INTRODUCTION
In recent times, there has been increasing use of herbal products for curing various ailments as a result of which active compounds obtained from medicinal plants have become very popular. In fact, several drugs for the treatment of various infectious diseases and anticancer agents are of plant origin. However, as a result of overexploitation, many of these plant resources are gradually declining, and some are in the verge of extinction. However, now, it is well established that these plants harbor some distinct microbes known as endophytes, which are defined as microbes colonizing healthy inner plant tissues asymptomatically and exert beneficial effect to its host. A number of these endophytic fungi have been reported to produce many new and interesting bioactive metabolites with wide therapeutic applications. During the recent years, endophytic fungi colonizing medicinal plants have gained special attention because of the fact that these microbes were found to produce similar molecules and sometime with more bioactivity as their respective hosts. Zhang et al. (2006) thought that fungal endophytes harboring medicinal plants could be good sources for the production of pharmaceutical products. At present, several endophytic fungi obtained from medicinal plants received significant attention because many new and interesting bioactive metabolites have been reported from them. Strobel and Daisy (2003) suggested that plants belonging to biodiversity-rich regions and home to numerous endemic species may be inhabited by potent endophytes that might produce interesting chemical compounds with significant bioactivity. The northeast region of India, which occupies a major part of Indo-Burma Belt, is rich in medicinal plants and is also home to a large number of ethnic tribes. These communities, through generations, possess ethnopharmacological experience and have vast knowledge on the effective treatment of various diseases using medicinal plants. This knowledge is kept secret and usually passed on to the next generations mostly through oral traditions as a secret within families. However, there is meager information on endophytic fungi associated with these plant resources, and metabolites that they produce have not been investigated so far for bioactivity. Houttuynia cordata Thunb., belonging to family Saururaceae, commonly known as “Fish mint” is an important folk medicinal plant for the ethnic communities of Northeast India. Yang and Jiang (2009) reported that H. cordata showed an extensive array of pharmaceutical properties that include anti-inflammatory, antibacterial, antiviral, anticancer, antioxidative as well as antimutagenic effects (Yang and Jiang, 2009). Recently, injectable H. cordata is being used clinically for the treatment of various infectious diseases, inflammation, and anaphylaxis. Pan et al. (2016) investigated the endophytic fungi from H. cordata of China and reported to have a wide-spectrum antifungal activity against plant pathogens (Pan et al., 2016). Here, we isolated endophytic fungi from the healthy leaf tissues of H. cordata, of Northeast India with an aim to determine the antimicrobial potential of the fungal strains against some human pathogenic test microorganisms and characterize the secondary metabolites produced by some of the potent isolates.
MATERIALS AND METHODS
Sample collection and isolation of endophytic fungi
Healthy leaves of H. cordata were collected from four different sites of Assam, Northeast India. The plant species was thoroughly identified, and a herbarium specimen was deposited at the herbaria of the Department of Botany, Gauhati University, with accession no. 18538. From the site, five individual healthy plants were selected randomly, and the leaves were collected in sterile polybags. The collected samples were immediately brought to the laboratory and processed for the isolation of endophytic fungi. The healthy leaf samples were washed thoroughly under running tap water, followed by washing with 10% mild biodetergent and finally with double distilled water to remove the surface debris. For the isolation of endophytic fungi, the leaves were surface sterilized following Tayung and Jena (2013) with slight modifications (Tayung and Jena, 2013). Surface sterilization was performed by sequentially dipping the leaf samples in 70% ethanol (3 minutes) followed by 0.5% sodium hypochlorite (2 minutese), then rinsed thoroughly with sterile distilled water (1 minute), and finally, kept for drying over sterile blotting paper under the laminar air flow chamber. The surface sterilized leaves were then punched into circular fragments of about 0.5 mm diameter using a sterile puncture. The surface-sterilized fragments were then inoculated into Potato Dextrose Agar (PDA) medium supplemented with streptomycin sulfate (50 μg/ml) and then incubated at 25°C ± 2°C for 2 weeks. The plated fragments were observed once a day for the growth of any endophytic fungal mycelium. Hyphal tips of endophytic fungi growing out of the surfaced sterilized leaf fragments were transferred to PDA slants and stored at 4°C for the further study.
