2005). Theses active compounds alone or in combinations are responsible for antioxidant activity (Pradayna et al., 2012). Moreover, the observed antioxidant and hypoglycemic effect are in accordance with previous findings. This neuroprotective effect of CO may be due to the presence of phytoconstituents, like glycosides and flavonoids, which contribute to the potent antioxidant along with antiglycation and antidiabetic activities.
CONCLUSION
The results suggest that administration of different doses (150, 300, and 600 mg/kg) of ethanolic and hydroalcoholic extracts of the rhizomes of C. orchioides produced significant amelioration in progression and development of STZ–NAD-induced DN. This amelioration may be attributed to the presence of terpenoids and phenolic compounds (which are proven to possess significant activity in attenuation of diabetes and its complications) in the extracts of CO.
LIST OF ABBREVIATIONS
ALR: aldose reductase; AGEs: advanced glycation end products; CO: Curculigo orchioides; DM: diabetes mellitus; DN: diabetic neuropathy; FBG: fasting blood glucose; GSH: reduced glutathione; MDA: malondialdehyde; MAPK: mitogen-activated protein kinases; PKC: protein kinase C; RAGE: receptor for advanced glycation end products; ROS: reactive oxygen species; SOD: superoxide dismutase; STZ: streptozotocin; TBARS: thiobarbituric acid reactive substances; TG: triglycerides; TC: total cholesterol; VLDL: very low density lipoprotein. DDW: double distilled water; NAD: nicotinamide; COEE: Curculigo orchioides ethanolic extract; COHAE: Curculigo orchioides hydroalcoholic extract; i.p.: intraperitonial.
CONFLICT OF INTEREST
Authors declared that they do not have any conflicts of interest.
FUNDING
This study did not receive any funding from any agency.
AUTHORS’ CONTRIBUTION
Randhir Singh conceptualized and supervised the study. Krishan Singla wrote the original draft. Krishan Singla and Randhir Singh contributed to writing and editing this review article.
REFERENCES
Adisakwattana S, Sompong W, Meeprom A, Ngamukote S, Yibchok–Anum S. Cinnamic acid and its derivatives inhibit fructose mediated protein glycation. Int J Mol Sci, 2012; 13:1778–89. CrossRef
Anandakirouchenanea E, Chandiranb IS, Kadalmani B. An investigation on preliminary phytochemicaland safety profiles of methanolic root extract of Curculigo orchioides. J Pharm Res, 2013; 7:692–6. CrossRef
Barrett EJ , Liu Z, Khamaisi M, King GL , Klein R, Klein BEK , Hughes TM , Craft S, Freedman BI , Bowden DW , Vinik AI , Casellini CM. Diabetic Microvascular disease: an endocrine society scientific statement. J Clin Endocrinol Metab, 2017; 102(12):4343–410. CrossRef
Berezin A. Metabolic memory phenomenon in diabetes mellitus: achieving and perspectives. Diabetes Metab Syndr, 2016; 10(2):S176–83. CrossRef
Boulton AJM. Management of diabetic peripheral neuropathy. Clin Diabetes, 2005; 23(1):9–15. CrossRef
Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys, 1959; 82:70–7. CrossRef
Evans WC. Trease and evans textbook of pharmacognosy. 15th edition, Elsevier, Amsterdam, Netherlands, vol 47, pp 279,454, 2002.
Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res, 2010; 107(9):1058–70. CrossRef
Greene DA, Levis RA, Lattimer SA, Brown MJ. Selective effects of myo-inositol administration on sciatic and tibial motor nerve conduction parameters in the streptozotocin-diabetic rat. Diabetes, 1982; 31:573–8 CrossRef
Inytska O, Lyzogubov VV, Stevens MJ, Drel VR, Mashtaalir N, Pacher P. Poly (ADP-ribose) polymerase inhibition alleviates experimental diabetic sensory neuropathy. Diabetes, 2006; 55:1686–94. CrossRef
Kaur N, Kishore L, Singh R. Antidiabetic effect of new chromane isolated from Dillenia indica L. leaves in streptozotocin induced diabetic rats. J Funct Foods, 2016; 22:547–55. CrossRef
Kaur N, Kishore L, Singh R. Chromane isolated from leaves of Dillenia indica improves the neuronal dysfunction in STZ-induced diabetic neuropathy. J Ethnopharmacol, 2017; 206:19–30. CrossRef
Kishore L, Kaur N, Singh R. Effect of kaempferol isolated from seeds of Eruca sativa on changes of pain sensitivity in streptozotocin-induced diabetic neuropathy. Inflammopharmacology, 2018; 26:993–1003. CrossRef
Kumar A, Kaundal RK, Iyer S, Sharma SS. Effects of resveratrol on nerve functions, oxidative stress and DNA fragmentation in experimental diabetic neuropathy. Life Sci, 2007; 80:1236–44. CrossRef
Kuwabara S, Misawa S. Pharmacologic intervention in axonal excitability: in vivo assessment of nodal persistent sodium currents in human neuropathies. Curr Mol Pharmacol, 2008; 1:61–7. CrossRef
Lauria G, Lombardi R. Small fiber neuropathy: is skin biopsy the holy grail? Curr Diab Rep, 2012; 12:384–92. CrossRef
Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem, 1972; 247:3170–5.
