INTRODUCTION
Derivation of name and historical aspects
There are about 90 species of the genus Bulbine, which are found in the southern and western provinces of South Africa and Australia (Van Jaarsveld, 2017). Most of the species in the genus have found traditional medicinal applications for the treatment of different diseases that possibly arise from microbial infections (Bezabih et al., 1997), among them is Bulbine natalensis Baker. B. natalensis (synonym Bulbine latifolia (L.f.) Spreng.) is a member of the family Asphodelaceae and a predominant species in the genus (Van Wyk and Gericke, 2000). It is an indigenous succulent herb, widely dispersed in the eastern and northern provinces of South Africa, and locally known as ibhucu (Zulu), ingcelwane in Xhosa, broad-leaved Bulbine (English), and rooiwortel (Afrikaans) (Pather et al., 2011; Yakubu and Afolayan, 2010). The ability of the plant to display a distinct orange color whenever the lower stem is damaged and exposed to sunlight made the Afrikaans call it rooiwortel, meaning red root (Van Wyk et al., 1997). Bulbine is bulb-like in nature, and all the members of this genus are succulent herbs, many of which are used for horticultural purposes while others are widely cultivated, for instance, Bulbine frutescens (stalked Bulbine or rankkopieva) is a popular groundcover (Eggli, 2001). A conclusion has been drawn based on the chemotaxonomic investigation on the presence of knipholone and its derivatives in Bulbinella, Bulbine, and Kniphofia species, and it was concluded that these three genera form a monophyletic unit within the family Asphodelaceae (Yenesew et al., 1994).
Botanical description
Bulbine natalensis is a hardy, evergreen, drought-resistant, nonedible, perennial succulent herb with bright green, soft fleshy leaves that form the basal rosette (Van Jaarsveld, 2005). It is a perennial, fast-growing, succulent plant, whose appearance closely resembles the aloe species, forming solitary rosettes up to 20 cm high and possesses fleshy roots that are yellowish and circular in cross-section (Eggli, 2001). Bulbine natalensis has frost-tender, evergreen, perennial, broad, sharp-pointed, fleshy, yellowish green leaves that are triangular-lanceolate (190–400 × 30–60 mm), green with faint lines; the firm, ascending, older leaves become recurved; the upper surface is flat and slightly channelled toward the end; the lower surface is flat to a somewhat acute rounded margin, bearing a minute fringe of hairs (Eggli, 2001). Bulbine natalensis has clusters of star-shaped yellow flowers (7–12 mm in diameter) that are held in densely packed spikes at the ends of long, gracefully arching, flowering stems throughout the year as shown in Figure 1 (Eggli, 2001). The plant’s inflorescence consists of 1–4 densely flowered racemes that are 400–1,017 mm tall, with flower stalks that are 12–14 mm long and terete, and they flower in spring. Bulbine natalensis has six petals that are yellow, spread out, and 7 mm long with bearded stamens that differentiate it from Bulbinella (Eggli, 2001). The plants have 6 mm long styles and most species can reach a size of up to 60 cm (Eggli, 2001). Bulbine natalensis has pollen and nectar-rich flowers that attract pollinating insects to the garden, making it a worthwhile container plant for landscaping (Eggli, 2001; Van Jaarsveld, 2005).
Distribution and habitat
Bulbine natalensis is indigenous to South Africa, and is usually found in dry river valleys, rocky grassland, and gorges. The plant is generally dispersed in the southeastern province of South Africa (the KwaZulu Natal and Eastern Cape), and Knysna in the Western Cape (Eggli, 2001). Bulbine natalensis grows quickly, hence perfect for new gardens, and thrives well in shale and sandstone-based soils and on well-drained sites (Eggli, 2001; Van Jaarsveld, 2005). The species is widespread in hot areas, prefers rainfall from 600 to 1,000 mm per annum, and is well adapted to disturbances, such as grazing and trampling, as the plants regenerate easily from the seed (Eggli, 2001; Van Jaarsveld, 2005). The plant has pointed, thickset leaves that can reserve water, thus making it drought-tolerant and an ideal water-wise garden plant. Moreover, its ability to adapt to wind dispersal depends on the presence of the climbing inflorescence, with fruiting capsules and winged seed (Eggli, 2001).
