INTRODUCTION
Rourea is a genus in the Connaraceae family. Genus Rourea has about 65 species and 129 varieties (The Plant List, 2013). Rourea is a climbing shrub or small tree, usually with prominent lenticels. The leaflets are small and imparipinnate. An unbranched inflorescence bears flowers of five petals in the calyx. They have longer petals than sepals. The fruits are curved and hairless. Rourea sp. is widely distributed in the Amazon, Pacific region, Africa, and Asia (Forero, 2009). Some of Rourea sp. are poisonous, while others are widely used in traditional medicine. There are several reports on the potential of Rourea sp. as hypoglycemic agents. Despite their wide application in ethnomedicine, very few scientific reports on their chemical constituents and biological activity are documented.
TRADITIONAL USES
In Malaysia, several Rourea species are used by the local communities. The decoction of the roots of R. regusa Planch, locally known as akar semeling, is traditionally used to treat respiratory diseases (Alsarhan et al., 2012). The roots decoction of R. concolor, locally known as akar semelit in Malaysia, is used by Temuan villagers to treat kidney diseases, diabetes (Ong et al., 2011a), lung tumor, and stomach tumor (Ong et al., 2011b). R. mimosoides or sembelit merah is traditionally used to treat bloody diarrhea, as diuretics (Grosvenor et al., 1995), and to treat bloody cough (Sabran et al., 2016). The roots decoction of R. humilis Blume or akar kayu mengecut is used to improve the contraction of the uterus (Jamal et al., 2011).
R. induta Planch is commonly known as chapeudinha, pau-de-porco, or campeira and is widely distributed in Brazil. It is traditionally used in folk medicine to treat rheumatisms and Chagas disease (Kalegari et al., 2014a). R. cuspidate Benth ex. Baker is commonly known in Brazil as miraruíra, cip′o miraruíra, and muiraruíra. It is traditionally used to treat diabetes (Laikowski et al., 2017).
R. coccinea Benth, commonly known as Tomigavi, is used in Togo for the treatment of paralyses and Alzheimer's disease (Kantati et al., 2016). R. coccinea is also utilized in Benin traditional medicine for the treatment of male and female infertility, sexual asthenia, blennorrhoea, snakebites, furuncles, and malaria (Bero et al., 2009). The leaves of R. minor are used as a styptic to treat minor abrasions and lesions in Chinese folk medicine. The stems and roots of R. minor are poisonous; however, they are widely used as tying material (He et al., 2006). R. volubis, R. orientalis, R. platysepala, and R. glabra are poisonous and they are often used to deter animals (Jeannoda et al., 1985; Oliveira et al., 2012). The roots of R. santaloides (Vahl.) Wight & Arnott is traditionally taken for the treatment of joint pains and asthma (Bargali et al., 2003). R. puberela Baker is used in Chazuta Valley of Peruvian Amazon for its diuretic property (Sanz-Biset &Canigueral, 2011).
PHYTOCHEMISTRY
Flavonoids
Isolation from ethanolic leaves extract of Rourea induta yielded quercetin 1, and three glycosylated derivatives, quercetin-3-O-α-arabinofuranoside 2, quercetin-3-O-β-xyloside 3, and quercetin-3-O-β-galactoside 4 (Kalegari et al., 2011). Procyanidin C1 5 was isolated from the aqueous leaves extract of R. induta (Kalegari et al., 2014b). HPLC titration of the aqueous leaves extract of R. induta revealed the presence of hyperin 6, as well as compounds 2–4 (Kalegari et al., 2014b). The phytochemical study on the chloroform fraction of ethanolic leaves extract of R. doniana led to the isolation of 7,4’-dimethylkaempferol 7 (Oliveira et al., 2012). Kaempferol 8 and rutin 9 were reported in R. microphylla (Zhang et al., 2008). Leucopelargonidin 10 was isolated from the roots of R. santoloides (Ramiah et al., 1976).
