INTRODUCTION
Evidences have shown that the oxidative stress possesses a vital role of in pathogenesis of many neurobiological diseases, such as stroke, Alzheimer’s, and Parkinson’s diseases, and also contributes to the aging process (Albarracin et al., 2012; Chen et al., 2011; Uttara et al., 2009). Several medicinal plants can target oxidative stress and mitochondrial dysfunction, and are known as good therapeutic and preventive candidates for such neural diseases. Plants, as reach sources of antioxidants, can potentially donate hydrogen to free radicals and inhibit molecular damages caused by oxidative stress (Albarracin et al., 2012). The whole plant extracts can be advantageous over individual phytochemicals because of the multitude of compounds involved in their structure, providing a mixture with variety of biological effects and much less toxicity (Nobili, 2009). Regarding the profound capacity of natural antioxidants versus the established adverse effects of the synthetic ones, it is necessary to find naturally occurring compounds protecting neuronal cells against oxidative damages.
Artemisia ciniformis Krasch & Popov ex Poljakov is one of the 34 Artmeisia species, which can be found in wide regions of Iran (Mozaffarian, 1996). The genus Artemisia (family Asteraceae) has about 500 species widely distributed in Europe, Asia, as well as North America (Bora and Sharma, 2011; Mucciarelli and Maffei, 2002). In the Iranian traditional medicine, different Artemisia species have long been known as a remedy for different disorders (Gorji and Ghadiri, 2001; 2002; Ghorbani, 2005; Zargari, 1995). For example, A. annua has been known as a species with hemostatic effect and a useful remedy for amelioration of constipation. Artemisia absinthium was believed to possess laxative properties and was used for parasite-induced intestinal disorders (Ghorbani, 2005). Furthermore, this species was a remedy for headache (Gorji and Ghadiri, 2002) and used for antiepileptic preparations in medieval Persia (Gorji and Ghadiri, 2001). Artemisia kopetdaghensis was believed to be effective in alleviation of parasite-induced intestinal disorders and colitis (Ghorbani, 2005). Several potentially therapeutic bioactivities, such as antimalarial, antioxidant, anti-inflammation, antitumor, antifungal, neuroprotective, and leishmanicidal effects, have been reported for different members of the genus Artemisia (Bora and Sharma, 2010; 2011; Emami et al., 2012; Hatami et al., 2014; Tayarani-Najaran et al., 2017). Besides, some of these species were found to have nutritive value as food additives (Kordali et al., 2005). Artemisia ciniformis has demonstrated in vitro effects, including apoptogenic and antiproliferative (Taghizadeh Rabe et al., 2011; Tayarani-Najaran et al., 2014), leishmanicidal (Emami et al., 2012), cardioprotective (Mojarrab et al., 2013), antimalarial (Mojarrab et al., 2015), free radical-scavenging and cytoprotective (Mojarrab et al., 2016), and antimicrobial effects (Taherkhani, 2016). These widespread pharmacological effects could be attributed to the variety of bioactive compounds and secondary metabolites in different parts of this species. The high content of polyphenols (Mojarrab et al., 2016) and other bioactive constituents like sesquiterpene lactones (Iranshahi et al., 2007) made this species to be of botanical and pharmaceutical interests. In addition, monoterpenoids such as camphor, myrcene, and linalool and sesquiterpenoids, such as davanone, have been reported as the main constituents in its volatile oil (Firouzni et al., 2008; Rustaiyan et al., 2007; Taherkhani et al., 2012). Recently, isochlorogenic acid isomers have been isolated from the hydroethanolic extract of the species (Nasseri et al., 2019).
Because of the notable distribution of different species of Artemisia in Iran (Naghavi et al., 2017), and with respect to neuroprotective potential of some species in the genus (Bora and Sharma, 2010; Lachenmeier, 2010) as well as the cytoprotective and antioxidant effects reported for A. ciniformis, (Mojarrab et al., 2013; 2016), it seems to be of value to examine whether different extracts of this species—in spite of lack of information on traditional use—can protect neuronal cells against oxidative stress. To the best of our knowledge, there has not been any similar cell-based study done on the neuroprotectivity of different extracts and fractions of this species and the mechanism underlying for their cytoptotective effect. As per the fact, that apoptosis is the common pathway for death of cells exposed to oxidative damage; this study has been designed to elucidate if antiapoptotic pathways are involved in inhibitory activity of this species.
