INTRODUCTION
Skin aging is a common and gradual degenerating physiological and biochemical process that affects the skin in several ways. During aging, skin becomes flimsy, thinner and gradually decreases its ability to maintain hydration and natural elasticity resulting in the formation of wrinkles (Pimentel et al., 2018; Wang et al., 2015). Biochemical processes such as enzymatic degradation (metalloproteinases) of elastin and collagen occur in the epidermal and dermal layers of the skin causing degeneration of the extracellular matrix. Besides, external factors such as ultraviolet radiation exposure results in the activation of tyrosinase and collagenases, resulting in melanin production, aging of skin, and formation of wrinkle (Wu et al., 2018). Thus, explorations on the use of naturally occurring source (such as seaweeds) of active ingredients in the formulation of personal care products that have beneficial effects in preventing skin aging are sought after in the pharmaceutical industry.
The value of the worldwide seaweed market in 2017 is about USD 11.48 billion and is estimated to attain USD 21.75 billion in 2025 with the Asia Pacific region to hold the largest global market share (Commercial Seaweed Market Size, 2019). The projected market growth of seaweed is at a rapid pace because of the health benefits associated with seaweed products, which motivates consumers to utilize these important resources. As reported by the Food and Agriculture Organization, a total of 23.8 million tons of seaweeds ($6.4 billion) are being harvested and utilized yearly (Pimentel et al., 2018). The leading countries for seaweed production are China and Indonesia generating 54% and 27%, respectively, of the total bulk production. Other Asia Pacific countries such as the Philippines, Korea, Japan, and Malaysia followed the leading countries with a total seaweed production share of 7.4%, 4.3%, 1.85%, and 1.39%, respectively (Pimentel et al., 2018). Conventionally, seaweeds are being utilized as food, ingredient for animal feeds, for medicinal purposes, or an alternative source of fertilizer. However, recent scientific evidence proved that these valuable resources could also be used for cosmetic application (Pimentel et al., 2018). The global need for cosmetic products is constantly increasing since there is a huge demand by millions of consumers for cosmetics and their active ingredients. In fact, the global cosmetic market is believed to increase to USD 429.8 billion by 2022 displaying a compound annual growth rate of 4.3% for the duration of 2016–2022 (Cosmetics Market by Category, 2019). Nowadays, the cosmetic industry is developing innovative personal care products that satisfy the needs and expectations of consumers by finding cheap, sustainable, and natural raw materials possessing diverse bioactive compounds as a source of active ingredients (Wu et al., 2018). Seaweeds are an excellent naturally occurring resource that can satisfy these prerequisites. These organisms are important marine resources that are known to possess biologically active substances such as lipids, polysaccharides, proteins, pigments, and polyphenol showing significant antioxidative, antibacterial, antitumor, tyrosinase inhibition, antiviral, and skin-lightening activities (Bourguiba et al., 2017). The integration of seaweed-derived constituents in the formulation of cosmetics has long been studied as several research findings on their potential beneficial attributes in preventing skin aging have been reported. Dolorosa et al. (2019) showed that the methanolic extract of Eucheuma cottonii and Sargassum plagyophyllum has a potent tyrosinase inhibition activity with an IC50 of 2,631.648 and 1,769.336 μg ml-1, respectively. Furthermore, biologically active substances with potential use in cosmetics such as terpenoids, saponins, steroids, alkaloids, flavonoids, and tannins were also observed from the extracts. Besides, Jiménez et al. (2010) reported the antioxidant, tyrosinase inhibition, and antibacterial (against Micrococcus luteus and Staphylococcus aureus) activities of brown seaweed Ascophyllum nodosum. The acetone extract of the macroalga was able to display an IC50 value of 0.1 mg ml-1 with 65.6% inhibition of tyrosinase. These findings give highlights on the possible use of several seaweed species as a source of substances with bioactive properties that can be used in the formulation of cosmetic products.
