INTRODUCTION
Cancer is a major disease in the world (Bray et al., 2018). The number of new cases of lung cancer occurred 11.8% and other cancer 53.9%. Lung cancer is the first rank of incidence, mortality, and prevalence by cancer site (WHO, 2018).
Several attempts including p53 gene-based therapy, antisense and siRNA, p53 vaccine, small molecule activating p53, inhibition of p53-mfm2 interaction, and combination of drugs have been tried to activate p53 (Lane et al., 2010). P53 has an important role in the chemotherapeutic drug response of cancer cells. Stabilizing p53, thus increasing in Bax and decreasing in bcl-2, by Vitamin C sensitized cervical carcinoma cells to cisplatin (Reddy et al., 2001). An introduction of p53 cDNA into ovarian cancer cells increased sensitivity to cisplatin (Jin et al., 2002).
Cisplatin has been shown to have efficacy in cancer treatment. A single high-dose cisplatin was more effective than the fractionated doses; however, side effects, such as vomiting and leukopenia still occurred (Li et al., 2019). Synergist treatment has been demonstrated to be an effective alternative to inhibit cancer. Rh-endostatin combined with vinorelbine plus cisplatin improved the survival rate of patients with advanced non-small-cell cancer (An et al., 2018). A combination of cyclophilin inhibitor and cisplatin has shown synergistic cytotoxicity in human hepatocellular carcinoma cells (Lee, 2010). In addition, a combination of cisplatin and photodynamic therapy has shown a synergistic cytotoxicity in HeLa cells (Wei et al., 2013).
Gene therapy has been depicted to synergize with chemotherapy. Cationic liposome-iNOS gene delivery enhanced the anticancer effect of cisplatin in human lung cancer xenograft mouse models (Ye et al., 2013). Cationic liposome bcl-2 antisense-coated human serum albumin increased the anticancer effect in oral carcinoma cells by the chemotherapeutic drug, doxorubicin (Weecharangsan et al., 2012).
The present study investigated a combined treatment of cationic liposome/p53 complexes and a chemotherapeutic drug, cisplatin on human carcinoma cell growth, HeLa, and A549 cells. This study had a novelty on the combined treatment of cationic liposome/p53 complexes and chemotherapeutic drug on human carcinoma cell growth.
MATERIALS AND METHODS
Materials
Dimethyldioctadecyl ammonium bromide (DDAB), polyethylenimine (PEI 0.8KDa, branched), egg phosphatidylcholine, and α-tocopherol polyethylene glycol 1000 succinate were purchased from Sigma-Aldrich (St. Louis, MO). pEGFP (enhanced green fluorescence protein)-C2 plasmid DNA was obtained from Clontech (Palo Alto, CA). Plasmid GFP-p53 encoding p53 protein was obtained from Addgene (plasmid # 12091, Tyler Jacks) (Boyd et al., 2000). Six- and 96-well plates were purchased from SPL Life Sciences (Gyeonggi-do, Korea). Cisplatin was purchased from Roche (Basel, Switzerland). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Bio Basic Inc. (Ontario, Canada). Fetal bovine serum, Minimum Essential Medium (MEM) and OptiMEM were purchased from Invitrogen (Grand Island, NY). Human cervical carcinoma (HeLa) and human lung adenocarcinoma (A549) cell lines were purchased from American Type Culture Collection (ATCC, Rockville, MD).
Cationic liposome/GFP-p53 complexes and characterization
Cationic liposomes were prepared with cationic lipid, DDAB as previously described (Maurer et al., 2001; Weecharangsan et al., 2014). Briefly, DDAB, egg phosphatidylcholine, and α-tocopherol polyethylene glycol 1000 succinate were dissolved in absolute ethanol at a molar ratio of 58:40:2, diluted into (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) (HEPES)-buffered solution at pH 7.4, and mixed for 30 minutes by vortexing at room temperature. Cationic liposomes/GFP-p53 complexes were prepared at weight ratios of 1.5, 3.1, 6.2, 12.5, and 25, and the complexes were initiated by mixing for 3–5 seconds and incubated at 25°C for 15 min. The amount of GFP-p53 used was 0.125–0.5 μg. Cationic liposome/PEI 0.8K/ GFP-p53 complexes were prepared by mixing PEI0.8K with GFP-p53 solution at a PEI:DNA ratio of 1.1:1 and then mixed with cationic liposomes. The formation of cationic liposome/GFP-p53 complexes and cationic liposome/PEI0.8K/GFP-p53 complexes were checked using gel electrophoresis (Weecharangsan et al., 2014).
