INTRODUCTION
The liver, which is a vital organ of metabolism, plays a major role in detoxification and elimination of toxicants (Saleem et al., 2018; Sultana et al., 2018). It is incessantly and extensively exposed to xenobiotics, hepatotoxins, and chemotherapeutic agents which can lead to impairment of its functions (Kumar and Veere, 2011; Preussmann, 1978). Hepatic cells are mainly damaged by hepatotoxic chemicals inducing lipid peroxidation and other oxidative damages (Chattopadhyay, 2003; Kumar and Veere, 2011). The synthetic drugs used in curing liver damages are therapeutically nonpromising and may lead to hepatotoxicity (Joshi and Shailajan, 2016). Carbon tetrachloride (CCl4), as a standout among the hepatotoxins, is generally utilized to measure the efficiency of many hepatoprotective drugs (Cheng et al., 2013; Recknagel et al., 1989; Zarezade et al., 2018; Zhao et al., 2018).
For the prevention and management of liver disease, plants and natural products have been used traditionally worldwide (Lahon and Das, 2011). Medicinal plants are great sources of natural compounds such as phenolic acids and flavonoids, which have antioxidative properties, and are able to scavenge free radicals and defend the liver from CCl4-induced hepatic injury (Azeem et al., 2010; Gnanadesigan et al., 2011; Gupta et al., 2011; Singhal and Gupta, 2012; Zarezade et al., 2018). Many plants have been reported to show the hepatoprotective activity, namely, Phaseolus trilobus (Fursule and Patil, 2010), Cassia abbreviata (Sobeh et al., 2018), Cichorium glandulosum (Tong et al., 2015), Cichorium intybus (Heibatollah et al., 2008), Parmelia perlata (Shailajan et al., 2014), Artocarpus lakoocha (Saleem et al., 2018), Avicennia marina (Joshi and Shailajan, 2016), Hibiscus vitifolius (Samuel et al., 2012), Solanum nigrum (Raju et al., 2003), Asteracantha longifolia (Shailajan et al., 2005), and Flemingia macrophylla (Hsieh et al., 2011).
Flemingia macrophylla has been reported to possess hepatoprotective effect due to the presence of flavonoids (Gahlot et al., 2011; Hsieh et al., 2011). A number of studies have proved that flavonoids are a class of compounds exhibiting hepatoprotective activity (Bratkov et al., 2016; Kondeva-Burdina et al., 2018; Krasteva et al., 2016; Mbemya et al., 2017; Pelissero et al., 1996; Pistelli, 2002). One of the most useful properties of this class of compounds is their ability to scavenge ROS and is considered more efficient than Vitamins C and E (Gao et al., 2001; Mbemya et al., 2017; Wang and Zheng, 1992). Flavonoids act by shielding membranes or by reducing their absorbency to bind with hepatotoxic substances (Gyr and Meier, 1991). Kaempferol, a flavonoid, has been reported to show strong hepatoprotective activity in CCl4-treated mice (Wang et al., 2015). Earlier publication from the laboratory has already shown the presence of kaempferol, in the ethanolic extract of Flemingia tuberosa (Shailajan and Mascarenhas, 2018). The hepatoprotective activity of the plant, however, has not been investigated scientifically.
Hence, this study aims to evaluate the hepatoprotective activity of the ethanolic extract of aerial parts of F. tuberosa against CCl4-induced hepatotoxicity in rats.
MATERIALS AND METHODS
Collection of plant material
The aerial parts of F. tuberosa were collected from Rajapur, Maharashtra, and authenticated by Dr. M. M. Lekhak (Shivaji University, Kolhapur, Maharashtra)—AUTH 85/2016. The material was shade dried for a week and further oven-dried at 37°C ± 2°C, powdered in a mixer grinder, sieved (85-mm mesh – BSS sieve), and stored in airtight plastic containers.
Apparatus and chemicals
CCl4 (GR grade, batch no.: IG8G580365, Merck Specialties Pvt. Ltd.) and Silybon tablets (Silymarin as silybon 70 mg, batch no. SIAD0025, Micro Labs Limited) were procured from the market. All other chemicals used were of analytical grade.
Quality control
The quality of the sample was evaluated for foreign matter, total ash, acid insoluble ash, alcohol-soluble extractive and water-soluble extractive, and moisture content (Bajwa et al., 2013; Khandelwal, 2008; Mukherjee, 2007; Pharmacopoeia, 2010).
