INTRODUCTION
Multiple sclerosis (MS) is a progressive autoimmune disease of the central nervous system (CNS), characterized by focal inflammation, demyelination, and axonal damage. Among autoimmune diseases, MS is the most prevalent, affecting about 2.5 million people worldwide, especially women. The MS symptoms are varied, showing progressive neuronal functional loss, including muscle and extremity weakness, ataxia, spasticity, fatigue, paresthesia, dizziness, visual and cognitive impairment, bowel and bladder dysfunction, emotional and sexual problems, leading to death (Elliott et al., 2012; Dias et al., 2014; Fontes et al., 2014).
Experimental autoimmune encephalomyelitis (EAE) is the established and widely accepted animal model for studying and understanding MS, since both EAE and MS present similar clinical and pathophysiological evolution. The study of immunopathogenesis of EAE and MS demonstrates that both are mediated by the processes orchestrated by T helper cells Th1 and Th17. Therefore, cytokines originating from Th1 cells, such as interferon gamma (INF-γ) and produced by Th17, such as IL-17, are triggers for inflammatory processes and lead to the production of inflammatory mediators, such as TNF-α and free radicals of oxygen, such as nitric oxide (NO) and hydrogen peroxide (H2O2), which are closely involved in the immunopathological process and tissue damage (Fontes et al., 2014; 2017; Murta and Ferrari, 2013).
β-caryophyllene (BCP) is a natural sesquiterpene found in several medicinal plants, being the main constituent of copaiba oil, a resin extracted from the native Brazilian tree (Copaifera landsdorfi), which is popularly use as antirheumatic, analgesic, and anti-inflammatory (Fernandes et al., 2007; Fontes et al., 2017; Galucio et al., 2016; Veiga Junior et al., 2007; Yamaguchi and Garcia, 2012). These observations have been corroborated experimentally, in which BCP demonstrated antioxidant and neuroprotective activities, suppressing neuroinflammatory responses, such as the secretion of cytokines by inhibiting the activation of nuclear factor kappa β (NF-κβ) by CNS cells, such as microglia (Gelmini et al., 2013; Guimarães-Santos et al., 2012; Paula-Freire et al., 2014).
Studies have also shown that BCP binds selectively with agonist activity to the cannabinoid receptor type 2 (CB2), but it did not demonstrate affinity with the CB1 receptors, presenting, therefore, a strong anti-inflammatory activity. Inhibition of NF-κβ by BCP in microglia leads to the reduction of inflammatory cytokines, such as TNF-α and IFN-γ, also significantly inhibiting the generation of reactive oxygen species in the mitochondria (Al Mansouri et al., 2014; Bahi et al., 2014; Gertsch, 2008; Guo et al., 2014; Klauke et al., 2014).
In our previous work (Fontes et al., 2017), we showed that the oral treatment with BCP inhibited cytokine production, such as TNF-α, IFN-γ , IL-17, and attenuated neurological damages in the CNS of EAE-mice, leading to clinical improvement of animals. In addition, the recent approval of BCP by the FDA, after demonstrating its safety for clinical uses, makes this substance even more promising and an excellent candidate for the treatment of inflammatory neurodegenerative diseases, such as MS (Gertsch, 2008; Varga et al., 2018).
To further explore the potential mechanisms of BCP on the animal model of MS, at the first time, the in situ effects on remyelinization, inflammatory infiltrate, IL-17 production, and the presence of Th1 and Th2 cells by expression of T-bet and GATA-3, C57BL/6 mice were induced for EAE and treated by gavage with different concentrations of BCP.
MATERIAL AND METHODS
Chemicals
Pertussis toxin, MOG35–55, β-caryophyllene, complete Freund’s adjuvant were acquired from Sigma-Aldrich (St. Louis, MO) and Mycobacterium tuberculosis H37RA was from Difco (Detroit, MI, USA). Imunohistochemistry assay kits were obtained from Santa Cruz Biotechnology (Santa Cruz, CA), while all other compounds were acquired from Sigma-Aldrich.
Animals
Female C57BL/6 mice (8–12 weeks old; 21–23 g of weight) were obtained from the biotereum of the Federal University of Juiz de Fora (CBR/UFJF) and kept in micro isolator cages. All the animal experimental protocols and care were approved by the Ethical Committee for Animal Care of the Federal University of Juiz de Fora (Protocol no. 039/2010).
