INTRODUCTION
Cattle raising is one of the most important sectors of the Egyptian agribusiness and consequently of the national economy. Rhipicephalus annulatus represents a major risk factor for the cattle industry in Egypt (Aboelhadid et al., 2016). Tick transmits crucial pathogens for cattle, including Babesia and Anaplasma species (Ferreira et al., 2018). Since the use of commercial drugs involves many problems, including drug-resistance shown by the most important parasites, the environmental damage, and the toxicity (Flamini, 2003); therefore, the direct application of effective plant extracts is a big demand to substitute the synthetic acaricides (Chungsamarnyart and Jansawan, 2001).
The use of the plant extracts, essential oils, and their major constituents for the control of several external and internal parasites have been carried out in different studies. The tested parasites were, for example, house dust mite (El-Zemity et al., 2006), mosquitos including Anopheles culicifacies, Anopheles stephensi, Culex quinquefaciatus, Aedes aegypti (Jayaraman et al., 2015), and sheep nematodes (Hussain et al., 2011), and different ticks, including Amblyomma americanum, Dermacentor variabilis, Ixodes scapularis, Rhipicephalus sanguineus (Flor-Weiler et al., 2011), Hyalomma dromedarii (Al-Rajhy et al., 2003), and R. annulatus (Moawad et al., 2017).
The inhibition of acetylcholinesterase (AChE) enzyme is the mode of action of organophosphate and carbamate pesticides consequently increasing neuronal excitation caused by acetylcholine which is followed by paralysis and death of the parasite (Tan et al., 2011). Some studies focused on measuring the in vitro acaricide and anticholinesterase activities to find a direct relation. The n-hexane extract of Calea serrata had acaricidal activity against larvae of Rhipicephalus (Boophilus) microplus and inhibition of AChE is a possible mechanism of action (Ribeiro et al., 2012). It was also reported that the AChE inhibitory activity of stigmasterol and hexacosanol is responsible for larvicidal and repellent properties of Chromolaena odorata (Gade et al., 2017). In the same regard, Santos et al. (2018) found that the hexanic and ethyle acetate extracts and hexanic extract fraction two obtained from the leaves of Digitaria insularison induced significant inhibition of AChE activity and acaricidal activity. Many other studies have revealed that the effect against AChE was implicated in the acaricidal mechanisms of many plant constituents and extracts ( Abdelgaleil et al., 2019; Badawy et al., 2010; Van Leeuwen et al., 2010).
Aizoaceae family has many plants with reported antifungal, antibacterial activity (Mohammed et al., 2012), acaricidal activity against R. annulatus (Moawad et al., 2017). Trianthema portulacastrum L. is a potential traditional herb belongs to the family Aizoaceae. It is rapidly growing, annual terrestrial weed. It showed interesting antiparasitic activities, including anthelmintic activity against sheep nematodes (Hussain et al., 2011) and also mosquito larvicidal activity against Anopheles culicifacies, Anopheles stephensi, Culex quinquefaciatus, and Aedes aegypti (Singh et al., 2011). Traditionally, it is also used for the treatment of various diseases, such as stomachic, laxative, analgesic, antianemia, antiulcer, and abortifacient (Yadav et al., 2016).
Aizoon canariensis is a perennial plant belonging to the family Aizoaceae. It is distributed in North Africa, Mediterranean, south Iran, Afghanistan, and Pakistan (Freije et al., 2013). It has poor literature concerning its chemical constituents and biological uses.
From the previously reported antiparasitic properties of Aizoaceae plants and previous reports about T. portulacastrum against internal and external parasites, it is interesting to evaluate the anticholinesterase and acaricidal activity of T. portulacastrum L. and A. canariensis against adult and larvae of R. annulatus tick.
MATERIALS AND METHODS
Materials for chromatographic study
Silica gel for column chromatography (CC) (E. Merck), Pre-coated silica gel G60 F254 thin layer chromatography (TLC) plates (20 × 20 cm) (Pharmacia Biotech AB, Uppsala). Solvents for chromatography were of analytical grade. Steroids were visualized by spraying TLC with p-anisaldehyde’s reagent, followed by warming with a heat gun. The chemical acaricide deltamethrin 50 μg/ml (Butox ® 50 Intervet International, The Netherlands) was used as a positive control. Fifty percent DMSO-EtOH was used as a vehicle for preparation of extracts for the acaricidal study. AChE, acetylthiocholine iodide, and 6, 6′-Dinitro-3, 3′-dithiodibenzoic acid were obtained from Sigma Chemical Company (Saint Louis, MO). All other chemicals are commercially obtained and are of analytical grade.
Nuclear magnetic resonance spectrometry
Nuclear magnetic resonance (NMR) was used for identification of steroids. Spectra were recorded using a Bruker Avance III 400 MHz (Bruker AG, Switzerland) with AEON Nitrogen-Free Magnet and BBFO Smart Probe. Data acquisition and processing was performed using Topspin 3.1 Software. CDCl3, an NMR solvent, was purchased from Cambridge Isotope Laboratories, Inc., (Andover, MA).