MATERIALS AND METHODS
Reagents
Petroleum ether (40–60), ethyl acetate (EA), Gallic acid, sodium carbonate, Folin–Ciocalteu’s phenol reagent, LiChroprep® RP-18 (15–25 μm), and H2O2 were purchased from Merck, Germany. Ethanol and dichloromethane (DCM) were procured from Scharlau, Spain. Quercetin, 2′,7′-dichlorofluorescin diacetate (DCF), Triton-X100, dimethylsulphoxide (DMSO), MTT [(3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide], and Rhodamine 123 were purchased from Sigma-Aldrich (St Louis, MO). Trypsin-EDTA was supplied from Bon Yakhteh, Iran. Fetal bovine serum and Penicillin/Streptomycin were purchased from Gabon, USA and Gibco, USA. Dulbecco's Modified Eagle's Medium (DMEM) was supplied from ATCC, USA.
Plant material
The aerial parts of A. ciniformis were collected from Tandooreh National Park (Razavi Khorasanprovince, Iran). Samples were identified by Dr. Valiollah Mozaffarian (Research Institute of Forest and Rangelands, Tehran, Iran). The voucher specimen (with the identification numbers of 12569) was deposited in the herbarium, Department of Pharmacognosy, Faculty of Pharmacy, Mashhad University of Medical Sciences, Mashhad, Iran.
Preparation of extracts and fractions
The subsequent maceration method was used for the preparation of different extracts. Ninety grams of shade-dried aerial parts of A. ciniformis were milled and then were subsequently macerated in solvents with different polarities; petroleum ether (40–60), dichloromethane, EA, ethanol, and ethanol-water (1:1 v/v), respectively. After filtering the obtained extracts, all of them were dried by rotary evaporator (Heidolph, Germany) under a reduced pressure condition and at the low temperature (≤45°C) to yield 4.13, 9.66, 0.38, 2.54, and 17.01 g of corresponding extracts, respectively.
The dried extracts were kept at −20ºC for further investigating. After preliminary cell-based assays, 360 mg of the most promising sample (EA extract of A. ciniformis) was fractionated by reversed-phase vacuum liquid chromatography on RP-18 (15–25 μm) with H2O containing increasing amounts (20%–100%) of MeOH to obtain six fractions (F1–F6). All the fractions were fully dried as described above to yield 0.071, 0.095, 0.029, 0.038, 0.066, and 0.048 g of corresponding fractions, respectively.
Estimation of total phenolic and flavonoid contents
The Folin–Ciocalteu method (Singleton et al., 1999) was used to determine total phenolic content (TPC). Each dried fraction of EA extract (30 mg) was mixed with 25 ml of distilled water and filtered through Whatman No. 1 paper. This solution (0.5 ml) was then mixed with 2.5 ml of 0.2-N Folin–Ciocalteu reagent for 5 minutes and 2 ml of 75 g/l sodium carbonate was then added. After standing in dark for 2 hours, the absorbance of the reaction mixture was measured at 760 nm. Gallic acid (0–100 mg/l) was used as a standard to produce the calibration curve. The mean of three readings was used and the TPC was expressed in mg of gallic acid equivalent (GAE)/g of dried fraction.
The total flavonoid content (TFC) was determined using the Dowd method as adapted by Meda et al. (2005). Briefly, 2 ml of 2% aluminum trichloride in methanol was mixed with the same volume of a methanolic solution (0.2 or 0.3 mg/ml) of each fraction. Absorption readings at 415 nm were taken after 15 minutes. TFC was determined using a standard curve with quercetin (0–50 mg/l) as the standard. The mean of three readings was used and expressed as milligrams of quercetin equivalent (QE)/g of dried fraction.
MTT assay
In this study, PC12 pheochromocytoma cells were supplied from Pasteur Institute of Iran (Tehran, Iran). The cell growth medium was prepared by dissolution of fetal bovine serum (10% v/v) and 1% penicillin/streptomycin (100 U/ml: 100 U/ml) in DMEM-F12 at 37°C in a humidified incubator containing 5% CO2. 15–20 × 103 PC12 cells were seeded in each well of a 96-well culture plate and then treated with different concentrations of each Artemisia extract in DMSO (6.25, 12.5, 25, and 50 μg/ml) and incubated for 24 hours. At appropriate time intervals, the medium was replenished with 0.5 mg/ml MTT solution and plates were further incubated for 3 hours at 37°C. Thereafter, 100 μl DMSO was added to the wells for solubilizing the crystals of formazan. The absorbance at 570 nm corresponding to different treatment groups and control was detected on Eliza micro plate reader (BioTek Instruments, USA). Cell viability percentage of each sample was calculated by dividing the absorbance at 570 nm, corresponding to each treatment group, to that of control group. The IC50 value was also defined as the concentration in which 50% of cells were killed. All the MTT assays were carried out in triplicate.