Preparation of plant extracts
The ethanolic extract of the aerial parts of F. tuberosa was prepared by adding 1000 mL of ethanol to 100 g of plant powder and agitated on a shaker for 4 hours (yield = 12.52%). The extract was standardized using a validated HPTLC method and the content of hepatoprotective marker, kaempferol (Wang et al., 2015). The kaempferol content was found to be 1.65%. The extract was further evaporated to dryness in a water-bath preset at 78°C to remove all traces of ethanol from the extract. Animals were dosed by weighing this ethanol-free dried extract accurately as per the weight of the animal and by suspending it in distilled water (total volume > 1 ml/100 g). A dose of 500 mg/kg body weight of animals was used in the study (Hsieh et al., 2011).
Chromatographic characterization
The powdered plant material (1.0 g) was extracted with ethanol (10.0 ml), and vortex mixed and sonicated for 1 and 20 minutes, respectively, followed by filtration through Whatman filter paper No. 1. The separation and quantitation of kaempferol were established using HPTLC.
Kaempferol as a biomarker
The presence of kaempferol as a biomarker in the ethanolic extract of F. tuberosa has been confirmed using a published method on HPTLC (Shailajan and Joshi, 2011; Shailajan and Mascarenhas, 2018) (Fig. 1).
Animals
Albino Wistar rats (female, 180–220 g) were procured from Bharat Serums and Vaccines Pvt. Ltd., Thane. The animals were maintained under standard laboratory conditions at an ambient temperature of 25°C ± 2°C and relative humidity of 50%–55% with 12-hour light and dark cycle in an animal house with standard facilities as per the CPCSEA guidelines (CPCSEA/315). They were fed with a commercially available standard pellet diet (AMRUT feed), and the filtered drinking water was provided ad libitum. After 1 week of acclimatization, the animals were subjected to experimental procedures. The approval from the Institutional Animal Ethics Committee of Ramnarain Ruia Autonomous College, Matunga, for the usage of animals in the experiment had been obtained as per the CPCSEA guidelines (Approval number: RM– 160110– 02).
Safety evaluation
The safety of the plant has been established on albino Swiss mice following the OECD guidelines (No. 420, fixed dose procedure) (Shailajan and Mascarenhas, 2018). The extract prepared was evaporated to dryness to remove all the traces of ethanol solvent before use in the animals.
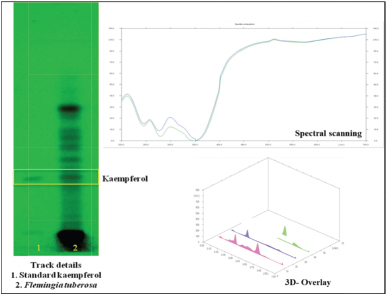 | Figure 1. HPTLC plate photo and chromatogram for kaempferol estimation from the ethanolic extract of F. tuberosa. [Click here to view] |
Evaluation of antioxidant activity
The antioxidant activity of the extracts, based on the scavenging activity of the stable 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical, was determined (Joshi and Shailajan, 2016; Narendhirakannan and Rajeswari, 2010), and the percentage inhibition (I) was calculated as follows:
where “A” is absorbance. EC50 is the concentration at which DPPH radical is scavenged by 50%. The ascorbic acid was used as a reference antioxidant.
Evaluation of hepatoprotective activity
For the study, albino Wistar rats were divided randomly into five groups with six animals in each (Table 1). The animals were kept fasting (water ad libitum) for 4 hours prior to dosing. Group I animals received an intraperitoneal injection of 0.7 ml/Kg BW liquid paraffin/animal on the 0th day of the study and were treated as normal controls. Animals from Groups II, III, IV, and V received an intraperitoneal injection of 0.7 ml/kg body weight of CCl4 (Frank et al., 2012; Joshi and Shailajan, 2016) in 0.5 ml liquid paraffin/animal on the 0th day of the study. The animals from Groups I, II, and III were dosed orally with 2 ml of distilled water (D/W) once daily. A dose of 70 mg/ kg Silymarin (Joshi and Shailajan, 2016, 1995) in the form of Silybon tablets suspended in 2 ml of D/W was administered orally to each animal of Group IV once daily, starting 24-hour post CCl4 induction. The animals from Group V were given an oral dose of the ethanolic extract of aerial parts of F. tuberosa suspended in distilled water (500 mg/kg body weight 24-hour post induction on the first day and further for next 4 days). The animals from Groups I, II, IV, and V were sacrificed on the fourth day (72 hours after first dosing), whereas Group III animals were sacrificed on seventh day of the study to evaluate the natural recovery in the study. The per diem records of body weight and consumption of food and water was maintained for each group.