Madhavan V, Joshi R, Murali A, Yoganarasimhan SN. Antidiabetic activity of Curculigo orchioides. Root Tuber. Pharm Biol, 2007; 45(1):18–21. CrossRef
Morani A, Bodhankar S. Neuroprotective effect of vitamin E acetate in models of mononeuropathy in rats. Neuroanatomy, 2008; 7:33–7.
Morani AS, Bodhankar SL. Early co-administration of vitamin E acetate and methylcobalamin improves thermal hyperalgesia and motor nerve conduction velocity following sciatic nerve crush injury in rats. Pharmacol Rep, 2010; 62:405–9. CrossRef
Oates Pj. Polyol pathyway and diabetic peripheral neuropathy. Int Rev Neurobiol, 2002; 50:325. CrossRef
Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem, 1979; 95:351–8. CrossRef
Oyaizu M. Studies on products of browning reactions: antioxidative activities of product of browning reaction prepared from glucosamine. Japan J Nutr, 1986; 44:307–15. CrossRef
Palsamy P, Sivakumar S, Subramanian S. Resveratrol attenuates hyperglycemia-mediated oxidative stress, proinflammatory cytokines and protects hepatocytes ultrastructure in streptozotocin-nicotinamide-induced experimental diabetic rats. Chem Biol Interact, 2010; 186(2):200–10. CrossRef
Pradayna O, Jitender B, Revan K. Evaluation of antioxidant activity of traditional formulation Giloy satva and hydroalcholic extrtact of Curculigo orchiodes. J Appl Pharm Sci, 2012: 02(06):209–13.
Ruch RJ, Cheng SJ, Klaunig JE. Carcinogenesis. Rely Chim Acta, 1989; 10:1003–8. CrossRef
Sabu MC, Ramadasan K. Anti-diabetic activity of medicinal plants and its relationship with their antioxidant property. J Ethnopharmacol, 2002; 81:155–60. CrossRef
Sensi M, Pricci F, Pugliese G, De Rossi MG, Petrucci AF, Cristina A, Morano S, Pozzessere G, Valle E, Andreani D. Role of advanced glycation end-products AGE in late diabetic complications. Diabetes Res Clin Pract, 1996; 28(1):289–17. CrossRef
Shaikh AS, Somani RS. Animal models and biomarkers of neuropathy in diabetic rodents. Indian J Pharmacol, 2010; 42:129–34. CrossRef
Shimada K, Fujikawa K, Yahara K, Nakamura T. Antioxidative properties of xanthone on the auto oxidation of soybean in cylcodextrin emulsion. J Agr Food Chem, 1992; 40:945–8. CrossRef
Singh R, Devi S, Gollen R. Role of free radical in atherosclerosis, diabetes and dyslipidemia: larger than life. Diab Metab Res Rev, 2015; 31:113–26. CrossRef
Singh R, Kaur N, Kishore L, Gupta GK. Management of diabetic complications: a chemical constituents based approach. J Ethnopharmacol, 2013; 150(1):51–70. CrossRef
Singh R, Kishore L, Kaur N. Diabetic peripheral neuropathy: current perspective and future directions. Pharmacol Res, 2014; 80:21–35. CrossRef
Smith AG, Singleton JR. Diabetic neuropathy. Continuum, 2012; 18:60–84. CrossRef
Stadtman ER, Levine RL.Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino acids, 2003; 25:207–18. CrossRef
Thakur M, Chauhan NS, Sharma V, Dixit VK, Bhargava S. Effect of Curculigo orchioides on hyperglycemia-induced oligospermia and sexual dysfunction in male rats. Int J Impot Res, 2012; 24:31. CrossRef
Toth C, Rong LL, Yang C, Matinez J, Song F, Ramji N. Receptor for advanced glycation end products RAGEs and experimental diabetic. Neuropathy Diabetes, 2008; 57:1002–17. CrossRef
Vlassara H, Cai W, Crandall J, Goldberg T, Oberstein R, Dardaine V, Peppa M, Rayfield EJ. Inflammatory mediators are induced by dietary glycotoxins, a major risk factor for diabetic angiopathy. Proc Natl Acad Sci U S A, 2002; 99(24):15596–601. CrossRef
Wu Q, Fu DX, Hou AJ, Lei GQ, Liu ZJ, Chen JK. Zhou TS. Antioxidative phenols and phenolic glycosides from Curculigo orchioides. Chem Pharm Bull (Tokyo), 2005; 53:1065–70. CrossRef
Xia P, Kramer RM, King GL. Identification of mechanism for inhibition of NA+ , K+, adenosine triphosphate by hyperglycemia involving activation protien kinase C and cytosolic phospholipase A2. J Clin Invest, 1995; 96:733–40. CrossRef