Determination of intracellular ROS
Intracellular reactive oxygen species (ROS) formation was determined using 7-dichlorofluorescein diacetate (DCF-DA), a non-florescent dye which is oxidation sensitive, and its reaction with ROS can make it fluorescent (in the form of dichlorofluorescein (DCF) molecules) (Bai et al., 2005). The intracellular ROS formation study was conducted on different groups of cells: (1) Control group, (2) Cells treated with H2O2 (62.5 μM) for 24 hours, and (3) Cells pretreated with non-toxic concentrations of different extracts (selected after MTT study) for 24 hours and subsequently treated with H2O2 (62.5 μM) for another 24 hours. After treatment period, these groups were incubated with 100 μl DCFDA at 37°C for 30 minutes. Thereafter, cells were lysed with Triton-X100 and the absorbance at wavelength of 488–510 nm was recorded using a fluorescence microplate reader (BioTek, H1M).
Determination of SOD activity
SOD activity was defined based on the inhibitory ability of these metallo enzymes against oxidation of hydroxylamine, which possesses a prominent role in the cellular antioxidant defense mechanism. In the present study, commercial SOD assay kit Cayman (USA) was utilized to determine SOD activity following the manufacturer’s protocol. In this method, detection of superoxide radicals (produced from the xanthine oxidase-hypoxanthine system) would be possible using a tetrazolium salt. One unit of SOD was defined as enzyme concentration required for dismutation of 50% of the superoxide radicals. The activity of SOD was expressed as U/mg protein.
Caspase-3 activity assay
In order to find out the mechanism in which samples exert anti-apoptogenic activity, caspase-3 activity was determined based on the instructions of sigma colorimetric caspase kit. For this purpose, PC12 cells were cultured in 6-well tissue culture plates and incubated for 24 hours. Similar to intracellular ROS formation study, three different groups of cells (control, H2O2-treated, and extracts-pretreated H2O2-treated groups) were prepared and incubated at the conditions similar to the previous section. Then, the treated cells were centrifuged for 5 minutes at 1,300 rpm and were lysed in 15 μl of the cell lysis buffer. The substrate reaction buffer containing caspase-3 substrate was added to the supernatant and incubated for 2 hours at 37°C. The absorbance was then measured at 405 nm using a plate reader (BioTek, H1M).
Measurement of mitochondrial membrane potential (MMP)
PC12 cells were treated with the selected extracts in 6-well tissue culture plates for 24 hours. After that, the IC50 concentration of H2O2 was added to the cells and incubated for another 24 hours. At the end of the treatment, cells were co-incubated with rhodamine 123 (15 μl, 20 μM) for 30 minutes at 37°C. Thereafter, 1-ml Triton-X100 was used for lysing the treated cells and the amount of their fluorescence was measured at the wavelengths in range of 488–510 nm using a fluorescence microplate reader (BioTek, H1M).
Statistical analysis
In the present study, all the experiments were conducted in triplicate and reported values represented as the mean value ± SD. The one-way analysis of variance (ANOVA) using Tukey’s test was performed to compare the results. The statistical significance of variations can be confirmed at p-values less than 0.05.
RESULTS
MTT assay results
Cell viability assay was performed to determine viability of PC12 cell after exposure of different extracts obtained from A. ciniformis. Another aim of this assay was also to investigate whether pretreatment of cells with non-toxic concentrations of these extracts can reduce the toxicity of H2O2 in PC12 cells. In order to find out the non-toxic concentration of each extract, which can suppress the H2O2-induced cytotoxicity on PC12 cells, we first examined the effect of different concentrations of each extract (0–50 μg/ml) on PC12 cell survival (Fig. 1a). A glance at Figure 1 obviously indicates that none of the A. ciniformis extracts possess cytotoxic activity toward these cells (viability percentage >75% even at the highest concentration). Accordingly, the best results could be assigned to the PE (25 μg/ml), EA (25 and 50 μg/ml), and DCM (25 μg/ml) extracts of A. ciniformis. Then, these extracts were selected for further studies. The protective study of these extracts against H2O2-induced cytoxicity was also performed. The concentration of H2O2 was chosen based on a preliminary MTT assay and the IC50 concentration (62.5 μM) was chosen for remained analyses (Fig. 1b).
