INTRODUCTION
A major conundrum in modern medicine and pharmacology is the widespread emergence and evolution of drug resistance among clinically important pathogens and threatens human and veterinary health, food security, and socio-economic conditions (Caniça et al., 2019; Idris et al., 2019). In terms of prevalence and diversity of the infections, bacteria and helminth parasites are at the forefront of this drug resistance dilemma. In fact, multi-drug resistance in bacteria has become a major factor in increased morbidity and mortality worldwide (Bogoch et al., 2019; Jenkins et al., 2017). Anthelmintic resistance is specifically pervasive among helminth parasites of livestock animals. Virtually all veterinary helminths have developed resistance to all available anthelmintic drugs (Brooker, 2018; Moser et al., 2019). It is for these reasons that recent vocal advocates for the pressing need for new and alternative medications are supportively justified (Clarke et al., 2018; Willing et al., 2018).
Imperata cylindrica (L.) Räuschel is a perennial and rhizomatous grass in the family Poaceae. It has a thin and lanceolate leaf, and a relatively short stem. The underground parts consist of rhizome and tiny roots that emerge from nodes on the rhizome. The plant is used in different traditional medicines of Southeast Asia for the treatment of various diseases. The flowers and roots have been used as antibacterial, diuretic, skin softening (emollient), antipyretic (febrifuge), salivating (sialagogue), anticoagulant (styptic), and soothing (tonic) agents (Townson, 1991). The roots are specifically used for the treatment of jaundice, edema, and hematological disorders such as blood urine (hematuria), blood vomit (hematemesis), and nosebleed (epistaxis). Bioactive compounds isolated from the leaves are also shown to have neuro-protective (Yoon et al., 2006) and vasodilative effects (Matsunaga et al., 1994).
To the Mizo people of India and Myanmar, the underground part (rhizome-root extract) is a familiar remedy for bacterial infections and has been used in the treatment of dermal wound, cholera, dysentery, and diarrhea. It is also known to be a good antifungal agent for ringworms and other dermatitis (Sawmliana, 2013). The most unique application is for deworming intestinal helminthiasis. The underground parts are crushed, and the juice is directly consumed (Lalthanpuii et al., 2018). The importance of this antiparasitic activity is that, while most anthelmintic compounds or drugs are helminth-specific, the plant extract is equally used for both tapeworm and roundworm species. Therefore, in the light of its medicinal properties, it is worthwhile to perform chemical profiling and test its antibacterial and antiparasitic potentials.
MATERIALS AND METHODS
Plant material
Imperata cylindrica was collected from Ngopa (located between 23.8861° latitude north and 93.2119° longitude east), a village in Champhai district, Mizoram, India. The specimen was identified at the Botanical Survey of India, Shillong, Meghalaya, and a voucher herbarium (PUC-I-2018-01) is maintained at Pachhunga University College, Aizawl, Mizoram. The underground parts consisting of rhizome and roots were selected and dried in shade at room temperature ranging from 21°C to 27°C.
Preparation of plant extracts
The dried specimens were pulverized to powder in an electric grinder. Methanol extract was prepared in a 5-liter Soxhlet apparatus. The extract was concentrated by recovering the methanol in a vacuum rotary evaporator (Buchi Rotavapor® R-215). The final plant extract was semi-solid mass and was kept at 4°C until further use.
Gas chromatography-mass spectrometry (GC-MS) analysis
The methanol extract of I. cylindrica was analyzed for chemical identification using the GC-MS system (Thermo Scientific TRACE™ 1300 ISQ™ LT). Sample was dissolved in acetonitrile. A non-polar column used was TR-5MS (260F142P) with a dimension of 30 m × 0.25 mm × 0.25 μm and film thickness of 0.25 μm. The injector port temperature was set at 250°C. The oven temperature was set at 70°C for 2 minutes and gradually raised at an increment of 10°C up to 250°C. Helium was used as the carrier gas and set at a constant flow rate of 1 ml/minute. The sample was injected at a volume of 1 μl in the split mode at a splitting ratio of 1:50. The mass spectrometer was run with an electron energy of 70 eV for ionization. Ion source and transfer line temperature were set at 250°C. Thermo Scientific™ Xcalibur™ software was used for data generation. The total running duration was 55 minutes. Compounds were identified based on retention time, mass spectra, and molecular weight from libraries of Wiley Registry™ and the National Institute of Standards and Technology database.