 | Figure 1. Flavonoids in Rourea sp. (Kalegari et al., 2011; 2014b; Oliveira et al., 2012; Ramiah et al., 1976; Zhang et al., 2008). [Click here to view] |
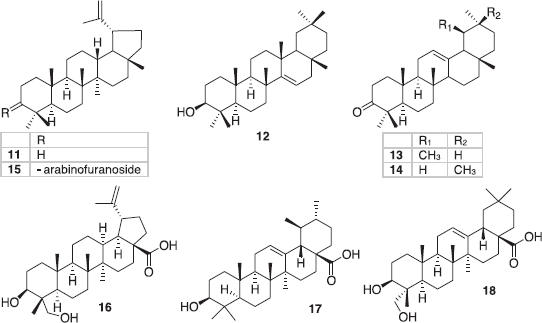 | Figure 2. Triterpenes in Rourea sp. (Oliveira et al., 2012; Zhang et al., 2008). [Click here to view] |
 | Figure 3. Phytosteroids in Rourea sp. (He et al., 2006; Oliveira et al., 2012). [Click here to view] |
 | Figure 4. Lipids in Rourea sp. (He et al., 2006; Zhang et al., 2008). [Click here to view] |
Triterpenes
Purification of hexane fraction of ethanolic leaves extract of R. doniana yielded lupeol 11, lupenone 12, α-amyrenone 13, β-amyrenone 14, and taraxerol 15 (Oliveira et al., 2012). Phytochemical study on R. microphylla gave 23-hydroxybetulinic acid 16, ursolic acid 17, and hederagenin 18 (Zhang et al., 2008).
Phytosteroids
Isolation on chloroform fractions of ethanolic leaves extract of R. doniana yielded β-sitosterol 19, stigmasterol 20, β-sitosteryl-3-O-β-D-glucopyranoside 21, and stigmasteryl-3-O-β-D-glucopyranoside 22 (Oliveira et al., 2012). β-sitosterol glucoside 23 was isolated from R. minor (He et al., 2006).
Lipids
1-Hentriacontanol 24 and 1-hexacosanoyl glycerol 25 were isolated from R. microphylla (Zhang et al., 2008). Isolation on chloroform soluble fraction of methanolic stems extract of R. minor gave 1-(26-hydroxyhexacosanoyl)-glycerol 26, 1-O-ß-D-glucopyranosyl-(2S,3R,4E-8Z)-2-N-(20-hydroxypalmitoyl)-octadecasphinga-4,8-dienine 27, rourimin 28, and 9S,12S, 13S-trihydroxy-10E-octadecenoic acid 29 (He et al., 2006).
 | Figure 5. Coumarin in Rourea sp. (Oliveira et al., 2012; Zhang et al., 2008). [Click here to view] |
Coumarin
Scopeletin 30 was purified from chloroform fraction of ethanolic leaves extract of R. doniana (Oliveira et al., 2012). Daphnetin 31 was reported from R. microphylla (Zhang et al., 2008).
Phenolic acid
Purification of the aqueous leaves extract of R. induta yielded chlorogenic acid 32 and neochlorogenic acid 33 (Kalegari et al., 2014b). (E)-Ferulic acid nonacosyl ester 34 was isolated from R. microphylla (Zhang et al., 2008).
Others
Purification of hexane fraction of ethanolic leaves extract of R. induta yielded n- tetracosane 35 (Kalegari et al., 2011). Ropanone 36 was purified from R. santoloides (Ramiah et al., 1976). Isolation of chloroform soluble fraction of methanolic stems extract of R. minor yielded dihydrovomifoliol-9-β-D-glucopyranoside 37 (He et al., 2006). Rourinoside 38 was isolated from the chloroform soluble fraction of methanolic stems extract of R. minor (He et al., 2006).
BIOLOGICAL ACTIVITY
Hypoglycemic activity
Rourea minor
The methanol roots extract of R. minor showed antihyperglycemic activity in a dose-dependent manner at 200 and 400 mg/kg when administered to streptozotocin-induced diabetic rats. In oral glucose tolerance test, no glucose lowering effect was observed at 30 and 60 minutes but the effect was quite significant after 90 and 120 minutes administration of the extract. Administration of 100, 200, and 400 mg/kg of methanol extract resulted in a significant reduction of hyperglycemia at days 4, 8, and 12 in a dose-dependent manner. Oral administration of methanol roots extract of R. minor at all doses significantly reduced glucose level in the diabetic rats (Chaudhary et al., 2012).