Research methodology
The literature search was conducted from April 2019 to February 2020, where a mixed-method review approach, which included combining quantitative and qualitative research, was used to compile the review. An assessment of all the information on B. natalensis and a systematic and comprehensive literature search was conducted using electronic research-based databases, such as Scopus, ScienceDirect, PubMed, Google Scholars, books, and theses. Important words, such as taxonomy, botany, distribution, ethnobotanical uses, and biological and chemical properties, in relation to Bulbine plants were used for the search. A detailed appraisal of the existing knowledge and literature on B. natalensis morphology, ecology, distribution, and economic importance were discussed. Although B. natalensis are highly valuable, they face rapid depletion. It is, therefore, imperative to document the indigenous knowledge, medicinal use, and pharmacological properties of this plant.
 | Table 1. Distribution of anthraquinones in B. natalensis (Asphodelaceae). [Click here to view] |
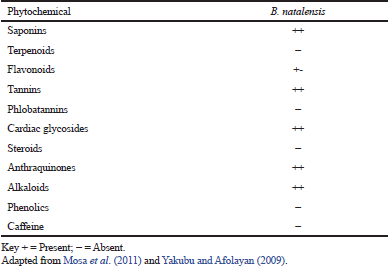 | Table 2. Phytochemical analysis of B. natalensis. [Click here to view] |
Ethnopharmacology and utilization
Chemical compounds
The phytochemical investigation has displayed many bioactive constituent(s) of plant extracts (Yakubu et al., 2007), and the first chemical analysis of the B. natalensis stem revealed the presence of chrysophanol, 10, 7′-bichrysophanol, knipholone, and isoknipholone (Van Wyk et al., 1995) as shown in Table 1. Similarly, the study of Nigussie (1999) revealed that the root extract of B. natalensis contained chrysophanol, knipholone anthrone, isoknipholone, knipholone, aloe-emodin, 8-hydroxy-1-methylnaphtho [2,3-c] furan- 4, 9- dione, and 5,8-dihydroxy-1-methylnaphtho[2,3-c] furan-4,9-dione. Research has shown that the B. natalensis tuber has some phytochemical constituents, such as saponins, anthraquinones, tannins, alkaloids, and cardiac glycosides (Table 2; Yakubu and Afolayan, 2009). Also, the study of Mosa et al. (2011) reported the total phenolic and flavonoid contents of the extracts of B. natalensis as shown in Table 3. Bulbine natalensis (rooiwortel) has a high content of sterols and sterolins and are inappropriately used in the treatment of HIV/AIDS in South Africa (Plessis-Stoman et al., 2009). Furthermore, an additional six compounds, namely Bulbnatalonoside A, Bulbnatalonoside B, Bulbnatalonoside C, Bulbnatalonoside D, Bulbnatalonoside E, and Bulbnatalone, have been isolated and characterized from the methanol extract of B. natalensis (Bae et al., 2019).