Identification of endophytic fungi
The endophytic fungal isolates were identified based on their morphological and microscopic characters by growing them on PDA medium referring to standard identification manuals (Barnett and Hunter, 1996; Damm et al., 2012; Gilman, 1971). Species confirmation of the endophytic isolates that showed prominent bioactivity was determined by internal transcribed spacer (ITS) rDNA sequence analysis.
Fungal cultivation and metabolite extraction
Pure and actively growing endophytic fungi were cultivated separately in Erlenmeyer flasks containing 100 ml of potato dextrose broth medium. These were then incubated at 28°C for 2–3 weeks in a BOD shaking incubator with periodic shaking at 120 rpm. After a specified incubation period, the fungal cultures were filtered through sterile Whatman filter paper to remove mycelia. The liquid broth was collected and extracted using an equal volume of ethyl acetate by vigorously shaking for 10–15 minute in a separating funnel. In the process, the medium and cell debris got separated, and the solvent thus obtained was collected. The solvent was then evaporated in a rotary evaporator, and the crude ethyl acetate extracts were obtained. The extracts were further concentrated and dried with MgSO4. The concentrated crude extracts were then dissolved in dimethyl sulfoxide (DMSO) and stored at 4°C for the determination of antimicrobial activity assay.
Determination of antimicrobial activity
The antimicrobial activities of the extracted crude metabolites were determined through agar cup diffusion method against some clinically significant human test pathogens. The test organisms included three bacterial pathogens, namely, Staphylococcus aureus (MTCC 737), Pseudomonas aeruginosa (MTCC 424), and Escherichia coli (MTCC 443) and one pathogenic fungus, Candida albicans (MTCC 227). The pathogenic test organisms were procured from the Institute of Microbial Technology, Chandigarh, India. The bacterial and fungal test pathogens were activated by cultivating them on freshly prepared nutrient agar (NA) and sabouraud dextrose agar (SDA) media, respectively. Meanwhile, sterile NA and SDA plates were prepared, and the plates were inoculated with 0.2 ml of overnight grown bacterial and fungal cultures containing 1.0 × 106 cells. A lawn culture was made over each plate by evenly spreading the inoculum with the help of a sterile cotton swab. Agar cups were made in the plates with a sterile cork borer (7 mm in diameter). The cups were then filled in 100 μl of the crude metabolites obtained from the endophytic fungi. Plates were then incubated at 37°C ± 1°C for 24 hours for bacterial and at 28°C ± 1°C for 48 hours for fungal pathogens. Antimicrobial activity was determined by the appearance of clear zones of inhibition against the test organism around the agar cups. DMSO was used as the negative control for the assay.
Characterization of fungal metabolites
The metabolites that showed significant antimicrobial activity were characterized by both fourier- transform infrared (FTIR) and gas chromatography- mass spectrometry (GCMS) analyses. FTIR analysis was performed using Thermo Nicolet iS10 FTIR Spectrometer (Thermo scientific) instrument. About 1 mg of dried ethyl acetate extract of endophytic mixed with 10 mg of anhydrous potassium bromide was used to prepare the KBr pellet. For 32 scans, the spectra were recorded from 400 cm−1 to 4,000 cm−1. The resolution used was 4 cm−1, and the spectra thus obtained were recorded and analyzed. The compounds were further identified using a Perkin Elmer TurboMass Spectrophotometer (USA) of model Claurus 680 Gas chromatography/Claurus 600 Mass spectrometer (GC with liquid autosampler). Perkin Elmer Elite-5MS capillary column was used, which had a length of 60 m and an internal diameter of 0.25 mm and composed of 5% diphenyl 95% dimethyl polysiloxane (low bleed). Helium at a flow rate of 1 ml/minute was used as the carrier gas. About 1 μl of the prepared 1% of extract diluted with methanol was injected in a splitless mode. The inlet temperature was maintained at 250°C. The oven temperature was set initially at 60°C for 5 minutes, then increased to 180°C at a rate of 10°C and holding time of about 5 minutes, and then again set at 280°C at a rate of 20°C for 5 minutes. The temperature of the MS transfer line was maintained at 200°C, and the source temperature was maintained at 180°C. Total run time was 37 minutes. GC-MS analysis was done using electron impact ionization at 70 eV. Data were evaluated using total ion chromatogram for compound identification and quantification. The identification of chemical compounds present in the extract was performed by comparing their respective retention time (RT) and mass spectra fragmentation patterns with data from those stored in the library of the National Institute of Standard and Technology (NIST) and also with available published literature. All the identified components were summarized in terms of relative peak area percentage.