Before sacrificing the animals, blood was collected from retro-orbital plexus into heparinized vials, and the plasma was separated and analyzed for biochemical parameters, such as glutamate oxaloacetate transaminase (GOT), glutamate pyruvate transaminase (GPT), alkaline phosphatase (ALP), direct bilirubin (DB) and total bilirubin (TB), cholesterol (CHO), triglycerides (TG), low-density lipoprotein (LDL), and high-density lipoprotein (HDL) content. The percentage protection for biochemical parameters was calculated as follows:
(Joshi and Shailajan, 2016; Rao et al., 2012).
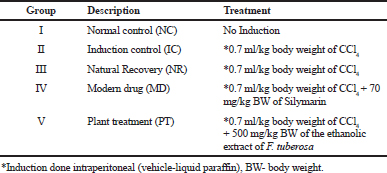 | Table 1. Grouping of animals. [Click here to view] |
Fresh liver tissue samples were also processed for the analysis of liver glycogen.
Statistical analysis
The results were expressed as mean ± SE. The statistical analysis was carried out using Microsoft Excel and GraphPad Prism 5.0 software for a one-way analysis of variance followed by Dunnett’s t-test. The p values < 0.001 were considered to be significant.
RESULTS AND DISCUSSION
A large number of plants and plant-based formulations have been claimed to cure liver disorders. The plants have been said to have hepatoprotective activity due to the presence of some phytoconstituents which have a potential antioxidant activity. The standardized ethanolic extract of F. tuberosa was found to be rich in kaempferol which has been reported to possess hepatoprotective in addition to antioxidant activity (Chen et al., 2018; Wang et al., 2018; Yin et al., 2018). The animals were administered with the dried extract using distilled water as the vehicle. The toxicity study showed that the extract is safe up to a dose of 2,000 mg/kg body weight in albino Swiss mice with no mortality or abnormality.
The antioxidant activity of the extract was assessed in DPPH model. To quantify the antioxidant activity, the IC50 value, i.e., the concentration of sample required to decrease the absorbance of specific free radical at specific λmax by 50%, was calculated (Acharya et al., 2012; Joshi and Shailajan, 2016). The extract showed an IC50 value of 20.95 μg/ml for DPPH. The results of the assay revealed that the free radicals were scavenged by the plant extract in a concentration-dependent manner. Figure 2(a) and (b) is the graphical representation of free radical scavenging activity of the plant extract in comparison with ascorbic acid. The data on regression analysis and IC50 values shown by the ethanolic extract of F. tuberosa are given in Table 2.
The extract of F. tuberosa exhibited free radical scavenging activity of 98.61% at a concentration of 60.0 μg/ml. The antioxidant activity displayed by the plant extract may be due to the presence of kaempferol. The extract of F. tuberosa showed a potential antioxidant property as observed from DPPH radical scavenging activity assay (Table 1). The antioxidant potential of the extract prevents the formation of trichloromethylperoxy radical, thereby reducing tissue damage, triggered by CCl4 treatment.
To have a good idea about the functional state of the liver, alkaline phosphatase, TB and DB were monitored (Joshi and Shailajan, 2016; Rao et al., 2012). Hepatic toxicity caused by the administration of CCl4 (0.7 ml/kg body weight) caused acute liver injury which was indicated by the sudden increase in the concentration of biochemical biomarkers, such as serum glutamic oxaloacetic transaminase (SGOT), serum glutamic pyruvic transaminase (SGPT), direct and total bilirubin (DB/TB), ALP, CHO, TG, LDL, and liver glycogen along with a decrease in the concentration of HDL in the induction control (IC) group. This was in comparison with normal control (NC) group. The levels of these biochemical parameters significantly came back to normal after the treatment of standardized ethanolic extract of the aerial parts of F. tuberosa (PT) and also in the animals treated with modern drug group (MD). The results are shown in Table 3, where the treatment with extract at 500 mg/Kg body weight showed a significant recovery (p < 0.001 with Dunnett’s test). These results proved the ability of the extract in reversing the damage caused by CCl4 and significantly enhanced the recovery process. This is indicated by the level of biochemical parameters in the treated group of animals (PT) (MD) as compared with those in the animals of the natural recovery group (NR).