Plant material
Entire herbs of T. portulacastrum L. (TP) and A. canariensis L. (AC) were collected and identified by Prof. Dr. Abdel Haleem Abdel Motagaly, Agriculture Museum, Giza, Egypt in the flowering stage in spring and summer 2015 and 2017, from Cairo - Suez Canal road, East desert, Egypt. The voucher specimen’s numbers (R-Trian-10) and (R-Aizo) given to T. portulacastrum L. and A. canariensis L., respectively, and deposited at the Botanic Herbarium, Agriculture Museum, Dokky, Egypt.
Preparation of plant extracts
Extraction
A small-scale extraction for preliminary screening is done for both plants. Fifty grams of each plant material were separately extracted with 70% ethanol to prepare the crude hydroalcoholic (CH) extracts. TP was found active while AC wasn’t so; large-scale extraction was performed for TP. A kilogram powder was successively extracted with 70% ethanol (3 × 3L). TP-CH extract was concentrated in vacuum using rotary evaporator at 40ºC. The residue (116 g) was suspended in distilled water and was subjected to solvent partitioning using n-hexane (HX) (3 × 500 ml), ethyl acetate (EA) (4 × 300 ml), and n-butanol (BT) (3 times × 200 ml). All solvents were dried under vacuum and the extract yields are shown in Figure 2. Five concentrations were prepared from different extracts (12.5, 25, 50, 100, and 150 mg/ml) in 50% DMSO-EtOH as a vehicle. The prepared concentrations were applied on adult ticks and unfed larvae.
Preparation of USM
Ten gm of n-hexane extract was saponified by refluxing with 100 ml 10% alcoholic KOH, 40 ml benzene for 24 hours (Kamal et al., 2017). After distillation of ethanol and benzene and dilution with water, the USM was extracted with ether till exhaustion to yield 1.5 g USM.
Isolation of the fatty acids from the saponifiable fraction
The aqueous mother liquor, left after extraction of USM, was acidified with 10% HCl and the liberated fatty acids were extracted with ether till exhaustion to yield 1.5 g FAs (Christie, 1993).
Chromatographic isolation of T. portulacastrum L. secondary metabolites
USM (3 g) was fractionated on Vacuum Liquid Chromatography (VLC) using silica gel for column (90 g, 25 × 3.5 cm) eluted with petroleum ether (PE) and increasing the polarity by adding 5% increments of EA and collecting 100 ml fractions. TLC using HX–EA (8:2) and spraying with p-anisaldehyde’s reagent followed by heating was done to collect similar subfractions. The subfraction eluted with PE-EA (90:10 to 80:20) (1.250 g) was chromatographed on silica column (30 g, 28 × 2 cm) eluted with PE-EA with 1% increments to give 400 mg white powder. The powder was found to be a (1:1) mixture of β-sitosterol and stigmasterol as shown by1D-NMR spectroscopy including 1H and 13CNMR spectra and comparison with literature (Chaturvedula and Prakash, 2012) and (Amin et al., 2017). EA extractive (3 g) was chromatographed over silica column (90 g, 88 × 2 cm). Elution was performed with MeOH/CH2Cl2 in gradient elution with 5% increment of MeOH and collecting 10 ml fractions. TLC of the fractions was done and similar ones were combined to get 100 mg yellow residue eluted with 15% MeOH/CH2Cl2 was rechromatographed on Sephadex - LH20 column eluted with MeOH to give compound (3) (20 mg). Compound (3) was found to be 20-hydroxyecdysone by1D-NMR spectroscopy, including 1H and 13CNMR spectra and comparison with literature (Girault and Lafont, 1988) which was the major steroid in EA and BT fractions. The amount isolated from 20-hydroxyecdysone was not sufficient for immersion test and only AChE inhibition test was performed on it. The structure of isolated compounds are shown in Figure 1.
AChE assay
AChE percent inhibition was detected based on the methods of Ellman et al. (1961) and Akkol et al. (2012) with little modification. The plant extracts, fractions, and constituents were dissolved in dimethyl sulphoxide (DMSO). Briefly, the test sample was prepared by adding 3 ml phosphate buffer (pH = 8), 0.1 ml AChE source (600 Units/ml) diluted 1:120, 100 μl plant extract, fraction, or constituent (dissolved in DMSO), and 20 μl acetylthiocholine iodide (0.075 M) in Wassermann tubes. The absorbance was measured just after addition and mixing at 15 minutes and ΔA was calculated for each test sample. Blank test was prepared by using 100 μl DMSO instead of the plant extract, fraction, or constituent, and ΔA for blank was also calculated in the same way. The percentage of inhibition of AChE was determined by applying the following equation: AChE percent inhibition = 100 – [ΔA for sample/ΔA for blank] × 100. Three final concentrations (0.25, 0.5, and 1 mg/ml) were used for each plant extract, fraction, or constituent, and three triplicates were applied for each concentration. A standard drug, galantamine, was used at the same concentrations.