Induction of EAE
Mice were subcutaneously (s.c.) injected at the both tail base with 100 μg of myelin oligodendrocyte glycoprotein peptide (MOG35–55), emulsified with complete Freund's adjuvant (CFA) (v/v), and supplemented with 400 μg of attenuated M. tuberculosis H37RA. Pertussis toxin at 300 ng/mice was injected intraperitoneally (i.p.) on the first day of immunization and 48 hours later (Alves et al., 2012; Corrêa et al., 2010). Non-immunized mice were used as myelin control.
In vivo treatment with BCP
After the induction of EAE, mice were divided into three groups (n = 5): MOG35–55 immunized group that received only vehicle (EAE group); MOG35–55 immunized group that were treated by gavage with BCP (Fig. 1), at the oral doses of 25 mg/kg/day (BCP 25 group); and MOG35–55 immunized group that were treated by gavage at doses of 50 mg/Kg/day (BCP 50 group). These doses were based on previous studies where BCP presented in vivo anti-inflammatory results (Fernandes et al., 2007). After the 10th day of immunization, when 80% of the animals demonstrated clinical aspects of EAE, the treatments were performed daily, for 9 days. At this point, on the day 19 after the induction (peak of EAE), mice were euthanized under deepening anesthesia (i.p.), brain and spinal cord were removed.
Histological assessment of CNS tissues
For histological analysis of CNS tissues, brain and spinal cord were obtained and dissected from the mice and fixed in 10% formalin. Following dissection, the CNS samples were dehydrated in three different concentrations of ethanol (70%, 90%, and 100%) in baths of 1 hour each, with another three additional baths in absolute alcohol. After this, the samples were then bleached in three baths of xylol (1 hour each) and finally underwent impregnation in paraffin in an autoclave at 58°C and inclusion in paraffin was made at room temperature. The blocks were then cut into 5 μm of thickness and, to allow the study of the inflammatory process and axonal damage, were stained by HE, as previously described (Fontes et al., 2017). Histopathological and immunohistochemistry examination were performed out by two different pathologists. Photomicrographs were taken with Axiostar plus (Camera Sony DCR-PC 100 e Software Axiovision release® version 4.8). After careful observation, the same significant anatomic were selected for digital capture in all the samples.
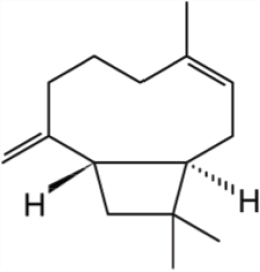 | Figure 1. Chemical structure of β-caryophyllene. [Click here to view] |
Weigert–Pal–Russel’s Iron Hematoxylin
After obtaining the histological sections and deparaffinization procedures, the Weigert–Pal–Russel staining was done for general visualization of the CNS, as well as for the distribution and organization of the myelin layer (Behmer, 2003). A digitalized capture of four fields per sample was made in anatomical areas pre-established by Axiostar plus (Camera Sony DCR-PC 100 e Software Axiovision release® version 4.8) (Fontes et al., 2017). The automatic morphometry was performed by software Zen2012, from the same company. The results were initially expressed in square micrometers (myelin area per field increasing by 200×). Subsequently, simple arithmetic averages were obtained per group. The mean myelinated area of the healthy group was considered as standard (100%) and from there the other mean values (Group EAE, BCP 25, and BCP 50) were evaluated comparatively.
Immunohistochemistry
Slices with 5 μm from paraffin blocks were placed on silanized slides (3-aminopropyltriethoxysilane; Sigma-Aldrich Inc., St. Louis, MO), deparaffinized in an autoclave at 60°C for 60 minutes, hydrated in sequence by baths in xylol, absolute alcohol, 80% alcohol, 70% alcohol, and distilled water for 5 minutes at room temperature. Tissue samples were immersed in citrate buffer (1 mM; pH = 6.0), blocked with 3% hydrogen peroxide for 30 minutes and incubated with primary antibodies (polyclonal rabbit anti-IL17, anti-T-bet, and anti-GATA-3, diluted 1:100—Santa Cruz, CA) for 1 hour. A second incubation with biotinylated antibodies for 30 minutes was performed, followed by another 30 minutes incubation with the avidin-biotin peroxidase-anti-peroxidase complex. The substrate for staining used was diaminobenzidine chromogen for 1 minute and the omission of the primary antibodies allowed the obtention of the negative control.