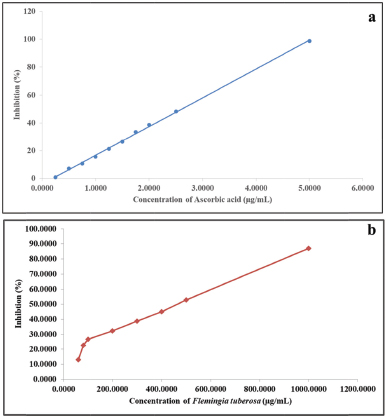 | Figure 2. Graphical representation of the effect of ascorbic acid (a) and ethanolic extract of F. tuberosa (b) on DPPH radical scavenging model. [Click here to view] |
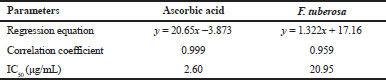 | Table 2. Data on regression analysis and IC50 value for the effect of ascorbic acid and ethanolic extract of F. tuberosa on DPPH radical scavenging model. [Click here to view] |
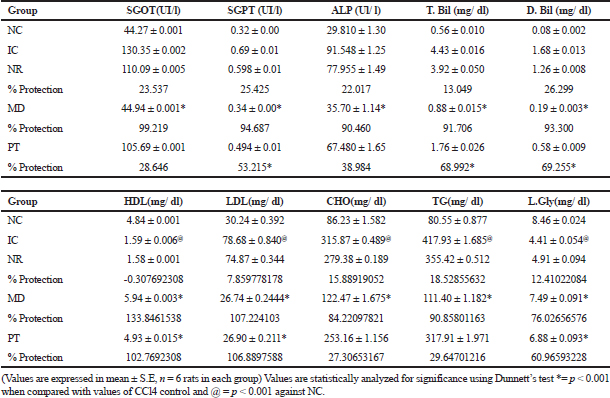 | Table 3. Effect of standardized extract on biochemical parameters. [Click here to view] |
The increase in the levels of alkaline phosphatase reflected the loss of functional integrity of cell membrane and cellular leakage, whereas the increase in both the DB and TB indicated the incidence of jaundice (Freitag et al., 2015; Joshi and Shailajan, 2016). These observations are supported by the electron microscopy results. The normalization of the biochemical marker enzymes (Table 3) after the plant treatment confirms its hepatoprotective potential. This may be attributed to its ability to expedite the regeneration of liver cells and maintain membrane integrity, whereby the leakage of marker enzymes into the bloodstream is reduced. The study also statistically affirms that the protection offered by the extract is compared to the modern drug used as a positive control, i.e., silymarin. The recovery toward normalcy of biochemical markers and that of tissue histoarchitecture caused after the treatment with plant extract was found to be less significant (p < 0.01) as that of silymarin (p < 0.001) treatment.
The hepatoprotective effect of the standardized extracts of the aerial parts of F. tuberosa was further evaluated by histopathological observations of the excised liver tissue using a light microscope and electron microscope. The administration of carbon tetrachloride in animals caused a disruption of rough endoplasmic reticulum, damage to mitochondria, centrilobular necrosis, fatty changes, and vacuolization indicating impairment of normal liver cytoarchitecture. The natural recovery group did not show significant improvement when compared to induction control group. The histoarchitecture of the liver in treated animals of plant extract group and modern drug group showed a significant recovery to normalcy in comparison to induction control group (Figs. 3 and 4). The plant-treated group showed recovery of cellular matrix at par with the positive control group. Treatment with the plant extract, however, is not as effective as that of the modern drug. This is evident in the microscopic observation (Fig. 3).