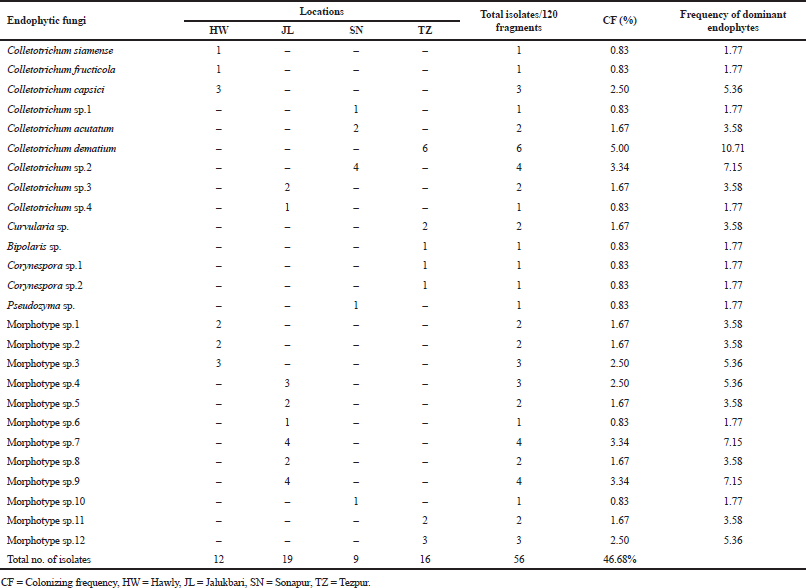 | Table 1. Composition of endophytic fungi in healthy leaf tissues of H. cordata isolated from four different sites of Assam. [Click here to view] |
RESULTS AND DISCUSSION
Isolation and identification of endophytic fungi
Endophytic fungi are ubiquitous and known to be distributed naturally in both temperate regions and tropical rainforests. Medicinal plants are known to harbor many endophytic fungi which have been reported to be involved in the co-production of active metabolites (Alvin et al., 2014). In this study, endophytic fungi associated with H. cordata, an ethnomedicinal plant of Northeast India, were investigated for antimicrobial metabolites. Altogether, 56 endophytic fungal strains were obtained from surface-sterilized leaf fragments of H. cordata in PDA medium. Previous workers from China have also isolated endophytic fungi from H. cordata on PDA medium (Pan et al., 2016). Furthermore, in many instances, PDA medium has been primarily used for the isolation of endophytic fungi. The isolated endophytes consisted of fungi belonging to genera Colletotrichum, Curvularia, Bipolaris, Corynespora, and Pseudozyma and non-sporulating fungi categorized as mycelia sterilia (Table 1). The dominant endophytic fungi comprised of fungal class Deuteromycetes. Such results were also obtained by many workers from several medicinal plants such as Withania somnifera and Achyranthes aspera (John and Mathew, 2017; Khan et al., 2010). The overall colonization frequency of endophytes was found to be 46.68%, of which the highest colonization frequency was shown by fungi belonging to mycelia sterilia (23.36%) followed by the genus Colletotrichum (19.19%), Curvularia (1.67%), Bipolaris (0.84%), Corynespora (1.67%), and Pseudozyma (0.84%) (Table 1).
In various instances, the genus Colletotrichum has been reported as dominant endophytic fungi from several medicinal plant species (John and Mathew, 2017; Siqueira et al., 2011; Thalavaipandian, 2011). However, this is the first report from H. Cordata leaf tissue. Pan et al. (2016) reported Chaetomium globosum as an endophyte from H. Cordata tissues collected from Yaan City, Southwest China (Pan et al., 2016). It was very interesting to note that unicellular ustilaginomycetous anamorphic yeast, Pseudozyma sp., was isolated from H. cordata leaves as an endophyte. Although the occurrence of Pseudozyma sp. as endophyte is rare, the association of this species as endophytic fungi has been reported from Hyoscyanus muticans, a medicinal plant collected from Yamaguchi, Japan (Abdel-Motaal et al., 2009). In this study, a variation in the colonization frequency of endophytic fungi was observed among the sites which might be due to various environmental factors. Many workers thought that endophytic fungal communities vary along with time and space and are also governed by climatic and environmental conditions (Coince et al., 2013; Fisher et al., 1994; Gao et al., 2005; Jumpponen and Jones, 2010; Peršoh, 2015).