Tissue sections were examined by light microscopy (400×), and 10 photomicrographs were collected per section (Zeiss Axiostar, Zeiss, Germany). Five out of ten representative anatomical and digitalized microscopic fields were selected and digitalized (Camera Sony DCR-PC 100 e Software Axiovision release® version 4.8; 400×). All the cells were counted (100%) and, subsequently, only cells considered positive (brown coloration in cytoplasm) were quantified to obtain the percentage. The results were expressed by simple arithmetic mean (± SD) per group.
Statistical analysis
For non-continuous data, we used the Kruskal–Wallis test followed by the Dunn’s post doc test. The analysis of significance level was set at 5% (p < 0,05). Analyses were obtained from program GraphPad Prism version 5:00 for Windows (GraphPad instat software, version 3, San Diego, CA).
RESULTS AND DISCUSSION
The medicinal application of copaiba oil is extensive, including for industries, because it shows diuretic, healing, anti-inflammatory, and anti-tumor effects. However, the anti-inflammatory activity of copaiba oil is undoubtedly the most widespread use in folk medicine and has been extensively investigated. In Brazil, mainly in Amazon region, it has already been used as an anti-inflammatory (Veiga Junior et al., 2007; Yamaguchi and Garcia, 2012).
Regarding the activity of copaiba oil in its various immunomodulatory activities, its effects were investigated in vitro in C57BL/6 mice induced for EAE. It was demonstrated that mice spleen cells, after stimulation with MOG35–55, showed an inhibition of the production of IFN-γ, TNF-α, IL-17, NO, and H2O2 when measured by ELISA (Dias et al., 2014).
Chemically, BCP is a sesquiterpene, the major constituent (approximately 50%) of the copaiba oil-resin. It is present in several plants and its concentration in this oil varies with the species and the region of its extraction (Fernandes et al., 2007; Veiga Junior et al., 2007; Yamaguchi and Garcia, 2012).
The anti-inflammatory effects of sesquiterpenes are known, for example, α-humulene, which leads to a significant increase in the release of anti-inflammatory mediators in mice’s allergic airways inflammation models (Rogerio et al., 2009).
According to Medeiros et al. (2007), BCP inhibits the migration of neutrophils induced by LPS in paw edema in rats. Another experiment demonstrated that in dextran-induced colitis, BCP reduced the inflammatory infiltrate in the colon, decreasing myeloperoxidase and IL-6 levels, leading to a significant improvement in the disease (Cho et al., 2007).
In relation to the inflammatory development of atherosclerosis, BCP presented an ability to inhibit the process by reducing the expression of vascular cell adhesion molecule 1 (VCAM-1), which are crucial for the migration of cells to the inflamed site (Zhang et al., 2017).
In addition, the anti-inflammatory and neuroprotective activities of BCP on EAE have been discussed in the literature. These effects were recently shown by Fontes et al. (2017), which showed that BCP in vitro (at concentrations of 20 and 40 μg/ml) is able to reduce NO production and some inflammatory cytokines in cultured splenocytes of C57BL/6 EAE-mice. Also, when orally administered in vivo, BCP (25 and 50 mg/kg/day) caused a reduction in the production of IFN-γ, TNF-α, IL-17, NO, and H2O2, as well as a decrease in inflammatory areas in the CNS. According to Fontes et al. (2017), the reduction in these mediators was correlated with the significant reduction of the clinical score and mean weight of the BCP-treated animals and, consequently, the severity of the symptoms.
Many authors reported that the anti-inflammatory activity of BCP is related to its capacity to selectively stimulate the type 2 cannabinoid receptor (CB2), which exhibits, after activation, antioxidant, and anti-inflammatory activities. Thus, there is a significant inhibition of the generation of reactive oxygen species and inactivation of NF-κappa β transcriptional factor in the microglia, which implies in the reduction of pro-inflammatory cytokines, such as TNF and IFN- (Cho et al., 2007; Fontes et al., 2017; Rogerio et al., 2009; Zhang et al., 2017).
Regarding the mechanism of BCP in EAE-mice, together with our previous work (Fontes et al., 2017), in this present study we analyzed, by immunohistochemistry, the in situ effects of BCP in EAE-mice.