Traditional uses of B. natalensis
The infusions of the roots of B. natalensis and other species of Bulbine are used orally for the management of illnesses, such as diabetes, diarrhea, blood disorders, convulsions, nausea (vomiting), rheumatism, urinary complaints, and venereal diseases (Table 4) (Erasto et al., 2005; Oyedemi et al., 2009; Van Wyk, 2011). The leaves or leaf sap of B. natalensis is applied directly to the skin and conventionally it is used topically to stop bleeding, thus it is widely used in the management of wounds, cracked lips, cuts, grazes, itches, mosquito bites, rashes, ringworm, sores, and herpes (Pather et al., 2011; Van Wyk, 2011). Furthermore, the leaves sap or root is employed in the treatment of dermatophytosis, burns, venereal diseases, and assists in the healing of postoperative scars (Pujol, 1990). Since 1995, the leaf gels’ branded products were developed and commercially exploited, such as the Montagu Museum Bulbine Crème and the Bulb Aloe Levine products. In a clinical study of nearly 300 patients, a patented product consisting of microporous paper tape infused with a combination of bulbine gel (12.5%–25%) with panthenol and asiaticoside exhibited some wound healing properties and accelerated scar maturation (Widgerow et al., 2000). The healing effects are probably due to the presence of polysaccharides and/or glycoproteins in the leaf gel, as well as the hydrating effects (Widgerow et al., 2000). The gel of B. natalensis is frequently utilized by both traditional healers and the local population, as well as the European descendants, in South Africa for its wound healing properties, especially for the treatment of bruises, sunburn, fever blisters, and sores among others (Pather et al., 2011). Similarly, the gel from the fresh leaf can be applied directly onto the wound to help recovery or it can be used in the form of a warm poultice in traditional medicine, particularly for quick relief when used on stings and bites (Pather et al., 2011).
Antimicrobial activity
The steroids, saponins, and tannins of B. natalensis exhibited antibacterial properties (Oyekunle et al., 2006) through the inhibition of enzymes, iron deprivation, and reduction in oxidative phosphorylation (Parekh and Chanda, 2007). Yakubu and Quadri (2012) investigated the antimicrobial activity of different extracts of the B. natalensis tuber at concentrations ranging between 0.1, 0.5, 1.0, 5.0, and 10 mg/ml. Accordingly, the ethanolic extracts showed significant inhibition against all the Gram-negative bacteria tested with 75% of the bacterial strains inhibited; likewise, the n-butanol fraction repressed almost 87.5% of the bacteria at MIC ranging from 3 to 10 mg/ml. While the ethyl acetate fraction produced 100% growth inhibition against all the test organism at MIC of 1 and 5 mg/ml, the water extract produced no growth inhibition (Yakubu and Quadri, 2012). According to Parekh and Chanda (2007), the stronger the extraction capability of organic solvents the more range of phytoconstituents responsible for the observed antibacterial activity.
An in vivo study by Pather et al. (2011) on the effects of leaf gel extracted from B. frutescens and B. natalensis on wound healing in pigs showed notable effects. The leaf gel extracts of both B. frutescens and B. natalensis displayed a beneficial effect on collagen synthesis and on wound contraction, hence resulting in faster healing than in untreated wounds (Pather et al., 2011). The study of Coopoosamy (2011) reported that the acetone and ethyl acetate extracts of the leaf, root, and rhizome of B. natalensis had more antibacterial activities against both Gram-positive and Gram-negative bacteria tested when compared to the water extracts. The phytosterols, such as ergosterol, stigmasterol, and sitosterol, from the chloroform extract of B. natalensis showed that the antifungal potential is more fungistatic rather than fungicidal against Aspergillus flavus, Penicillium digitatum, and Fusarium verticilloides (Mbambo et al., 2012).
