INTRODUCTION
Pediatric population constitutes a significant portion of total population. Unlike the overall perception, pediatric population is a diverse group comprising of different subgroups, categorized differently by agencies across the world. The American Academy of Pediatrics (AAP) considers the pediatric group from fetus up to the age of 21 (AAP, 1988). The British National Formulary for Children categorizes the separate dosage regimens for neonates (under 1 month of age), for children from 1 month to 4 years, and for children from 4 years to 10 years (British National Formulary for Children, 2006). Other agencies do not follow this age division. For example, the US FDA classifies neonate into newborn (1 month of age), infant (1 month–2 years of age), children (2 years–12 years of age), and adolescent (12 years–16 years of age) (US Department of Health and Human Services FDA CDER, 2014). However, generally accepted subcategories, as per the International Conference on Harmonization of Technical Requirements for the Registration of Pharmaceuticals for Human Use and endorsed by World Health Organization (WHO), are as follows: preterm neonates, full-term newborn infants (birth until 27 days), infants and toddlers – 28 days until 23 months of age, children – 2 years until 11 years of age, and adolescent – 12 years until 16–18 years of age (depends on region) (WHO Technical Report Series No. 970, 2012). A pediatric population is heterogeneous. Therefore, sometimes, the difference between dosage of an adolescent and that of preterm neonate can go up to 100-fold. Similarly, a typical average pediatric dose could be 10% of adult’s dose; however, when compared to preterm neonate, it can be 10 times the appropriate dose (Grissinger, 2015). Currently, there are very limited medications designed and developed, especially for pediatric population. The WHO in its working document emphasizes on timely development of medicines for the safe and effective pharmacotherapy of pediatric patients and related information on proper use of medicine with respect to the age, body size, and physiological condition of the child available. This report also acknowledges the widespread use of off-label and unlicensed medicines for treating children. In the absence of age-appropriate formulations, the medicines which are not licensed for children are used. Such medicines are not properly studied for their effects on children. Considering this, there is a need of formulations developed, which could be suitable for children (WHO Technical Report Series No. 970, 2012).
Medication errors are the result of the above mentioned challenges in this population. For several reasons, the pediatric patients have some unique diseases and specific medical conditions and additionally have a higher risk of adverse drug events. Medication errors affecting pediatric patients and unique challenges in this special population have been discussed in detail in the literature (Grissinger, 2015).
Pediatric expert emphasizes on the fact that a successful drug delivery to pediatric patients can be realized only after overcoming the basic differences between children and adults. The preferred administration route, i.e., oral dosage forms may not always be palatable or available in doses appropriate for children. Thus, dosage forms, such as pills and tablets are often manipulated in ways that are not ideal for delivering the safe, effective, and consistent doses. Compounding pharmacist and health workers can help, but their practices may differ, and hence, the results are not always reproducible. Furthermore, these services may not be always available, especially in undeveloped parts of the world. Patients often follow the methods such as dividing doses, crushing and dissolving them in liquids (water, juices, etc.), and administering drugs in quantities that have not been adequately tested (Meyers and Myers, 2016).
In pediatric pharmacotherapy newsletter, the author reports that optimization of oral drug delivery has been one of the biggest challenges in pediatric pharmacology. For most of the children over 6 years of age, swallowing solid dosage forms can be taught, and many children remain uncomfortable with it until adolescence. In one study on children, 54% between the age of 6 and 11 years described inability to swallow a tablet easily (Buck, 2013).
Children could not be considered as small adults and require establishing the proper dose, safety, and efficacy of a medicine in that population through pediatric trials. The method of conduct and design aspects of these pediatric trials, however, recently improved due to the increasing involvement of pediatric expertise. The important changes introduced in the US legislation instructed the pharmaceutical industry to study medicines in children as a requirement, for which financial incentives were offered. This has resulted in a significant increase in the number of pediatric trials conducted since 1997. Based on the similar framework of incentives and requirements, in 2007, the European Union adopted pediatric medicinal regulations, which have further stimulated pediatric drug development, especially in Europe (Dunne et al., 2010). Due to the WHO’s efforts, some success is achieved for “tropical” diseases such as malaria and tuberculosis, where pediatric patient-friendly suspension and dispersible dosage forms are becoming available, but the availability of such dosages is still awaited for antiretroviral drugs as HIV/AIDS are the major challenges in these countries (Quique Bassat, 2015). At present, fixed-dose combinations (FDCs) and once daily solid dosage forms are helping adolescents and older children, but suitable dosage forms are lacking for younger children and infants, which results in poor adherence, viral resistance, and decreased survival of HIV-positive children (Adrienne et al., 2016).
Pediatric population differs in various physiological aspects as compared to the adult population. These differences in physiology affect absorption, distribution, metabolism, and excretion processes which control the overall availability of the drug in blood at the site of action. Neonates in the early stage of less than 2 weeks could not produce acid in the stomach (achlorhydria), which can significantly affect the drug release and absorption of drugs. In addition, gastric emptying time in these neonates is found to be prolonged, irregular, and difficult to predict. This increased gastric emptying time may result in higher degradation of the drug due to increased contact time with gastric contents. Pancreatic enzyme activity is low in early ages and develops gradually, thereby affecting the drug bioavailability of those drugs, which are sensitive to those enzymes. Absorption of lipid-soluble drugs may also be decreased in neonates due to the lower release of bile acids and lipase.
Total body water ratio in infants is higher than adults (94% in fetus, 78% in full term infant, and 60% in adults), which affects the distribution volume of hydrophilic drugs toward higher side and lower for lipophilic drugs. Infants not only have low plasma protein level of albumin but also have protein binding characteristics that are modified which increase the competition for binding endogenous substances. Drug distribution is affected by the major factors such as perfusion rate, tissue permeability, and tissue drug binding, which are altered in pediatric population. Drug metabolism is reduced due to low liver volume and poor regional blood flow of the liver.
The drug metabolism in the liver is mostly carried by enzyme cytochrome P-450. Less maturation of various metabolic pathways like these within infants is another reason to have substantially slower drug metabolism among pediatric population in infants in comparison to higher age children and adults. The efficacy of the renal excretion of drug is the function of various related processes, such as glomerular filtration, tubular secretion, and tubular reabsorption determining the efficacy of renal excretion, which are poorly developed in the 1st year of birth (Mannan et al., 2018). In infants, glomerular filtration rate is about 2–4 ml/minute/1.73 m2, whereas the normal values for adults are largely over 60 ml/minute/1.73 m2 (Delanaye et al., 2012).