.png) | Figure 1. The cytotoxic activity of different extracts obtained from (a) A. ciniformis and (b) H2O2 toward PC12 cells. [Click here to view] |
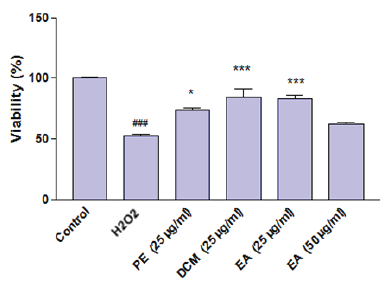 | Figure 2. The PC12 cell viability percentage after exposure of H2O2 in presence and absence of selected extracts of A. ciniformis. [Click here to view] |
A comparison between the results of cell viability of H2O2-treated cells and those pretreated with selected A. ciniformis extracts (Fig. 2) exhibited that all of these extracts (except EA 50 μg/ml) attenuated the cytotoxicity induced by H2O2 on PC12 cells and significantly protected these cells against H2O2-induced cellular death. Although EA extract (50 μg/ml) increased the cell survival, its cytoprotective effect was not-significant (p > 0.05). The most protective samples were found to be DCM (25 μg/ml) and EA (25 μg/ml) extracts which caused more than 30% increase in the percentage of viable cells.
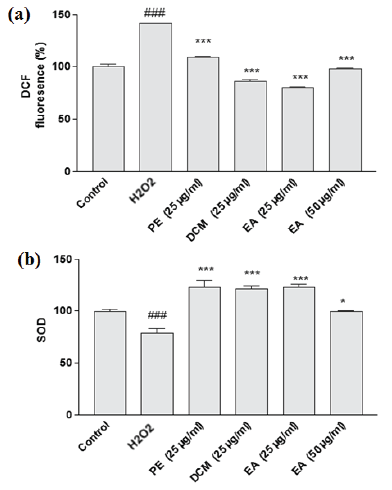 | Figure 3. The effect of selected extracts of A. ciniformis on the (a) H2O2-induced overproduction of ROS and (b) SOD enzymatic activity in PC12 cells. [Click here to view] |
Effect of selected extracts on the H2O2-induced intracellular ROS production
The intracellular ROS accumulation was also assessed for H2O2-treated PC12 cells and compared to the corresponding cells pretreated with different A. ciniformis extracts. As expected, in the presence of H2O2 the level of intracellular ROS prominently increased (more than 40%), indicating the significant induction of free radicals by H2O2 in cells (Fig. 3a). In all the cell groups co-incubated with A. ciniformis extracts, overproduction of ROS was diminished. However, the highest scavenging activity could be assigned to EA (25 μg/ml) extract (more than 70% decrease) following by DCM (25 μg/ml) and EA extract (50 μg/ml). These three extracts not only attenuated the oxidative stress caused by H2O2, but also reduced the level of free radicals in comparison with control group, indicating their protective potential toward H2O2-induced oxidation.
.png) | Figure 4. The effect of H2O2 and selected extracts of A. ciniformis on (a) caspase-3 activity and (b) H2O2-induced mitochondrial membrane potential collapse in PC12 cells. [Click here to view] |
Effect of selected extracts on SOD enzymatic activity
Endogenous antioxidant enzymes, such as SOD, can promisingly eliminate or decrease the quantity of free radicals and attenuate oxidative stress. Oxidants and toxic metabolites, such as H2O2, are believed to cause a significant decline in expression/activity of the endogenous antioxidant enzymes like SOD. In order to examine whether co-culture of selected extracts can exert noticeable effect on the deficiency of SOD expression/activity, induced by H2O2, the SOD activity of co-cultured cells and corresponding H2O2-treated cells were compared (Fig. 3b). As expected, H2O2 significantly reduced the SOD enzymatic activity of PC12 cells. It is obvious from the first glance that this effect has been inhibited in the cells co-cultured with different A. ciniformis extracts. All the three extracts (dose 25 μg/ml) markedly increased the SOD activity in comparison with control cells.
Effect of selected extracts on the caspase-3 activity
As a biomarker of mitochondrial-dependent apoptotic pathway, caspase-3 activity has been determined to examine whether these extracts can inhibit or reduce apoptosis induced by oxidative stress in PC12 cells (Fig. 4a). The noticeable increase (about 140%) in capsase-3 activity of H2O2-treated cells is not uncommon or surprising because this oxidant causes cellular death via apoptosis by induction of oxidative stress in these cells. Upon addition of selected extracts to the cells, the apoptogenic activity of H2O2 was attenuated. All of them successfully reduced the caspase-3 activity. However, DCM and EA extracts were the most active ones and more than 180% reduction in caspase-3 activity (in comparison with H2O2-treated cells) was attributed to these extracts.