Antibacterial activity
The antibacterial activity of the plant extract was studied using the Kirby–Bauer test. An aerobic Gram-negative bacterium Pseudomonas aeruginosa ATCC® 10145™ and an anaerobic Gram-negative bacterium Klebsiella pneumoniae ATCC® 10031™, and an aerobic Gram-positive bacterium Bacillus subtilis ATCC® 11774™ were used. The bacteria were grown in culture dishes containing Mueller–Hinton agar. The plant extracts were prepared in two concentrations, namely, 10 and 20 mg/ml. They were impregnated on absorbent disks (Whatman Antibiotic Assay Disks). Negative control contained only the bacteria in agar medium. 20 mg/ml of ceftriaxone was used as a standard positive antibiotic. The assays were performed in triplicates. The culture dishes were maintained at 37°C ± 1°C in a microbiological incubator for 20 hours. After incubation, the sizes of the inhibition zones were measured.
Antiparasitic test
Antiparasitic test was performed on two intestinal worms, namely, a tapeworm Raillietina echinobothrida Mégnin, 1880, and a roundworm Ascaridia galli Schrank, 1788. Ethical clearance for the experiment was issued by Pachhunga University College (PUC-IAEC-2016-Z2 of 10/08/2016). The parasites were collected from a freshly sacrificed local fowl, Gallus gallus domesticus Linnaeus, 1857. One hour before the experimental assay, solutions of incremental concentrations such as 5, 10, and 20 mg/ml of the plants extract were prepared in culture plates by dissolving them in 0.9% neutral phosphate-buffered saline (PBS) supplemented with 1% dimethylsulfoxide (DMSO). Corresponding concentrations were also prepared for albendazole as standard references. Control experiment consisted only of PBS with DMSO. The parasites were incubated at 37°C ± 1°C in each of the test media. Batches of three worms were selected for each test, and each test was further performed in triplicates. Efficacy was assessed in terms of survival in the culture media. Death was defined as no further motor activity even after stimulation with tepid PBS (45°C). The times of death were recorded, and data were generated as statistical means ± standard deviation. Significance of the antiparasitic activity was determined using unpaired Student’s t-test, and the level of significance was considered when the p-value was <0.05.
RESULTS
Extraction and detection of phytocompounds
The underground parts of I. cylindrica yielded 19.75 g of methanol extract from 110 g of the plant sample, i.e., with an extractive value of 17.95%. Standard chemical detection using GC-MS revealed important compounds in the methanol extract of I. cylindrica as shown in Table 1. Compounds were identified from the chromatogram as given in Figure 1. The presence of 14 volatile compounds was established, while compounds with low probabilities were excluded. n-Hexadecanoic acid and (Z)-18-octadec-9-enolide were the most abundant at 86.2% each. In addition, methyl 9-cis, 11 trans-octadecadienoate (36.8), hexadecanoic acid, methyl ester (35.8%), octadecanoic acid (35.8%), 6-methylenebicyclo [3.2.0] hept-3-en-2-one (17%), phenol,2,4-bis (1,1-dimethylethyl) (16.8%), 2-methoxy-4-vinylphenol (16.4%), and (E)-4-(3-hydroxyprop-1-en-1-yl)-2-methoxyphenol (15.8) were the major constituents.
Antibacterial activity
Ceftriaxone at 20 mg/ml was effective on the growth inhibition of all bacteria tested, producing inhibition zones of 13 mm on B. subtilis, 14 mm on P. aeruginosa, and 12 mm on K. pneumonia. The methanol extract of I. cylindrica was also effective on all bacteria tested. At 10 and 20 mg/ml, the plant extract produced inhibition zones of 9 and 10 mm on B. subtilis, 7 and 8 mm on P. aeruginosa, and 6 and 8 mm on K. pneumonia, respectively. Thus, the relative susceptibility of the bacteria species for the plant extract was in an order B. subtilis > P. aeruginosa > K. pneumonia.
 | Table 1. Chemical compounds identified from the methanol extract of I. cylindrica underground parts using GC-MS. [Click here to view] |
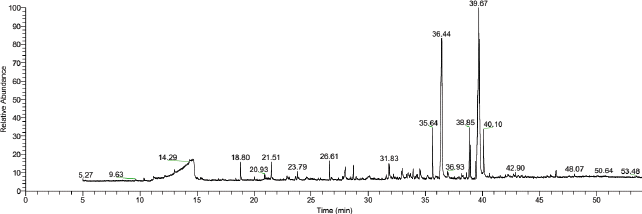 | Figure 1. GC-MS chromatogram of the methanol extract of I. cylindrica underground parts. [Click here to view] |
Antiparasitic activity
The methanol extract of I. cylindrica was effective against both the tapeworms and roundworms. As given in Table 2, control tapeworms survived up to 74.03 ± 1.89 hours. Albendazole was highly effective and killed R. echinobothrida in 14.99 ± 0.43, 12.07 ± 0.49, and 8.99 ± 0.45 hours at the concentrations of 5, 10, and 20 mg/ml, respectively. At similar concentrations, I. cylindrica extract took 60.69 ± 4.81, 48.19 ± 4.89, and 30.58 ± 3.15 hours to completely kill the worms. A. galli survived much longer, up to 187.01 ± 6.77 hours in the plant extract (Table 3). In albendazole, they survived for 56.94 ± 1.76, 18.01 ± 2.73, and 15.97 ± 1.99 hours at 5, 10, and 20 mg/ml, respectively. Imperata cylindrica extract was effective only at 10 and 20 mg/ml.