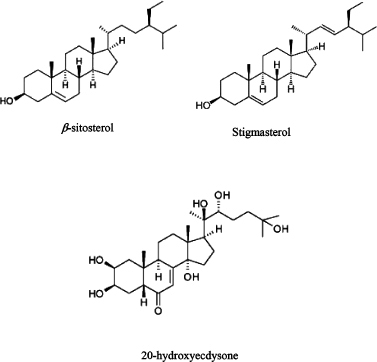 | Figure 1. Structure of steroids isolated from T. portulacastrum L herb. [Click here to view] |
Acaricidal study
Sampling of R. annulatus
The collection of fully engorged female R. annulatus ticks was performed during Summer 2017 from naturally infested cattle making sure that they had not received any tick treatments for at least 20 days according to Rodriguez-Vivas et al. (2006). The ticks were kept in clean plastic bottles with lids containing small holes. The collected ticks were transported to the Parasitology Laboratory, Faculty of Veterinary Medicine, Beni-Suef University for identification and experimental application. The freshly collected females were separated, carefully washed, and then dried on an absorbent paper. Engorged females weighing not less than 140 mg with no signs of injury were used in the study.
Adult immersion test (AIT)
AIT was performed as described by Sharma et al. (2012) with little modifications. Ticks were divided into 10 groups (10 ticks each) to evaluate the activity of TP and AC extracts and TP major compounds. The groups were: negative control group (50% DMSO-EtOH), AC CH extract, and TP CH extract. TP-hexane fraction, TP-EA fraction, TP-butanol fraction, TP USM, TP-fatty acids, β-sitosterol–stigmasterol mixture, and finally the positive control deltamethrin (50 μg/ml). All experiments were done in triplicates in clean-labeled Petri dishes. The different groups of ticks were immersed in 5 ml of each treatment. After 2 minutes, the liquid was poured off and the ticks were transferred to a filter paper for drying and then kept separately in clean Petri dishes. Simultaneously, the ticks in the control group were treated with 50% DMSO-EtOH. The treated ticks were kept in biochemical oxygen demand (B.O.D.) incubator at a temperature of 27 ± 2ºC and relative humidity of 80 ± 10%. The mortality was recorded after 72-hour post treatment. Mortality % = (number of dead tick in treated groups-number of dead tick in control groups) × 100/Total number of treated ticks. Oviposition was estimated by measuring the weight of eggs (mg) laid by each group (10 females).
Larval immersion test (LIT)
The different concentrations of the products were screened against the unfed (15-day old) larvae. One milliliter of each solution was transferred to1.5 ml microcentrifuge tubes and then approximately 100 larvae were added to each one. Control solutions were prepared adding one ml 50% DMSO-EtOH. Immediately after addition of larvae, tubes were closed and shaken vigorously for 30 seconds and then gently for 10 minutes (Klafke et al., 2006). The tubes were then opened, and the larvae transferred with a paint brush to a filter paper. After drying, paper was folded and closed with clips forming a packet. The packets were incubated at 27ºC–28ºC and 80%–90% relative humidity for 24 hours, and then the mortality was determined.
 | Figure 2. Scheme for bioassay-guided isolation of acaricidal steroids from Trianthema protulocastrum; % mortality of adult tick followed by % inhibition of AChE are between brackets. [Click here to view] |
Statistics
For acaricidal study, statistical analysis of data was performed using Statistical Package for Social Science (SPSS for Windows (IBM), version 22, Chicago, IL) to determine if variables differed between treatments. ANOVA tests and subsequent Duncan’s multiple range tests were applied to determine the differences between means. Results were presented as means. Probability values of less than 0.05 (p < 0.05) was considered as significant. The effective concentration (EC50) with 95% Confidence Interval (95%CI) was calculated by non-linear regression and probit analysis (SPSS version 22). For AChE, ANOVA followed by least significant difference (LSD) test were applied for the analysis of data by using the IBM SPSS version 22 and PC-STAT programs.
RESULTS
The two plants of the family Aizoaceae: TP and AC were assessed for potential acaricidal activity against R. annulatus tick using immersion tests and the anticholinesterase activity to find a mechanism for the acaricidal activity.
The effect of the used materials on adult R. annulatus
The CH extracts of both plants were tested. Mortalities and deposited egg mass of AC-CH group against R. annulatus ticks were non-significantly different from negative control group even at its highest concentration, (Table 1).
On the contrary, TP-CH extract showed significant mortalities at 100 and 150 mg/ml that showed non-significant difference with deltamethrin-treated group; ticks died quickly without egg deposition and showed black coloration and shrinkage of the cuticle. Also, egg mass was reduced significantly (p ≤ 0.05) in concentrations 50, 25, and 12.5 mg/ml compared to untreated control group (Table 1).
In TP-HX group, the highest two concentrations (100 and 150 mg/ml) achieved the same adulticidal findings of TP-CH extract. At 50 mg/ml concentration; TP-HX showed 70% mortalities which still significantly different from negative control group, while the lower concentrations were not. TP-HX was the most active among CH fractions, so saponification was done to prepare USM and FA fractions.