The Philippine coast has suitable environmental conditions and several coastal water with a diverse number of marine seaweeds that may have lead bioactive compounds with potential use in the cosmetic industry yet to be explored. Relatively, few studies are known about the anti-melanogenesis, antioxidant, and antibacterial potential of Philippine seaweeds, and to date, the scientific investigations of its biological activities for the formulation of cosmetics are still limited in the country (Arguelles et al., 2019). Previously, Corpuz et al. (2013) studied the beneficial effects of Sargassum siliquosum extract as a potential source of naturally occurring antioxidants. The results suggest that the seaweed extract has bioactive substances that can act as a free radical scavenger with antioxidant and anticancer properties. This study is the first report in the Philippines exploring the biological activities of a brown macroalga, S. siliquosum J. Agardh, with potential use for cosmetic application. The current study sought to do screening for antibacterial, tyrosinase inhibition, and antioxidative activities as well as know the total phenolic content of S. siliquosum (making use of gallic acid as a reference standard). Furthermore, the correlation among phenolic content and antioxidant activity of the seaweed was analyzed.
MATERIALS AND METHODS
Collection of seaweed
The marine brown macroalga S. siliquosum J. Agardh was freshly collected on 24 November 2019 from Catanauan (Lat. 13° 36’ 20.88’ N; Long. 122° 14” 18.24’ E), Quezon, Philippines. The seaweed was identified based on morphotaxonomic features according to Trono (1992) and Algae Base (web site: www.algaebase.org) (Guiry and Guiry, 2017). The collected macroalga was immediately washed with seawater using soft brush bristles to remove sand particles, animal castings, and attached detritus. The necrotic parts of the seaweed were also removed. The cleaned seaweed was immediately transferred to the laboratory using sterilized polythene bags. The seaweed sample was then washed several times with sterile tap water to take away excess salt on the external portion of the alga. The algal sample was then placed on a clean tissue paper to wipe off the water residues. The sample was air-dried (for 6 days), cut into small portions, and pulverized into a fine-grained powder before extraction and/or isolation of bioactive components.
Seaweed extract preparation
The powdered algal biomass (1 g) was soaked in 30 ml of acidified methanol (1 HCl:80 CH3OH:10 H2O), extracted for 30 minutes using an ultrasonic bath, and stirred for 1 hour. The seaweed extract was centrifuged at a speed of 12,000 rpm at 20°C for 20 minutes. The collected seaweed extract was dried out by means of a rotary evaporator subjected to reduced pressure (at 40°C) until a concentrated crude macroalgal extract was gathered. The extract was kept at 4°C to maintain its activity before using it in a different assay (Arguelles et al., 2019).
Determination of total phenolic content
The phenolic content of S. siliquosum was estimated using the Folin–Ciocalteu reagent as given by Nuñez Selles et al. (2002). A portion (0.5 ml) of the diluted seaweed extract was thoroughly mixed with an equal volume of Folin– Ciocalteu reagent and 10% sodium carbonate solution for 1 minute. The mixture was then set aside for 5 minutes, and its volume was adjusted by adding 5 ml of sterile distilled water. The optical density (OD) reading of the reaction mixture was taken at 720 nm using an Ultraviolet-Visible spectrophotometer. A calibration curve was constructed using gallic acid with a prepared range of concentrations from 20 to 100 μg ml-1. The total phenolic concentration (TPC) of the seaweed extract was presented as mg gallic acid equivalents (GAE) g-1 of the macroalgal samples.
Diphenyl-1, 2-picrylhydrazyl (DPPH) radical scavenging assay
The DPPH scavenging assay was performed based on the methods of Ribeiro et al. (2008) with few alterations. A portion (100 μl) of the algal extract was thoroughly mixed with 5 mM of DPPH solution. The solution was set aside at room temperature for 20 minutes, and the OD for each solution was noted at 517 nm by means of a spectrophotometer. Ascorbic acid (prepared in different concentrations: 0.08, 0.16, 0.24, 0.32, and 0.40 mg ml-1) was used as the positive control in this study. The percentage inhibition of DPPH was estimated using the following equation:
where Acontrol is the OD reading of the control, and Asample is the OD reading of the algal sample. IC50 was calculated to denote the concentration (expressed in mg GAE ml-1) of the macroalgal extract that can exhibit a 50% scavenging of the DPPH radical.
Copper reduction antioxidant capacity (CUPRAC) assay
The CUPRAC assay was done following the method described by Alpinar et al. (2009). Briefly, 1 ml each of 0.01 M CuCl2, 0.0075 M neocuproine, and 1 M ammonium acetate buffer (pH 7) solutions were thoroughly mixed in a test tube. Subsequently, 0.5 ml of the prepared seaweed extract at varying concentrations (5, 10, 15, 20, and 25 μg GAE ml-1) as well as ascorbic acid standard solutions was combined in the initial mixture. The total volume for each concentration was adjusted up to 4.1 ml by adding sterile distilled water. The mixtures were set aside at ambient temperature for 30 minutes, and the OD of each tested sample was measured at 450 nm.