Effect of selected extracts of A. ciniformis on the H2O2-induced dissipation of MMP
It was expected that A. ciniformis extracts would potentially inhibit the depolarization of mitochondria induced by H2O2. To address this assumption, we further assessed the role of selected extracts on MMP level of H2O2-treated PC12 cells, by determining the fluorescence intensity of rhodamine 123. Upon addition of H2O2 a rapid reduction, about 23%, in the fluorescence intensity of rhodamine 123 was observed. Pretreating the cells with EA extract (25 and 50 μg/ml) could significantly stabilize MMP (27% and 21% increase in MMP, respectively); however, PE and DCM extracts markedly potentiated the dissipation of MMP caused by H2O2 (Fig. 4b).
Cytoprotective effect of fractions of EA extract
First, the cytotoxic effect of fractions was evaluated in PC-12 cells by MTT method. The MTT results of these fractions are demonstrated in Figure 5a. According to the results, none of them were able to decrease PC12 viability compared to control. When the protective effect of fractions was assayed, it was observed that F3 is strongly able to protect PC12 cells against cytotoxicity induced by H2O2. Pretreatment of cells with the selected fraction (F3) at concentration of 25 μg/ml significantly suppressed the cytotoxicity of H2O2, and about 30% increase was observed in the number of viable cells (Fig. 5b). Further investigation showed that overproduction of ROS, induced by H2O2, was successfully attenuated by F3 (12.5 and 25 μg/ml). As per the illustration (Fig. 5c), ROS plays a prominent role in H2O2-induced cellular death and F3 at both concentrations significantly overcame this alteration. Almost 85% and 170% decrease in ROS level was the result of pretreating PC12 cells with F3 (12.5 and 25 μg/ml, respectively).
Total phenolic and flavonoid contents of fractions of EA extract
TPC (mg of GAE/g of each fraction) varied from 4.36 ± 1.15 (F6) to 263.08 ± 1.23 (F2) (Table 1) using the standard curve of gallic acid (R2 =0.999, y = 0.016x + 0.074). Regression equation of the calibration curve of quercetin (R2 =0.999, y = 0.030x − 0.011) was used to calculate the TFC of fractions (mg of QE/g of each fraction). Large variations in TFC of the samples were found, ranging from 2.82 ± 0.26 (F6) to 129.64 ± 3.57 (F2) (Table 1).
DISCUSSION
Nervous system is believed to be particularly vulnerable to oxidative stress and based on its high content of poly unsaturated fatty acids; one of the most important reasons for nervous cellular damage is the over generation of oxygen species (Gitto et al., 2002; Xu et al., 2012). Oxidative stress, caused by ROS such as hydrogen peroxide, can be taken into account as one of the vital factors involved in the development of several neurodegenerative diseases by disrupting the cellular function and eventually mediating cellular death (Farzaei et al., 2016). The overproduction of ROS negatively modifies the organelles, such as mitochondria, and causes damaging their function (Avery, 2011). In this study, significant cellular death (almost 55% of cells) was detected during the oxidative stress, upon exposure of PC12 cells to H2O2. As per the fact, this oxidant mostly induces ROS generation in PC12 cells, the critical is attenuating the overproduction of ROS to overcome the apoptosis and cellular death caused by H2O2. Artemisia ciniformis was shown in the present study to be a promising natural source of phytochemicals, effective in inhibition of H2O2-mediated cellular death.
 | Figure 5. The cytotoxic effect of different fractions isolated from (a) EA extract of A. ciniformis against PC12 cells and (b) the role of selected fraction on H2O2-induced toxicity, and (c) inhibitory effect of selected fraction on H2O2-induced generation of ROS in PC12 cells. [Click here to view] |
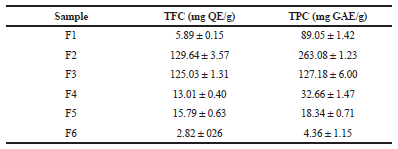 | Table 1. Total phenolic and flavonoid contents of the fractions from EA extract of A. ciniformis. [Click here to view] |
Different extracts of A. ciniformis (EA, DCM, and PE extracts) inhibited the cytotoxic effect of H2O2 toward PC12 cells. The cell viability significantly increased in the group of cells pretreated with these extracts. The H2O2-induced apoptotic rate of these cells was also found to be affected by these extracts. The ROS-scavenging activity and the inhibitory effect on the H2O2-induced reduction of SOD activity, the substantial role in suppression of caspase-3 activity, and promising effect in maintaining MMP were all exerted by these three extracts. EA extract was the most promising one, demonstrating the highest antioxidant activity against oxidative injury induced by H2O2.