These physiological parameters not only found different when compared to the adult population but also vary over the entire age range among pediatric population itself. This makes the pediatric drug development very challenging in terms of unifying the dosages and dosage forms. Pediatric drug development process has other factors contributing to make the situation more challenging and complex. Some of these factors include, but not limited to, the following (Fig 1):
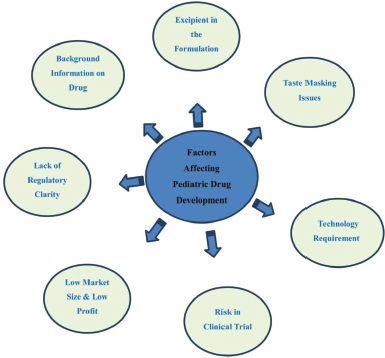 | Figure 1. Various factors affecting pediatric drug development. [Click here to view] |
1. Insufficient background information on drug molecule in target population.
2. Excipients for pediatric formulations
3. Taste-masking issues
4. Technology requirement
5. Challenges/Risks involved in clinical trials (CTs)
6. Low market size and low profitability
7. Lack of regulatory clarity
Insufficient Background Information on Drug Molecule in Pediatric Population
Historically, drugs have been developed with focus on mainly adult population. Hence, information obtained for the drug molecule is not readily applicable for direct use in pediatric population given the fact that nonlinearity exists on various aspects across pediatric age range. The validated biomarkers that could be used for pediatric population are limited in number. Therefore, application of the available adult biomarkers directly to children to gather pediatric data is often carried out. In this approach, various impacts on these biomarkers are not considered, which occurs due to the development and potential atypical pathophysiology of the diseases in the pediatric age group (Mulugeta et al., 2017). Majority of drugs prescribed for children have not been adequately tested in pediatric population, especially with younger age group, as less information is available, which is required to conduct the pediatric study.
Clinicians and consumers get an important information about the appropriate use of drugs and associated risks from the product label which is derived from the controlled clinical studies. However, as a limited number of drugs are tested in pediatric patients, the product label lacks specific information for many drugs used to treat children. This situation creates challenges for clinicians who treat children of entire pediatric range, i.e., infant to adolescent (Vanchieri et al., 2008). This leaves the clinician and end user with no other option than to use the available adult formulation in the market for pediatric population. To make such formulation be conveniently administered, it is modified by one or the other way from its parent form, which is termed as “extemporaneous.”
Excipients for Pediatric Formulations
In general, excipient forms the major bulk of almost all dosage forms. Hence, this is a relatively large component which goes into the body along with the active drug. Although excipients are essentially nontoxic and most of them are generally recognized as safe, not all of them can be considered safe for children. There is only limited knowledge available on the acceptability and safety of formulation excipients in relation to the age and development status of the child (EMA, 2006).
The factors such as age, weight, poorly developed organ systems, presence or absence of various enzymes, and their different levels in pediatric population also affect the excipient metabolism. Hence, an adverse reaction in children due to the excipients used in the medicines is an additional challenge which is not experienced by adults or is not seen to the same extent. The literature shows a limited amount of data on adverse reactions due to the excipients, and the available data are variable in quality (WHO technical report series No. 970, 2012). Some of the excipient categories along with available data are explained below:
Antimicrobial preservatives
Metabolic pathways for benzoic acids are poorly developed, especially in neonates who cannot conjugate benzoic acid effectively. This is of great importance as benzyl alcohol is used as an excipient in this age group, which metabolizes to toxic benzoic acid and can accumulate due to weak metabolism. Excipients such as propylene glycol are hygroscopic in nature and when used, for example, in rectal dosage forms, it may irritate rectal mucosa, which needs special consideration while developing dosage forms (EMA, 2006).
Sweeteners
Sweeteners such as sucrose, fructose, sorbitol, xylitol, and aspartame need to be cautiously used while considering some of their effects. Formulations with high amount of sucrose should be avoided as it lowers the pH of dental plaque which dissolves tooth enamel and promotes dental caries. Similarly, fructose in high amounts can lead to laxative effects in children. Sorbitol and xylitol may cause osmotic diarrhea though xylitol offers the protection from dental caries (Afaque et al., 2015). Artificial sweetener aspartame is harmful in some patients with phenylketonuria and homozygous autosomal recessive diseases and can cause cross-reactivity with sulfonamides (Pawar and Kumar, 2002). Lactose intolerant children can be more sensitive to lactose. Considering varied lactose sensitivities, even small amount (less than 3g) may trigger described symptoms in lactose intolerant population. In the US, some of the sweeteners are either banned or restricted by providing warning on label such as cyclamates and saccharin, respectively, which requires consideration while developing the formulations catering to those countries (Afaque et al., 2015).
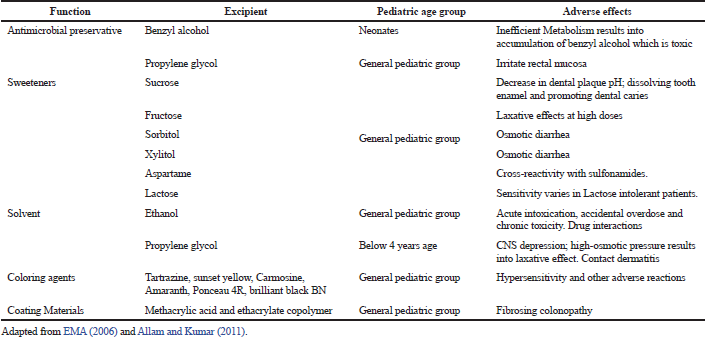 | Table 1. Examples of excipients and their effects in pediatric patients. [Click here to view] |
Solvents
Ethanol is commonly used solvent in oral liquid preparations. However, when it is used in products for pediatric populations, there are several toxicity concerns which can be acute intoxication due to accidental overdose and chronic toxicity on long-term routine use for medical conditions (AAP, 1997). The co-administration of ethanol containing products with some drugs has a potential to affect drug absorption or metabolism and may result in drug interaction. If blood ethanol concentration reaches up to 1–100 mg/100 ml, adverse effects in the central nervous system are commonly reported. However, the long-term effect on the hepatorenal function of higher ethanol concentration has never been studied in the pediatric population (Fiocchi et al., 1999). Propylene glycol is another solvent used commonly in oral, topical, and injectable formulation. However, it can accumulate in the body as pediatric patients below 4 years have a limited metabolic pathway (alcohol dehydrogenase). Depression of the central nervous system is the main toxic effect. In addition, laxative effects due to high osmotic pressure may be observed. Hence, the products containing high propylene glycol levels are not suitable for children, especially below 4 years of age. Even topical use of propylene glycol reported to cause contact dermatitis (EMA, 2006).
Colors and coating materials
To improve the overall feel of the product and to have product appeal, most of the pediatric compositions have bright colors, which are commonly preferred by children. However, the use of the colors should be discouraged unless it is required similar to the cases to conceal unpleasant color of a drug in a liquid product. Hypersensitivity and other adverse reactions have been associated with many coloring agents. Various side effects of coloring agents in the pediatric population are reported in the literature (AAP, 1997; Ibero et al., 1982; Kumar et al., 1996; Pawar and Kumar, 2002). For young children (less than 1-year old), acceptable daily values are presented in the European Commission report. With coating materials such as methacrylic acid and ethacrylate copolymer, the cases of fibrosing colonopathy have also been reported in children (EMA, 2006).