Tests microorganisms
Type cultures of three pathogenic Gram-negative bacteria (Pseudomonas aeruginosa BIOTECH 1824, Enterobacter aerogenes BIOTECH 1145 and Escherichia coli BIOTECH 1825) and two Gram-positive bacteria (Staphylococcus epidermidis BIOTECH 10098 and S. aureus BIOTECH 1823) were acquired from the Philippine National Collection of Microorganisms, National Institute of Molecular Biology and Biotechnology (BIOTECH), University of the Philippines Los Banños (UPLB). The type cultures of pathogenic organisms were precultured using Luria–Bertani medium with shaking overnight at 37°C. The morphological and biochemical tests were checked continuously to ensure purity (Arguelles, 2018).
Microdilution antibacterial assay
The minimum inhibitory concentration (MIC) of the macroalgal extract was analyzed by using a two-fold serial dilution technique according to the method done by Arguelles (2018), with few modifications. Using a 96-well microtiter plate, 100 μl of pathogenic bacterial cultures (cell density of 1 × 105 cells ml-1) were placed to a 100 μl of seaweed extract prepared in varying dilutions (1,000–7.8125 μg ml-1). The acidified methanol was also included as the negative control. The experimental plate was stored at 35°C in an incubator for 24 hours, after which MICs were noted. MIC is the minimum concentration of the test seaweed extract that can cause the inhibition of bacterial growth after an incubation time of 12 hours. On the other hand, MBC was analyzed by plating a loopful of sample from each MIC well that showed the inhibition of growth into newly prepared culture (tryptic soy agar) medium (Arguelles, 2018). The culture plates were stored at 35°C for 24 hours and were checked for visible colony growth or lack of growth for each dilution subculturing. No bacterial growth would mean that the seaweed extract was bactericidal at that particular dilution. MBC value is the minimum extract concentration, at which no observable bacterial growth on agar subculture was noted (Arguelles et al., 2019).
Tyrosinase inhibition assay
The tyrosinase inhibitory activity of S. siliquosum extract was assayed in in vitro conditions by using a microplate reader following the procedures described by Hapsari et al., (2012) with few alterations. The solutions of 5 mM DOPA (3,4-dihydroxy-L-phenylalanine, Sigma D-9628), 0.1 M potassium phosphate buffer, pH 6.5, and mushroom tyrosinase (250 units ml-1, Sigma T-3824) were prepared. To 40 μl of DOPA (3,4-dihydroxy-L-phenylalanine), 40 μl of seaweed extract and 40 μl of buffer (in the case of the control) were mixed in a microtiter well plate. Phosphate buffer was added to the solution to come up with a total volume of 160, and finally, 40 μl of tyrosinase was added. The blank used was the OD without the enzyme solution. After 15 minutes, the OD at 490 nm was noted using a microplate reader. Tyrosinase inhibition activity was calculated using the following equation:
where Ac is the OD of the control, Ab is the OD of the blank, and As is the OD of the sample.
Statistical analyses
Each experiment run was done at three experimental replicates and the results are shown as means ± standard deviations (mean ± SD). The statistical tests for the linear correlation coefficient needed for correlation analysis were analyzed using MS Office Excel 2007.
RESULTS AND DISCUSSION
Total phenolic content (TPC)
The TPC detected in the seaweed extract was analyzed employing the Folin–Ciocalteu method and was presented in GAE (calibration curve equation: y = 0.0682x-0.0214, R2 = 0.997). The result indicated that the TPC in the analyzed S. siliquosum extract is 30.34 ± 0.00 mg GAE g-1. The amount of phenolic substances obtained in this study is greater than that observed by Kim et al. (2005) from Korean seaweeds such as Ecklonia cava, Ishige okamurae, and Sargassum siliquastrum with TPC of 2.27, 0.63, and 0.29 mg GAE g-1, respectively. Similarly, Arguelles et al. (2019) reported a lower total phenolic content (10.13 ± 0.166 mg GAE g-1) for Sargassum vulgare from Lobo, Batangas. The total phenolic content of a sample is highly reliant on the polarity of extractant (solvent) utilized during the process of extraction. Normally, polyphenols have high solubility in polar solvents (acidified methanol), which results in recovering a high concentration of these desired compounds in the sample extracts (Arguelles et al., 2019).