Particle size and zeta potential measurements
Particle size and zeta potential of cationic liposome/GFP-p53 complexes and cationic liposome/PEI0.8K/GFP-p53 complexes were measured by photon correlation spectroscopy using a Zetasizer (Malvern). The complexes were prepared in DI water, diluted to 1 ml, and measured at 25°C using aqueous flow cell. The amount of cationic liposome/PEI0.8K/GFP-p53 used was 6.25–12.5/1.1/1 μg.
Cell culture
Human cervical carcinoma (HeLa) and human adenocarcinoma (A549) cell lines were used in this study. The cells were grown in MEM with 100 μg/ml streptomycin and 100 U/ml penicillin, 1% amphotericin B, and 10% fetal bovine serum. Cells were incubated in 95% atmosphere and 5% CO2 at 37°C.
Intracellular delivery and gene expression
pEGFP was transferred into HeLa and A549 cells by cationic liposomes/pEGFP complexes and cationic liposome/PEI0.8K/pEGFP complexes. Cells were seeded in a 6-well plate at 1.5 × 105 cells/well for HeLa and 9 × 104 cells/well for A549. After 24 hours, the cells were treated with OptiMEM having cationic liposomes/pEGFP complexes and cationic liposome/PEI0.8K/pDNA complexes for 4 hours. Cells incubated with pEGFP and without complexes were used as controls. After 4-hour incubation, the cells were rinsed and incubated with the growth media for 24 hours. Following this, cells were rinsed and trypsinized. The number of GFP expressing cells was measured by flow cytometer InCyte software (Guava, Merck, Germany).
Cell growth inhibition
Cell growth inhibition by cationic liposomes/GFP-p53 complexes and cationic liposome/PEI0.8K/GFP-p53 complexes was determined using MTT assay. HeLa cells were cultured in growth media and seeded into 96-well plate (5 × 103 cells/well), and let them grown. After 24 hours, the cells were rinsed, and the cationic liposomes/GFP-p53 complexes and cationic liposome/PEI0.8K/GFP-p53 complexes prepared in 62.5 μl OptiMEM at a weight ratio of 6.25:1 and 6.25:1.1:1, respectively, were transferred to the cells. After 4-hour incubation, the cells were rinsed, growth medium was added, and the cells were further incubated for 24 hours. The cell growth inhibition was measured by MTT assay using a microplate reader (SpectraMax M3, Molecular Devices, San Jose, CA). The cell growth inhibition (%) was calculated by the absorbency of the treated cells relative to the untreated cells.
Chemosensitization of cisplatin to HeLa and A549 cells
Cells were seeded into 96-well plate at 5 × 103 cells/well for HeLa cells and 3 × 103 cells/well for A549 in growth media. After 24-hour incubation, cells were rinsed and incubated with cationic liposomes/GFP-p53 complexes and cationic liposome/PEI0.8K/GFP-p53 complexes prepared in 62.5 μl OptiMEM at a weight ratio of 6.25:1 and 6.25:1.1:1, respectively, for 4 hours. After incubation, cells were rinsed and incubated with growth media for 24 hour under 95% atmosphere and 5% CO2. After 24-hour incubation, the growth media were replaced with growth media having 4 μM cisplatin and further incubated for 24 hours. The cell growth inhibition was measured by MTT assay as described in cell growth inhibition.
Statistical analysis
The statistical analysis was determined by one-way analysis of variance following with Fisher's Least Significant Difference (LSD) post hoc test. A comparison between means was determined using t-test. The significant level was set at p < 0.05.