DISCUSSION
GC-MS analysis of the underground parts of I. cylindrica revealed the presence of interesting volatile compounds. Of the different compounds detected from I. cylindrica, some are already known to have important biological effects. For example, 2-methoxy-4-vinylphenol has been isolated from pine (Pinus species) needles and is demonstrated to have potent anticarcinogenic activity by specifically blocking the hyper-phosphorylation of retinoblastoma protein in vitro (Jeong and Jeong, 2010). It also promotes anti-inflammatory response suppression of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and mitogen-activated protein kinase (MAPK) activation, and acetylation of histone H3 (Jeong et al., 2011). 6-Methylenebicyclo [3.2.0] hept-3-en-2-one is also isolated from Allium tuberosum and is shown to be an important defensive molecule for the plant against infection with the root-knot nematode, Meloidogyne species (Huang et al., 2016). Phenol,2,4-bis (1,1-dimethylethyl) is reported from avocado (Persea americana) and acts as an effective antifungal compound against fungal pathogens such as Aspergillus and Phytophthora cinnamomi (Rangel-Sánchez et al., 2014).
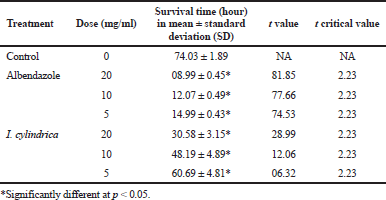 | Table 2. Efficacy of the methanol extract of I. cylindrica underground parts on the tapeworm, R. echinobothrida. (n = 6). [Click here to view] |
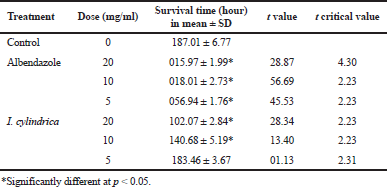 | Table 3. Efficacy of the methanol extract of I. cylindrica underground parts on the roundworm, A. galli (n = 6). [Click here to view] |
Imperata cylindrica underground parts are traditionally familiar to the Mizo people as anti-infectious agents. They are used as broad-spectrum antimicrobials and anthelmintics for different microbial and helminth infections. Our study shows that the methanol extract is quite effective against pathogenic bacteria, such as B. subtilis, P. aeruginosa, and K. pneumonia. It was most effective on B. subtilis on which its efficacy was comparable to that of tetracycline. As secondary metabolites of plants are a good source of antimicrobial agents (Chandra et al., 2017), as such further investigation of this plant is a compelling task to fully explore its pharmacological potential on microbial pathogens of medical importance.
Validating the traditional application, this study also shows that I. cylindrica is an effective antiparasitic agent. There are serious concerns in the management of parasitic infections such as drug resistance and adverse effects. These factors raise the need for novel drugs (Clarke et al., 2018). In this context, the plant extract in the present study is remarkable in that it is effective on both the tapeworms and roundworms. This is important because tapeworms and roundworms have contrasting structural and physiological properties (Abongwa et al., 2017).
Tapeworms are soft-bodied helminths without the digestive system so that absorption of nutrients or drugs is direct through the body surface called tegument (Taman and Azab, 2014). Whereas roundworms are hard-bodied helminths covered with tough cuticle and nutrients or drugs are ingested into a digestive tract. The course of drug action is therefore prolonged and requires complex interaction with motor and signaling proteins (Greenberg, 2014). For these reasons, drugs are usually specific for each group of helminths. The effectiveness of I. cylindrica extract against both tapeworms and roundworms as demonstrated in this study is, therefore, an evidence that the plant is a promising source of novel anthelmintic molecules, which exert broad-spectrum activity.
CONCLUSION
GC-MS analysis revealed that the methanol extract of I. cylindrica underground part contains important volatile compounds, some of which are known to be pharmacologically bioactive compounds. This is not an exhaustive analysis and the plant is likely to contain more interesting compounds. Validating the medicinal use as antibacterial and antiparasitic agents, the plant extract was effective against three bacteria including Gram-negative bacteria such as P. aeruginosa and K. pneumoniae, and a Gram-positive bacterium B. subtilis. It was an interesting observation that the plant extract was also effective against intestinal parasites such as the tapeworm R echinobothrida and the roundworm A. galli.