Therefore, special consideration should be given to the use of excipients in pediatric medicines and should be guided by functional requirement and justified through a thorough risk-based assessment. Various factors such as frequency of dosing, pediatric age group, and duration of treatment should be taken into account (WHO Technical report series no. 970, 2012) Table 1.
Taste-masking Issues
Taste and odor are important senses for the oral route of administration and are developed at very early stage of the life. Newborns not only have taste buds but also they are more in numbers compared to adults. However, their sensitivity to some taste such as salts develops after 5 months. The sense of smell is also present right from the beginning, which helps baby to localize odors (Babycenter, 2019). There are in-depth and interesting research conducted in the area of the taste of medicines. Some of the researchers even found the reasons for differences in the bitterness profile of the drug perceived by the children over the age of 4. They attributed the reason for different taste perceptions to the presence of specific gene, i.e., bitter taste receptor gene, TAS2R38, and it has several forms. They are the most studied receptor among reported 25 members in the TAS2R family of bitter taste receptors. Genetically, these receptors are extremely diverse and show a selective sensitivity to particular compounds (Mennella et al., 2015).
As a route of administration, oral route is the most preferred by both caretakers and pediatric patients due to its convenience. Liquid dosage forms are the first choice among other dosage forms for oral administration in pediatric population. Most of the medicines are bitter, and this causes noncompliance issues which are of paramount importance when it comes to pediatric population. Parents and caretakers in hospitals often struggle to tackle the issue of bitter drug administration. Nonacceptance of such bitter formulations leads to the missing doses or repeated doses due to spillage or vomiting. This has a serious impact on the overall treatment regimen. Sometimes, this may force physicians to adopt an invasive route for administration, which is not only painful but also not risk-free.
Formulating such bitter drugs poses a major challenge for formulators and hence requires special consideration. Liquid dosage form, by its nature, exposes a higher surface of the drug(s) covering kids’ tongue, which already have higher taste buds (compared to adult) providing increased bitterness profile or perception. Evaluation of bitterness has always been a challenge, especially for pediatric population, as it is hard to predict the bitterness profile of their age specifically because tasting panel comprises of adults. In addition, traditional taste evaluation by expert panel with the scale of 1–10 is very vague estimation of taste. However, recently, artificial taste sensors are available where bitterness intensity scores can be evaluated and formulations can be compared (Ishizaka et al., 2004). Its applicability in children remains to be proved.
In one of the surveys reported by the American Academy of Pediatrics, over 800 pediatricians revealed that unpleasant taste of medication is a major impediment to compliance for 90.8% of patients with acute illness and 83.9% of patients with chronic illness. Thus, the formulation and palatability have affected the acceptability of medicines in children, and the compliance rates have been found to range from 11% to 93%. To overcome the unpleasant taste, in most of the cases to facilitate dosing, patients and carers dilute or mask the taste of a medicinal product by mixing or sprinkling it in food/beverages. However, this approach carries certain risks such as nonconsumption of entire quantity of food or beverage due to too large volume or due to poor taste masking. Hence, this approach of mixing with food or beverage should not be the primary means of taste-masking formulation (Walsh et al., 2014). Sometimes, compounding of medications is needed due to the lack of proper formulations or poor taste and odor. Such compounding medications can compromise safety and efficacy due to the lack of related data (Sarah et al., 2019).
To overcome the unpleasant taste masking of the bitter medicines, various approaches have been used and are still evolving. Some of the techniques are as simple as masking the bitter taste with sweeteners and flavors. Increasing viscosity and use of cosolvents can also help to mask the taste to some extent. However, not all bitter drugs can be formulated with these simple and traditional techniques. Some of the modern approaches include the use of complexation with other molecules such as cyclodextrins that encapsulate the bitter molecule and ion exchange resins that work by exchanging the ions with drug molecule. Other approaches include the use of barrier coating of drugs with polymers or related inert materials such as lipids.
Technology Requirements for Drug Delivery
Due to swallowing issues and patient noncompliance in the smaller age groups, many drugs are required to be available in liquid dosage forms and even in other convenient and patient friendly dosage forms such as soluble films and soluble mini tablets. Some of these dosage forms require special techniques, and these technology requirements may not be always readily available and need to be either indigenously developed or procured from third parties having cost implications.
Considering the diverse age range of pediatric patients, formulation development approach to develop one single formulation that fits all may not be appropriate. Thus, flexible technology platforms are required, which enables the preparation of formulations with different active pharmaceutical ingredients (APIs), dose strengths, and/or release profiles. Some of these technologies may include the production of tablets with multiple splits, and more recently, to achieve the better accuracy of the divided doses, inert drug-free layer is provided at splitting line. The development of the solid dosage pen is another novel drug delivery, which consists of a cylindrical rod manufactured by mass extrusion and incorporated into a pen-like device. Using this handy device, dosing adjustments can be easily made by cutting small tablet-like slices of the required length (Lopez et al., 2015).
Promising and flexible platform technologies are now available to manufacture solid multiparticulate dosage forms (e.g., mini-tablets and pellets) which can be dispersed in liquids or sprinkled on food, fulfilling the requirement of oral medicines to achieve precise dose measurement. This platform technology can provide the flexibility to prepare fixed-dose combination products. These multidrug FDCs are useful, especially for chronic diseases such as HIV or tuberculosis. In addition, new devices as modified feeding bottles and pacifiers are developed, which contain drug reservoir that not only assist the oral delivery of liquids to small children but also improve the palatability of oral solutions by using a dose-sipping technology. Similarly, pulp spoon containing single dry dose of medicine helps to increase the product stability (Ivanovska et al., 2014).
Swallowing the whole tablet has always been cumbersome, especially with pediatric patients, where orodispersible tablets (ODTs) can be preferred as it is designed to disintegrate in the oral cavity quickly, within seconds. It can be also designed to allow the disintegration and dissolution sufficiently fast, where the need of water can be avoided. ODTs can be formulated using various approaches including direct compression, lyophilization, flash heat processing, tablet molding, and recently 3D printing technology. However, manufacturing methods, such as direct compression and lyophilization are most commonly used. Although generally lyophilized tablet offers quicker disintegration (often less than 10 seconds), they are mechanically more fragile than compressed ODTs and therefore necessitate special packaging to keep it intact. Another limitation of lyophilized ODTs is the relatively less amount of dose loading (<400 mg for low water-soluble drugs and ~60 mg for water-soluble drugs). On the other hand, while compressed ODTs have a good mechanical strength, it lacks quick disintegration compared to lyophilized tablets, and hence, the development of compressed ODT formulation is tedious as it has to incorporate both quick disintegration and sufficient mechanical strength. Further, most of the production techniques of ODTs are controlled by patents (Lopez et al., 2015).