RESULTS AND DISCUSSION
Cationic liposomes/pDNA complexes and cationic liposome/PEI0.8K/pDNA complexes
Figure 1 shows the analysis of DPT liposome/GFP-p53 and DPT liposome/PEI 0.8 kDa/GFP-p53 complexes on 1% agarose gel. DPT liposome/GFP-p53 formed complete complexes at a lipid-to-DNA ratio of 6.25:1 (Fig. 1A). DPT liposome/PEI 0.8 kDa/GFP-p53 complexes formed complete complexes at a lipid-to-DNA ratio of 1.5:1.1:1. PEI 0.8 kDa/GFP-p53 complexes formed complete complexes at the PEI-to-DNA ratio of 1.1:1 (Fig. 1B). DPT liposome/GFP-p53 complexes at lipid-to-DNA ratios of 6.25:1 and 12.5:1 had a particle size of 319 ± 19–352 ± 27 nm. DPT liposome/PEI 0.8 kDa/GFP-p53 complexes at a lipid-to-PEI-to-DNA ratio of 6.25:1.1:1 had a particle size of 754 ± 109 nm. PEI 0.8 kDa/GFP-p53 complexes at PEI-to-DNA ratios of 1.1:1 had a particle size of 2,072 ± 142 nm. DPT liposome/GFP-p53 complexes at a lipid-to-DNA ratio of 6.25:1 had a particle size which is not different from that of 12.5:1. PEI 0.8 kDa/GFP-p53 complexes had particle size bigger than DPT liposome/PEI 0.8 kDa/GFP-p53 complexes (Fig. 2A). DPT liposome/GFP-p53 complexes at lipid-to-DNA ratios of 6.25:1 and 12.5:1 had a zeta potential of –15.7 ± 2.8–+27.0 ± 0.9 mV. DPT liposome/PEI 0.8 kDa/GFP-p53 complexes at a lipid-to-PEI-to-DNA ratio of 6.25:1.1:1 had a zeta potential of +3.8 ± 0.7 mV nm. PEI 0.8 kDa/GFP-p53 complexes at the PEI-to-DNA ratio of 1.1:1 had a zeta potential of –1.0±0.1. Lipid-to-DNA ratios of 6.25:1 to 12.5:1 and the presence of PEI 0.8 kDa increased the zeta potential of DPT liposome/GFP-p53 complexes (Fig. 2B).
 | Figure 1. Gel electrophoresis analysis of (A) DPT liposome/GFP-p53. Lane 1: free GFP-p53 0.25 μg. Lanes 2-: DPT liposome/GFP-p53 at lipid-to-DNA ratios of 1.5:1, 3.125, 6.25:1, 12.5:1 and 25:1, respectively, and (B) DPT liposome/PEI 0.8 kDa/GFP-p53. Lane 1: free GFP-p53 (0.25 μg). Lanes 2: PEI 0.8 kDa/GFP-p53 at PEI-to-DNA ratio of 1.1. Lanes 3-7: DPT liposome/PEI 0.8 kDa/GFP-p53 at lipid-to-PEI-to-DNA ratios of 1.5:1.1:1, 3.125:1.1:1, 6.25:1.1:1, 12.5:1.1:1 and 25:1.1:1, respectively. [Click here to view] |
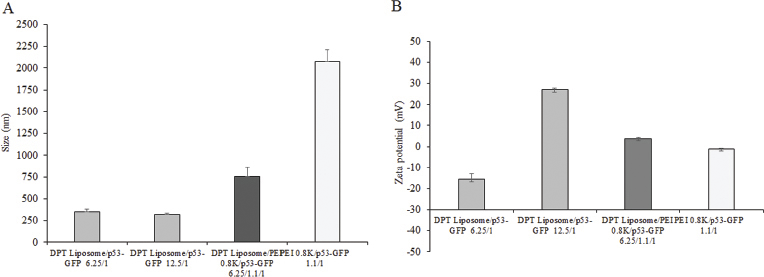 | Figure 2. Particle size (A) and zeta potential (B) of DPT liposome/GFP-p53 at lipid-to-DNA ratios of 6.25:1 and 12.5:1, DPT liposome/PEI 0.8 kDa/GFP-p53 at lipid-to-PEI-to-DNA ratio of 6.25:1.1:1, and PEI 0.8 kDa/GFP-p53 at PEI-to-DNA ratio of 1.1:1. [Click here to view] |
Intracellular delivery and gene expression
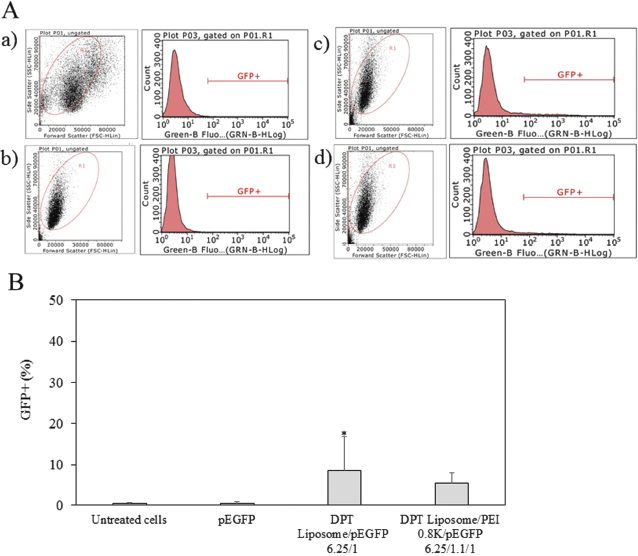