The potent radical scavenging activity of this extract in comparison with two other extracts (DCM and PE) could be probably due to the higher content of phenolic compounds (Mojarrab et al., 2016). In another study, the most potent extract of Artemisia turanica against H2O2-induced cytotoxicity was also the EA extract (Hosseinzadeh et al., 2018). The common belief is that antioxidant activity of medicinal plants is in direct correlation with their TPC, which has been examined and corroborated in several studies (Hatami et al., 2014; Ishige et al., 2001; Jing et al., 2015; Kim, 2010; Tian et al., 2017). In our previous study, we have examined the radical scavenging and ferrous iron chelating activity of different extracts obtained from A. ciniformis and amount of natural phenolic compounds have been estimated as well. Among the three extracts examined in this study, EA extract possessed the highest TPC (55.94 ± 0.59 mg GAE/g), followed by DCM (15.80 ± 0.48 mg GAE /g), and PE (1.98 ± 0.22 mg GAE/g) extracts, respectively (Mojarrab et al., 2016). This order was consistent with the order of cytoprotective efficacy of extracts in the present study. It is worth noting that EA extract showed the highest ferrous ion chelating ability among the samples in previous study (Mojarrab et al., 2016).
Beside the powerful antioxidant activity of EA extract, the considerable anti-apoptogenic effect was another reason making it promising for neuroprotective applications. SOD enzymatic activity is directly correlated with antioxidant and anti-apoptogenic effects. The higher endogenous SOD activity was shown to be corresponded with the higher anti-apoptotic ability (Avery, 2011). Our results suggested the prominent role of EA extract on elevation of SOD activity and decrease of ROS level, implying that this extract protects PC12 cells from apoptosis. Among the studied extracts, this extract was the only one preventing the depolarization of mitochondria induced by H2O2. The mitochondria possess a prominent role in activating apoptosis in mammalian cells. The rapid collapse of MMP, as a result of oxidative stress, was remarkably prevented by EA extract. The MMP results in association with the results of caspase-3 activity identified that EA extract inhibited the apoptosis of PC12 cells via a mitochondria-dependent pathway and intrinsic mitochondrial pathway can be the possible mechanism for inhibition of apoptosis. Apart from these findings, different fractions (F1–F6) were obtained from EA extract of A. ciniformis, while they all were safe toward PC12 cells. Fraction F3, which was estimated to be rich in flavonoids showed promising neuroprotective potential against oxidative injury caused by H2O2. When the protective effect of F3 in PC12 cells was examined, it was observed that pretreating the cells with this fraction considerably prevented the cellular death caused by H2O2. An immense reduction in H2O2-induced overproduction of intracellular ROS indicated the promising antioxidant effect of F3 against oxidative injury in PC12 cells.
Flavonoids can protect neuronal cells from oxidative stress (Ishige et al., 2001), as they have been isolated and identified as the major components of EA fraction with neuroprotective potential from different plant species such as Orostachys japonicus (Park et al., 2017), Hypericum afrum, Cytisus villosus (Larit et al., 2018), as well as Diospyros kaki (Jeong et al., 2018). In the current study, the highest ratio of flavonoid to non-flavonoid phenolic compounds was calculated for F3; but, based on the lack of complete phytochemical analyses on the isolated fraction; we cannot certainly discuss which bioactive components are responsible for the powerful protective effect of F3. It should be noted that although the antioxidant activity of medicinal plants is mostly attributed to their polyphenol constituents, there are several studies demonstrating the powerful antioxidative and neuroprotective activities of non-phenolic compounds, such as α-pinene and 1,8-cineole (Porres-Martínez et al., 2015), DL-3-n-butylphthalide (Li et al., 2009), panaxydol (Zhu et al., 2008) , tetrahydropalmatine (Yu et al., 2010), tanshinone IIA (Jia et al., 2012), and catalpol (Wang et al., 2008). Thus, the promising neuroprotective activity of EA extract of A. ciniformis cannot be certainly assigned to its phenolic constituents, and it still remains a highly possible hypothesis. The antioxidant activity-guided phytochemical investigation of A. ciniformis, based on the results of cell-free assays (Mojarrab et al., 2016) has led to isolation and identification of the known isomers of dicaffeoylquinic acid (DCQ), including 3,5-DCQ (isochlorogenic acid A), 3,4-DCQ (isochlorogenic acid B), and 4,5-DCQ (isochlorogenic acid C) (Nasseri et al., 2019), which is consistent with the previous reports on the presence of caffeoylquinic acids as the major antioxidant and neuroprotective components in the genus Artemisia (Lee et al., 2011; Kang et al., 2016; Zhang et al., 2016) Our further studies would be beneficial to elucidate the profile of active constituents of the selected fraction of EA extract of A. ciniformis to provide a definite conclusion and elucidate the neuroprotective mechanisms, unequivocally. Taken together, the results of the present study indicated the potency of different extracts of A. ciniformis as neuroprotective agents. In agreement with these results, similar studies have shown the neuroprotective potential of the extracts and phytochemicals obtained from other Artemisia species, such as A. asiatica (Kim et al., 2000), A. turanica and A. turcomanica (Hosseinzadeh et al., 2018), A. princeps (Lee et al., 2011), and A. absinthium L. (Hallal and Kharoubi, 2016), which considerably inhibited the intracellular ROS production and exerted antiapoptotic activity. These results altogether demonstrates the neuroprotective efficacy of A. ciniformis and introduces this plant species as a useful candidate to be further examined against neurodegenerative diseases.