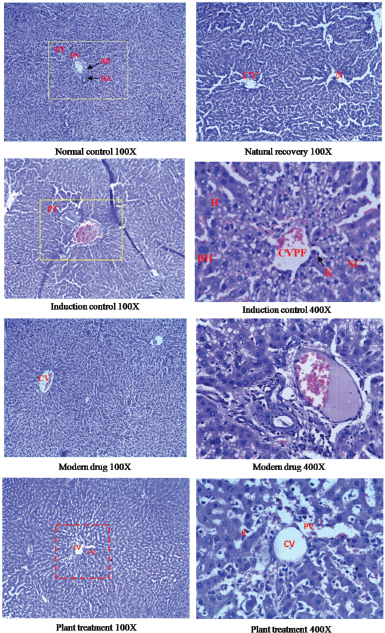 | Figure 3. Histopathological observation using light microscopy. [Click here to view] |
The results are confirmed in electron microscopic observations too. Lipid accumulation, ballooned mitochondria, reduction in rER activity, loss of microtubules, and membrane integrity are observed in hepatocytes after CCl4 treatment (Fig. 4). These cytoarchitectural changes are reversed to normalcy after treatment with plant extract and modern drug (Fig. 4). The recovery after treatment with plant extract is less as compared to that observed in the hepatocytes after treatment with the modern drug. An increase in treatment period or increase in the dose of plant extract could be a possible alternative strategy.
The modern drug showed a significant protection in terms of the physical, biochemical, and histological parameters of the hepatic tissue than the plant extract. A similar kind of trend was followed in the results of biochemical assays as well. Thus, F. tuberosa can be considered as a promising plant to be used as a hepatoprotective agent (Jahan et al., 2015; Kheiripour et al., 2019; Mahli et al., 2015; Saeed et al., 2017).
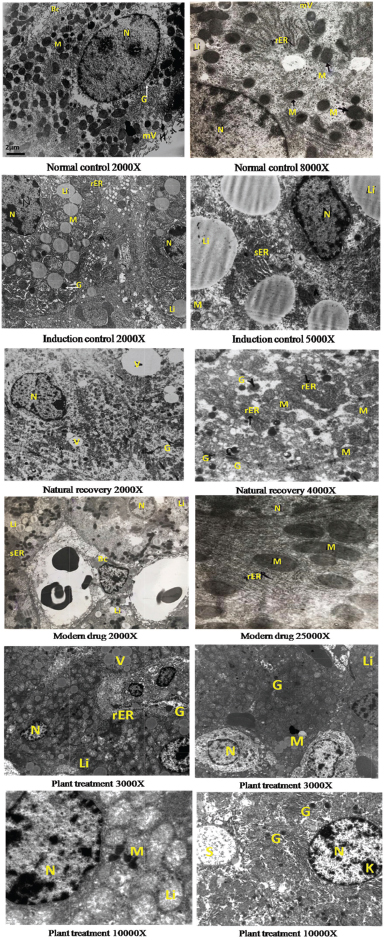 | Figure 4. Histopathological observation using electron microscopy. [Click here to view] |
CONCLUSION
The antioxidant activity of the plant extract in terms of the free radical scavenging activity has been established in vitro. The results of this study indicate that the standardized ethanolic extract of the aerial parts of F. tuberosa showed a significant hepatoprotective activity at the dose of 500 mg/kg body weight. The plant extract brought the levels of biochemical markers to normalcy and showed significant (p < 0.01) recovery in histopathological statistics. The results are not significant when compared to the modern drug, silymarin (p < 0.001). The hepatoprotective activity of the plant may have to be enhanced either by increasing the dosage or by extending the duration of the treatment in order to use it as an effective liver tonic. This study also provides scientific data for ethnomedicinal use of the plant and can create awareness in cultivating this plant to help its proliferation and avoid its eradication. The plant is important as it is an endemic species to the Western Ghats of Maharashtra, and this work is a preliminary step in conserving a medicinally important species, F. tuberosa Dalzell.
ACKNOWLEDGMENT
The authors would like to thank Jaslok Hospital and Research Centre for their help, assistance, and support for electron microscopy. A part of this work was presented at the 13th Maharashtra State Inter-University Research Convention: Avishkar 2018 held at Gondwana University, Gadchiroli, on January 14–17, 2019, and was awarded a Silver Medal.
FINANCIAL SUPPORT
None.
CONFLICT OF INTEREST
Authors declare that there are no conflicts of interest.
REFERENCES
Acharya SR, Acharya NS, Bhangale JO, Shah SK, Pandya SS. Antioxidant and hepatoprotective action of Asparagus racemosus Willd. root extracts. Indian J Exp Biol, 2012; 50(11):795–801.