An intracellular delivery of DPT liposome/PEI 0.8 kDa/pEGFP complexes was examined by GFP gene expression. Plasmid DNA delivered into HeLa cells. Figure 3 shows the flow cytometric analysis and GFP expression of HeLa cells by DPT liposome/PEI 0.8 kDa/GFP-p53 complexes. HeLa cells treated with pEGFP had GFP+ = 0.6%. Cells treated with DPT liposome/PEI 0.8 kDa/pEGFP complexes had GFP+ = 6.1%. Cells treated with DPT liposome/pEGFP complexes had GFP+ = 5.4%. HeLa cells treated without pEGFP had GFP+ = 0.2%. GFP expression of HeLa cells treated with DPT liposome/PEI 0.8 kDa/pEGFP and DPT liposome/pEGFP complexes was significantly higher than HeLa cells treated with pEGFP (p < 0.05). GFP expression of HeLa cells treated with DPT liposome/PEI 0.8 kDa/pEGFP and DPT liposome/pEGFP complexes was not significantly different. Plasmid DNA delivered into A549 cells. Figure 4 shows the flow cytometric analysis and GFP expression of A549 cells by DPT liposome/PEI 0.8 kDa/GFP-p53 complexes. The A549 cells treated with and without pEGFP had GFP+ = 0.3%. Cells treated with DPT liposome/PEI 0.8 kDa/pEGFP complexes had GFP+ = 5.5%, whereas cells treated with DPT liposome/pEGFP complexes had GFP+ = 8.5%. GFP expression of A549 cells treated with DPT liposome/pEGFP complexes was significantly higher than A549 cells treated with DPT liposome/PEI 0.8 kDa/pEGFP complexes and cells treated with pEGFP (p < 0.05).
 | Figure 3. Flow cytometry analysis (A) and GFP+ (%) (B) of HeLa cells treated with media (a) pEGFP (b) DPT liposome/pEGFP at lipid-to-DNA ratio of 6.25:1 (c), and DPT liposome/PEI 0.8 kDa/pEGFP at lipid-to-PEI-to-DNA ratio of 6.25:1.1:1 (d). [Click here to view] |
DPT liposome/GFP-p53 complexes and DPT liposome/PEI 0.8 kDa/GFP-p53 complexes were formulated and delivered into HeLa cells and A549 cells. This study showed that DPT liposome and DPT liposome/PEI 0.8 kDa could deliver plasmid DNA into cells. The intracellular delivery and cytotoxicity of the carrier/nucleic acid complexes have been demonstrated to be dependent on the characteristic of carrier and carrier/plasmid DNA ratio and the amount of nucleic acid (Weecharangsan et al., 2017; Zhang et al., 2018)
Growth inhibition of HeLa cells by cisplatin
Figure 5 shows the growth inhibition of HeLa cells by cisplatin at a concentration of 0–50 μM. The growth inhibition of HeLa cells increased when the concentration of cisplatin increased from 2.5 to 50 μM. At a cisplatin concentration of 4 μM, growth inhibition of HeLa cells was 8%–10%. Therefore, cisplatin at a concentration of 4 μM was used to evaluate the sensitivity of cancer cells by DPT liposome/PEI 0.8 kDa/GFP-p53 complexes.