Drug-loaded orodispersible films (ODFs) are manufactured using polymeric matrices and can disintegrate rapidly in the mouth releasing the active ingredient. ODFs possess elegant appearance and are likely to be preferred by pediatric patients. These films also offer advantage over tablets such as increased dose flexibility, as different strengths can easily be dispensed by special dispenser, which simply cuts the films of the prescribed size, and require minimal or no water. However, ODFs suffer from technological challenges such as taste masking and controlled release. The oral mucosa has a limited drug absorption capacity, for which the controlled release ODFs become poor candidate, and hence, the controlled release ODFs are best suited for topical delivery rather than systemic delivery. Another limitation of ODFs is the amount of drug that can be loaded that is typically low (<60–70 mg) due to its limited size (2–9 cm2) and thickness (25 μm–2 mm). However, because of novel technologies, it is possible to incorporate the higher drug doses of >100 mg, this amount is still limited, and thus, only potent drugs with specific physicochemical properties can be successfully delivered (Lopez et al., 2015; Visser et al., 2017).
It is recommended to pack each ODF separately to improve stability and to avoid the films sticking together which may lead to overdosing. It also demands sophisticated multidose dispensers using which patient or caregiver could cut the strips of prescribed length. Due to these requirements, the development and production costs incurred on ODFs are higher and may increase the risk of dosing errors. The need for specialized manufacturing and packaging equipment may reduce the viability of the ODF technologies. In fact, due to the manufacturing issues and poor revenue, several commercially available ODF products have been discontinued in the past. However, ODFs still lead the market in over-the-counter medicines including vitamins and food supplements, breath fresheners, antihistamines, and cough suppressants. Ondansetron oral-soluble film was the first prescription-only ODF to reach the market, indicated for adults and children from 4 years of age in the USA (Lopez et al., 2015).
Chewable formulations that include chewable tablets, chewing gums, and soft chews are designed to mechanically process in the mouth to aid disintegration and/or dissolution of the APIs. Similarly, metoclopramide formulation in the form of soft candy (as a jelly) was prepared to mask the bitterness and to improve the patient compliance. This drug is routinely prescribed as an antiemetic during chemotherapy to children in the form of dispersible tablet, and the soft candy is claimed to be superior and acceptable than dispersible tablet. These products offer advantages as it avoids requirement of water and gets rid of swallowing of whole dose unit. Chewable dosage forms are patient friendly due to their esthetic properties and hence may be preferred by patients over other formulations. However, when compared to conventional tablets, chewable products do not offer an advantage in terms of dose flexibility. Further, chewable dosage forms are not suitable for taste masking and controlled release by coating techniques because it is subjected to a great mechanical stress on administration. Further, chewing ability of patients varies which can result in variable drug release and may potentially impact therapeutic effect. This can increase intra- and inter-individual variability with this type of formulations (Ivanovska et al., 2014; Karaiskou et al., 2019).
Although 3D printing technology is relatively new in the field of medicine, it is increasing its share in this area. Spritam® (Levetiracetam) tablets (250/500/750/1000 mg) was the first drug approved by the USFDA, which is produced using 3D printing technology indicated for epilepsy in children and adults (CDER, 2015). It is ODTs and disintegrates within 27 seconds when taken with a sip of liquid. This technology does not require compression to bind the powder together (Printed Labeling for SPRITAM (Levetiracetam) Tablets, 2015). In one of the studies, the patient-specific flexi dosage forms such as chewable jelly was produced by embedded 3D printing technique using model drug ink (containing ibuprofen and paracetamol) which is extruded into soft, sweetened edible gelatine matrix (Preis et al., 2017; Rycerz et al., 2019). Similarly, ODFs of narrow therapeutic index drug such as warfarin were also prepared using various 3D printing techniques allowing dispensing by hospital pharmacist as per the individual patient-specific doses (Heidi et al., 2019). This technique requires material with specific properties. Such excipients and polymers in pharmaceuticals are limited in number. Some of the extensively studied 3D printing techniques, such as fused filament fabrication require drug and polymer to be thermostable (Heidi et al., 2019). Other typical challenges include stability and capacity for large doses (Rycerz et al., 2019).
Some of the examples of the above discussed dosage forms are shown in Table 2.
Existing or evolving innovative drug delivery technologies which are originally targeted for adults could potentially benefit pediatric population. Many novel experimental treatments are now being increasingly available but not limited to nanoparticle-targeted therapy, polymer-based drug delivery systems, new chemical entities (e.g., dendrimers), and remote triggering devices for an adult cancers, infections, and asthma. Once proven effective and safe, these treatments could be applied in children. However, because these innovative technologies are costly, the major challenge remains to extend these technologies for developing new pediatric formulations and makes them available on the market and in daily practice, given that the pediatric market size is limited (Ivanovska et al., 2014).
Challenges of Pediatric Clinical Trials (CTs)
Risks involved
Historically CTs have always focused adults and rarely included children. This has resulted in a scarcity of pediatric labeled formulation and constitutes an underserved market for children. However, the absence of CTs has various reasons. Given the large market size of adults, treatments are generally developed for adults, and hence, CTs always prioritized accordingly. The pediatric subjects belong to the large age group of highly fragmented population based on age, weight, and related factors that are found in adult population. Designing the study and its subsequent interpretation is complicated as children are dynamic population and can change over the course of a single study. In addition, there are ethical concerns while obtaining consent which is expected often from both parents and subjects, who must agree to participate (Aumock et al., 2013).
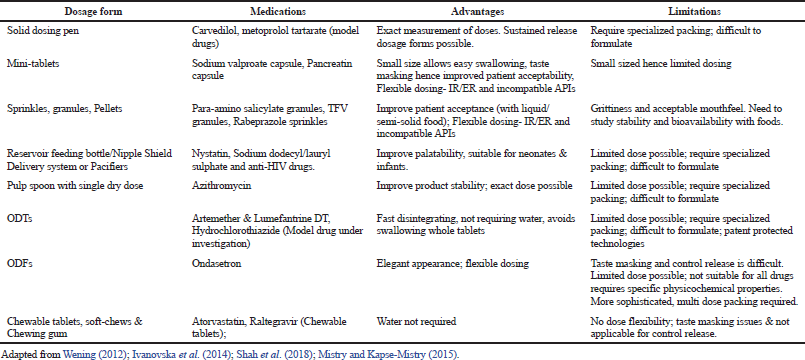 | Table 2. Overview of varied type of dosage forms available for children. [Click here to view] |
Requisites
Some of the requirements for conducting pediatric CTs include novel approaches obtained from rare disease experience, and especially developed clinical research infrastructure to take care of pediatric age group operational challenges (Vanchieri et al., 2008). The preclinical studies to identify appropriate animal models should be a research priority. Further to facilitate pediatric drug development, adult in vitro models, e.g., gastrointestinal models need appropriate adjustment to study the bioavailability in children, and the extrapolation of adult efficacy data requires refined criteria. Ongoing technological advancements should be coupled with appropriate patient outcome studies and clinical feedback on patient compliance, preferences, safety, and efficacy, presently which seem to be lacking. The healthcare professionals and caregivers can play a key role in evaluating novel formulations by collecting relevant information on the basis of practice-based evidence. This could provide additional support for the development of pediatric medicines with clear clinical advantages (Ivanovska et al., 2014).