TP-USM achieved 85% adult mortality at 50 mg/ml, while TP-FA achieved only 20% mortality at the same concentration. Egg mass was insignificant from deltamethrin group in TP-USM, while in TP-FA it was insignificantly different from negative control group. TP-USM showed significant adulticidal activity at p ≤ 0.05; Therefore, GC-MS analysis was performed, and chromatographic isolation was done.
 | Table 1. Adult mortality, egg mass and larval mortality of adult females of Rhipicephalus annulatus tick exposed to the immersion test with the extracts and fractions of AC and TP. [Click here to view] |
TP-EA extract achieved 70% and 50% adult mortality at 100 and 150 mg/ml concentration, respectively, which were significantly different from both positive and negative control groups. At lower concentrations, it was inactive. Moreover, egg masses reached 250.00 ± 0.00 mg/10 females at 1.25% concentration and decreased gradually by increasing concentration till reached 43.3 ± 31.8 mg/10 females at 15% concentration. Egg mass was reduced significantly with the untreated (p ≤ 0.05) in all used concentrations but still significantly larger than deltamethrin group
TP-BT fraction showed 53% mortalities (p ≤ 0.05) at 150 mg/ml. However, no lethal effect at lower concentrations was recorded. Egg mass was reduced significantly with the negative control (p ≤ 0.05) in all tested concentrations but still significantly larger than deltamethrin group.
B-sitosterol + stigmasterol (50 mg/ml) showed 50.0 ± 5% adulticidal activity. Egg mass was reduced significantly with the untreated (p ≤ 0.05) to 100 mg/10 females. However, Egg masses were noticed even at the highest concentrations of EtOAc, BT, 50 mg/ml β-sitosterol+stigmasterol, and deltamethrin 50 μg/ml, the deposited egg mass decreased significantly (p ≤ 0.05) at all treatments of T. portulacastrum L. In the control untreated group, mortality was 0% and ticks deposited egg mass about 347.00 ± 0.00 mg/10 females after 72 hours (Fig. 2, Table 2). EC50 of adult ticks was 69.4, 45.7, and 114.9, 164.5 mg/ml in TP-CH, TP-HX, TP-EA, and TP-BT group, respectively.
The effect on larvae of R. annulatus
Findings of T. portulacastrum L. were matched with its adulticidal activity against R. annulatus. The mortality% was significantly (p ≤ 0.05) variable among different extracts. It reached 100% (p ≤ 0.05) in TP-CH and TP-HX fraction at 100 and 150 mg/ml and decreased to 50% at 25 mg/ml. Also, TP-EA fraction achieved good larvicidal activity at the highest concentration (70.0 ± 0.0%) and 50.0 ± 0.0% at 100 mg/ml (p ≤ 0.05). While, larvicidal activity of TP-EA was 10.0 ± 0.0% and non-significantly different with untreated control group at lower concentrations. However, butanol had 50.0 ± 0.0 % (p ≤ 0.05) larvicidal activity at its highest concentration. On the contrary, no lethal effect was noticed in larvae that were subjected to BT at concentration of (10%, 5%, 2.5%, and 1.25%). β-sitosterol+stigmasterol (50 mg/ml) showed 50.0 ± 0.0% larvicidal activity (p ≤ 0.05), while the positive control Deltamethrin 50 μg/ml showed 95.00% ± 0.0 (p ≤ 0.05). On the contrary, non-significant larval mortalities were noticed in ticks that were subjected to A. canariensis CH extract at 100 and 150 mg/ml concentrations and the untreated control group (50% DMSO-ETOH). EC50 of larvae were 24, 34.6, 114.7, and 169 mg/ml in TP-CH, TP-HX, TP-EA, and TP-BT group, respectively.
 | Table 2. Percentage of inhibition of the AChE enzyme (mean + standard deviation) using extracts of AC and TP in comparison with galantamine. [Click here to view] |
Effect of tested plant extracts, fractions or constituents on AChE activity
As indicated in Figure 2, Table 2, TP-CH, TP-HX and 20-hydroxyecdysone produced the most potent inhibitory effect on AChE activity (99.33 ± 0.34%, 98.21 ± 1.3%, and 96.47 ± 0.44% inhibition at highest concentration) which was concentration dependent; these effects were comparable to the effects of standard AChE inhibitor, galantamine. According to the classification of Vinutha et al. (2007), >50% inhibition of AChE activity is classified as a potent inhibitor.
TP-EA fraction and TP-BT are arranged in the second order as the potent AChE activity inhibitors. AC-CH was the less potent in affecting AChE inhibitory activity, especially at concentrations 0.25 and 0.5 mg/ml. The TP-CH at three different concentrations (0.25, 0.5, and 1 mg/ml) produced a highly significant increase in AChE percent inhibition as compared with the corresponding concentrations of AC-CH. TP-HX was more effective than TP-EA and TP-BT fractions at the three different concentrations. The TP-USM of hexane extract seemed to be more effective on AChE percent inhibition than TP-FA at concentrations 0.25, 0.5 and 1 mg/ml; however, the effect was not significant (p > 0.05; LSD) at concentrations 0.25 and 1 mg/ml. The β-sitosterol-stigmasterol mixture induced a significant dose dependent anti-AChE effect (p ≤ 0.05; LSD). Concerning one-way ANOVA, the effect between plant ingredients on AChE activity percent inhibition at different concentrations was highly significant (p ≤ 0.01; F-probability).