Azeem AK, Mathew M, Nair C. Hepatoprotective effect of Averrhoea carambola fruit extract on carbon tetrachloride induced hepatotoxicity in mice. Asian Pac J Trop Med, 2010;3(8):610–13. CrossRef
Bajwa RK, Kulkarni SS, Tekale PP, Shinde NM, Bagwe KA. Proximate analysis of Phyllanthus amarus. Int J Res Pharm Chem, 2013; 3:221–7.
Bratkov VM, Shkondrov AM, Zdraveva PK, Krasteva IN. Flavonoids from the genus Astragalus: Phytochemistry and biological activity. Pharmacogn Rev, 2016; 10(19):11. CrossRef
Chattopadhyay R. Possible mechanism of hepatoprotective activity of Azadirachta indica leaf extract: part II. J Ethnopharmacol, 2003; 89(2–3):217–9. CrossRef
Chen X, Qian J, Wang L, Li J, Zhao Y, Han J, Khan Z, Chen X, Wang J, Liang G. Kaempferol attenuates hyperglycemia-induced cardiac injuries by inhibiting inflammatory responses and oxidative stress. Endocrine, 2018; 60(1):83–94. CrossRef
Cheng N, Ren N, Gao H, Lei X, Zheng J, Cao W. Antioxidant and hepatoprotective effects of Schisandra chinensis pollen extract on CCl4-induced acute liver damage in mice. Food Chem Toxicol Int J Publ Br Ind Biol Res Assoc, 2013; 55:234–40. CrossRef
Frank PR, Suresh V, Arunachalam G, Kanthlal SK, Ziaudheen VM. Evaluation of hepatoprotective effect of Adiantum incisum forsk leaf extract against CCl4 induced hepatotoxicity in rats. Int Res J Pharm, 2012; 3(3):230–4.
Freitag AF, Cardia GFE, da Rocha BA, Aguiar RP, Silva-Comar FM de S, Spironello RA, Grespan R, Caparroz-Assef SM, Bersani-Amado C, Cuman RKN. Hepatoprotective effect of silymarin (Silybum marianum) on hepatotoxicity induced by acetaminophen in spontaneously hypertensive rats. Evid Based Complement Alternat Med, 2015; 2015(3):538317. CrossRef
Fursule RA, Patil SD. Hepatoprotective and antioxidant activity of Phaseolus trilobus, Ait on bile duct ligation induced liver fibrosis in rats. J Ethnopharmacol, 2010; 129(3):416–9. CrossRef
Gahlot K, Lal VK, Jha S. Phytochemical and pharmacological potential of Flemingia Roxb. ex WT Aiton (Fabaceae). Int J Phytomed, 2011; 3(3):294.
Gao Z, Huang K, Xu H. Protective effects of flavonoids in the roots of Scutellaria baicalensis Georgi against hydrogen peroxide-induced oxidative stress in HS-SY5Y cells. Pharmacol Res, 2001; 43(2):173–8. CrossRef
Gnanadesigan M, Ravikumar S, Inbaneson SJ. Hepatoprotective and antioxidant properties of marine halophyte Luminetzera racemosa bark extract in CCL4 induced hepatotoxicity. Asian Pac J Trop Med, 2011; 4(6):462–5. CrossRef
Gupta RK, Hussain T, Panigrahi G, Das A, Singh GN, Sweety K, Faiyazuddin M, Rao CV. Hepatoprotective effect of Solanum xanthocarpum fruit extract against CCl4 induced acute liver toxicity in experimental animals. Asian Pac J Trop Med, 2011; 4(12):964–8. CrossRef
Gyr K, Meier R. Flumazenil in the treatment of portal systemic encephalopathy—an overview. Intensive Care Med, 1991; 17(1):S39–42. CrossRef
Heibatollah S, Reza NM, Izadpanah G, Sohailla S. Hepatoprotective effect of Cichorium intybus on CCl4-induced liver damage in rats. Afr J Biochem Res, 2008; 2(6):141–4.
Hsieh P-C, Ho Y-L, Huang G-J, Huang M-H, Chiang Y-C, Huang S-S, Hou WC, Chang YS. Hepatoprotective effect of the aqueous extract of Flemingia macrophylla on carbon tetrachloride-induced acute hepatotoxicity in rats through anti-oxidative activities. Am J Chin Med, 2011; 39(02):349–65. CrossRef
Jahan S, Khan M, Imran S, Sair M. The hepatoprotective role of Silymarin in isoniazid induced liver damage of rabbits. J Pak Med Assoc, 2015; 65(6):620–3.