ACKNOWLEDGMENT
The research is supported by the Science and Engineering Research Board (SERB), Government of India, through research project no. EMR/2016/004053 of 23/03/2017. PBL is a Senior Research Fellow under the project. GC-MS facility was provided by the Directorate of Forensic Science Laboratory, Government of Mizoram.
CONFLICT OF INTEREST
None declared.
REFERENCES
Abongwa M, Martin RJ, Robertson AP. A brief review on the mode of action of antinematodal drugs. Acta Vet, 2017; 67:137–52. CrossRef
Bogoch II, Utzinger J, Lo NC, Andrews JR. Antibacterial mass drug administration for child mortality reduction: Opportunities, concerns, and possible next steps. PLoS Negl Trop Dis, 2019; 13:e0007315. CrossRef
Brooker SJ. Soil-transmitted helminth treatment: multiple-drug regimens. Lancet Infect Dis, 2018; 18:698–9. CrossRef
Caniça M, Manageiro V, Abriouel H, Moran-Gilad J, Franz CM. Antibiotic resistance in foodborne bacteria. Trends Food Sci Technol, 2019; 84:41–4. CrossRef
Chandra H, Bishnoi P, Yadav A, Patni B, Mishra A, Nautiyal A. Antimicrobial resistance and the alternative resources with special emphasis on plant-based antimicrobials—a review. Plants, 2017; 6:16(1–11). CrossRef
Clarke NE, Doi SA, Wangdi K, Chen Y, Clements AC, Nery SV. Efficacy of anthelminthic drugs and drug combinations against soil-transmitted helminths: a systematic review and network meta-analysis. Clin Infect Dis, 2018; 68:96–105. CrossRef
Greenberg RM. Ion channels and drug transporters as targets for anthelmintics. Curr Clin Microb Rep, 2014; 1:51–60. CrossRef
Huang YH, Mao ZC, Xie BY. Chinese leek (Allium tuberosum Rottler ex Sprengel) reduced disease symptom caused by root-knot nematode. J Integr Agric, 2016; 15:364–72. CrossRef
Idris OA, Wintola OA, Afolayan AJ. Helminthiases; prevalence, transmission, host-parasite interactions, resistance to common synthetic drugs and treatment. Heliyon, 2019; 5:e01161. CrossRef
Jenkins HE, Yuen CM, Rodriguez CA, Nathavitharana RR, McLaughlin MM, Donald P, et al. Mortality in children diagnosed with tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis, 2017; 17:285–95. CrossRef
Jeong JB, Jeong HJ. 2-Methoxy-4-vinylphenol can induce cell cycle arrest by blocking the hyper-phosphorylation of retinoblastoma protein in benzo [a] pyrene-treated NIH3T3 cells. Biochem Biophys Res Commun, 2010; 400:752–7. CrossRef
Jeong JB, Hong SC, Jeong HJ, Koo JS. Anti-inflammatory effect of 2-methoxy-4-vinylphenol via the suppression of NF-κB and MAPK activation, and acetylation of histone H3. Arch Pharmacal Res, 2011; 34:2109–16. CrossRef
Lalthanpuii PB, Zarzokimi, Lalchhandama K. Imperata cylindrica: a noxious weed of pharmacological potentials. In Lalchhandama K (ed.). Advances in engineering research: perspective and trends in the development of science education and research. Atlantis Press, Paris, France, pp 173–7, 2018. CrossRef
Matsunaga K, Shibuya M, Ohizumi Y. Graminone B, a novel lignan with vasodilative activity from Imperata cylindrica. J Nat Prod, 1994; 57:1734–6. CrossRef
Moser W, Schindler C, Keiser J. Drug combinations against soil-transmitted helminth infections. Adv Parasit, 2019; 103:91–115. CrossRef
Rangel-Sánchez G, Castro-Mercado E, García-Pineda E. Avocado roots treated with salicylic acid produce phenol-2, 4-bis (1, 1-dimethylethyl), a compound with antifungal activity. J Plant Physiol, 2014; 171:189–98. CrossRef
Sawmliana M. The Book of Mizoram Plants. P. Zakhuma, Aizawl, India, p 143, 2013.
Taman A, Azab M. Present-day anthelmintics and perspectives on future new targets. Parasitol Res, 2014; 113:2425–33. CrossRef
Townson JK. Imperata cylindrica and its control. Weed Abstr, 1991; 40:457–68.
Willing BP, Pepin DM, Marcolla CS, Forgie AJ, Diether NE, Bourrie BC. Bacterial resistance to antibiotic alternatives: a wolf in sheep’s clothing? Anim Front, 2018; 8:39–47. CrossRef
Yoon JS, Lee MK, Sung SH, Kim YC. Neuroprotective 2-(2-phenylethyl) chromones of Imperata cylindrica. J Nat Prod, 2006; 69:290–1. CrossRef