Antioxidant activity
A versatile antioxidant system is important in search for a seaweed-based formulation of cosmetic products. When studying the potential antioxidant activity of seaweeds, the use of only one assay is inadequate for determining some of the involved mechanisms of antioxidant activity. Therefore, two methods of antioxidant assays were used to verify the antioxidative potential of the seaweed extract used in this investigation. The antioxidant activity of S. siliquosum extract was evaluated using CUPRAC assay and DPPH radical scavenging activity assay.
DPPH radical scavenging activity
DPPH radical scavenging assay uses DPPH to check the ability of the antioxidative compounds (algal extract) acting as radical scavengers of proton. Substances possessing antioxidative activity will bring about the change of color from purple chromogen radical to the pale yellow hydrazine (Arguelles et al., 2017; Goh et al., 2010). This method has been used considerably as an initial method for screening of novel antioxidants because of its simplicity, reproducibility, and stability (Chen et al., 2008; Jiménez et al., 2010). Sargassum siliquosum exerted a potent radical scavenging activity against DPPH free radical showing inhibition percentage that is dose dependent (Table 1 and Fig. 1). The activity of the seaweed extract to scavenge DPPH increases when the tested algal extract concentration is also increased. Furthermore, S. siliquosum extract exhibited greater antioxidant activity than the positive control (ascorbic acid) with an IC50 of 0.19 mg GAE ml-1 and 0.23 mg GAE ml-1, respectively.
The coefficient of correlation (R2) among phenolic concentration and antioxidant activity of S. siliquosum using DPPH scavenging assay is shown in Figure 1. It is evident from this result that a good positive correlation (R2 = 0.93208) exists between TPC and free radical scavenging activity for the acidified methanolic extract of S. siliquosum. This proves that the antioxidant properties of the seaweed extract are enhanced by phenolic compound present in the sample. The result of this investigation agrees with the earlier studies done by Arguelles et al. (2019) and Jiménez et al., (2010) on antioxidant properties (using DPPH radical scavenging assay) of brown seaweeds, A. nodosum and S. vulgare, where a direct relationship between phenolic concentration and antioxidant activity occurs. Also, IC50 (0.19 mg GAE ml-1) obtained by S. siliquosum extract exhibited a greater antioxidant activity compared to A. nodosum (IC50 = 1.55 mg ml-1) and S. vulgare (IC50 = 37.2 mg GAE ml-1).
Copper reduction antioxidant capacity assay
The CUPRAC assay is a spectrophotometric method of determining the antioxidant activity of an extract by evaluating the capacity of the sample extract to reduce Cu (II) to Cu (I). Reducing power of varying concentration of S. siliquosum extract and ascorbic acid is shown in Table 2. Sargassum siliquosum extract showed a concentration-dependent ability of copper ion reduction. In this method, a higher absorbance reading denotes a greater antioxidant capacity. The highest reducing activity was noted at 25 μg GAE ml-1 of prepared phenolic concentration as compared to other lower concentration. The trend observed in this assay is similar to those obtained from the DPPH assay, in which 0.5 mg GAE ml-1 exhibited the strongest antioxidant activity. The study observed that there is an increase in the antioxidant activity when the seaweed extract concentration was also increased. Furthermore, the investigation reveals that S. siliquosum extract contains bioactive metabolites of good antioxidant potential attributed from its copper reducing ability. It was reported that brown seaweeds possess bioactive compounds (mainly fucoxanthin and phlorotannins) with reduction potential such as antioxidative properties (Chakraborty et al., 2013). These compounds when present in high concentration exhibit a strong antioxidant property better than the commonly used antioxidant (carotenes, ascorbic acid, and α-tocopherol) in the food and pharmaceutical industry.