GC-MS analysis of USM
GC-MS analysis of TP-USM revealed the presence of trimethy-l2-pentadecanone (13.83%), 2-phenyldodecane (7.70%), 5-phenylundecane (4.07%), as major hydrocarbons and phytol (4.67%) which is acyclic diterpene, stigmasterol (2.59%), β- and sitosterol (1.67%) as major sterols as shown in Table 3.
DISCUSSION
Terrestrial plants and their products are promising source of effective acaricides (Pavela et al., 2016). Being a mixture of several compounds, it is hard for the insect to develop resistance against the plant extracts, because of several mechanisms of killing. In addition, being eco-friendly, easily degradable are additional advantages. Here, we investigate the activity of hydro alcoholic extracts of two herbs of the family Aizoaceae: T. portulacastrum L. and A. canariensis L. against R. annulatus (the bovine tick in Egypt). A biologically-guided isolation of active plant metabolites was our strategy as shown in Figure 1. In this strategy, chromatography and bioassay are parallel; fractionation and purification of only active fractions are done.
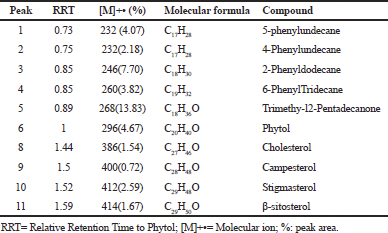 | Table 3. Chemical compounds identified by GC-MS analyses of the USM of the n-hexane fraction obtained from Trianthema portulacastrum L herb. [Click here to view] |
In this study, TP-CH was active while AC-CH was not, so the TP only was extracted on large scale and fractionated using solvents of different polarities (HX, EA, and BT) to divide the natural constituents into groups according to their polarities. The prepared extracts are monitored by bioassay. TP-HX and TP-EA showed promising activities at 150 mg/ml (100% and 70%, respectively) in both adult and larval immersion tests. The most effective fraction (TP-HX) was then subjected to saponification to separate the USM (steroids, triterpenoids and hydrocarbons) from the fatty acids. Both USM and FA were retested for their acaricidal activity where USM showed 85 ± 5% adult mortality which still insignificantly different from deltamethrin; the used chemical pyrethroid. The lowest EC 50 of adult ticks was 69.4 and 45.7 mg/ml in TP-CH and TP-HX, respectively. Meanwhile, in TP-EA and TP-BT groups, the EC 50 of adult ticks were 114.9, and 164.5 mg/ml, respectively.
TP-USM was subjected to GC-MS analysis (Table 3) and phytol was present which was previously reported to contribute to the pesticidal and nematicidal activity of Cynodon dactylon with palmitic acid, ethyl hexadecanoate, and ethyl stearate (Jananie et al., 2011). In addition, stigmasterol (2.59%) and β-sitosterol (1.67%) were present as major sterols. Chromatographic purification of TP-USM was performed to isolate major constituents for evaluation of acaricidal activity. A mixture of β-sitosterol-stigmasterol (1:1) was isolated as shown by NMR spectroscopy and measuring the integration of the proton signals at δH 5.03 ppm characteristic for stigmasterol and δH 5.35 ppm for each stigmasterol and β-sitosterol. Calculation of the percentage was done through comparing both integration values. and then the mixture was used in immersion tests. In our previous study, β-sitosterol (25 mg/mL) showed 86.66 ± 5% and 91.66 ± 2.88% adulticidal and larvicidal activity, respectively (Moawad et al., 2017). It is the same concentration of β-sitosterol present in this mixture (50 mg/ml of a mixture of two compounds with equal proportions). Since the mixture was less active than β-sitosterol alone, this indicates that the presence of stigmasterol in the mixture reduced the activity. Stigmasterol is Δ24 β-sitosterol which might produce antagonistic effect with β-sitosterol due to structure similarity and they are a common non-separable mixture of natural products (Lokadi Pierre, 2015). Our finding is also supported by the study on Culex quinquefasciatus larvae in which a mixture of phytosterols were more active when compared to stigmasterol alone (Gade et al., 2017). Steroids are important constituent in tick cuticle and the observed shrinkage in TP-CH, TP-HX, and TP-USM may be due to interference of steroids synthesis by the phytosterols of T. portulacastrum herb. The high lipophilicity of TP-USM constituents may contribute to their acaricidal effect through facilitating the penetration of the cuticle of adult tick that is composed mainly of waxes or lipids (Diehl et al., 1982).
On the other hand, TP-EA was also subjected to chromatographic isolation and 20-hydroxyecdysone was isolated (Banerji et al., 1971) but its amount was not sufficient for immersion test and only AChE inhibition assay was performed. TP-BT showed adulticidal activity only at the highest concentration but no activity at lower concentrations. This also may be due to its 20-hydroxyecdysone content.
20-hydroxyecdysone is a main representative of ecdysteroids which are arthropod steroid hormones controlling development and reproduction and are synthesized from cholesterol (Rewitz and Gilbert, 2008). Ecdysteroids bind to ecdysone receptor and many chemicals may be agonists or antagonists (Minakuchi et al., 2003).