 | Figure 6. Phenolic acids in Rourea sp. (Kalegari et al., 2014b; Zhang et al., 2008). [Click here to view] |
 | Figure 7. Other compounds in Rourea sp. (He et al., 2006; Kalegari et al., 2011; Ramiah et al., 1976). [Click here to view] |
 | Table 1. Bioactivities of Rourea sp. [Click here to view] |
In another study, hypoglycemic activity was observed over 120 minutes in streptozotocin-induced diabetic rats treated with ethanolic and aqueous roots extract of R. minor at 400 mg/kg as compared to diabetic control rats. After 15 days, treatment with ethanolic and aqueous extracts reduced glycemia significantly at 43.1% and 34.8%, respectively. The diabetic rats treated with ethanol extracts showed higher insulin secretion at 19.7 μU/ml as compared to those treated with water extract, which showed insulin secretion at 17 μU/ml. The insulin secretion of diabetic control rats and glibenclamide treated rats (10 mg/kg) were less than 5 and 22 μU/ml, respectively. Both extracts reversed the elevated lipid parameters and normalized them significantly to near normal values (Kulkarni et al., 2014).
Rourea cuspidata
Oral administration of hydroalcoholic stems extract of R. cuspidata at 200 mg/kg significantly reduced the glucose level in streptozotocin-induced diabetic rats comparable to glibenclamide. Hydroalcoholic extract contains flavonoids as major compounds. The extract showed a significant hepatoprotective effect on the rat’s liver as shown by reduction of AST level from 253 to 49 U/l (Laikowski et al., 2017).
Antibacterial activity
The chloroform and ethyl acetate fractions of ethanol leaves extract of R. induta showed potential antibacterial activity against Staphylococcus aureas and S. epidermidis at 1,000 μg/ml. Chloroform fractions showed inhibition against S. epidermidis and S. aureas with average inhibition halos of 12.3 and 7.6 mm, respectively. Ethyl acetate fraction showed antibacterial activity against S. epidermidis and S. aureas with average inhibition halos of 15.0 and 7.6 mm, respectively. Antibacterial activity of the ethanol extract could be due to the presence of hyperin 6, which showed antibacterial activity against S. epidermis at 1,000 and 500 μg/ml with average inhibition halos of 9.3 and 7.0 mm, respectively (Kalegari et al., 2012).
Hepatoprotective activity
Administration of 500 mg/kg of ethanolic leaves extract of R. induta caused a significant reduction in AST and ALT activities and TB level in the CCl4 treated group comparable to Legalon. The weight of the liver of the treated group was also smaller as compared to the non-treated control group. Treatment with the extract also normalized the hepatic oxidative stress markers CAT, SOD, GPx, and GSH as compared to the non-treated control group, although Legalon showed a stronger effect. The endogenous antioxidant defense was restored and lipid peroxidation in the liver was reversed over 7 days post-treatment with the extract, similar to the effects shown by Legalon. The hepaprotective activity could be due to the presence of flavonoids 2, 3, and 6 (Kalegari et al., 2014b).
Antinociceptive activity
Treatment of aqueous leaves extract of R. induta on mice showed a significant antinociceptive effect on different pain models without affecting the motor activity and corporal temperature of the mice, and the extract did not depend on the opioid system. The aqueous extract inhibited the neurogenic (0–5 minutes) and inflammatory (15–30 minutes) phases of formalin-induced licking at 30, 100, and 100 mg/kg. The marker compound, hyperin 6, showed comparable result at 100 mg/kg at the neurogenic phase of the test. The mice in the extract treated group showed a significant reduction (60%–65%) of the mechanical sensitivity on the ipsilateral paw when induced with intraplantar injection of Complete Freund’s Adjuvant. The treatment of the extract reduced the level of IL-1β and TNF-α in the skin of the hind paw by 22% and 50%, respectively, as compared to the non-treated control group. The treated group showed a significant reduction in biting behavior caused by TNF-α (0.1 pg/site i.t.) but no effect was observed on IL-1β-induced biting response. It was concluded that the antinociceptive effect of the aqueous leaves extract of R. induta is due to decrease synthesis or release of pro-inflammatory cytokines, such as TNF-α and IL-1β (Kalegari et al., 2014a).
Antiplasmodial activity
Rourinoside 38, rouremin 28, and 1-(26-hydroxyhexacosanoyl)-glycerol 26 isolated from R. minor showed antiplasmodial activity in vitro against chloroquine sensitive (D6) and chloroquine resistant (W6) Plasmodium falciparum with the IC50 values of 3.7/2.1 μM, 5.1/4.5 μM, and 9.5/12.7 μM, respectively (He et al., 2006).