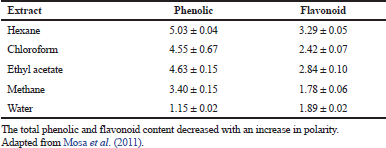 | Table 3. Total phenolic and flavonoid content of the extracts of B. natalensis (mg/g dry plant material). [Click here to view] |
 | Figure 1. Bubline natalensis. (A) Whole plant; (B) matured flower; and (C) root. Source: Top Tropicals (2020). [Click here to view] |
Stimulant effect
Due to the positive impact of B. natalensis on penile erection and sexual behavior parameters, the stem of this plant is extensively used in the treatment of male sexual dysfunctions (Ajao et al., 2019; Malviya et al., 2011; Yakubu and Afolayan, 2009). An ethnobotanical survey has documented the traditional uses of B. natalensis plants as an aphrodisiac which can be used to arouse sexual instinct, induce general desire, and increase pleasure and performance (Malviya et al., 2011). Bulbine natalensis is commonly called as “testosterone booster”, and this is presented in the study of Yakubu and Afolayan (2010), where the effect of aqueous stem extracts of B. natalensis and its anabolic and androgenic effects in male Wistar rats were investigated. An experiment of 60 rats was equally clustered into four (A–D), with group A being the control receiving 0.5 ml of distilled water and groups B–D was administered 0.5 ml of distilled water containing 25, 50, and 100 mg/kg of body weight of the extract (Yakubu and Afolayan, 2010). The findings revealed that, at doses 25 and 50 mg/kg body weight, there was a significant increase in the serum testosterone and luteinizing hormone concentrations. Moreover, the frequencies of mount, intromission, ejaculation, and ejaculatory latency in the rats increased (Carlos, 2020; Yakubu and Afolayan, 2010). Penile reflexes and copulatory performance of male rats were significantly enhanced, which may be attributed to the increase in serum testosterone concentration. Testosterone is considered to contribute to the improvement in sexual function, libido, and penile erection (Carlos, 2020; Gauthaman et al., 2002), thus B. natalensis is being sold as various brands of health supplements because of its effect on boosting the testosterone level in men (Hofheins et al., 2012; Patel, 2018; Widgerow et al., 2000). Bioactive agents, such as saponins and alkaloid, are responsible for aphrodisiac activity (Yakubu et al., 2005). While saponins enhance androgen production (Gauthaman et al., 2002), alkaloids increase the dilation of blood vessels in the sexual organs (JianFeng et al., 2012).
 | Table 4. Uses of B. natalensis. [Click here to view] |
Anti-platelet aggregation
Bulbine natalensis is mostly used by Zulu traditional healers in South Africa because of its notorious anti-platelet aggregation bioactivity and its aptitude to impede thrombin, ADP, and epinephrine-induced aggregation of platelets (Lazarus, 2012; Mosa et al., 2011). The extracts of B. natalensis exhibited a concentration-dependent anti-platelet aggregation activity, with the chloroform extract of B. natalensis exhibiting the highest activity (IC50 of 0.43mg/ml), which was observed on the epinephrine-induced platelet aggregation (Mosa et al., 2011). A previous study by Mosa et al. (2011) supports the use of B. natalensis in folk medicine and in the management of blood-clotting-related diseases. Likewise, the study of Lazarus (2012) revealed that the chloroform extract of B. natalensis exhibited 100% inhibition of ADP-induced clotting at doses of 1 and 3 mg/ml with IC50 values of 5.32 mg/ml.
Free radicals are known to stimulate platelet aggregation by interfering with several key steps of platelet functions (Bakdash and Williams, 2008). Bulbine natalensis has been validated as more effective anti-platelet aggregation agents of natural origin (Mosa et al., 2011) and literature reports that the presence of tannins must be responsible for the anticoagulant or anti-platelet aggregation activity (Kee et al., 2008; Kim and Choi, 2008). However, Mosa et al. (2011) suggested that the anti-platelet aggregation activity of the plant could be attributed to its high phenolic and flavonoid contents.
Apoptosis
Different parts of the Bulbine species, such as the stems, corms, leaves, and roots are known to contain anticancer compounds, namely anthraquinones, chrysophanol, and knipholone. These compounds have been proven by various studies to be cytotoxic against cancer cell lines and antibacterial against various bacterial strains (Coopoosamy, 2011; Kasumbwe and Reddy, 2010; Padayachee and Reddy, 2009). Singh and Reddy (2012) reported that the aqueous and organic fractions of B. natalensis induced the expression of caspase-3, which plays a significant role in apoptosis. The variation in bax gene expression that regulates apoptosis indicated that HEp-2 cell death was due to apoptosis and other unknown forms of cell death that may or may not activate caspase-3 gene expression.