The tissue expression of IL-17, T-bet, and GATA-3 transcriptional factors was investigated in EAE-mice orally treated with BCP (25 and 50 mg/kg/day) in comparison with untreated EAE-mice. These effects were evaluated in the separate regions of the CNS (brain, cerebellum, and medulla) for a better understanding of their histopathological aspects.
In EAE model, the literature indicates TCD4 + cells with a self-responsive Th17 profile as the major responsible for the immunopathogenic development. Also, demyelinating lesions are triggered, among other factors, by cytokines, such as IL-17 and chemokines, with high levels of IFN-γ and, therefore, potent activation of macrophages, with high production of NO and H2O2 (Ivanov et al., 2006; Machado, 2012; Teniente-Serra et al., 2017; Yang et al., 2008).
After immunohistochemistry, the tissue aspects for IL-17, T-bet, and GATA-3 present in Figure 2, while Figure 3 shows the remyelination results. As described in Material and Methods, these stains are from brain, medulla, and cerebellum, and the statistical analyzes that will be described for each marker represent the mean number of labeled cells/field representative of five fields/animal in each group.
We found a statistically significant (p < 0.05) dose-dependent decrease of IL-17 tissue expression in the BCP-treated groups in all the regions of the CNS (brain, cerebellum, and medulla) (Fig. 4).
Corroborating with our results that showed lower expression of IL-17 in situ, previous reports also showed lower levels of IL-17 by splenocytes both with copaiba oil (which presents BCP as the main constituent) (Dias et al., 2014) and BCP (Fontes et al., 2017). Also, Fontes et al. (2017) suggested a positive correlation of low levels of IL-17 with the reduction of inflammatory infiltrate in the CNS in BCP-treated mice.
In addition, the literature confirms that these data may be associated with the best clinical performance of the animals and the decrease of the inflammatory process demonstrated by HE staining.
IL-17 presents well-established encephalopathogenic actions, such as BBB breaking, as well as direct action on various cell types (microglia, endothelial cells, epithelial cells, synoviocytes, fibroblasts, and myeloid cells), leading to the production of inflammatory cytokines and stimulation of neutrophil infiltration (Guo et al., 2014; Ivanov et al., 2006; Kroenke et al., 2008).
The Th1 population profile is historically recognized as a stimulator of the inflammatory process in EAE and MS, especially at the spinal cord (Goverman, 2009). In our research, this was investigated by immunohistochemistry through its transcriptional factor, T-bet. Our results showed a significant reduction (p < 0.05) in the expression of T-bet cells in medulla and cerebellum in the groups orally treated with 50 mg/kg/day of BCP (Fig. 5). Although the brain was not found statistically significant differences, in our experiments, there is a clear tendency in reduction of the T-bet expression for all BCP-treated groups. In this way, considering the pathophysiology of MS, the literature reports the minor importance of the Th1 profile in the brain in comparison with Th17 response profile (Goverman, 2009).
Th1 cells is known to produce cytokines, such as TNF-α, IFN-γ, and IL-2, which can directly attack myelin layer and promote demyelination by activation of macrophages, astrocytes, and glia cells. When Th1 and Th17 profiles are inhibited, the literature shows the reduction in levels of TNF-α and oxygen radicals, such as NO and H2O2. These radicals are closely associated with oxidative stress, the inflammatory process, and demyelination (Goverman, 2009; Machado, 2012).
