INTRODUCTION
Encephalopathy is an acute inflammation of the brain parenchyma. Metabolic encephalopathy is a diverse category that describes abnormalities of electrolytes, vitamins, water retention, and the other chemicals related to the brain functions (Berisavac et al., 2017).
Azilsartan is a newly launched antihypertensive drug that belongs to the angiotensin II receptor blockers (ARB) class. ARBs involve in selective blocking of angiotensin II to AT1 receptors, therefore causing an increase in vasodilation and a decrease in aldosterone effects (Kurtz and Kajiya, 2012; Zaiken and Cheng, 2011). However, the side effect profile of traditional ARBs is quite significant, including hyperkalemia (Bandak et al., 2017). In our opinion, this is the first case enlightening the adverse effect profile of azilsartan in a hypertensive patient. This case represents the clinical course, treatment, and prognosis of azilsartan-related metabolic encephalopathy.
CASE PRESENTATION
A 58-year-old female presented to the emergency department of a tertiary care center with the chief complaints of vomiting and disorientation and altered sensorium. A detailed history revealed that the patient had generalized fatigability, nausea, and multiple episodes of vomiting for 1 week. She also had reduced appetite and irregular bowel movements for a few weeks. On physical examination, the patient was restless, but there was no neck stiffness; Kernig’s and Brudzinski’s signs were negative.
The patient had a history of hypertension and diabetes mellitus for more than 5 years. Four years back, she had a non-ST-elevation myocardial infarction, where the left anterior descending artery (LAD) was diseased. She underwent percutaneous coronary intervention (PCI) with drug-eluting stent to LAD. An Echocardiogram (ECHO) was showing normal left ventricle (LV) function. The patient was started on antiplatelet drugs, i.e., aspirin (loading dose of 325 mg and then 150 mg once daily as a maintenance dose), clopidogrel (loading dose of 300 mg and then 75 mg once daily as a maintenance dose), along with atorvastatin of 40 mg per day, antihypertensive agents (ramipril 2.5 mg once daily and metoprolol extended release 25 mg once daily), and anti-hyperglycemic drug (glimepiride 1 mg daily).
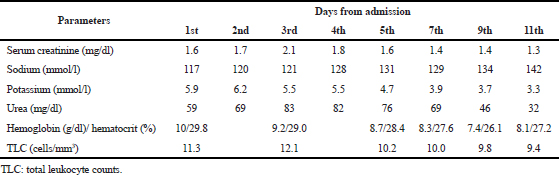 | Table 1. The course of abnormal laboratory parameters from admission to discharge. [Click here to view] |
One year back, 3 years after LAD-PCI, she again present with acute pulmonary edema and angina, and a coronary angiogram showed calcific left main coronary artery and double vessel disease with left circumflex and LAD restenosis. At this admission, she underwent coronary artery bypass grafting with three grafts. ECHO showed mild-to-moderate LV dysfunction with ejection fraction (EF) of 44%. The patient was continued on aspirin with atorvastatin and newly added ivabradine, isosorbide dinitrate, and hydralazine combination for LV dysfunction. Glimepiride 1 mg daily was continued for diabetic status. After a few months of this admission, the patient stopped the medicines for 1 week and developed acute shortness of breath, progressive in nature. She admitted again, and ECHO showed a severe LV dysfunction with EF 20% and moderate-to-severe mitral regurgitation (MR), severe pulmonary arterial hypertension (PAH), and right ventricle dysfunction. The patient was treated with a diuretic, oxygen therapy, and inotropes for heart failure. The high blood sugar levels were managed with insulin and oral hypoglycemic agents (glimepiride 1 mg once daily). Ramipril 2.5 mg once daily and metoprolol extended release 25 mg once daily were continued for hypertensive history and current high blood pressure.
One month back, the patient came for the follow-up to the outpatient department, where her BP was >140/90 mmHg, and ramipril 2.5 mg was substituted by azilsartan 40 mg orally twice daily. At the present admission, the patient presented with the above mentioned signs and symptoms. The laboratory investigations were done, which showed deranged renal parameters. Liver enzymes and thyroid-stimulating hormone were normal. Hematological parameters show low hemoglobin and hematocrit and slight high total leukocyte counts (TLCs) (Table 1).