Anti-diabetic activity
Bulbine natalensis has been reported through the ethnomedicinal survey to possess anti-diabetic properties (Afolayan and Sunmonu, 2010; Odeyemi and Bradley, 2018), but till date, the anti-diabetic activity of the plant has not been scientifically proven.
CONCLUSION
Bulbine natalensis has displayed a broad-spectrum of antimicrobial potential and this review has summarized its traditional use in male reproductive healthcare, wound healing, and treatment of various ailments in South Africa. Since primary healthcare in most communities in South Africa is dependent on traditional healers and herbalists, it is, therefore, imperative to validate the herbal remedies, as well as preserving the indigenous heritage for the benefit of these communities. Even though the effectiveness of B. natalensis in traditional medicine is well known, supplementary studies are required to isolate and characterize the active components of the extracts and to elucidate their mechanisms of action. In order to strengthen the total body of evidence, more research needs to be carried out in terms of scientifically assessing the traditional medicine and learning how to combine these therapies with modern medicine and exploration toward producing low cost-effective topical medications for various ailments.
ACKNOWLEDGMENTS
The authors would like to express their gratitude to the Govan Mbeki Research and Development Centre (GMRDC), University of Fort Hare, for financial support to conduct this research.
AUTHORS’ CONTRIBUTIONS
Both the authors contributed in experimentation, data collection, analysis of the data, writing, and approved the final manuscript.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
REFERENCES
Abdillahi HS, Van Staden J. South African plants and male reproductive healthcare: conception and contraception. J Ethnopharmacol, 2012; 143(2):475–80. CrossRef
Afolayan AJ, Sunmonu TO. In vivo studies on antidiabetic plants used in South African herbal medicine. J Clin Biochem Nutr, 2010; 47(2):98–106. CrossRef
Ajao AA, Sibiya NP, Moteetee AN. Sexual prowess from nature: a systematic review of medicinal plants used as aphrodisiacs and sexual dysfunction in sub-Saharan Africa. S Afr J Bot, 2019; 122:342–59. CrossRef
Bae JY, Zulfiqar A, Yan-Hong W, Amar GC, Ahmed AZ, Alvaro MV, Ikhlas AK. Anthraquinone-based specialized metabolites from rhizomes of Bulbine natalensis. J Nat Prod, 2019; 82(7):1893–901; doi:10.1021/acs,jnatprod.9b00187
Bakdash N, Williams MS. Spatially distinct production of reactive oxygen species regulates platelet activation. Free Radic Biol Med, 2008; 45:158–66. CrossRef
Bezabih M, Motlhagodi S, Abegaz BM. Isofuranonaphthoquinones and phenolic and knipholone derivatives from the roots of Bulbine capitata. Phytochem, 1997; 46:1063–7. CrossRef
Carlos T. Bulbine Natalensis Benefits + Side effects & reviews. Available via https://selfhacked.com/blog/bulbine-natalensis/ (Accessed 20 April 2020).
Coopoosamy RM. Traditional information and antibacterial activity of four Bulbine species (Wolf). Afr J Biotechnol, 2011; 10:220–4.
Eggli U. Sukkulenten lexicon. Band 1. Einkeimblaattrige pflanzen (Monocotyledonen), Springer, Berlin, Germany, 2001.
Erasto P, Adebola PO, Grierson DS, Afolayan AJ. An ethnobotanical study of plants used for the treatment of diabetes in the Eastern Cape Province, South Africa. Afr J Biotechnol, 2005; 4(12):1458–60.