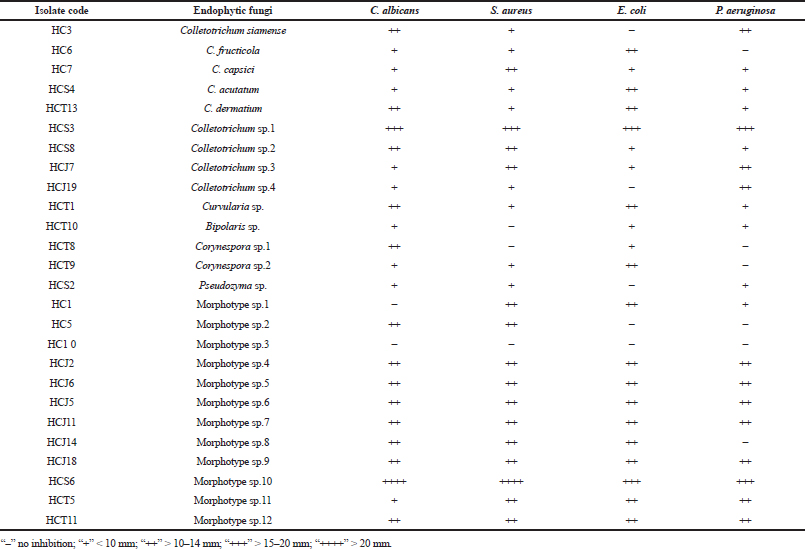 | Table 2. Antimicrobial activity of endophytic fungal isolates of H. cordata against some human test pathogens. [Click here to view] |
Antimicrobial activity of endophytic fungi
There are substantial evidences that endophytic fungi isolated from medicinal plants produce bioactive metabolites with antimicrobial and anticancer properties (Deng, 2009; Xiaoli et al., 2008). Therefore, in this study, the endophytic fungal isolates were evaluated for antimicrobial activity against some human test pathogenic organisms of clinical importance. The study revealed that 73.07% of the isolates showed an antimicrobial activity inhibiting at least one of the test pathogens in varying degrees, whereas 92.32% of them showed anticandida activity against C. albicans (Table 2). Pan et al. (2016) also isolated several endophytic fungi from fresh, symptomless H. cordata tissues of Southwest China (Pan et al., 2016). However, those endophytes were evaluated for antifungal activity against plant pathogens. The antimicrobial activity of the endophytic fungi may be attributed to the medicinal properties of H. cordata. There are numerous reports of endophytic fungi isolated from medicinal plants to display the antimicrobial activity inhibiting human pathogenic microorganisms (Li et al., 2005; Tong et al., 2011). Among the endophytes, two isolates showed a significant antimicrobial activity by inhibiting all of the test pathogens (Table 2 and Figs. 1 and 2). The isolates were designated as Colletotrichum sp.1 strain “HCS3” and a non-sporulating fungus designated as Morphotype sp.10 strain “HCS6.” These isolates were later identified to be Colletotrichum coccodes and Phyllosticta capitalensis through ITS rDNA sequence analysis.
In many instances, endophytic Colletotrichum sp. isolated from medicinal plants has been reported to show the antimicrobial activity against a broad range of pathogenic microbes, and thus, the results collaborate with the findings of many previous workers (Gond et al., 2005; Siqueira et al., 2011; Zou et al., 2000). Ethyl acetate extracts of both the endophytic fungi under optimized conditions showed a significant antimicrobial activity against the pathogenic test organisms (Table 3).