ACKNOWLEDGMENT
The authors would like to thank the Research Council of Kermanshah University of Medical Sciences for the financial support (grant no.: 94383).
FINANCIAL SUPPORT
None.
CONFLICT OF INTEREST
The authors declared that they have no conflict of interest for this study.
REFERENCES
Albarracin SL, Stab B, Casas Z, Sutachan JJ, Samudio I, Gonzalez J, Gonzalo L, Capani F, Morales L, Barreto GE. Effects of natural antioxidants in neurodegenerative disease. Nutrl Neurosci, 2012; 15(1):1–9. CrossRef
Avery SV. Molecular targets of oxidative stress. Biochem J, 2011; 434(2):201–10. CrossRef
Bai XC, Lu D, Liu AL, Zhang ZM, Li XM, Zou ZP, Zeng WS, Cheng BL, Luo SQ. Reactive oxygen species stimulates receptor activator of NF-κB ligand expression in osteoblast. J Biol Chem, 2005; 280(17):17497–506. CrossRef
Bora KS, Sharma A. Neuroprotective effect of Artemisia absinthium L. on focal ischemia and reperfusion-induced cerebral injury. J Ethnopharmacol, 2010; 129(3):403–9. CrossRef
Bora KS, Sharma A. The genus Artemisia: a comprehensive review. Pharm Biol, 2011; 49(1):101–9. CrossRef
Chen H, Yoshioka H, Kim GS, Jung JE, Okami N, Sakata H, Maier CM, Narasimhan P, Goeders CE, Chan PH. Oxidative stress in ischemic brain damage: mechanisms of cell death and potential molecular targets for neuroprotection. Antioxid Redox Signal, 2011; 14(8):1505–17. CrossRef
Emami SA, Rabe SZT, Ahi A, Mahmoudi M. Inhibitory activity of eleven Artemisia species from Iran against Leishmania major parasites. Iran J Basic Med Sci, 2012; 15(2):807.
Farzaei MH, Bahramsoltani R, Rahimi R, Abbasabadi F, Abdollahi M. A systematic review of plant-derived natural compounds for anxiety disorders. Cur Topic Med Chem, 2016; 16(17):1924–42. CrossRef
Firouzni A, Vahedi H, Sabbaghi F, Bigdeli M. Composition of the essential oil of Artemisia ciniformis, A. kopetdaghensis, and A. khorasanica in Iran. Chem Nat Comp, 2008; 44(6):804–6. CrossRef
Ghorbani A. Studies on pharmaceutical ethnobotany in the region of Turkmen Sahra, north of Iran: (Part 1): general results. J Ethnopharmacol, 2005; 102(1):58–68. CrossRef
Gitto E, Reiter RJ, Karbownik M, Tan DX, Gitto P, Barberi S, Barberi I. Causes of oxidative stress in the pre-and perinatal period. Biol Neonate, 2002; 81(3):146–57. CrossRef
Gorji A, Ghadiri MK. History of headache in medieval Persian medicine. Lancet Neurol, 2002; 1(8):510–5. CrossRef
Hallal N, Kharoubi O. Evaluation of oxidative stress and neuroinflammation after mercuric-chloride and Artemisia absinthium L. administration. Toxicol Lett, 2016; 258:S246. CrossRef
Hatami T, Emami SA, Miraghaee SS, Mojarrab M. Total phenolic contents and antioxidant activities of different extracts and fractions from the aerial parts of Artemisia biennis Willd. IJPR, 2014; 13(2):551.
Hosseinzadeh L, Malekshahi A, Ahmadi F, Emami SA, Hajialyani M, Mojarrab M. The protective effect of different extracts of three Artemisia species against H(2)O(2)-induced oxidative stress and apoptosis in PC12 neuronal cells. Pharmacog Res, 2018; 10(1):64–71.