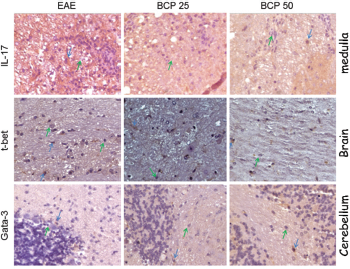 | Figure 2. Tissue expression of IL-17 (spinal cord), T-bet (brain), and GATA-3 (cerebellum) in the CNS of C57BL/6 mice induced for EAE, treated with BCP at concentrations of 25 and 50mg/ kg/day. The photos represent immunohistochemical expression of IL-17, T-bet, and GATA-3 in the CNS of the vehicle-treated EAE group, BCP 25 and 50 mg/kg/day groups. Green arrows: negative control of the reaction; Blue arrows: positive reaction control (Camera Sony DCR-PC 100 e Software Axiovision release® version 4.8). [Click here to view] |
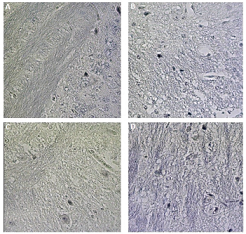 | Figure 3. Distribution of myelin stained by Weigert–Pal–Russel Hematoxylin in the CNS (brain) of normal C57BL/6 mice (A), induced for EAE with administration of vehicle (B), treated with BCP at concentrations of 25 mg/kg/day (C), and 50 mg/kg/day (D) (Camera Sony DCR-PC 100 e Software Axiovision release® version 4.8). [Click here to view] |
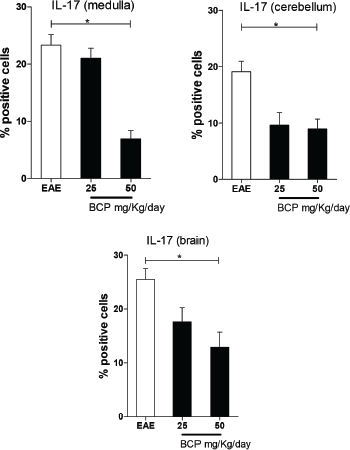 | Figure 4. Percentage of positive cells in labeling for IL-17 expression in medulla, cerebellum, and brain (p <0.005). [Click here to view] |
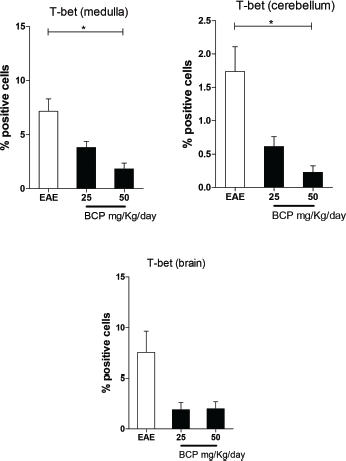 | Figure 5. Percentage of positive cells in labeling for T-bet expression in medulla, cerebellum, and brain (p <0.005). [Click here to view] |
Therefore, our results (Figs. 4 and 5) showed a clear in situ reduction in the expression of the Th1 (T-bet) and Th17 (IL-17) profiles. These findings corroborate with the literature, which showed the lower production of cytokines associated with these markers in splenocytes culture (Dias et al., 2014; Fontes et al., 2017). It is also important to note that the present work was innovative in showing the effect of BCP directly at the CNS level.
The Th2 profile was also investigated by the expression of transcriptional factor GATA-3 (Fig. 6). The literature correlates the regulation of the inflammatory process in the EAE with the increase of Treg and Th2’s cytokines in the CNS (Balabanov et al., 2006; Fontes et al., 2017; Machado, 2012; Yang et al., 2008). Our results showed a significant (p < 0.05) increase in GATA-3 expression in animals treated with the highest dose of BCP in cerebellum (Fig. 6).
CD4 + T cells are belonging to the Th2 population and pointed out by several authors as important modulator of Th2 response and, consequently, as of great relevance for decreasing the inflammatory process in EAE and MS. Recent data have shown that the animals treated with glatiramer acetate and dimethylfumarate demonstrated an elevation of Th2 expression in tissues. However, some drugs to treat MS, such as interferon, natalizumab, fingolimod, alemtuzumab, and teriflunomide did not demonstrated this profile (Bar-Or et al., 2014; Chiarini et al., 2015; Machado, 2012; Pinschewer et al., 2011).
In addition to our results for the Th17, Th1, and Th2 profiles, our research verified the effect of BCP on the myelin slayer in animals induced with EAE (Fig. 7). After stained by Weigert–Pal–Russel Iron Hematoxylin, our findings showed an intense remyelination (Fig. 7) along with an evident better organization in CNS for BCP-treated groups. Significant areas of remyelination were found in both treated groups (p < 0.001), without a dose-dependence response.
The remyelination process often occurs due to the action of cells from the innate and adaptive immune response (Dittmer et al., 2018; Dombrowski et al., 2017). Among these cells, T cells, which due to their phenotypic and functional variations, are shown to be effective in myelin damage but are also necessaries for the successful of the remyelination process. The regeneration of myelin in the CNS involves the differentiation of oligodendrocyte progenitor cells (OPC). During MS, despite the large amount of OPC, the remyelination process may fail, suggesting a compromise during the process of oligodendrocyte differentiation. Recent work has discussed stimuli for remyelination and proliferation of oligodendrocytes in EAE, demonstrating the importance of this process in the clinical recovery of diseased animals and prognostic evolution (Dittmer et al., 2018; Dombrowski et al., 2017).