The anticholinesterase activity of the active extracts and fractions were evaluated in order to find the possible relation between the acaricidal effect and the AChE inhibitory property. The AChE inhibition Activity was correlated to immersion test results and it may be one of the mechanisms of acaricidal activity.
Our results indicated that the TP-CH and TP-HX as well as 20-hydroxyecdysone produced the most potent inhibitory effects on AChE activity which are comparable with the standard AChE inhibitory drug, galantamine. On the other hand, A. canariensis crude hydroethanolic extract (AC-CH) showed lower AChE inhibitory activity. In parallel with this result, Sesuvium portulacastrum (Aizoaceae) showed 50% inhibitory activity to AChE as indicated by Suganthy et al. (2009). The inhibition of AChE may prolong the neuronal excitation induced by acetylcholine, which may in turn result in neuromuscular paralysis and death of the parasite. This attribution was supported by Tan et al. (2011). T. portulacastrum exhibited potent cholinesterase inhibitory activity attributable to the presence of the isolated sterols and of significance in the acaricidal activity. On comparison with data in literature, sterol mixture was better AChE inhibitor than its pure compound although stigmasterol has shown a good inhibitory effect on the enzyme (Gade et al., 2017).
CONCLUSION
Steroids, located in the n-hexane, EA and BT fractions, are part of the bioactive compounds responsible for T. portulacastrum acaricidal effects which may be mediated, at least in part, via AChE inhibitory activities. With the previously reported in vivo safe intake of T. portulacastrum L. herb; it provides a promising herb for veterinary use for control of vector-borne diseases and as safer alternatives in the control of tick populations. Additional studies are necessary for the evaluation of 20-hydroxyecdysone activity and in vivo studies are also needed for the confirmation of the in-vitro effect
ACKNOWLEDGMENT
The parasitology work done in this study was financially supported by a grant funded by Project Funding and Granting Unit, Scientific Research Development Unit, Beni-Suef University.
CONFLICT OF INTEREST
The authors indicate that there is no conflict of interest
AUTHORS’ CONTRIBUTIONS
This work was carried out in collaboration between all the authors. Abeer Moawad, Hala Abuzaid, Hetta, and Rabab Mohammed wrote the protocol, performed the chromatographic isolation, and spectroscopic analysis. Waleed M. Arafa designed and performed the acaricidal study. Osama Ahmed designed and performed the Anticholinesterase study. All authors wrote the first draft of the manuscript and managed the analyses of the results and the literature searches. All authors read and approved the final manuscript.
REFERENCES
Abdelgaleil SAM, Badawy ME, Mahmoud NF, Marei, AEM. Acaricidal activity, biochemical effects and molecular docking of some monoterpenes against two-spotted spider mite (Tetranychus urticae Koch). Pestic Biochem Physiol, 2019; 156:105–15. Available via https://doi.org/10.1016/j.pestbp.2019.02.006. CrossRef
Aboelhadid SM, Mahran HA, El-Hariri HM, Shokier KM. Rhipicephalus annulatus (Acari: Ixodidae) control by Nigella sativa, Thyme and spinosad preparations. J Arthropod Borne Dis, 2016; 2:148–58.
Akkol EK, Orhan IE, Yeilada E. Anticholinesterase and antioxidant effects of the ethanol extract, ethanol fractions and isolated flavonoids from Cistus laurifolius L. leaves. Food Chem, 2012; 131:626–31. Available via https://doi.org/10.1016/j.foodchem.2011.09.041. CrossRef
Al-Rajhy DH, Alahmed AM, Hussein HI, Kheir SM. Acaricidal effects of cardiac glycosides, azadirachtin and neem oil against the camel tick,Hyalomma dromedarii (Acari: Ixodidae). Pest Manag Sci, 2003; 59:1250–54. Available via https://doi.org/10.1002/ps.748. CrossRef
Amin E, Moawad A, Hassan H. Biologically-guided isolation of leishmanicidal secondary metabolites from Euphorbia peplus L. Saudi Pharm J, 2017; 25:236–40. Available via https://doi.org/10.1016/j.jsps.2016.06.003. CrossRef
Badawy MEI, El-Arami SA, Abdelgaleil SAM. Acaricidal and quantitative structure activity relationship of monoterpenes against the two-spotted spider mite, Tetranychus urticae. Experiment Appl Acarol, 2010; 52:261–74. CrossRef
Banerji A, Chintalwar GJ, Joshi NK, Chadha MS. Isolation of ecdysterone from indian plants. Phytochemistry 1971; 10:2225–26. Available via https://doi.org/10.1016/S0031-9422(00)97227-3. CrossRef
Chaturvedula VSP, Prakash I. Isolation of stigmasterol and β-sitosterol from the dichloromethane extract of Rubus suavissimus. Int Curr Pharm J, 2012; 1:239–42. Available via Christie WW. Preparation of ester derivatives of fatty acids for chromatographic analysis. In: William WC (ed.). Advances in lipid methodology – two, Oily Press, Dundee, The Scottish Crop Research Institute, Invergowrie, Dundee, Scotland, pp. 69–111, 1993. CrossRef
Chungsamarnyart N, Jansawan W. Effect of Tamarindus indicus L. Against the Boophilus microplus. Kasetsart J (Nat Sci), 2001; 35:34–9.