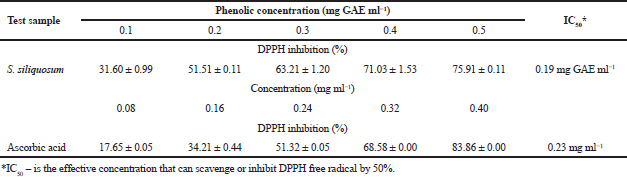 | Table 1. DPPH radical scavenging activity of phenolics from S. siliquosum extract. [Click here to view] |
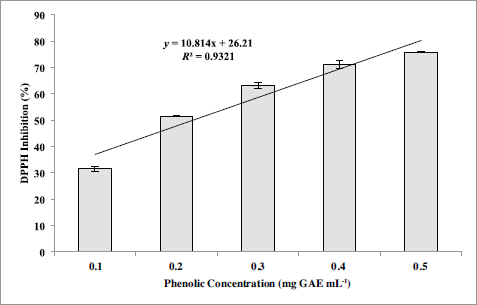 | Figure 1. Simple regression correlation between phenolic content and antioxidant activity via DPPH radical scavenging assay of S. siliquosum extract. [Click here to view] |
The positive correlation (R2) between phenolic concentration and antioxidant activity of S. siliquosum using CUPRAC assay (R2 = 1.00) shows that phenolic substances serve a vital part in the antioxidant capacity of the macroalga (Fig. 2). Besides, S. siliquosum extract exhibited a greater antioxidant activity than the positive control (ascorbic acid) with an IC50 value of 18.50 and 46.30 μg GAE mg ml-1, respectively. The potent antioxidant activity of S. siliquosum extract against ascorbic acid proves its potential as a natural source of active ingredients used in the development of cosmetic products.
Tyrosinase inhibition assay
The tyrosinase inhibitory effect of S. siliquosum extract was determined spectrometrically using mushroom tyrosinase (Table 3). The results of the assay demonstrated that S. siliquosum extract is considered more potent in inhibiting tyrosinase enzyme with an IC50 value of 65 μg GAE ml-1 than kojic acid (positive control) with an IC50 value of 109.8 μg GAE ml-1. Such result proves that the seaweed extract contains anti-melanogenic substances that are of potential use as skin-lightening active ingredient. Furthermore, comparing the activity of the acidified methanolic extract of S. siliquosum against other seaweed extracts, such as the brown algae Ecklonia stolonifera, A. nodosum, and Turbinaria conoides, showed a greater tyrosinase inhibition activity with an IC50 value of 0.345 mg mL-1, 0.1 mg ml-1, and 188.85 μg ml-1, respectively (Jimenéz et al., 2010; Kang et al., 2004; Sari et al., 2019).
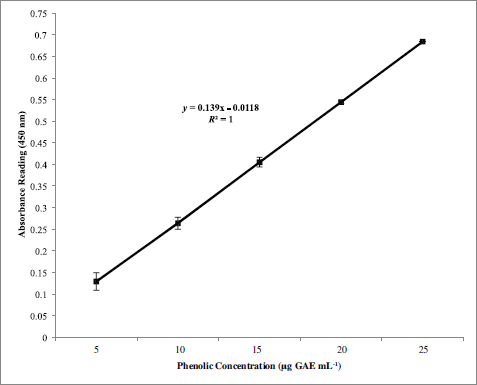 | Figure 2. Simple regression correlation between phenolic content and antioxidant activity via CUPRAC assay of S. siliquosum extract. [Click here to view] |
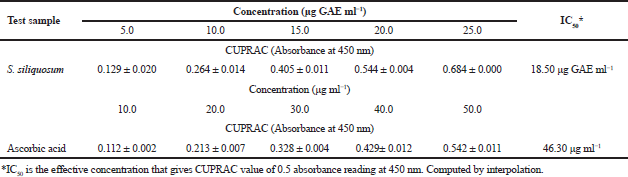 | Table 2. Copper reduction antioxidant capacity of phenolics from S. siliquosum extract. [Click here to view] |
The inhibitory activity exhibited by the algal extract in this assay can be ascribed to the hydroxyl groups present in phenolic substances in S. siliquosum extract that can lead to the formation of hydrogen bonds to the active site of the target enzyme (tyrosinase) causing stearic hindrances and changes in the enzyme conformation which results to lower activity. Furthermore, the presence of other known tyrosinase inhibitors such as fucoidan, fucoxanthin, terpenoids, and other polyphenols may also serve a crucial role in the inhibition of tyrosinase using the seaweed extract. These compounds have antiaging effects and are known to have a competitive enzymatic inhibitory effect against tyrosinase. Furthermore, these substances cause the suppression of the polymerization of metabolic intermediates needed for melanin synthesis (Jimenéz et al., 2010; Kim et al., 2008; Namjooyan et al., 2019).