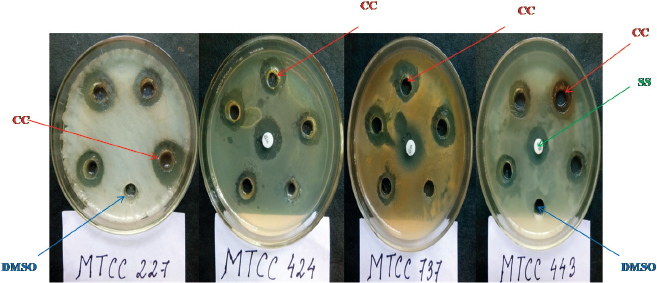 | Figure 1. Antimicrobial activity of the crude extract of C. coccodes HCS3 against all the test pathogens. CC = C. coccodes, SS = Streptomycin sulfate, DMSO = Dimethyl sulfoxide. [Click here to view] |
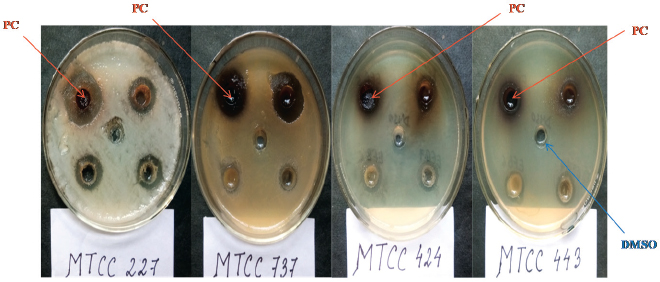 | Figure 2. Antimicrobial activity of the crude extract of P. capitalensis HCS6 against all four test pathogens. PC = P. capitalensis, DMSO = Dimethyl sulfoxide. [Click here to view] |
Characterization of secondary metabolites
Endophytes are considered as chemical synthesizers inside their respective host (Owen and Hundley, 2004). Currently, several endophytic fungi inhabiting medicinal plants have been reported to possess unique chemical diversity and bioactivities for exploitation as therapeutic agents. We, therefore, investigated the metabolites obtained from the two potent endophytic fungi by FTIR and GCMS analyses. FTIR spectroscopic analysis of the ethyl acetate extract obtained from the endophytic fungus, C. coccodes (HCS3), showed presence of different functional groups as revealed from the spectra at different wavelengths. Among different compound classes, wavenumber obtained at 1,746 cm−1 showed the presence of the ester functional group, which is known to be δ-lactones. This compound class is reported to have antitumor property (Yixi, 2015). Similarly, FTIR analysis of the ethyl acetate extract of P. capitalensis (HCS6) also showed the presence of various function groups at different wavelengths. Some of them were alkenes, amines, aromatic esters, and nitro compounds.
The metabolites were further characterized by GCMS analyses. The chromatogram of the metabolite obtained from C. coccodes indicated the presence of several peaks at different RTs. The compounds were identified based on their peak area, RT, molecular weight, and molecular formula. For interpretation of the mass spectrum, the database of NIST was used along with available published literature. The major compounds present in the extract were identified as geranylgeraniol, farnesol, and squalene at a peak value of 53.121 (Fig. 3). Available works of literature revealed that these compounds have bioactive properties. Geranylgeraniol has been reported to possess antibacterial activity against S. aureus (Kobayashi et al., 2005). Another compound, namely, farnesol is reported to be an antiquorum sensing molecule, reducing the virulence factor of C. albicans (Derengowski et al., 2009). It should be noted that the extract showed a considerable antimicrobial activity against S. aureus and C. albicans in the in vitro antimicrobial assay. Thus, the antimicrobial activity of the crude extract might be attributed to the presence of these compounds. The extract also contains squalene, which is known to be a phytosterol, and this compound is reported to have antioxidant and cytotoxic activities against human cancer cells (De Los Reyes, 2015). However, no studies have been undertaken so far to evaluate the anticancer potential of endophytes isolated from H. cordata although the extract of this plant is reported to have anticancer activity (Lai et al., 2010). Further, the GCMS analysis of the ethyl acetate extract of P. capitalensis revealed the presence of one major compound identified as 1-Octacosanol in the chromatogram (Fig. 4). This compound has been reported to exhibit good antioxidant and antibacterial activity (Sengupta et al., 2018).