Diehl PA, Aeschlimann A, Obenchain FD. Tick reproduction: oogenesis and oviposition. Physiol Ticks, 1982; 1:277–350. Available via https://doi.org/10.1016/B978-0-08-024937-7.50014-7. CrossRef
Ellman GL, Courtney KD, Andres V, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol, 1961; 7:88–95. Available via https://doi.org/10.1016/0006-2952(61)90145-9. CrossRef
El-Zemity S, Rezk H, Farok S, Zaitoon A. Acaricidal activities of some essential oils and their monoterpenoidal constituents against house dust mite, Dermatophagoides pteronyssinus (Acari: Pyroglyphidae). J Zhejiang Univ Sci B, 2006; 7:957–62. Available via https://doi.org/10.1631/jzus.2006.B0957. CrossRef
Ferreira FM, Delmonte CC, Novato TLP, Monteiro CMO, Daemon E, Vilela FMP, Amaral, MPH. Acaricidal activity of essential oil of Syzygium aromaticum, hydrolate and eugenol formulated or free on larvae and engorged females of Rhipicephalus microplus. Med Vet Entomol, 2018; 32:41–47. Available via https://doi.org/10.1111/mve.12259. CrossRef
Flamini G. Acaricides of natural origin, personal experiences and review of literature (1990-2001). Stud Nat Prod Chem, 2003; 28:381–451. Available via https://doi.org/10.1016/S1572-5995(03)80146-1. CrossRef
Flor-Weiler LB, Behle RW, Stafford KC. Susceptibility of four tick species, Amblyomma americanum, Dermacentor variabilis, Ixodes scapularis, and Rhipicephalus sanguineus (Acari: Ixodidae), to nootkatone from essential oil of grapefruit. J Med Entomol, 2011; 48:322–26. CrossRef
Freije A, Alkhuzai J, Al-Laith AA. Fatty acid composition of three medicinal plants from Bahrain: new potential sources of γ-linolenic acid and dihomo-γ-linolenic. Ind Crops Prod, 2013; 43:218–24. Available via https://doi.org/10.1016/j.indcrop.2012.07.021. CrossRef
Gade S, Rajamanikyam M, Vadlapudi V, Nukala KM, Aluvala R, Giddigari C, Karanam, NJ, Barua NC, Pandey R, Upadhyayula VSV, Sripadi P, Amanchy R, Upadhyayula SM. Acetylcholinesterase inhibitory activity of stigmasterol & hexacosanol is responsible for larvicidal and repellent properties of Chromolaena odorata. Biochim Biophys Acta - Gen Subj, 2017; 1861:541–50. Available via https://doi.org/10.1016/j.bbagen.2016.11.044. CrossRef
Girault JP, Lafont R. The complete 1H-NMR assignment of ecdysone and 20-hydroxyecdysone. J Insect Physiol, 1988; 34:701–6. Available via https://doi.org/10.1016/0022-1910(88)90080-7. https://doi.org/10.3329/icpj.v1i9.11613. CrossRef
Hussain A, Khan MN, Iqbal Z, Sajid MS, Khan MK. Anthelmintic activity of Trianthema portulacastrum L. and Musa paradisiaca L. against gastrointestinal nematodes of sheep. Vet Parasitol, 2011; 179:92–9. Available via https://doi.org/10.1016/j.vetpar.2011.02.022. CrossRef
Jananie R, Priya V, Vijayalakshmi K. Determination of bioactive components of Cynodon dactylon by GC-MS analysis. New York Sci J, 2011; 4:16–20.
Jayaraman M, Senthilkumar A, Venkatesalu V. Evaluation of some aromatic plant extracts for mosquito larvicidal potential against Culex quinquefasciatus, Aedes aegypti, and Anopheles stephensi. Parasitol Res, 2015; 114:1511–18. Available via https://doi.org/10.1007/s00436-015-4335-0. CrossRef
Kamal AM, Ziada AA, Soliman RF, Selim MA. Chemical investigation of lipoidal matter of Ficus craterostoma. J Adv Pharm Res, 2017; 547:150–54. CrossRef
Klafke GM, Sabatini GA, De Albuquerque TA, Martins JR, Kemp DH, Miller RJ, Schumaker TTS. Larval immersion tests with ivermectin in populations of the cattle tick Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) from State of Sao Paulo, Brazil. Vet Parasitol, 2006; 142:386–90. Available via https://doi.org/10.1016/j.vetpar.2006.07.001. CrossRef
Lokadi Pierre L. Isolation and characterisation of stigmasterol and β-sitosterol from Odontonema Strictum (Acanthaceae). J Innov Pharm Biol Sci, 2015; 2;88–95.