Antibacterial activity
In the Philippines, there are few studies on the use of Sargassum for its pharmaceutical properties with great potential for medicinal and cosmetic application, and thus, this area was studied in this investigation. The results of the analysis on the antibacterial properties of the acidified methanolic extract of S. siliquosum against common bacterial pathogens are shown in Table 4. Of the five pathogenic bacteria tested, two test organisms were inhibited by the seaweed extract. Sargassum siliquosum showed a pronounced activity against common skin pathogen such as S. aureus and S. epidermidis having MIC of 125 and 250 μg ml-1, respectively. However, no inhibitory activity was observed against E. aerogenes, E. coli, and P. aeruginosa. Minimum bactericidal concentration against S. aureus is greater than that of S. epidermidis, having 250 and 500 μg ml-1, respectively. These antibacterial activities exhibited by S. siliquosum are in agreement with those earlier antimicrobial studies of Sargassum (Arguelles et al., 2019; Osman et al., 2010; Rani et al., 2016).
The genus Sargassum is known in producing bioactive metabolites such as terpenoids, polyphenols, polysaccharides, and steroids (Arguelles et al., 2019). Therefore, Sargassum species are regarded as candidate marine organism with good bioactivities that can be used as an alternative raw material of active ingredients for the formulation of skincare products. Besides, this study shows that the algal extract is more potent for its antagonistic activity toward Gram-positive bacteria as compared to that of the Gram-negative bacteria. Such observation can be attributed to the complex cell wall composition of Gram-negative bacteria in contrast to that of the Gram-positive bacterial cells. In general, Gram-negative bacteria are characterized by a multilayered cell wall structure affecting the penetration of active compounds within the bacterial cells giving added protection for the bacteria (Arguelles et al., 2019; Amaro et al., 2011). Variations in the antibacterial activities between species and strains of Sargassum can be attributed to factors such as the kind of solvent used for extraction that can result in differences in the type of recovered bioactive substances and varying methods of antimicrobial assay (Al-Judaibi, 2014). Thus, additional studies must be done on the optimization, purification, and identification of these bioactive substances for cosmetic and pharmaceutical application.
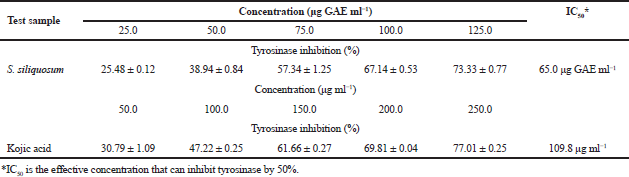 | Table 3. Tyrosinase inhibition activity of phenolics from S. siliquosum extract. [Click here to view] |
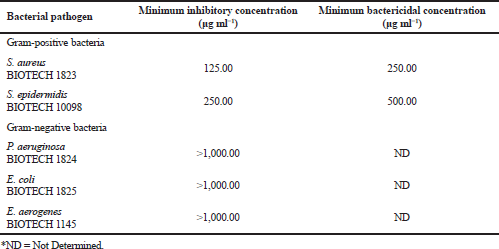 | Table 4. Antibacterial activities of S. siliquosum extract. [Click here to view] |
CONCLUSIONS
Sargassum siliquosum is capable of producing polyphenolic compounds with direct relevance to cosmetic application. The brown macroalga was able to exhibit good tyrosinase inhibition, antioxidant, and antibacterial properties that can be utilized for large-scale production. Additional studies focusing on the identification and purification of phenolic substances using high-performance liquid chromatography must be done to better understand the mechanisms of action of the active substances found in the seaweed extract. The results of this study are beneficial for the effective use of S. siliquosum extract as a source of polyphenolic compounds important for the development of novel skin-lightening ingredient for pharmaceutical and cosmetic industry.
ACKNOWLEDGMENTS
The authors would like to acknowledge the Philippine National Collection of Microorganisms and Food Laboratory of the National Institute of Molecular Biology and Biotechnology (BIOTECH), University of the Philippines Los Baños, for the provision of materials and equipment used for the conduct of the experimental assays. Also, the authors are thankful for the anonymous reviewers for their significant suggestions for the improvement of the manuscript.