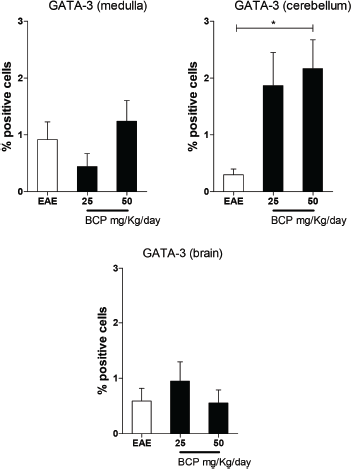 | Figure 6. Percentage of positive cells in labeling for GATA-3 expression in medulla, cerebellum, and brain (p <0.005). [Click here to view] |
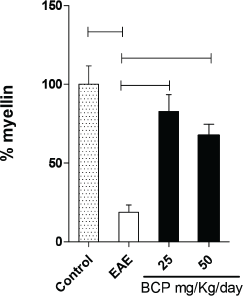 | Figure 7. Percentage of remyelination of the treated groups and EAE in comparison to the normal control group (100% myelin). [Click here to view] |
CONCLUSION
The present work showed the immunomodulatory effect of BCP on the inflammatory aspects of EAE in C57BL/6 mice in situ. The effects on decreasing IL-17 cytokine expression, T-bet transcription factor associated with increasing in GATA-3 (cerebellum), and the strong remyelination observed demonstrated, at tissue level, the unquestionable therapeutic potential of BCP for the treatment of the inflammatory and demyelinating diseases, such as EAE and MS.
ACKNOWLEDGMENTS
The authors would like to thank FAPEMIG (Grant numbers # #PPM-00296-16; APQ 04257/10; APQ 0171/11; APQ 02015/14), CNPq (Grant number # 487221/2012-5), as well as to CAPES, PIBIC/CNPq/UFJF, and CNPq for fellowships.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
REFERENCES
Al Mansouri S, Ojha S, Al Maamari E, Al Ameri M, Nurulain SM, Bahi A. The cannabinoid receptor 2 agonist, β-caryophyllene, reduced voluntary alcohol intake and attenuated ethanol-induced place preference and sensitivity in mice. Pharmacol Biochem Behav, 2014; 124:260–8. CrossRef
Alves CC, Castro SB, Costa CF, Dias AT, Alves CJ, Rodrigues MF, Teixeira HC, Almeida MV, Ferreira AP. Anthraquinone derivative O,O'-bis-(3'-iodopropyl)-1,4-dihydroxyanthraquinone modulates immune response and improves experimental autoimmune encephalomyelitis. Int Immunopharmacol, 2012; 14(2):127–32. CrossRef
Bahi A, Al Mansouri S, Al Memari E, Al Ameri M, Nurulain SM, Ojha S. β-Caryophyllene, a CB2 receptor agonist produces multiple behavioral changes relevant to anxiety and depression in mice. Physiol Behav, 2014; 135:119–24. CrossRef
Balabanov R, Strand K, Kemper A, Lee JY, Popko B. Suppressor of cytokine signaling 1 expression protects oligodendrocytes from the deleterious effects of interferon-gamma. J Neurosci, 2006; 26(19):5143–52. CrossRef
Bar-Or A, Pachner A, Menguy-Vacheron F, Kaplan J, Wiendl H. Teriflunomide and its mechanism of action in multiple sclerosis. Drugs, 2014; 74(6):659–74. CrossRef
Behmer OA. Manual de técnicas para histologia normal e patológica. 2nd edition. Barueri, Manole, Bulgaria, 2003.