Data sharing issues
In most of the cases, the pediatric clinical trial data largely remain unavailable, and hence, physicians have no choice but to consider if children are just “small adults” with a smaller weight, and accordingly, the doses are adjusted by interpreting the adult data. However, according to the statistics, such an approach works only in 6% of the cases (Shelley, 2015). Access to raw data has been major challenge, for which the National Institute of Health (US Department of Health Sciences) has mandated to share the data by cross-collaborating among investigators. The challenge still remains for the lack of uniformity in data collection methods and data fields across investigators (Mulugeta et al., 2017). At present, there are few and dotted attempts to collect the available data, which can be helpful for the future study. One of such attempts is done in the USA in 2010, where the pediatric trials network (PTN) is working under the sponsorship of the National Institute of Child Health and Human Development. The PTN is an alliance of clinical research sites cooperating in the design and conduct of pediatric CTs. PTN research provides evidence for optimal dosing of commonly used medications in infants and children with the objective of improving health care for these patients. It conduct the studies designed to determine the dosing, safety, and efficacy of drugs that have been approved for adults but lack information for the pediatric population and developed a repository of the electronic health record data gathered from nearly 265,000 pediatric patients to guide research (Julia, 2018). Similar efforts were made in Europe in 2011 by establishing the European Network of Pediatric Research at the European Medicines Agency (Enpr-EMA). Enpr-EMA is a network of research networks, which includes academia, pharmaceutical industry, regulators, and the pediatric committee (PDCO) members from within and outside the EU, facilitating collaboration with members. However, there are no publications on industry expectations of networks or other pediatric clinical trial networks’ capabilities, funding, and resources related to industry services (Lepola et al., 2016).
Low Market Size, Hence Low Profitability
Until now, the pharmaceutical companies have focused almost exclusively on adult indications because of varied cost and ethical issues involved with testing in pediatric population. However, there are a wide variety of diseases, for which not only treatment to be developed for children but also gather knowledge about children’s response to them (Shelley, 2015).
Apart from ethical concerns, an economic barrier also exists. The pharmaceutical companies have always been hesitating to invest in pediatric drug development because of the primary reason of relatively small market size which reduces financial outcomes. However, there are various other concerns with respect to pediatric studies which require various special considerations compared to adult studies. Some of them include a requirement of different endpoints; more innovative statistical design is warranted due to limited sample volume or requires multisite or even global studies to incorporate sufficient patients; and the additional safety concerns must be taken into account, such as issues of growth and development (Vanchieri et al., 2008).
Until recently except for therapies created expressly to treat children (such as childhood vaccines or perhaps for type I diabetes), drug companies hardly had any incentive to undertake the added effort and risk to carry out the pediatric CTs. The reasons include a limited market potential for any specific pediatric indication of a given drug, the cost and complexity of pediatric CTs, and the inherent logistical, clinical, and ethical challenges of enrolling children in CTs (Shelley, 2015).
Cost implications are multifold as a lack of availability of a large number of subjects necessitates a need for conducting studies at multiple locations (or geographies). This is further influenced by the ethical perception to protect vulnerability (Leibson and Koren, 2015).
The pediatric drug development is associated with numerous challenges, including methodological and ethical requirements for pediatric trials, high developmental costs, and a small and fragmented market. As a result of these challenges in pediatric drug development, there have hardly been any research efforts done to adapt medicines according to pediatric needs. Thus, only one-third of all medicines approved by the European Medicines Agency over the period of 1995–2005 were licensed for use in children. Higher but still unsatisfactory rates were reported in New Zealand (35%), Australia (38%), and the United States (54%). It is not only about the lower numbers but also about limited therapeutic area such as anti-infectives, hormones, and medicines for the respiratory and central nervous system. In addition, dermal preparations and medicines specifically aimed at younger age groups for the cardiovascular system, sensory organs, and cancers are rarely available. Moreover, especially in younger children and neonates, even authorized pediatric medicines may not always be age appropriate with respect to dosing, suitability of dosage forms, and excipients (Ivanovska et al., 2014).
Lack of Regulatory Clarity
When pediatric formulation is not available, healthcare professionals have no alternative but to use adult medicines in an off-label or unlicensed manner. This trend is common as 45%–60% of all medicines given to children in European Union are off-label. For neonates and infants, such use reaches to 90%, particularly in pediatric intensive care units. Not surprisingly, off-label use is common for antiarrhythmics, antihypertensives, proton-pump inhibitors, H2-receptor antagonists, antiasthmatic agents, and some antidepressants. In the United States, two-thirds of medicines used in pediatrics are off-label. Even worldwide, this proportion is as high as up to three-quarters (Ivanovska et al., 2014).
Such off-label use of medicines is not authorized, and most of the formulations were not designed for pediatric populations and hence “extemporaneously” used. This unsafe practice continued as there was a lack of regulatory clarity. In the US in 1994, the Pediatric Labeling Rule was introduced and updated to pediatric rule in 1998. However, this has not resulted in sufficient success until the introduction of FDA modernization act (1997) and introduction of Best Pharmaceutical for Children Act (BPCA) (2002) with 6 months of exclusivity. Although BPCA offered incentives, it depends on the drug sponsors to opt for it or opt out. Considering this, in 2003, Pediatric Research Equity Act (PREA) introduced which mandates new drug development to include the studies on pediatric population. In 2012, the FDA safety and innovation act passed by the US congress, where both BPCA and PREA were converted to permanent laws. However, these acts do not cover orphan drugs as they are exempted. In 2017, the FDA reauthorization act added orphan therapies for pediatric cancers to PREA mandate list (Treatment Action Group, 2019). These recent efforts have improved the situation; however, still, most of the rare diseases are waived. Other challenges such as timely completion of pediatric studies and availability of pediatric study results to end point users remain (Bourgeois et al., 2017). Efforts were initiated in Europe in 1997 with agreement of incentive for pediatric clinical trial (1998) and related guideline (2001). Actual legislation was introduced in 2007 by setting up the PDCO and Pediatric Investigation Plan (PIP).
Insufficient regulatory clarity, especially in the area of pediatric CTs, is another impediment for pediatric drug development. Obtaining informed consent from both child and parents is delicate and challenging. Furthermore, for younger ones, the ever changing status of adolescence is still difficult to translate to informed consent (Leibson and Koren, 2015).