FUNDING
Nil.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
Al-Judaibi A. Antibacterial effects of extracts of two types of red sea algae. J Biosci Med, 2014; 2:74–82. CrossRef
Alpinar K, Özyurek M, Kolak U, Guclu K, Aras Ç, Altun M, Celik SE, Berker KI, Bektasoglu B, Ampal R. Antioxidant capacities of some food plants wildly grown in Ayvalik of Turkey. Food Sci Technol Res, 2009; 15(1):59–64. CrossRef
Amaro HM, Guedes AC, Malcata FX. Antimicrobial activities of microalgae. In: Méndez-Vilas A (ed.). Science against microbial pathogens: communicating current research and technological advances. Formatex Research Center, Badajoz, Spain, pp 1272–80, 2011.
Arguelles EDLR. Proximate analysis, antibacterial activity, total phenolic content and antioxidant capacity of a green microalga Scenedesmus quadricauda (Turpin) Brébisson. Asian J Microbiol, Biotechnol Environ Sci, 2018; 20(1):150–8.
Arguelles EDLR, Laurena AC, Martinez-Goss MR, Monsalud RG. Antibacterial activity, total phenolic content and antioxidant capacity of a green microalga Desmodesmus sp. (U-AU2) from Los Baños, Laguna (Philippines). J Nature Stud, 2017; 16(2):1–13.
Arguelles EDLR, Monsalud RG, Sapin AB. Chemical composition and In Vitro antioxidant and antibacterial activities of Sargassum vulgare C. Agardh from Lobo, Batangas, Philippines. J ISSAAS, 2019; 25(1):112–22.
Bourguiba I, Zahlila A, Bouaïcha N, Amri M, Mezghani S. Antioxidant effect of the marine green alga Ulva rigida ethanolic precipitate in yeast cells and zebrafish embryos. S Afr J Bot, 2017; 113:253–60. CrossRef
Chen Y, Cai L, Zhao C, Xu H-C, Cao C-Y, Liu Y, Jia L, Yin H-X, Chen C, Zhang H. Spectroscopic, stability and radical-scavenging properties of a novel pigment from gardenia. Food Chem, 2008; 109:269–77; doi:10.1016/j.foodchem.2007.10.023 CrossRef
Chakraborty K, Praveen NK, Vijayan KK, Rao GS. Evaluation of phenolic contents and antioxidant activities of brown seaweeds belonging to Turbinaria spp. (Phaeophyta, Sargassaceae) collected from Gulf of Mannar. Asian Pac J Trop Biomed 2013; 3(1):8–16. CrossRef
Commercial Seaweed Market Size. Share and industry analysis by product type (red seaweed, brown seaweed, green seaweed), by form (flakes, powder, liquid), by end user (food and beverage, agricultural fertilizer, animal feed additives, pharmaceuticals, cosmetics and personal care), and regional forecast 2018–2025. 2019. Available at: https://www.fortunebusinessinsights.com/industry-reports/commercial-seaweed-market-100077. [Accessed 12 January 2020]
Corpuz MJAT, Osi MO, Santiago LA. Free radical scavenging activity of Sargassum siliquosum J. G. Agardh. Int Food Res J, 2013; 20(1): 291-297.
Cosmetics Market by Category. (Skin and sun care products, hair care products, deodorants, makeup and color cosmetics, fragrances) and by distribution channel (general departmental store, supermarkets, drug stores, brand outlets) – global opportunity analysis and industry forecast, 2014–2022. 2019. Available via https://www.alliedmarketresearch.com/cosmetics-market. (Accessed 13 January 2019)
Dolorosa MT, Nurjana, Purwaningsih S, Anwar E, Hidayat T. Tyrosinase inhibitory activity of Sargassum plagyophyllum and Eucheuma cottonii methanol extracts. IOP Conf Series: Earth Environ Sci, 2019; 278:2–8. doi:10.1088/1755-1315/278/1/012020 CrossRef
Goh SH, Yusoff FM, Loh SP. A comparison of the antioxidant properties and total phenolic content in a diatom, Chaetoceros sp. and a green microalga, Nannochloropsis sp. J Agri Sci, 2010; 2(3):123–30. CrossRef
Guiry MD, Guiry GM. AlgaeBase. World-wide electronic publication, National University of Ireland, Galway, Republic of Ireland, 2017. Available via: http://www.algaebase.org; searched on 14 December 2019.