Growth inhibition of HeLa cells by DPT liposome/PEI 0.8 kDa/GFP-p53 complexes and DPT liposome/GFP-p53 complexes
The growth inhibition of HeLa cells by DPT liposome and GFP-p53 was not different from cells treated with a medium. DPT liposome/GFP-p53 complexes significantly inhibited HeLa cell growth (p < 0.05) (Fig. 6). An increasing amount of GFP-p53 from 0.125, 0.25, and 0.5 μg increased the growth inhibition of HeLa cells by DPT liposome/PEI 0.8 kDa/GFP-p53 complexes and significantly at the amount of GFP-p53 of 0.5 μg (p < 0.05) (Fig. 7). Using PEI 0.8 kDa in DPT liposome/PEI 0.8 kDa/GFP-p53 complexes did not increase the growth inhibition of HeLa cells. The growth inhibition of HeLa cells by DPT liposome/GFP-p53 complexes was higher than that of by DPT liposome/PEI 0.8 kDa/GFP-p53 complexes and by only GFP-p53 (p < 0.05) (Fig. 8).
 | Figure 4. Flow cytometry analysis (A) and GFP+ (%) (B) of A549 cells treated with media (a) pEGFP (b) DPT liposome/pEGFP at lipid-to-DNA ratio of 6.25:1 (c), and DPT liposome/PEI 0.8 kDa/pEGFP at lipid-to-PEI-to-DNA ratio of 6.25:1.1:1 (d). [Click here to view] |
 | Figure 5. Growth inhibition of HeLa cells by cisplatin at a concentration of 0-50 μM. [Click here to view] |
 | Figure 6. Growth inhibition of HeLa cells by DPT liposome/GFP-p53; â–¡ = DPT liposome, â¹ = DPT liposome/GFP-p53 at lipid-to-DNA ratio of 6.25:1; *p < 0.05 when compared with cells treated with medium, cells treated with GFP-p53, and cells treated with DPT liposome. [Click here to view] |
DPT liposome/GFP-p53 complexes and DPT liposome/PEI 0.8 kDa/GFP-p53 complexes could inhibit HeLa and A549 cell growth. The growth inhibition of DPT liposome/PEI 0.8 kDa/GFP-p53 complexes was dependent on the concentration of GFP-p53. Song et al. (2012) revealed that cationic lipid-coated PEI/DNA polyplex increased the transfection efficiency and reduced the cytotoxicity in mesenchymal stem cells. However, effective delivery of plasmid DNA was dependent on the characteristic of PEI and the polymer and plasmid DNA ratio (Costa et al., 2018). This study showed that DPT liposome/PEI 0.8 kDa/GFP-p53 complexes did not show more effective intracellular delivery and gene expression and growth inhibition than DPT liposome/GFP-p53 complexes in HeLa and A549 cells. In this study, PEI 0.8 kDa resulted in a large particle size of DPT liposome/PEI 0.8 kDa/GFP-p53 complexes and did not improve intracellular delivery of GFP-p53 from DPT liposome/GFP-p53 complexes, thus not increasing the growth inhibition of cationic liposome/GFP-p53 complexes in HeLa and A549 cells. The particle size of liposome/PEI/DNA complexes could be dependent on the molecular weight and the structure of PEI.
 | Figure 7. Growth inhibition of HeLa cells by DPT liposome/PEI 0.8 kDa/GFP-p53; â–¡ = DPT liposome, â¹ = DPT liposome/PEI 0.8 kDa/GFP-p53 at lipid-to-DNA ratio of 6.25:1.1:1; *p<0.05 when compared with cells treated with medium, cells treated with GFP-p53, and cells treated with DPT liposome/PEI 0.8 kDa. [Click here to view] |
 | Figure 8. Growth inhibition of HeLa cells by DPT liposome/PEI 0.8 kDa/GFP-p53 at lipid-to-PEI-to-DNA ratio of 6.25:1.1:1 and DPT liposome/GFP-p53 at lipid-to-DNA ratio of 6.25:1; â–¡ = liposomes, â¹ = liposome/GFP-p53 ; *p<0.05 when compared with cells treated with medium, cells treated with GFP-p53, and cells treated with DPT Liposome; # p < 0.05 when compared with untreated cells. [Click here to view] |
Growth inhibition of HeLa and A549 cells by DPT liposome/PEI 0.8 kDa/GFP-p53 complexes in combination with cisplatin
Further treatment with cisplatin, growth inhibition of HeLa cells was slightly increased by DPT liposome/PEI 0.8 kDa/GFP-p53 complexes at a lipid-to-PEI-to-DNA ratio of 6.25:1.1:1 and DPT liposome/GFP-p53 complexes at a lipid-to-DNA ratio of 6.25:1 (Fig. 9). In A549 cells, growth inhibition was significantly different by DPT liposome/GFP-p53 complexes at lipid-to-DNA ratios of 6.25:1 treated with cisplatin as compared to cells treated with DPT liposome/GFP-p53 complexes at the same lipid-to-DNA ratio and no cisplatin (*p < 0.05) (Fig. 10).