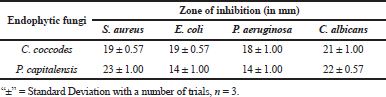 | Table 3. Antimicrobial activities of ethyl acetate extracts obtained from C. coccodes (HCS3) and P. capitalensis (HCS6) against selected test pathogens. [Click here to view] |
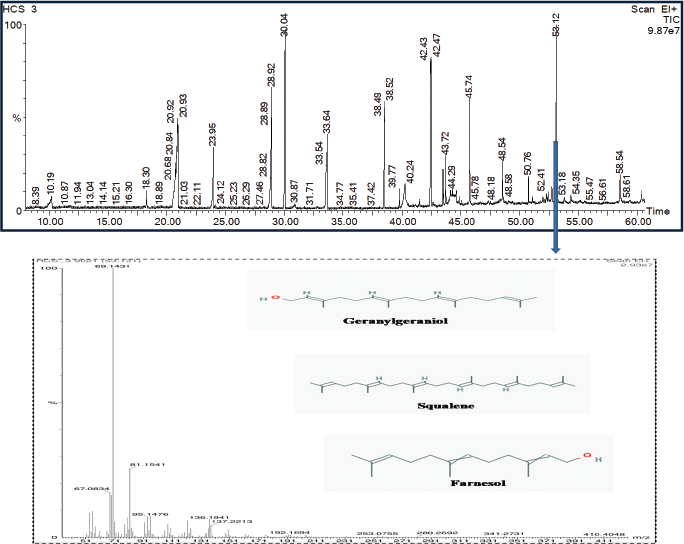 | Figure 3. GCMS chromatogram of ethyl acetate extract of C. coccodes (HCS3) showing the mass spectrum of peak 53.121 with three major identified compounds. [Click here to view] |
Endophytic fungi inhabiting medicinal plants have been reported to produce the same or analogous bioactive secondary metabolites as their hosts. This has been exemplified by the first report of endophyte, Taxomyces andreanae, which produces identical bioactive secondary metabolite “taxol” (paclitaxel) similar to its host, Taxus brevifolia (Stierle et al., 1993). Thereafter, numerous studies have revealed that plant-derived secondary metabolites are produced by endophytes (Zhao et al., 2011). In this study, the extracts derived from endophytic fungi isolated from H. cordata showed similar biological properties with the host plant. Liang et al. (2005) reported that the antimicrobial activity of H. cordata has been attributed to its volatile oil components (Liang et al., 2005). This has generated speculation in the research so as to whether bioactive compounds originating from plant sources are actually produced by plants only or their endophytic microorganisms or both of the partners facilitate the production of active metabolites. However, if the microbial origin of the bioactive metabolites is obtained, it would be easier to derive the active molecules without exploiting the plant. This study thus indicates that ethnomedicinal plants are colonized by distinct endophytic microbes, which could be explored for bioactive metabolites for use as therapeutic agents in future research programmes.
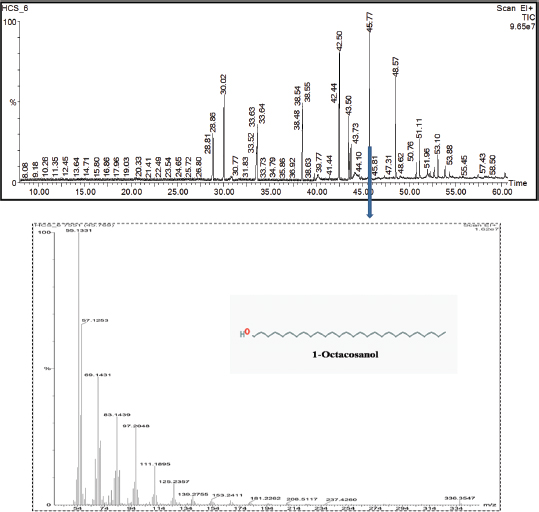 | Figure 4. GCMS chromatogram of ethyl acetate extract of P. capitalensis (HCS6) showing the mass spectrum of peak 45.769 with one major identified compound. [Click here to view] |
CONCLUSION
The result of this study indicated the presence of diverse endophytic fungal communities in the healthy leaf tissues of H. cordata and their capability to produce antimicrobial metabolites for future drug discovery. Among the endophytic fungi, two isolates identified as C. coccodes (HCS3) and P. capitalensis (HCS6) displayed a significant antimicrobial activity. The characterization of the potent isolates revealed the presence of various bioactive compounds. Such findings provide a promising opportunity for the development of antimicrobial drugs from medicinal plant-associated endophytic fungi.