Larvicidal activity
The hexane stems extract of R. doniana showed potential antilarvicidal activity with the LD50 value of 12.1 μg/ml. The chloroform leaves extract and hexane stems extract of R. doniana caused 88.9% mortality rate of the Aedes aegyptii larvae at the concentration of 250 μg/ml (Oliveira et al., 2010).
Acute toxicity
No acute toxicity was observed when rats were given 100, 200, and 400 mg/kg of methanolic roots extract of R. minor (Chaudhary et al., 2012). In other study on ethanolic and aqueous roots extracts of R. minor, the rats showed good tolerance up to 3 g/kg and no lethality was observed (Kulkarni et al., 2014). Ethanolic leaves extract of R. induta and its fractions showed no potential toxicity in brine shrimp assay and hemolytic test (Oliveira et al., 2012).
Antioxidant activity
DPPH radical scavenging activity
The chloroform and ethyl acetate fractions of ethanol leaves extract of R. induta showed significant DPPH radical scavenging activity with the IC50 values of 5.3 and 3.2 μg/ml, respectively (Kalegari et al., 2012).
Phosphomolybdenum complex method
Hexane, chloroform, and ethyl acetate fractions of ethanolic leaves extract of R. induta showed more than 100% activity in relation to rutin and vitamin C. Hyperin 6 also demonstrated antioxidant activity more than 127.8 % in relation to rutin but only more than 42.3% in relation to vitamin C (Kalegari et al., 2012).
CONCLUSION
Rourea sp. is widely used in traditional medicine for various health complaints. Scientific investigation on the plants yielded secondary metabolites of different classes. Several plants of Rourea sp. showed potential bioactivity, especially hypoglycemic and antinociceptive activities.
ACKNOWLEDGMENTS
The authors would like to express appreciation to Atta-ur-Rahman Institute for Natural Product Discovery, Universiti Teknologi MARA, for the facilities provided.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
FINANCIAL SUPPORT AND SPONSORSHIP
The authors would like to thank Universiti Teknologi MARA for financial support through Bestari Perdana Grant (600-IRMI/PERDANA 5/3 BESTARI (093/2018)).
REFERENCES
Alsarhan A, Sultana N, Kadir MRA, Aburjai T. Ethnopharmacological survey of medicinal plants in malaysia, the Kangkar Pulai region. Int J Pharmacol, 2012; 8:679–86.
Bargali S, Shrivastava SK, Dixit VK, Bargali K. Some less known ethno botanical plants of Jagdalpur district of Chhattisgarh state. Botanica, 2003; 53:192–7.
Bero J, Ganfon H, Jonville M-C, Frédérich M, Gbaguidi F, DeMol P, Moudachirou M, Quetin-Leclercq J. In vitro antiplasmodial activity of plants used in Benin in traditional medicine to treat malaria. J Ethnopharmacol, 2009; 122:439–44.
Chaudhary A, Bhandari A, Pandurangan A. Anti-hyperglycemic potential of Rourea minor roots in streptozotocin (STZ) induced diabetic rats. Int J Pharm Res, 2012; 4:59–62.
Forero E. Neotropical connaraceae. In: Milliken W, Klitgård B, Baracat A. (eds.). Neotropikey—Interactive key and information resources for flowering plants of the Neotropics. 2009. Available via http://www.kew.org/science/tropamerica/neotropikey/families/Connaraceae.htm (Accessed 20 Aug 2018).
Grosvenor PW, Gothard PK, McWilliam NC, Supriono A, Gray DO. Medicinal plants from Riau Province, Sumatra, Indonesia. Part 1: uses. J Ethnopharmacol, 1995; 45:75–95.
He Z-D, Ma C-Y, Tan GT, Sydara K, Tamez P, Southavong B, Bouamanivong S, Soejarto DD, Pezzuto JM, Fong HH, Zhang HJ. Rourinoside and rouremin, antimalarial constituents from Rourea minor. Phytochemistry, 2006; 67:1378–84.
Jamal JA, Ghafar ZA, Husain K. Medicinal plants used for postnatal care in Malay traditional medicine in the Peninsular Malaysia. Pharmacogn J, 2011; 3:15–24.