 | Figure 9. Growth inhibition of HeLa cells by DPT liposome/PEI 0.8 kDa/GFP-p53 at lipid-to-PEI-to-DNA ratio of 6.25:1.1:1 and DPT liposome/GFP-p53 at lipid-to-DNA ratio of 6.25:1; â–¡ = no cisplatin, â¹ = plus cisplatin. [Click here to view] |
 | Figure 10. Growth inhibition of A549 cells by DPT liposome/GFP-p53 at lipid-to-DNA ratio of 6.25:1; â–¡ = no cisplatin, â¹ = plus cisplatin; *p<0.05 when compared with cells treated with DPT liposome/GFP-p53 at the same lipid-to-DNA ratio and no cisplatin. [Click here to view] |
DPT liposome/GFP-p53 complexes and DPT liposome/PEI 0.8 kDa/GFP-p53 complexes inhibit HeLa cell growth in combination with cisplatin. DPT liposome/GFP-p53 complexes in combination with cisplatin significantly inhibit the A549 cell growth. The combination of DPT liposome/GFP-p53 complexes and cisplatin significantly inhibits A549 cell growth. Liu et al. (2017) depicted that the survival rate of human lung adenocarcinoma cell line was reduced by p53 alpha gene and increased the sensitivity to cisplatin treatment. Ye et al. (2013) showed that cationic liposome/pVAX-iNOS complex enhanced the cisplatin to inhibit cell proliferation of A549 human lung cancer cells and suppression of subcutaneous tumor growth. Liu et al. (2013) demonstrated that cisplatin synergized the TRAIL-induced apoptosis in breast cancer cell lines. Li et al. (2014) showed that the downregulation of p53 by Vasohibin 2 decreased the cisplatin sensitivity to hepatocarcinoma cells.
CONCLUSION
This study concluded that the sensitivity to the cancer cells of cisplatin was based on the cell line and the ratio of cationic liposome and GFP-p53. This study suggests that GFP-p53 expression delivered by cationic liposome/GFP-p53 complexes would be useful to increase the effect of cisplatin on the treatment of cancer cells.
CONFLICT OF INTEREST
The authors declared that they have no conflict of interest.
FUNDING
This study was funded by Srinakharinwirot University Grant, number 581/2560.
REFERENCES
An J, Lv W. Endostar (rh-endostatin) versus placebo in combination with vinorelbine plus cisplatin chemotherapy regimen in treatment of advanced non-small cell lung cancer: A meta-analysis. Thorac Cancer, 2018; 9:606–12. CrossRef
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre L.A, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin, 2018; 68:394–424. CrossRef
Boyd SD, Tsai KY, Jacks T. An intact HDM2 RING-finger domain is required for nuclear exclusion of p53. Nat Cell Biol, 2000; 2:563–8. CrossRef
Costa D, Valente AJM, Queiroz JA, Sousa A. Finding the ideal polyethylenimine-plasmid DNA system for co-delivery of payloads in cancer therapy. Colloids Surf B Biointerfaces, 2018; 170:627–36. CrossRef
Jin Z, Guan T, Li S. Effects of wild-type p53 gene on the chemotherapy sensitivity of ovarian cancer SKOV-3 cells to cisplatin. Zhonghua yi xue yi chuan xue za zhi, 2002; 19:218–20.