There are ongoing regulatory challenges for the pharmaceutical industry. This was highlighted in a report to the European Commission for the year 2014 on companies and products that have benefited from the rewards and incentives of the Pediatric Regulation. However, companies who have failed to comply with obligations highlighted some of the regulatory challenges which are seen in relation to PDCO decisions, resulting in delayed timelines, PIPs, and annual reporting of the deferred PIP trials or planned PIP progress (Ivanovska et al., 2014; Lepola et al., 2016; Shelley, 2015). In the EMA annual report (2013), the proportion of delayed PIP applications (6 months or more) was about 20%, and the time lag for submissions and full waiver applications was over 2 years on average. In addition, 31% of PIPs scheduled to be completed by June 2013 were not completed in time and did not have any justifications or agreement to change the timelines. The annual report cites several problems as reasons for PIP deferrals, such as recruitment difficulties (28.4%), other (nonspecified) reasons (19.4%), problems with national competent authorities (9.1%), and similar problems with ethics committees (7.5%). In addition, safety concerns were reported in few cases (4.7%) (EMA, 2014).
Failures to noncompliance with these regulations had not been because of shortcomings on the part of investigators, members of the institutional review boards (IRBs), or others, rather, it had been attributed to the ambiguity and lack of clarity in the regulations themselves. There had been attempts to clarify some key terms and concepts in the regulations. Chapter 4 of the report also recommends that the government provides a more interpretive guidance and provides the examples of procedures and studies that illustrate the permissible research involving infants, children, and adolescents. Such guidance can help investigators and IRBs to better understand their responsibilities and the boundaries between acceptable and unacceptable practices (Field and Berman, 2004).
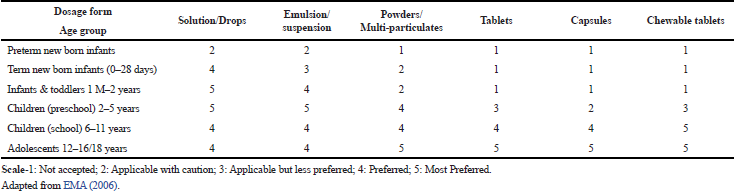 | Table 3. Preferences in children of different age groups for various oral dosage forms. [Click here to view] |
In the latest EMA annual report of 2018, a review of 10 years of the EU Pediatric Regulation is carried out and agrees that the changes brought about by the regulations have not been as effective in the therapeutic areas such as oncology or neonatology. Some of the areas that were benefited include infectious diseases and rheumatological conditions. Further actions proposed include identifying pediatric needs, strengthening cooperation between decision-makers, ensuring timely completion between PIPs, improving the handling of PIP applications, and increasing transparency around pediatric medicines. These recommendations are expected to improve the regulatory processes and thereby encourage the availability of pediatric medicines (EMA, 2018). Addendum (R1) to Guidance for Industry (2018) Clinical Investigation of Medicinal Products in the Pediatric Population was published recently (2018), where additional guidance on various aspects required to be considered during CTs are discussed (Guidance for Industry, 2018).
All these regulatory efforts for pediatric drug development are mostly initiated in the developed countries of Europe, the United States, and Australia, where strong regulatory and legal framework exists. However, in most of the developing countries, the situation is worrisome as, barring a few, such a strong regulatory and legal framework hardly exists. A lack of awareness or interest among healthcare professionals may be the reason for the lack of pediatric clinical or safety data from developing countries. These conditions may result in prescribing unlabeled or extemporaneously prepared drugs despite of having safe and effective alternatives at hand, which exposes children to dangerous and unproven therapies. Hence, it is of utmost importance that the physicians in the developing countries need to be aware of or sensitized to the issue of unlicensed and off-label drug use in children (Oberoi, 2015). In the past few years, some of the developing countries such as China and India have initiated regulatory reforms and related guidelines to encourage the pediatric drug development and to address the ethical issues related to it. In low-income countries in Africa, although specific pediatric regulations do not exist, a training program (African Pediatric Fellowship Program) has been initiated to provide training to healthcare professional dealing with children (Gerrard et al., 2019).
Pediatric drug delivery
Before the evaluation of various challenges involved in pediatric drug delivery, the formulators’ focus remains on delivering the dosage forms, which can trade if the challenges meet with the benefits. There are different dosage forms available. Available dosage forms can be divided based on the type of dosage forms and the route of administration. Among various routes of administration, oral route is the first and foremost choice for the children as it is noninvasive, painless and does not need special skill. In general, for oral route, solid dosage forms are the most economical and convenient dosage forms among larger adult population. However, solid dosage form is not good choice for the pediatric patients, especially smaller age children. Hence, special dosage forms are required to be provided to this section of population. Furthermore, dosage forms, which are suitable for the different age groups within pediatric population, require special considerations as the groups are diverse. An overview of preferences for different oral dosage forms is given in Table 3. This reflects that lower age group, i.e., infants and preschool children up to the age of 5 years prefers liquid dosage forms. Solid dosage forms (tablets and capsules) are more suitable for school-age children and adolescents.
Given the fact that the liquid dosage forms are the preferred choice of dosage forms for pediatric population, not all drugs are available in the liquid form. Wherever liquid dosage forms are available, many of them are not labeled for pediatric populations as they are not tested and approved in such population. Hence, they are called “off-label.” On the other hand, few of the medications which are labeled for pediatrics are not available in the appropriate dosage forms. In cases when there are no liquid dosage forms available, the suitable dosage forms are “especially” made or “extemporaneously” prepared from the existing/available dosage forms. Mostly, tablets and capsules are converted into either oral liquids or powders (Auilina, 2013). These “specials” and “extemporaneous” dosage forms are, therefore, considered as unlicensed dosage forms. “Specials” are products which have been specially manufactured or imported for the treatment of an individual patient after being ordered by a physician, dentist, nurse-independent prescriber, pharmacist-independent prescriber, and supplementary prescriber (Standing and Tuleu, 2005). Figure 2 shows the decision pathway for providing the oral doses to children, for whom whole tablet/capsules are unsuitable.