ACKNOWLEDGMENTS
The authors are grateful to Guwahati Biotech Park, IIT Guwahati, and Department of Biotechnology, Government of India, for providing all the necessary laboratory facilities to accomplish this particular research work.
CONFLICT OF INTEREST
All the authors declare that the research has been conducted with no financial or commercial relationship that could be interpreted as a potential conflict of interest.
REFERENCES
Abdel-Motaal FF, El-Zayat SA, Kosaka Y, El-Sayed MA, Nassar MSM, Ito S. Four novel ustilaginomycetous anamorphic yeast species isolated as endophytes from the medicinal plant Hyoscyamus muticus. Asian J Plant Sci, 2009; 8:526–35. CrossRef
Alvin A, Miller KI, Neilan BA. Exploring the potential of endophytes from medicinal plants as sources of antimycobacterial compounds. Microbiol Res, 2014; 169(7–8):483–95. CrossRef
Barnett HL, Hunter BB. Illustrated genera of imperfect fungi. APS Press, St. Paul, MN, 1996.
Coince A, Caël O, Bach C, Lengellé J, Cruaud C, Gavory F, Morin E, Murat C, Marçais B, Buée M. Below-ground fine-scale distribution and soil versus fine root detection of fungal and soil oomycete communities in a French beech forest. Fungal Ecol, 2013; 6(3):223–35. CrossRef
Damm U, Johnston PR, Weir BS. The Colletotrichum gloeosporioides species complex. Stud Mycol, 2012; 73:115–80. CrossRef
De Los Reyes MM, Oyong GG, Ebajo Jr VD, Ng VAS, Shen CC, Ragasa CY. Cytotoxic Triterpenes and Sterols from Pipturus arborescens (Link) C.B. Rob. J Appl Pharm Sci, 2015; 5(11):023–30. CrossRef
Deng BV, Liu K H, Chen WQ, Ding XW, Xie XC. Fusarium solani, Tax-3, a new endophytic taxol-producing fungus from Taxus chinensis. World J Microbiol Biotechnol, 2009; 25:139–43. CrossRef
Derengowski LS, De Souza Silva C, Braz SV, Sousa TM, Báo SN, Kyaw CM, Silva-Pereira I. Antimicrobial effect of farnesol, a Candida albicans quorum sensing molecule, on Paracoccidioides brasiliensis growth and morphogenesis. Ann Clin Microbiol Antimicrob, 2009; 8:13. CrossRef
Fisher PJ, Petrini O, Petrini LE, Sutton BC. Fungal endophytes from the leaves and twigs of Quercus ilex L. from England, Majorca and Switzerland. New Phytol, 1994; 127:133–7. CrossRef
Gao XX, Zhou H, Xu DY, Yu CH, Chen YQ, Qu LH. High diversity of endophytic fungi from the pharmaceutical plant, Heterosmilax japonica Kunth revealed by cultivation-independent approach. FEMS Microbiol Lett, 2005; 249:255–66. CrossRef
Gilman JC, 1971. A manual of soil fungi. 2nd edition, Iowa State College Press, Ames, IA.
Gond SK, Mishra A, Sharma VK, Verma SK, Kumar J, Kharwar RN, Kumar A. Diversity and antimicrobial activity of endophytic fungi isolated from Nyctanthes arbor-tristis, a well-known medicinal plant of India. Mycoscience, 2012; 53:113–21. CrossRef
John R, Mathew L. Endophytic fungal assemblage in Achyranthes aspera Linn. revealed by internal transcribed spacer region of nuclear ribosomal RNA genes. 3 Biotech, 2017; 7(2):109. CrossRef
Jumpponen A, Jones KL. Seasonally dynamic fungal communities in Quercus macrocarpa phyllosphere differ among urban and rural environments. New Phytol, 2010; 186:496–513. CrossRef
Khan R, Shahzad S, Choudhary MI, Khan SA, Ahmad A. Communities of endophytic fungi in medicinal plant Withania Somnifera. Pak J Bot, 2010; 42(2):1281–7.