Chiarini M, Sottini A, Bertoli D, Serana F, Caimi L, Rasia S, Capra R, Imberti L. Newly produced T and B lymphocytes and T-cell receptor repertoire diversity are reduced in peripheral blood of fingolimod-treated multiple sclerosis patients. Mult Scler, 2015; 21(6):726–34. CrossRef
Cho JY, Chang HJ, Lee SK, Kim HJ, Hwang JK, Chun HS. Amelioration of dextran sulfate sodium-induced colitis in mice by oral administration of β-caryophyllene, a sesquiterpene. Life Sci, 2007; 80(10):932–9. CrossRef
Corrêa JO, Aarestrup BJ, Aarestrup FM. Effect of thalidomide and pentoxifylline on experimental autoimmune encephalomyelitis (EAE). Exp Neurol, 2010; 226(1):15–23. CrossRef
Dias DS, Fontes LB, Crotti AE, Aarestrup BJ, Aarestrup FM, da Silva Filho AA, Corrêa JO. Copaiba oil suppresses inflammatory cytokines in splenocytes of C57Bl/6 mice induced with experimental autoimmune encephalomyelitis (EAE). Molecules, 2014; 19(8):12814–26. CrossRef
Dittmer M, Young A, O'Hagan T, Eleftheriadis G, Bankhead P, Dombrowski Y, Medina RJ, Fitzgerald DC. Characterization of a murine mixed neuronglia model and cellular responses to regulatory T cell-derived factors. Mol Brain, 2018; 11(1):25. CrossRef
Dombrowski Y, O'Hagan T, Dittmer M, Penalva R, Mayoral SR, Bankhead P, Fleville S, Eleftheriadis G, Zhao C, Naughton M, Hassan R, Moffat J, Falconer J, Boyd A, Hamilton P, Allen IV, Kissenpfennig A, Moynagh PN, Evergren E, Perbal B, Williams AC, Ingram RJ, Chan JR, Franklin RJM, Fitzgerald DC. Regulatory T cells promote myelin regeneration in the central nervous system. Nat Neurosci, 2017; 20(5):674–80. CrossRef
Elliott C, Lindner M, Arthur A, Brennan K, Jarius S, Hussey J, Chan A, Stroet A, Olsson T, Willison H, Barnett SC, Meinl E, Linington C. Functional identification of pathogenic autoantibody responses in patients with multiple sclerosis. Brain, 2012; 135(Pt 6):1819–33. CrossRef
Fernandes ES, Passos GF, Medeiros R, da Cunha FM, Ferreira J, Campos MM, Pianowski LF, Calixto JB. Anti-inflammatory effects of compounds alpha-humulene and (−)-trans-caryophyllene isolated from the essential oil of Cordia verbenacea. Eur J Pharmacol, 2007; 569(3):228–36. CrossRef
Fontes LB, Dos Santos Dias D, de Carvalho LS, Mesquita HL, da Silva Reis L, Dias AT, Da Silva Filho AA, do Amaral Corrêa JO. Immunomodulatory effects of licochalcone A on experimental autoimmune encephalomyelitis. J Pharm Pharmacol, 2014; 66(6):886–94. CrossRef
Fontes LBA, Dias DDS, Aarestrup BJV, Aarestrup FM, Da Silva Filho AA, Corrêa JODA. β-Caryophyllene ameliorates the development of experimental autoimmune encephalomyelitis in C57BL/6 mice. Biomed Pharmacother, 2017; 91:257–64. CrossRef
Galucio CS, Benites CI, Rodrigues RAF, Maciel MRW. Recuperação de sesquiterpenos do óleo-resina de copaíba a partir da destilação molecular. Quim Nova, 2016; 39(7):795–800.