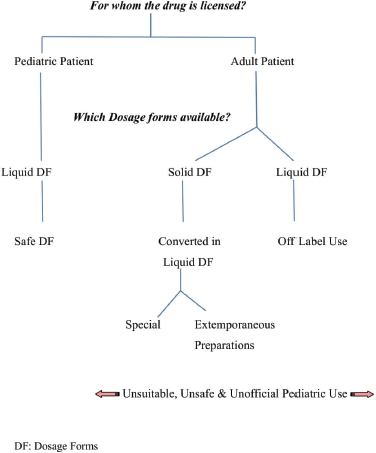 | Figure 2. Decision pathway for providing oral doses to children for whom whole tablet/capsules are unsuitable (Standing and Tuleu, 2005). [Click here to view] |
Clinicians and pharmacists are forced to use the alternative solutions due to the widespread lack of pediatric format oral products which are not typically backed by supporting bioavailability, stability, and safety studies. Most of the dosage forms which are originally designed for adults are, therefore, extemporaneously used by crushing the tablets or removing the capsule content and are mixed with food or drink. These delivery methods are not only inconsistent but they are also prone often to errors in dosing, reduced bioavailability or efficacy, and noncompliance because of foul-tasting APIs (Teresk et al., 2017). The nonavailability of pediatric friendly dosage forms leaves the physician with no choice but to prescribe solid dosage forms, which are associated with dosing errors, resulting due to poor division or due to extemporaneous way of dispensing as described earlier. This becomes even more critical for the antibiotics, which are most widely prescribed class of medicines in children. Thus, errors in antibiotic doses have a potential to either cause toxicity or inefficacy leading to antibiotic resistance which is already pandemic fuelled due to poor compliance, low regulations, and inadequate care taker resources for children in low- and middle-income countries (Kirsty et al., 2015). Although the level of relevant research in this field is on the rise, only a fraction of the available therapies in adults has been adequately evaluated in pediatric populations to assess age-appropriate dosing, tolerability, and efficacy (Bucci-Rechtweg, 2017).
CONCLUSION
The pediatric dosage forms have never been focus of the mainstream development of pharmaceuticals. The reason lies in the multifold challenges with respect to this segment of pediatric age group, some of which are discussed in this review. Industry, technology, and regulations should be the key factors affecting the overall development of pediatric population. Even today, traditional dosage forms are most widely used in pediatric patients as they are economical for mass production and do not require new settings compared to the novel dosage forms. As the acceptance of the formulations by the children is first step to the success of the therapy, newer devices and taste-masking techniques have been evolving to help existing traditional dosage forms to be more patient friendly. Novel dosage form development requires separate considerations for pediatric and cannot be seen in the same way as development for adult population, given the volumes of challenges. Although the latest development in the technology has helped in the recent past to improve the situation, the technological advantage has not been fully utilized for the pediatric dosage form. To improve this situation further, the regulatory agencies are updating the pertaining acts and regulations in this regards. Incentives offered by the approving agencies in terms of exclusivity should work as a stimulus for the pharmaceutical industries to consider the pediatric dosage form development as a “serious” business.
FINANCIAL SUPPORT
None.
CONFLICTS OF INTEREST
Authors declare that they do not have any conflicts of interest.
REFERENCES
AAP (American Academy of Pediatrics), Committee on Drugs. Inactive ingredients in pharmaceutical products: update (subject review). Pediatrics, 1997; 99:268–78. CrossRef
AAP (American Academy of Pediatrics). Age Limits of Pediatrics. Pediatrics, 1988; 81(5):736.
Adrienne FS, Andrew RD, Rachel CV. The need for pediatric formulations to treat children with HIV. AIDS Res Treat, Volume 2016; 1654938. CrossRef
Afaque RMA, Saddamhusen JM, Gosavi JP. Review on artificial sweeteners used in formulation of sugar free syrups. Int J Adv Pharm, 2015; 4(2):2,4.
Allam KV, Kumar GP. Colorants- The cosmetics for the Pharmaceutical dosage forms. Int J Pharm PharmSci, 2011; 3:20.
Auilina A. The extemporaneous compounding of pediatric medicines at Mater Die Hospital. J Malta Coll Pharm Pract, 2013; 19:28–30.
Aumock N, Smith J, Townsend S. Do incentive drive pediatric research? McKinsey Center for Government. McKinsey & Company, New York, NY, 2013.
Babycenter. Your newborn’s development (Birth to 12 Month). 2019. Available via https://www.babycenter.com/0_your-newborns-development-birth-to-12-mo_1477143.bc (Accessed 21 August 2019).
BNF (British National Formulary) for Children, 2006. London: BMJ Publishing Group, RPS Publishing Society of Great Britain, and RCPCH Publications.
Bourgeois, FT, Hwang TJ. The pediatric research equity act moves into adolescence. JAMA, 2017; 317(3):259. CrossRef
Bucci-Rechtweg C. Enhancing the pediatric drug development framework to deliver better pediatric therapies tomorrow. Clin Ther, 2017; 39(10):1920. CrossRef
Buck M. Alternative forms of oral drug delivery for pediatric patients. Pediatr Pharmacotherapy, 2013; 19(3):1.
CDER. Approval Package for Application no. 207958Orig1s000. 2015. Available via https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/207958Orig1s000Approv.pdf. (Accessed 28 November 2019)
Delanaye P, Schaeffner E, Ebert N, Cavalier E, Christophe M, Krzesinski JM, Moranne O. Normal reference values for glomerular filtration rate: what do we really know? Nephrol Dial Transpl, 2012; 27:2664. CrossRef
Dunne J, Margaryants L, Murphy MD, Myers AM, Avant D, Rodriguez WJ. Globalization facilitates pediatric drug treatment in the 21st Century. Ther Innov Regul Sci, 2010; 44(6):757–65. CrossRef
EMA (European Medicine Agency). Annual Report—The European Medicines Agency’s contribution to science, medicines and health in 2018, pp 23–4, 2018.
EMA (European Medicine Agency). Report to the European Commission. Human Medicines Research and Development Support Division, EMA/24516/2014 Corr.3, p 35, 2014.
EMA (European Medicines Agency). Reflection paper: formulations of choice for the pediatric population (CHMP). Committee for Medicinal Products for Human Use, 3, p 22, 2006.
Field MJ, Berman RE. The ethical conduct of clinical research involving children. Institute of Medicine of National Academics. National Academic Press,Washington, DC, p 272, 2004.
Fiocchi A, Riva E, Giovannini M. Ethanol in medicines and other products intended for children. Nutr Res, 1999; 19(3):373–9. CrossRef
Gerrard SE, Walsh J, Bowers N, Salunke S, Hershenson S. Innovations in pediatric drug formulations and administration technologies for low resource settings. Pharmaceutics, 2019; 11:518. CrossRef
Grissinger M. Medication errors affecting pediatric patients: unique challenges for this special population. Penn Patient Safety Authority, 2015; 12(3):96–102.
Guidance for Industry. E11 (R1) Addendum: Clinical Investigation of Medicinal Products in the Pediatric Population. US Dep. of Health and Human Services. FDA. CDER. CBER. ICH, 2018.
Heidi Ö, Erica S, Maria R, Niklas S. Towards printed pediatric medicines in hospital pharmacies: comparison of 2D and 3D-Printed orodispersible warfarin films with conventional oral powders in unit dose sachets. Pharmaceutics, 2019; 11:334. CrossRef
Ibero M, Eseverri JL, Barroso C, Botey J. Dyes, preservatives and salicylates in the induction of food intolerance and/or hypersensitivity in children. Allergol Immunopathol, 1982; 10:263–8.