Joshi M, Shailajan S. Hepatoprotective and antioxidative activity of Avicennia marina Forsk. Medwin Publ, 2016; 2(1):1–9. CrossRef
Khandelwal, K. Practical pharmacognosy techniques and experiments. Nirali Prakashan Publishers, Pune, India, 2008.
Kheiripour N, Karimi J, Khodadadi I, Tavilani H, Goodarzi MT, Hashemnia M. Hepatoprotective effects of silymarin on liver injury via irisin upregulation and oxidative stress reduction in rats with type 2 diabetes. Iran J Med Sci, 2019; 44(2):108.
Kondeva-Burdina M, Shkondrov A, Simeonova R, Vitcheva V, Krasteva I, Ionkova I. In vitro/in vivo antioxidant and hepatoprotective potential of defatted extract and flavonoids isolated from Astragalus spruneri Boiss. (Fabaceae). Food Chem Toxicol, 2018; 111:631–40. CrossRef
Krasteva I, Shkondrov A, Ionkova I, Zdraveva P. Advances in phytochemistry, pharmacology and biotechnology of Bulgarian Astragalus species. Phytochem Rev, 2016; 15(4):567–90. CrossRef
Kumar KVA, Veere GK. Evaluation of hepatoprotective and antioxidant activity of Flemingia strobilifera R. Br. against experimentally induced liver injury in rats. Int J Pharm Pharm Sci, 2011; 3(3):115–9.
Lahon K, Das S. Hepatoprotective activity of Ocimum sanctum alcoholic leaf extract against paracetamol-induced liver damage in Albino rats. Pharmacogn Res, 2011; 3(1):13–8. CrossRef
Mahli A, Koch A, Czech B, Peterburs P, Lechner A, Haunschild J, Muller M, Hellerbrand C. Hepatoprotective effect of oral application of a silymarin extract in carbon tetrachloride-induced hepatotoxicity in rats. Clin Phytosci, 2015; 1(1):5. CrossRef
Mbemya GT, Vieira LA, Canafistula FG, Pessoa ODL, Rodrigues APR. Reports on in vivo and in vitro contribution of medicinal plants to improve the female reproductive function. Reprodução Clim, 2017; 32(2):109–19. CrossRef
Mukherjee, P. Quality control of herbal drugs-an approach to evaluation of botanicals. Business Horizons Publishers, New Delhi, India, 2007.
Narendhirakannan RT, Rajeswari K. In vitro antioxidant properties of three varieties of Allium sativum L. extracts. J Chem, 2010; 7(S1):S573–9. CrossRef
Pelissero C, Lenczowski MJP, Chinzi D, Davail-Cuisset B, Sumpter JP, Fostier A. Effects of flavonoids on aromatase activity, an in vitro study. J Steroid Biochem Mol Biol, 1996; 57(3–4):215–23. CrossRef
Pharmacopoeia, I. Government of India “Ministry of Health & Family Welfare” The Controller & Publication, New Delhi, India, pp 2186–8, 2010.
Pistelli LF. Secondary metabolites of genus Astragalus: structure and biological activity. In: Studies in natural products chemistry. Elsevier, Karachi, Pakistan, pp 443–545, 2002. CrossRef
Preussmann R. Hepatocarcinogens as potential risk for human liver cancer. In: Remmer H, Bolt HM, Bannasch P, Popper H (eds.). MTP Press, Lancaster, UK, pp 11–29, 1978.
Raju K, Anbuganapathi G, Gokulakrishnan V, Rajkapoor B, Jayakar B, Manian S. Effect of dried fruits of Solanum nigrum LINN against CCl4-induced hepatic damage in rats. Biol Pharm Bull, 2003; 26(11):1618–9. CrossRef
Rao BG, Rao YV, Rao TM. Hepatoprotective activity of Spillanthes acmella Extracts against CCl4-induced liver toxicity in rats. Asian Pac J Trop Dis, 2012; 2:S208–11. CrossRef
Recknagel RO, Glende Jr EA, Dolak JA, Waller RL. Mechanisms of carbon tetrachloride toxicity. Pharmacol Ther, 1989; 43(1):139–54. CrossRef
Saeed M, Babazadeh D, Arif M, Arain MA, Bhutto ZA, Shar AH, Kakar MU, Manzoor R, Chao S. Silymarin: a potent hepatoprotective agent in poultry industry. Worlds Poult Sci J, 2017; 73(3):483–92. CrossRef
Saleem M, Asif A, Akhtar MF, Saleem A. Hepatoprotective potential and chemical characterization of Artocarpus lakoocha fruit extract. Bangladesh J Pharmacol, 2018; 13(1):90. CrossRef
Samuel AJSJ, Mohan S, Chellappan DK, Kalusalingam A, Ariamuthu S. Hibiscus vitifolius (Linn.) root extracts shows potent protective action against anti-tubercular drug induced hepatotoxicity. J Ethnopharmacol, 2012; 141(1):396–402. CrossRef
Shailajan S, Chandra N, Sane RT, Menon S. Effect of Asteracantha longifolia Nees. against CCl4 induced liver dysfunction in rat. Indian J Exp Biol, 2005; 43(1):68–75.