Minakuchi C, Nakagawa Y, Kamimura M, Miyagawa H. Binding affinity of nonsteroidal ecdysone agonists against the ecdysone receptor complex determines the strength of their molting hormonal activity. Eur J Biochem, 2003; 270:4095–104. Available via https://doi.org/10.1046/j.1432-1033.2003.03801.x. CrossRef
Moawad A, Mohammed R, Arafa W. Biologically-guided Isolation of acaricidal phytosterols: an in vitro study against Rhipicephalus (B.) annulatus ticks infesting cattle in Egypt. Eur J Med Plants Brazil, 2017; 18:1–9. Available via https://doi.org/10.9734/EJMP/2017/32702. CrossRef
Mohammed R, El-Hawary SS, Abo-Youssef AM. Biological investigation of some wild aizoaceae and chenopediaceae species growing in Egypt. J Nat Prod (India), 2012; 5:193–206.
Pavela R, Canale A, Mehlhorn H, Benelli G. Application of ethnobotanical repellents and acaricides in prevention, control and management of livestock ticks: a review. Res Vet Sci, 2016; 109:1–9. Available via https://doi.org/10.1016/J.RVSC.2016.09.001. CrossRef
Rewitz KF, Gilbert LI. Daphnia Halloween genes that encode cytochrome P450s mediating the synthesis of the arthropod molting hormone: evolutionary implications. BMC Evol Biol, 2008; 8:1–8. Available via https://doi.org/10.1186/1471-2148-8-60. CrossRef
Ribeiro VLS, Vanzella C, Moysés F dos S, Santos JC dos, Martins JRS, Poser GL von, Siqueira IR. Effect of Calea serrata Less. n-hexane extract on acetylcholinesterase of larvae ticks and brain Wistar rats. Vet Parasitol, 2012; 189:322–26. Available via https://doi.org/10.1016/j.vetpar.2012.04.033. CrossRef
Rodriguez-Vivas RI, Alonso-Díaz, MA, Rodríguez-Arevalo F, Fragoso-Sanchez H, Santamaria VM, Rosario-Cruz R. Prevalence and potential risk factors for organophosphate and pyrethroid resistance in Boophilus microplus ticks on cattle ranches from the State of Yucatan, Mexico. Vet Parasitol, 2006; 136:335–42. Available via https://doi.org/10.1016/j.vetpar.2005.05.069. CrossRef
Santos FO, Lima HG, de Souza S, Rosa S, das Mercês NB, Serra TM, Uzeda RS, Reis IMA, Botura MB, Branco A, Batatinha MJM. In vitro acaricide and anticholinesterase activities of digitaria insularis (Poaceae) against Rhipicephalus (Boophilus) microplus. Vet Parasitol, 2018; 255:102–106. Available via https://doi.org/10.1016/j.vetpar.2018.04.003. CrossRef
Sharma AK, Kumar R, Kumar S, Nagar G, Singh NK, Rawat SS, Dhakad ML, Rawat AKS, Ray DD, Ghosh S. Deltamethrin and cypermethrin resistance status of Rhipicephalus (Boophilus) microplus collected from six agro-climatic regions of India. Vet Parasitol, 2012; 188:337–45. Available via https://doi.org/10.1016/j.vetpar.2012.03.050. CrossRef
Singh SP, Kamaraju R, Thomas T. Mosquito larvicidal properties of aqueous and acetone extracts of Trianthema portulacastrum Linn. (Family: Aizoaceae) against vector species of mosquitoes. J Commun Dis, 2011; 43:237–41.
Suganthy N, Pandian SK, Devi KP. Cholinesterase inhibitory effects of Rhizophora lamarckii, Avicennia officinalis, Sesuvium portulacastrum and Suaeda monica: mangroves inhabiting an Indian coastal area (Vellar Estuary). J Enzyme Inhib Med Chem, 2009; 24:702–07. Available via https://doi.org/10.1080/14756360802334719. CrossRef
Tan F, Wang L, Wang J, Wu X, Zhu H, Jiang L, Tao S, Zhao K, Yang Y, Tang X. Enhanced pesticide sensitivity of novel housefly actylcholinesterases: a new tool for the detection of residual pesticide contamination. Bioprocess Biosyst Eng, 2011; 34:305–14. Available via https://doi.org/10.1007/s00449-010-0472-0. CrossRef
Van Leeuwen T, Vontas J, Tsagkarakou A, Dermauw W, Tirry L. Acaricide resistance mechanisms in the two-spotted spider mite Tetranychus urticae and other important Acari: a review. Insect Biochem Mol Bio, 2010; 40:563–72. CrossRef
Vinutha B, Prashanth D, Salma K, Sreeja SL, Pratiti D, Padmaja R, Radhika S, Amit A, Venkateshwarlu K, Deepak M. Screening of selected Indian medicinal plants for acetylcholinesterase inhibitory activity. J Ethnopharmacol, 2007; 109:359–63. Available via https://doi.org/10.1016/j.jep.2006.06.014. CrossRef
Yadav E, Yadav PK, Verma A. Trianthema portulacastrum (L.): an important traditional herb. Ann Pharm Pharmaceut Sci, 2016; 7:46–52. Available via https://doi.org/10.15740/HAS/APPS/7.1/46-52. CrossRef