Ishizaka T1, Miyanaga Y, Mukai J, Asaka K, Nakai Y, Tsuji E, Uchida T. Bitterness evaluation of medicines for pediatric use by a taste sensor. Chem Pharm Bull (Tokyo), 2004; 52(8):943–8. CrossRef
Ivanovska V, Rademaker CMA, van Dijk L, Mantel-Teeuwisse. AK. Pediatric drug formulations: a review of challenges and Progress. Pediatrics, 2014; 134(2):366. CrossRef
Julia. PTN creates data repository to aid in pediatric research. Pediatric Trial Network Newsletter. Sponsored by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), 2018. Available via https://pediatrictrials.org/ptn-creates-data-repository-to-aid-in-pediatric-research/ (Accessed 11 August 2018).
Karaiskou SG, Kouskoura MG, Markopoulou CK. Modern pediatric formulations of the soft candies in the form of a jelly: determination of metoclopramide content and dissolution. Pharm Dev Technol, 2019; 25(1):20–7. CrossRef
Kirsty L D, Barker CI, Irwin A, Sharland M. Improving antibiotic prescribing for children in the resource-poor setting. Br J Clin Pharmacol, 2015; 79(3):446–55. CrossRef
Kumar A, Aitas AT, Hunter AG, Beaman DC. Sweeteners, dyes and other excipients in vitamin and mineral preparations. Clin Pediatr, 1996; 35:443–50. CrossRef
Leibson T, Koren G. Informed consent in pediatric research. Pediatr Drugs, 2015; 17:5. CrossRef
Lepola P, Tansev S, Dicks P, Preston J, Dehlinger-Kremer M. Pharmaceutical industry and pediatric clinical trial networks in Europe—how do they communicate? Appl Clin, 2016. Available via http://www.appliedclinicaltrialsonline.com/pharmaceutical-industry-and-pediatric-clinical-trial-networks-europe-how-do-they-communicate?pageID=3 (Accessed 11 August 2018).
Lopez FL, Ernest TB, Tuleu C, Gul MO. Formulation approaches to pediatric oral drug delivery: benefits and limitations of current platforms. Expert Opin Drug Deliv, 2015; 12(11):1728–9. CrossRef
Mannan A, Jabeen A, Mubeen H, Nasiha AW. Challenges and advances in pediatric pharmaceutical dosage forms. Int J Pharm Biol Sci, 2018; 8(1):5. CrossRef
Mennella JA, Roberts KM, Mathew PS, Reed DR. Children’s perceptions about medicines: individual differences and taste. BMC Pediatrics, 2015; 15:131. CrossRef
Meyers R, Myers NJ. Patient-centric drug design: a clinical & academic perspective. Dosage form development supplement 2016. American Pharm Review, 2016; 14–5.
Mistry A, Kapse-Mistry S. Innovative pediatric drug delivery systems. J Harmo Res Pharm, 2015; 4(2):127.
Mulugeta Y, Zajicek A, Barrett J, Sachs HC, McCune S, Sinha V, Yoa L. Development of drug therapies for newborns and children - the scientific and regulatory imperatives. Pediatr Clin N Am, 2017; 64:1191–2. CrossRef
Oberoi S. Regulating off-label drug use in India: the arena for concern. Perspect Clin Res, 2015; 6(3):129–33. CrossRef
Pawar S, Kumar A. Issues in the formulation of drugs for oral use in children. Pediatr Drugs, 2002; 4:371–9. CrossRef
Preis, M, Öblom, H. 3D-printed drugs for children—are we ready yet? AAPS PharmSciTech, 2017; 18(2):303–8. CrossRef
Printed Labelling for SPRITAM (levetiracetam) Tablets. 2015. Available via https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/207958Orig1s000lbl.pdf (Accessed 12 March 2019).
Quique Bassat. The unmet needs of pediatric therapeutics in poor countries. J Trop Pediatr, 2015; 61:403–6. CrossRef
Rycerz K, Stepien KA, Czapiewska M, Arafat BT, Habashy R, Isreb A, Peak M, Alhnan MA. Embeded 3D printing of novel bespoke soft dosage form concept for pediatrics. Pharmaceutics, 2019; 11:630. CrossRef
Sarah E, Karen E, Joseph L, Ann MP. Rational and irrational use of nonsterile compounded medications. ACCP white paper. J Am Coll Clin Pharm, 2019; 2:189–97. CrossRef
Shah BA, Patel AS, Patel BJ, Patel DJ, Qu A. Mini-Tablet drug delivery system for pediatric dosage form: a review of manufacturing process. Int J Drug Dev Res, 2018; 10(3):048.
Shelley S. Updated 2016 Feb 22. Pediatric drug market finds its own pathway. Pharmaceutical Commerce. New York, NY, 2015. Available via http://pharmaceuticalcommerce.com/brand-marketing-communications/pediatric-drug-market-finds-its-own-pathway/ (Accessed 25 March 2019).
Standing JF, Tuleu C. Pediatric formulations-Getting to the heart of the problem. Int J Pharm, 2005; 300(1–2):57. CrossRef
Teresk MG, Berkland CJ, Dormer NJ. Deficiencies in traditional oral dosage forms and the emergence of controlled-release powder manufacturing. KONA Powder Part J, 2017; 34:91. CrossRef
Treatment Action Group. Ensuring Treatment for Children with Orphan Diseases: Ending exemptions from the Pediatric Research Equity Act (PREA), 2019. Available via http://www.treatmentactiongroup.org/sites/default/files/prea_brief_2019_final.pdf (Accessed 12 March 2019).
US Department of Health and Human Services FDA CDER. Clinical Pharmacology, (Draft) Guidance for Industry – General Clinical Pharmacology Considerations for Pediatric studies for Drugs and Biological products, 2014.
Vanchieri C, Butler AS, Knusten A, Rapporteurs. Addressing the barriers to pediatric drug development. Workshop Summary. Forum on Drug Discovery, Development, and Translation. Institute of Medicine, National Academies Press, Washington, DC, vol 1, pp 3–4, 2008.
Visser JC, Woerdenbag HJ, Hanff LM, Frijlink HW. Personalized medicine in pediatrics: the clinical potential of orodispersible films. AAPS Pharm Sci Tech, 2017; 104(5):1292–300. CrossRef
Walsh J, Cram A, Woertz K, Breitkreutz J, Winzenburg G, Turner R, Tuleuc C. Playing hide and seek with poorly tasting pediatric medicines: do not forget the excipients. Adv Drug Deliv Rev, 2014; 73:15. CrossRef
Wening K, Laukamp EJ, Thommes M, Breitkreutz J. Individual oral therapy with immediate release and effervescent formulations delivered by the solid dosage pen. J Pers Med, 2012; 2:219. CrossRef
WHO Technical Report Series No. 970. Development of Pediatric medicines: WHO Annexure Points to be considered in formulation, 2012.