Hapsari R, Elya B, Amin J. Formulation and evaluation of antioxidant and tyrosinase inhibitory effect from gel containing the 70% ethanolic Pleurotus ostreatus extract. Int J Med Arom Plants, 2012; 2(1):135–40.
Kim SJ, Woo S, Yun H,Yum S, Choi E,Do JR,Jo JH,Kim D,Lee S, Lee TK.Total phenolic contents and biological activities of Korean seaweed extracts. Food Sci Biotechnol, 2005; 14:798–802.
Jiménez JT, O’Connell S, Lyons H, Bradley B, Hall M. Antioxidant, antimicrobial, and tyrosinase inhibition activities of acetone extract of Ascophyllum nodosum. Chem Pap, 2010; 64(4):434–42. CrossRef
Kang HS, Kim HR, Byun DS, Son BW, Nam TJ, Choi JS. Tyrosinase inhibitors isolated from the ed- ible brown alga Ecklonia stolonifera. Arch Pharm Res, 2004; 27:1226–32; doi:10.1007/BF02975886. CrossRef
Kim YJ, Kang KS, Yokozawa T. The anti-melanogenic effect of pycnogenol by its anti-oxidative actions. Food Chem Toxicol, 2008; 46:2466–71; doi:10.1016/j.fct.2008.04.002. CrossRef
Namjooyan F, Farasat M, Alishahi M, Jahangiri A, Mousavi H. The anti-melanogenesis activities of some selected brown macroalgae from northern coasts of the Persian Gulf. Braz Arch Biol Technol, 2019; 62:e19180198. Available via www.scielo.br/babt. CrossRef
Nuñez Selles A, Castro HTV, Aguero JA, Gonzalez JG, Naddeo F, De Simone F, Pastrelli L. Isolation and quantitative analysis of phenolic antioxidants, free sugars and polyols from mango (Mangifera indica L.) stem bark aqueous decoction used in Cuba as a nutritional supplement. J Agric Food Chem, 2002; 50: 762–6. CrossRef
Osman MEH, Abushady AM, Elshobary ME. In vitro screening of antimicrobial activity of extracts of some macroalgae collected from Abu-Qir bay Alexandria, Egypt. Afr J Biotechnol, 2010; 9(12):7203–8.
Pimentel FB, Alves RC, Rodrigues F, MBPP Oliveira. Macroalgae-derived ingredients for cosmetic industry–an update. Cosmetics, 2018; 5:2; doi:10.3390/cosmetics5010002 CrossRef
Rani V, Jawahar P, Shakila RJ, Srinivasan A. Antibacterial activity of some brown seaweeds of gulf of Mannar, south east coast of India. J Pharma BioSci, 2016; 4(3):14–21.
Ribeiro SMR, Barbosa LCA, Queiroz JH, Knodler M, Schieber A. Phenolic compounds and antioxidant capacity of Brazilian mango (Mangifera indica L.) varieties. Food Chem, 2008; 110(3):620–6. CrossRef
Sari DM, Anwar E, Nurjanah, Arifianti AE. Antioxidant and tyrosinase inhibitor activities of ethanol extracts of brown seaweed (Turbinaria conoides) as lightening ingredient. Pharmacog J, 2019; 11(2):379–82; doi:10.5530/pj.2019.11.58 CrossRef
Trono Jr., GC. The genus Sargassum in the Philippines. In: Abbott IA (ed.). Taxonomy of economic seaweeds with reference to some Pacific and western Atlantic species. Vol. III. California Sea Grant College, La Jolla, CA, pp 43–94, 1992.
Wang H-MD, Chen C-C, Huynh P, Chang J-S. Exploring the potential of using algae in cosmetics. Bioresour Technol, 2015; 184:355–62. CrossRef
Wu L, Chen C, Cheng C, Dai H, Ai Y, Lin C, Chung Y. Evaluation of tyrosinase inhibitory, antioxidant, antimicrobial, and antiaging activities of Magnolia officinalis extracts after Aspergillus niger fermentation. BioMed Res Int, 2018; 2018:11, Article ID 5201786; doi:10.1155/2018/5201786 CrossRef