Azilsartan was stopped in view of symptoms and deranged electrolytes and renal parameters. A neurology consultation was sought who opined nil intervention. Magnetic resonance imaging of brain was normal. A nephrologist suggested the initial stage of cardiorenal syndrome, which was improved later. Hyponatremia and hyperkalemia were improved within 1 week. ECHO showed a moderate LV dysfunction and moderate MR along with moderate PAH. The patient was discharged on the previous combination of aspirin, clopidogrel, and statin with the same dose and frequency, teneligliptin 20 mg once daily (switched from glimepiride because of an acute rise in creatinine level), and carvedilol 3.125 mg once daily (azilsartan was stopped, and metoprolol was switched to carvedilol based on the LV dysfunction). The patient was doing well on a 30-day follow-up, and the electrolyte reports and renal parameters were in the normal range. Hemoglobin was also noted more than 10 g/dl.
DISCUSSION
Encephalopathy is any diffuse disease of the brain, which alters the structure or function of the brain. Diverse etiologies are discovered, including infectious agents, tumor, increased exposure to toxic elements (such as certain metals and drugs), trauma, and cerebral ischemia. Neurological symptoms may include progressive loss of memory and cognitive ability, nystagmus, and seizures. One of the complications of encephalopathy is metabolic encephalopathy (Butterworth and Layrargues, 1990). Few cases have shown an increase in serum ammonia levels to patients on ARB treatment, due to the reduced renal excretion. Serum ammonia levels are a direct indication of hepatic encephalopathies. Hepatic encephalopathy is one of the causes of metabolic encephalopathy (Ong et al., 2003).
Metabolic encephalopathy can be defined as a potentially reversible abnormality of the brain function caused by processes of extracerebral origin. The most common symptom is delirium. However, mood and orientation disorder thought, memory disorder, intellectual deterioration, dementia, and depression may also occur. Metabolic encephalopathy can also include drug ingestions or medication side effects that affect the chemical transmitters in the brain. If left untreated, however, it may result in secondary structural damage to the brain (Fraser and Arieff, 1997). Still, the pathophysiological mechanisms have not been completely understood. However, an inflammation could trigger the endothelial activation in the brain, which leads to the malfunctioning of the blood–brain barrier and could result in the activation of cytokines and chemokines, which enter and damage the cellular metabolism. This initiates the mitochondrial dysfunction as well as oxidative stress, resulting in the disruption of neurotransmission and apoptosis (Tan et al., 2019). ARBs can be thought to have similar actions as they, too, disrupt the neurotransmission (Raebel, 2012). Metabolic encephalopathies are of two major types – those due to the lack of glucose, oxygen, or metabolic cofactors and those due to peripheral organ damage. Low-grade fever, anemia, mild-sedative intoxication, and minimal renal dysfunction are usually found in various combinations (Berisavac et al., 2017). Anemia, hyperkalemia, and hyponatremia were noted in the present patient also, which indicate the metabolic disturbances.
Azilsartan is distinguished from other sartans in the matter of efficacy and 24-hour blood pressure control (Kurtz and Kajiya, 2012). Similar to losartan, an AT1 receptor antagonist azilsartan inhibits the augmentation of noradrenergic neurotransmission, sympathetic tone enhancement, and biological effects of angiotensin-II such as vasopressin release, pressor responses, and the release of aldosterone and adrenal catecholamines. Azilsartan is also selective and potent (Das et al., 2015). In this case presentation, the patient might develop the sign and symptoms by the same mechanism. Azilsartan as an ARB can develop hyperkalemia as its side effect and induces an electrolyte imbalance (Georgiopoulos et al., 2016). The involvement of the renin–angiotensin–aldosterone system increases the serum of potassium levels by interfering with aldosterone secretion mediated by angiotensin II (Pradhan et al., 2019; Weir and Rolfe, 2010). Hyperkalemia could cause hyperexcitation of the neurons and, hence, could be the cause of deliriums. According to a study conducted in Guangxi Medical University, China, hyperkalemia could also ameliorate a brain injury by alleviating calcium overload, inhibiting the activity of NCX1, and lowering the concentration of Ca2+ (Tan et al., 2019).