Jeannoda VLR, Rakoto-Ranoromalala DAD, Valisolalao J, Creppy EE, Dirheimer G. Natural occurrence of methionine sulfoximine in the Connaraceae family. J Ethnopharmacol, 1985; 14:11–7.
Kalegari M, Cerutti ML, Macedo-Júnior SJ, Bobinski F, Miguel MD, Eparvier V, Santos AR, Stien D, Miguel OG. Chemical composition and antinociceptive effect of aqueous extract from Rourea induta Planch. leaves in acute and chronic pain models. J Ethnopharmacol, 2014a; 153:801–9.
Kalegari M, Gemin CAB, Araújo-Silva G, Brito NJNd, López JA, Oliveira Tozetto SD, das Graças Almeida M, Miguel MD, Stien D, Miguel OG. Chemical composition, antioxidant activity and hepatoprotective potential of Rourea induta Planch. (Connaraceae) against CCl4-induced liver injury in female rats. Nutrition, 2014b; 30:713–8.
Kalegari M, Miguel MD, Dias JDFG, Lordello ALL, Lima CPD, Miyazaki CMS, Zanin SM, Verdam MC, Miguel OG. Phytochemical constituents and preliminary toxicity evaluation of leaves from Rourea induta Planch. (Connaraceae). Brazil J Pharm Sci, 2011; 47:635–42.
Kalegari M, Miguel MD, Philippsen AF, de Fátima Gaspari Dias J, Zanin SMW, de Lima CP, Miguel OG. Antibacterial, allelopathic and antioxidant activity of extracts and compounds from Rourea induta Planch. (Connaraceae). J Appl Pharm Sci, 2012; 2:061–6.
Kantati YT, Kodjo KM, Dogbeavou KS, Vaudry D, Leprince J, Gbeassor M. Ethnopharmacological survey of plant species used in folk medicine against central nervous system disorders in Togo. J Ethnopharmacol, 2016; 181:214–20.
Kulkarni P, Patel V, Shukla ST, Patel A, Kulkarni V. Antidiabetic potential of Rourea minor (Gaertn.) root in streptozotocin—induced diabetic rats. Oriental Pharm Exp Med, 2014; 14:69–76.
Laikowski MM, dos Santos PR, Souza DM, Minetto L, Girondi N, Pires C, Alano G, Roesch-Ely M, Tasso L, Moura S. Rourea cuspidata: chemical composition and hypoglycemic activity. Asian Pac J Trop Biomed, 2017; 7:712–8.
Oliveira PV, Ferreira JC, Moura FS, Lima GS, de Oliveira FM, Oliveira PES, Conserva LM, Giulietti AM, Lemos RP. Larvicidal activity of 94 extracts from ten plant species of northeastern of Brazil against Aedes aegypti L. (Diptera: Culicidae). Parasitol Res, 2010; 107:403–7.
Oliveira PVD, Lemos RPL, Conserva LM. Chemical constituents of Rourea doniana. Rev Brasil Farmacogn, 2012; 22:451–4.
Ong HC, Ahmad N, Milow P. Traditional medicinal plants used by the Temuan villagers in Kampung Tering, Negeri Sembilan, Malaysia. Studies Ethno Med, 2011a; 5:169–73.
Ong CH, Chua S, Milow P. Ethno-medicinal plants used by the Temuan villagers in Kampung Jeram Kedah, Negeri Sembilan, Malaysia. Studies Ethno Med, 2011b; 5:95–100.
Ramiah N, Prasad NBR, Abraham K. Rapanone and leucopelargonidin from the roots of Rourea santaloides. J Inst Chem (India), 1976; 48:196–7.
Sabran SF, Mohamed M, Abu Bakar MF. Ethnomedical knowledge of plants used for the treatment of tuberculosis in Johor, Malaysia. Evid Based Complement Altern Med, 2016; 1–12.
Sanz-Biset J, Cañigueral S. Plant use in the medicinal practices known as “strict diets” in Chazuta valley (Peruvian Amazon). J Ethnopharmacol, 2011; 137:271–88.
The Plant List. Version 1.1. Published on the Internet. 2013. Available via http://www.theplantlist.org/ (Accessed 28 December 2018).
Zhang KM, Jiang JQ, Kong L. Chemical constituents from Rourea microphylla. Chinese J Nat Med, 2008; 6:345–7.