Gauthaman K, Adaikan PG, Prasda RNV. Aphrodisiac properties of Tribulus terrestis extract (protodioscin) in normal and castrated rats. Life Sci, 2002; 71:1385–96. CrossRef
Ghuman S, Coopoosamy RM. Crude sample preparation, extraction and in vitro screening for antimicrobial activity of selected wound healing medicinal plants in KwaZulu-Natal, South Africa: a review. J Med Plants Res, 2011; 5:3572–6.
Hofheins JE, Habowski SM, Ziegenfuss TN, Lopez HL. Short term safety of Bulbine natalensis supplementation in healthy men. J Int Soc Sports Nutr, 2012; 9(Suppl 1):1–33. CrossRef
JianFeng C, PengYing Z, ChengWei X, TaoTao H, YunGui B, KaoShan C. Effect of aqueous extract of Arctium lappa L.(burdock) roots on the sexual behavior of male rats. BMC Complement Altern Med, 2012; 12(1):8. CrossRef
Kasumbwe K, Reddy L. Screening of traditional South African plants for anticancer activity. B. Tech. Project. Durban University of Technology, Department of Biotechnology and Food Technology, Durban, South Africa, 2010.
Kee NL, Mnonopi N, Davids H, Naudé RJ, Frost CL. Antithrombotic/anticoagulant and anticancer activities of selected medicinal plants of South Africa. Afr J Biotechnol, 2008; 7:217–23.
Kim JM, Yun-Choi HS. Antiplatelet effect of flavonoid and flavonoid-glycosides from Sophora japonica. Arch Pharm Res, 2008; 31:886–90. CrossRef
Lazarus GG. In vitro anti-platelet aggregation activity of the extracts of Bulbine natalensis. Doctoral Dissertation, LAP LAMBERT Academic Publishing, 2012.
Mabona U, Van Vuuren SF. Southern African medicinal plants used to treat skin diseases. S A J Bot, 2013; 87:175–93. CrossRef
Malviya N, Jain S, Gupta VB, Vyas S. Recent studies on aphrodisiacs herbs for the management of male sexual dysfunction- a review. Acta Pol Pharm, 2011; 68:3–8.
Mbambo B, Odhav B, Mohanlall V. Antifungal activity of stigmasterol, sitosterol and ergosterol from Bulbine natalensis Baker (Asphodelaceae). J Med Plants Res, 2012; 6:5135–41. CrossRef
Mosa RA, Lazarus GG, Gwala PE, Oyedeji AO, Opoku AR. In vitro anti-platelet aggregation, antioxidant and cytotoxic activity of extracts of some Zulu medicinal plants. J Nat Prod, 2011; 136–46
Nigussie, G. Phytochemical investigations of Bulbine abyssinica, Bulbine natalensis and Trachyandra Dwaricata. Doctoral dissertation, Addis Ababa University, Addis Ababa, Ethiopia, 1999.
Odeyemi S, Bradley G. Medicinal plants used for the traditional management of diabetes in the Eastern Cape, South Africa: pharmacology and toxicology. Molecules, 2018; 23(11):2759. CrossRef
Oyedemi S, Bradley G, Afolayan AJ. Ethnobotanical survey of medicinal plants used for the management of diabetes mellitus in the Nkonkobe municipality of South Africa. J Med Plants Res, 2009; 3:1040–4.
Oyekunle MA, Aiyelaagbe OO, Fafunso MA. Evaluation of the antimicrobial activity of saponin extract of Sorghum bicolor L. Moench. Afr J Biotechnol, 2006; 5:2405–7.
Padayachee B, Reddy L. Apoptotic studies of traditional South African plants Chlorophytum comosum and Bulbine natalensis in vitro. B.Tech. Project. Durban University of Technology, Department of Biotechnology and Food Technology, Durban, South Africa, 2009.
Parekh J, Chanda S. In vitro antibacterial activity of the crude methanol extract of Woodfordia fruticose Kurz. Flower (Lythaceae). Braz J Microbiol, 2007; 38:204–7. CrossRef
Patel K. Bulbine natalensis Research breakdown. 2018. Available via https://examine.com/supplements/bulbine-natalensis/research/#citations. (Accessed 20 April 2020).