Hyponatremia caused by azilsartan may be explained by reduced aldosterone release and renal tubular sodium reabsorption mediated by angiotensin II (produced by AT1 receptor inhibition) (Das et al., 2015). This case did not present any evidence of adrenaline insufficiency, hypothyroidism, or established cardiorenal syndrome, which augments the condition to metabolic disturbances in the brain; there is a clear indication that the metabolic encephalopathy was induced by azilsartan use and the discontinuation of the drug improved it.
CONCLUSIONS
The current case suggests that azilsartan is associated with metabolic encephalopathy caused by electrolyte imbalance in a chronic hypertensive patient. The patient was initiated on a high dose of azilsartan, and starting with a low dose may help in avoiding such conditions. Metabolic encephalopathy is reversible in the primary stage and, therefore, should not be allowed to progress, which would cause the secondary structural changes to the brain. The elderly patients should be followed thoroughly to notice any imbalances and rectify immediately.
ACKNOWLEDGMENT
None.
STATEMENT OF ETHICS
Ethical approval (informed consent) was received for publication before preparing the manuscript.
CONFLICT OF INTEREST
None of the authors declare any conflict of interest.
REFERENCES
Bandak G, Sang Y, Gasparini A, Chang AR, Ballew SH, Evans M, et al. Hyperkalemia after initiating renin–angiotensin system blockade: The Stockholm Creatinine Measurements (SCREAM) project. J Am Heart Assoc, 2017; 6(7):e005428. CrossRef
Berisavac II, Jovanović DR, Padjen VV, Ercegovac MD, StanarÄević PD, Budimkić-Stefanović MS, et al. How to recognize and treat metabolic encephalopathy in Neurology intensive care unit. Neurol India, 2017; 65(1):123. CrossRef
Butterworth R, Layrargues G. Hepatic encephalopathy. Humana Press, Totowa, NJ, 1990. CrossRef
Das S, Bandyopadhyay S, Ramasamy A, Prabhu VV, Pachiappan S. A case of losartan-induced severe hyponatremia. J Pharmacol Pharmacother, 2015; 6(4):219. CrossRef
Fraser CL, Arieff AI. Epidemiology, pathophysiology, and management of hyponatremic encephalopathy. Am J Med, 1997; 102(1):67–77. CrossRef
Georgiopoulos G, Katsi V, Oikonomou D, Vamvakou G, Koutli E, Laina A, et al. Azilsartan as a potent antihypertensive drug with possible pleiotropic cardiometabolic effects: a review study. Front Pharmacol, 2016; 7:235. CrossRef
Kurtz TW, Kajiya T. Differential pharmacology and benefit/risk of azilsartan compared to other sartans. Vasc Healt Risk Manag, 2012; 8:133. CrossRef
Ong JP, Aggarwal A, Krieger D, Easley KA, Karafa MT, Van Lente F, et al. Correlation between ammonia levels and the severity of hepatic encephalopathy. Am J Med, 2003; 114(3):188–93. CrossRef
Pradhan A, Tiwari A, Sethi R. Azilsartan: current evidence and perspectives in management of hypertension. Int J Hypertension, 2019; 2019:1–8. doi:10.1155/2019/1824621 CrossRef
Raebel MA. Hyperkalemia associated with use of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. Cardiovasc Ther, 2012; 30(3):e156–66. CrossRef
Tan XF, Qin T, Li N, Yang YG, Zheng JH, Xie L, Chen MH. High-potassium preconditioning enhances tolerance to focal cerebral ischemia-reperfusion injury through anti-apoptotic effects in male rats. J Neurosci Res, 2019; 97(10):1253–65. CrossRef
Weir MR, Rolfe M. Potassium homeostasis and renin-angiotensin-aldosterone system inhibitors. Clin J Am Soc Nephrol, 2010; 5(3):531–48. CrossRef
Zaiken K, Cheng JW. Azilsartan medoxomil: a new angiotensin receptor blocker. Clin Ther, 2011; 33(11):1577–89. CrossRef