Shailajan S, Joshi H. Optimized separation and quantification of Pharmacologically active markers Quercetin, Kaempferol, ß-sitosterol and Lupeol from Cuscuta reflexa Roxb. J Pharm Res, 2011; 4:1851–3.
Shailajan S, Joshi M, Tiwari B. Hepatoprotective activity of Parmelia perlata (Huds.) Ach. against CCl4 induced liver toxicity in Albino Wistar rats. J Appl Pharm Sci, 2014; 4(2):70–4.
Shailajan S, Mascarenhas R. Chromatographic evaluation of kaempferol from Flemingia tuberosa Dalzell: an endemic plant of Western Ghats. J Appl Biol Biotechnol, 2018; 6(6):51–7. CrossRef
Silymarin Clinical Update, Profile on Liver Herb. Scientific Communications International Limited, Hong Kong, 1995.
Singhal KG, Gupta GD. Hepatoprotective and antioxidant activity of methanolic extract of flowers of Nerium oleander against CCl4-induced liver injury in rats. Asian Pac J Trop Med, 2012; 5(9):677–85. CrossRef
Sobeh M, Mahmoud MF, Abdelfattah MA, Cheng H, El-Shazly AM, Wink M. A proanthocyanidin-rich extract from Cassia abbreviata exhibits antioxidant and hepatoprotective activities in vivo. J Ethnopharmacol, 2018;213:38–47. CrossRef
Sultana B, Yaqoob S, Zafar Z, Bhatti HN. Escalation of liver malfunctioning: a step toward herbal awareness. J Ethnopharmacol, 2018; 216:104–19. CrossRef
Tong J, Yao X, Zeng H, Zhou G, Chen Y, Ma B, Wang Y. Hepatoprotective activity of flavonoids from Cichorium glandulosum seeds in vitro and in vivo carbon tetrachloride-induced hepatotoxicity. J Ethnopharmacol, 2015; 174:355–63. CrossRef
Wang L, Luo Y, Wu Y, Xia F, Wu Z. Quickly verifying the antioxidant contribution of the individual composition in natural antioxidants by HPLC-free radical scavenging detection. LWT, 2018; 96:461–8. CrossRef
Wang P-F, Zheng R-L. Inhibitions of the autoxidation of linoleic acid by flavonoids in micelles. Chem Phys Lipids, 1992; 63(1–2):37–40. CrossRef
Wang Y, Tang C, Zhang H. Hepatoprotective effects of kaempferol 3-O-rutinoside and kaempferol 3-O-glucoside from Carthamus tinctorius L. on CCl4-induced oxidative liver injury in mice. J Food Drug Anal, 2015; 23(2):310–7. CrossRef
Yin P, Wang Y, Yang L, Sui J, Liu Y. Hypoglycemic effects in alloxan-induced diabetic rats of the phenolic extract from Mongolian Oak cups enriched in ellagic acid, kaempferol and their derivatives. Molecules, 2018; 23(5). CrossRef
Zarezade V, Moludi J, Mostafazadeh M, Mohammadi M, Veisi A. Antioxidant and hepatoprotective effects of Artemisia dracunculus against CCl4-induced hepatotoxicity in rats. Avicenna J Phytomed, 2018; 8(1):51–62.
Zhao Z-W, Chang J-C, Lin L-W, Tsai F-H, Chang H-C, Wu C-R. Comparison of the hepatoprotective effects of four endemic Cirsium species extracts from Taiwan on CCl4-induced acute liver damage in C57BL/6 mice. Int J Mol Sci, 2018; 19(5). CrossRef