Gelmini F, Beretta G, Anselmi C, Centini M, Magni P, Ruscica M, Cavalchini A, Maffei Facino R. GC-MS profiling of the phytochemical constituents of the oleoresin from Copaifera langsdorffii Desf. and a preliminary in vivo evaluation of its antipsoriatic effect. Int J Pharm, 2013; 440(2):170–8. CrossRef
Gertsch J. Anti-inflammatory cannabinoids in diet: towards a better understanding of CB(2) receptor action? Commun Integr Biol, 2008; 1(1):26–8. CrossRef
Goverman J. Autoimmune T cell responses in the central nervous system. Nat Rev Immunol, 2009; 9(6):393–407. CrossRef
Guimarães-Santos A, Santos DS, Santos IR, Lima RR, Pereira A, de Moura LS, Carvalho RN, Lameira O, Gomes-Leal W. Copaiba oil-resin treatment is neuroprotective and reduces neutrophil recruitment and microglia activation after motor cortex excitotoxic injury. Evid Based Complement Alternat Med, 2012; 2012:918174. CrossRef
Guo K, Mou X, Huang J, Xiong N, Li H. Trans—caryophyllene suppresses hipóxia—induced neuroinflammatory responses by inhibiting NF-κB activation in microglia. J Mol Neurosci, 2014; 54(1):41–8. CrossRef
Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differenciation program of proinflammatory IL – 17+ T helper cells. Cell, 2006; 126(6):1121–33. CrossRef
Klauke AL, Racz I, Pradier B, Markert A, Zimmer AM, Gertsch J, Zimmer A. The cannabinoid CB receptor-selective phytocannabinoid beta-caryophyllene exerts analgesic effects in mouse models of inflammatory and neuropathic pain. Eur Neuropsychopharmacol, 2014; 24(4):608–20. CrossRef
Kroenke MA, Carlson TJ, Andjelkovic AV, Segal BM. IL-12 and IL-23 modulated T cells induce distinct types of EAE based on histology, CNS chemokine profile, and response to cytokine inhibition. J Exp Med, 2008; 205(7):1535–41. CrossRef
Machado S. Recomendações esclerose múltipla. Omnifarma, São Paulo, Brazil, 2012.
Medeiros R, Passos GF, Vitor CE, Koepp J, Mazzuco TL, Pianowski LF, Campos MM, Calixto JB. Effect of two active compounds obtained from the essential oil of Cordia verbenacea on the acute inflammatory responses elicited by LPS in the rat paw. Br J Pharmacol, 2007; 151(5):618–27. CrossRef
Murta V, Ferrari CC. Influence of Peripheral inflammation on the progression of multiple sclerosis: evidence from the clinic and experimental animal models. Mol Cell Neurosci, 2013; 53:6–13. CrossRef
Paula-Freire LI, Andersen ML, Gama VS, Molska GR, Carlini EL. The oral administration of trans-caryophyllene attenuates acute and chronic pain in mice. Phytomedicine, 2014; 21(3):356–62. CrossRef
Pinschewer DD, Brinkmann V, Merkler D. Impact of sphingosine 1-phosphate modulation on immune outcomes. Neurology, 2011; 76(8 Suppl 3):S15–9. CrossRef
Rogerio AP, Andrade EL, Leite DF, Figueiredo CP, Calixto JB. Preventive and therapeutic anti-inflammatory properties of the sesquiterpene alpha-humulene in experimental airways allergic inflammation. Br J Pharmacol, 2009; 158(4):1074–87. CrossRef
Teniente-Serra A, Ramo-Tello C, Martinez-Caceres EM. Imunomonitoring lymphocyte subpopulations in multiple sclerosis patients. In: Zagon IS, McLaughlin PJ (eds.). Multiple sclerosis: perspectives in treatment and pathogenesis. Codon Publications, Brisbane, AU, pp 139–54, 2017. CrossRef
Varga ZV, Matyas C, Erdelyi K, Cinar R, Nieri D, Chicca A, Nemeth BT, Paloczi J, Lajtos T, Corey L, Hasko G, Gao B, Kunos G, Gertsch J, Pacher P. β-Caryophyllene protects against alcoholic steatohepatitis by attenuating inflammation and metabolic dysregulation in mice. Br J Pharmacol, 2018; 175(2):320–34. CrossRef
Veiga Junior VF, Rosas EC, Carvalho MV, Henriques MG, Pinto AC. Chemical composition and anti-inflammatory activity of copaiba oils from Copaifera cearensis Huber ex Ducke, Copaifera reticulata Ducke and Copaifera multijuga Hayne-A comparative study. J Ethnopharmacol, 2007; 112(2):248–54. CrossRef
Yamaguchi MH, Garcia RF. Óleo de copaíba e suas propriedades medicinais: revisão bibliográfica. Rev Saúde Pesq, 2012; 5(1):137–46.
Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, Watowich SS, Tian Q, Jetten AM, Dong C. T helper 17 lineage differenciation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity, 2008; 28(1):29–39. CrossRef
Zhang Z, Yang C, Dai X, Ao Y, Li Y. Inhibitory effect of trans-caryophyllene (TC) on leukocyte—endothelial attachment. Toxicol Appl Pharmacol, 2017; 329:326–33. CrossRef