Pather N, Viljoen AM, Kramer B. A biochemical comparison of the In vivo effects of Bulbine frutescens and Bulbine natalensis on cutaneous wound healing. J Ethnopharmacol, 2011; 133:364–70. CrossRef
Plessis-Stoman DD, Downing TG, Van de Venter M, Govender S. Traditional herbal medicines: potential degradation of sterols and sterolins by microbial contaminants. S Afr J Sci, 2009; 105:147–50. CrossRef
Pujol J. Naturafrica-the herbalist handbook. Jean Pujol Natural Healers’ Foundation, Johannesburg, South Africa, 1990.
Singh R, Reddy L. Apoptosis in the human laryngeal carcinoma (HEp-2) cell line by Bulbine natalensis and B. frutescens fractions. Int J Biol Pharm Res, 2012; 3:862–74.
Top Tropicals. Available via https://toptropicals.com/catalog/uid/Bulbine_natalensis.htm (Accessed 3 May 2020).
Van Jaarsveld EJ. Bulbine dewetii, a New Cliff-Dwelling bulbine species (Asphodelaceae) from the Western Cape. Haseltonia, 2017; 23:53–6. CrossRef
Van Jaarsveld EJ. Bulbline latifolia (L.f) Roem.et Schult. Kirstenboch National Botanical Garden, Cape town, South Africa, 2005.
Van Wyk BE. The potential of South African plants in the development of new medicinal products. S Afr J Bot, 2011; 77(4):812–29. CrossRef
Van Wyk BE, Gericke N. People’s plants. Briza Publications, Pretoria, South Africa, 2000.
Van Wyk BE, Van Oudtshoorn B, Gericke N. Medicinal plants of South Africa. Briza Publications, Pretoria, South Africa, pp 64–5, 1997.
Van Wyk BE, Van Oudtshoorn B, Gericke N. Medicinal plants of South Africa, seconded. Briza Publications, Pretoria, South Africa, 2009.
Van Wyk BE, Yenesew A, Dagne E. Chemotaxonomic significance of anthraquinones in the roots of Asphodeloideae (Asphodelaceae). Pergamon Biochem Syst Ecol, 1995; 23:277–81. CrossRef
Widgerow AD, Chait LA. Treatment of post operative scars with a tape containing a gel from Bulbine frutescens. United States patent US 6,159,494, Irvine, CA, p 12, 2000.
Yakubu MT, Afolayan AJ. Anabolic and androgenic activities of Bulbine natalensis stem in male Wistar rats. Pharm Biol, 2010; 48:568–76. CrossRef
Yakubu MT, Afolayan AJ. Effect of aqueous extract of Bulbine natalensis (Baker) stem on the sexual behaviour of male rats. Int J Androl, 2009; 3:629–36. CrossRef
Yakubu MT, Akanji MA, Oladiji AT. Aphrodisiac potentials of aqueous extract of Fadogia agrestis (Schweinf. Ex Heirn) stem in male albino rats. Asian J Andrology, 2005; 7:399–404. CrossRef
Yakubu MT, Oladiji AT, Akanji MA. Evaluation of biochemical indices of male reproductive function and testicular histology in Wistar rats following chronic administration of aqueous extract of Fadogia agrestis (Schweinf. Ex Heirn) stem. Afr J Biochem Res, 2007; 1:156–63. CrossRef
Yakubu MT, Quadri AL. Garcinia Kola seeds: is the aqueous extract a true aphrodisiac in male wistar rats? Afr J Tradit Complement Altern Med, 2012; 9:530–5.
Yenesew A, Dagne E, Muller M, Steglich, W. An anthrone, anthraquinone and two oxyanthrones from Kniphofia foliosa. Phytochem, 1994; 37:525–8. CrossRef