Kobayashi S, Hamashima H, Hirose K, Shiraishi A, Hada T, Inoue Y. Biphasic Effects of Geranylgeraniol, Teprenone, and Phytol on the Growth of Staphylococcus aureus. Antimicrob Agents Ch, 2005; 49(5):1770–4. CrossRef
Lai KC, Chiu YJ, Tang YJ, Lin KL, Chiang JH, Jiang YL, Jen HF, Kuo YH, Agamaya S, Chung JG, Yang JS. Houttuynia cordata Thunb extract inhibits cell growth and induces apoptosis in human primary colorectal cancer cells. Anticancer Res, 2010; 30(9):3549–56.
Li Y, Song YC, Liu JY, Ma YM, Tan, RX. Anti-Helicobacter pylori substances from endophytic cultures. World J Microbiol Biotechnol, 2005; 21:553–8. CrossRef
Liang M, Qi M, Zhang C, Zhou S, Fu R, Huang J. Gas chromatography-mass spectrometry analysis of volatile compounds from Houttuynia cordata Thunb after extraction by solid-phase microextraction, flash evaporation and steam distillation. Anal Chim Acta, 2005; 531:97–104. CrossRef
Owen NL, Hundley N. Endophytes-the chemical synthesizers inside plants. Sci Prog, 2004; 87:79–99. CrossRef
Pan F, Liu ZQ, Chen Q, Xu YW, Hou K, Wu W. Endophytic fungus strain 28 isolated from Houttuynia cordata possesses wide-spectrum antifungal activity. Braz J Microbiol, 2016; 47(2):480–8. CrossRef
Peršoh D. Plant-associated fungal communities in the light of meta’omics. Fungal Divers, 2015; 75(1):1–25. CrossRef
Sengupta S, Nandi I, Bhattacharyya DK, Ghosh M. Anti-Oxidant and Anti-Bacterial properties of 1-Octacosanol isolated from rice bran wax. J Plant Biochem Physiol, 2018; 6:206. CrossRef
Siqueira VM, Conti R, Araujo JM, Souza-Motta CM. Endophytic fungi from the medicinal plant Lippia sidoides Cham. and their antimicrobial activity. Symbiosis, 2011; 53:89–95. CrossRef
Stierle A, Strobel G, Stierle D. Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific yew. Science, 1993; 260:214. CrossRef
Strobel GA, Daisy B. Bioprospecting for Microbial Endophytes and Their Natural Products. Microbiol Mol Biol Rev, 2003; 67:491–502. CrossRef
Tayung K, Jena SK. Endophytic fungal communities associated with two ethno-medicinal plants of Similipal Biosphere Reserve, India and their antimicrobial prospective. J Appl Pharm Sci, 2013; 3(4S-1):S7–17.
Thalavaipandian A. Diversity of fungal endophytes in medicinal plants of Courtallam hills, Western Ghats, India. Mycosphere, 2011; 2:575–82. CrossRef
Tong WY, Darah I, Latiffah Z. Antimicrobial activities of endophytic fungal isolates from medicinal herb Orthosiphon stamineus. Benth J Med Plant Res, 2011; 5(5):831–6.
Xiaoli L, Mingsheng D, Xiaohong C, Mei J, Xin L, Jianzhong Z. Antimicrobial activity of an endophytic Xylaria sp. YX-28 and identification of its antimicrobial compound 7-amino-4- methylcoumarin. Applied Microb Biotechnol, 2008; 78:241–7. CrossRef
Yang L, Jiang JG. Bioactive components and functional properties of Hottuynia cordata and its applications. Pharm Biol, 2009; 47(12):1154–61. CrossRef
Yixi L. Isolation and structure elucidation of anticancer and antimalarial natural products. Ph D thesis. Virginia Polytechnic Institute and State University, Blacksburg, VA, 2015;1–201.
Zhang HW, Song YC, Tan RX. Biology and chemistry of endophytes. Nat Prod Rep, 2006; 23:753–71. CrossRef
Zhao J, Shan T, Mou Y, Zhou L. Plant derived bioactive compounds produced by endophytic fungi. Mini Rev Med Chem, 2011; 11:159–68. CrossRef
Zou WX, Meng JC, Lu H, Chen GX, Shi GX, Zhang TY, Tan RX. Metabolites of Colletotrichum gloeosporioides, an endophytic fungus in Artemisia mongolica. J Nat Prod, 2000; 63:1529–30. CrossRef