Lane DP, Cheok CF, Lain S. p53-based cancer therapy. Cold Spring Harb Perspect Biol, 2010; 2:a001222. CrossRef
Lee J. Novel combinational treatment of cisplatin with cyclophilin A inhibitors in human heptocellular carcinomas. Arch of Pharm Res, 2010; 33:1401–9. CrossRef
Li Z, Tu M, Han B, Gu Y, Xue X, Sun J, Ge Q, Miao Y, Qian Z, Gao W. Vasohibin 2 decreases the cisplatin sensitivity of hepatocarcinoma cell line by downregulating p53. PLoS One, 2014; 9:e90358. CrossRef
Li X, Zhao X, Abbas M, Wang L, Li C, Liu S, Feng J, Shi M. Comparative effectiveness study of single high-dose cisplatin with fractionated doses cisplatin in first-line therapy for treatment-naive Chinese patients with advanced non-small-cell lung cancer. Curr Probl Cancer, 2019; 43(6):100466. CrossRef
Liu K, Gao W, Lin J. Effect of the p53alpha gene on the chemosensitivity of the H1299 human lung adenocarcinoma cell line. Oncol Lett, 2017; 14:1411–8. CrossRef
Liu X, Wang J, Wang H, Liu S, Liang Y, Lv Z, Zhou Q, Ding W. Combination of Ad-sTRAIL with the chemotherapeutic drug cisplatin synergistically enhances their pro-apoptotic ability in human breast cancer cells. Oncol Rep, 2013; 30:1913–9. CrossRef
Maurer N, Wong KF, Stark H, Louie L, McIntosh D, Wong T, Scherrer SC, Cullis, PS. Spontaneous entrapment of polynucleotides upon electrostatic interaction with ethanol-destabilized cationic liposomes. Biophys J, 2001; 80:2310–26. CrossRef
Reddy VG, Khanna N, Singh N. Vitamin C augments chemotherapeutic response of cervical carcinoma HeLa cells by stabilizing P53. Biochem Biophys Res Commun, 2001; 282:409–15. CrossRef
Song H, Wang G, He B, Li L, Li C, Lai Y, Xu X, Gu Z. Cationic lipid-coated PEI/DNA polyplexes with improved efficiency and reduced cytotoxicity for gene delivery into mesenchymal stem cells. Int J Nanomed, 2012; 7:4637–48. CrossRef
Weecharangsan W, Lee RJ. Growth inhibition and chemosensitization of human carcinoma cells by human serum albumin-coated liposomal antisense oligodeoxyribonucleotide against bcl-2. Drug Deliv, 2012; 19:292–7. CrossRef
Weecharangsan W, Opanasopit P, Niyomtham N, Yingyongnarongkul BE, Kewsuwan P, Lee RJ. Synergistic inhibition of human carcinoma cell growth via co-delivery of p53 plasmid DNA and bcl-2 antisense oligodeoxyribonucleotide by cholic acid-modified polyethylenimine. Anticancer Res, 2017; 37:6335–40. CrossRef
Weecharangsan W, Opanasopit P, Yingyongnarongkul BE, Kewsuwan P, Lee RJ. Co-delivery of plasmid DNA and antisense oligodeoxyribonucleotide into human carcinoma cells by cationic liposomes. Curr Pharm Biotechnol, 2014; 15:790–9. CrossRef
Wei XQ, Ma HQ, Liu AH, Zhang YZ. Synergistic anticancer activity of 5-aminolevulinic acid photodynamic therapy in combination with low-dose cisplatin on Hela cells. Asian Pacific journal of cancer prevention. Asian Pac J Cancer Prev, 2013; 14:3023–8. CrossRef
World Helath Organization. The Global Cancer Observatory: International Agency for Research on Cancer. [ONLINE] Available via https://gco.iarc.fr/today/data/factsheets/populations/900-world-fact-sheets.pdf (Accessed 27 February 2018).
Ye S, Yang W, Wang Y, Ou W, Ma Q, Yu C, Ren J, Zhong G, Shi H, Yaun Z, Su X, Zhu W. Cationic liposome-mediated nitric oxide synthase gene therapy enhances the antitumor effects of cisplatin in lung cancer. Int J Mol Med, 2013; 31:33–42. CrossRef
Zhang M, Zhang M, Wang J, Cai Q, Zhao R, Yu Y, Tai H, Zhang X, Xu C. Retro-inverso follicle-stimulating hormone peptide-mediated polyethylenimine complexes for targeted ovarian cancer gene therapy. Drug Deliv, 2018; 25:995–1003. CrossRef