Iranshahi M, Emami SA, Mahmoud-Soltani M. Detection of sesquiterpene lactones in ten Artemisia species population of Khorasan provinces. Iran J Basic Med Sci, 2007; 10(3):183–8.
Ishige K, Schubert D, Sagara Y. Flavonoids protect neuronal cells from oxidative stress by three distinct mechanisms. Free Rad Biol Med, 2001; 30(4):433–46. CrossRef
Jia LQ, Yang GL, Ren L, Chen WN, Feng JY, Cao Y, Zhang L, Li XT, Lei P. Tanshinone IIA reduces apoptosis induced by hydrogen peroxide in the human endothelium-derived EA. hy926 cells. J Ethnopharmacol, 2012; 143(1):100–8. CrossRef
Jeong DW, Cho CH, Lee JS, Lee SH, Kim T, Kim DO. Deastringent peel extracts of Persimmon (Diospyros kaki Thunb. cv. Cheongdo-Bansi) protect neuronal PC-12 and SH-SY5Y cells against oxidative stress. J Microbiol Biotechnol, 2018; 28(7):1094–104.
Jing L, Ma H, Fan P, Gao R, Jia Z. Antioxidant potential, total phenolic and total flavonoid contents of Rhododendron anthopogonoides and its protective effect on hypoxia-induced injury in PC12 cells. BMC Complement Alternat Med, 2015; 15:287. CrossRef
Kang JY, Park SK, Guo TJ, Ha JS, Lee DS, Kim JM, Lee U, Kim DO, Heo HJ. Reversal of trimethyltin-induced learning and memory deficits by 3, 5-dicaffeoylquinic acid. Oxid Med Cell Longev, 2016; 2016:13. CrossRef
Kim YC. Neuroprotective phenolics in medicinal plants. Arch Pharmacol Res, 2010; 33(10):1611–32. CrossRef
Kim H-KK, Shinate D-H. Inhibitory effect of Artemisia asiatica alkaloids on acetylcholinesterase activity from rat PC12 cells. Mol Cells, 2000; 10(3):253–62.
Kordali S, Cakir A, Mavi A, Kilic H, Yildirim A. Screening of chemical composition and antifungal and antioxidant activities of the essential oils from three Turkish Artemisia species. J Agric Food Chem, 2005; 53(5):1408–16. CrossRef
Larit F, Elokely KM, Chaurasiya ND, Benyahia S, Nael MA, León F, Abu-Darwish MS, Efferth T, Wang YH, Belouahem-Abed D, Benayache S, Tekwani BL, Cutler SJ. Inhibition of human monoamine oxidase A and B by flavonoids isolated from two Algerian medicinal plants. Phytomedicine, 2018; 40:27–36. CrossRef
Lachenmeier DW. Wormwood (Artemisia absinthium L.)—a curious plant with both neurotoxic and neuroprotective properties? J Ethnopharmacol, 2010; 131(1):224–7. CrossRef
Lee SG, Lee H, Nam TG, Eom SH, Heo HJ, Lee CY, Kim DO. Neuroprotective effect of caffeoylquinic acids from Artemisia princeps Pampanini against oxidative stress-induced toxicity in PC-12 cells. J Food Sci, 2011; 76(2):C250–6. CrossRef
Li L, Zhang B, Tao Y, Wang Y, Wei H, Zhao J, Huang R, Pei Z. DL-3-n-butylphthalide protects endothelial cells against oxidative/nitrosative stress, mitochondrial damage and subsequent cell death after oxygen glucose deprivation in vitro. Brain Res, 2009; 1290:91–101. CrossRef
Meda A, Lamien CE, Romito M, Millogo J, Nacoulma OG. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem, 2005; 91(3):571–7. CrossRef
Mojarrab M, Jamshidi M, Ahmadi F, Alizadeh E, Hosseinzadeh L. Extracts of Artemisia ciniformis protect cytotoxicity induced by hydrogen peroxide in H9c2 cardiac muscle cells through the inhibition of reactive oxygen species. Adv Pharmacol Sci, 2013; 2013:141683. CrossRef
Mojarrab M, Naderi R, Afshar FH. Screening of different extracts from Artemisia species for their potential antimalarial activity. Iran J Pharm Res, 2015; 14(2):603.
Mojarrab M, Nasseri S, Hosseinzadeh L, Farahani F. Evaluation of antioxidant and cytoprotective activities of Artemisia ciniformis extracts on PC12 cells. Iran J Basic Med Sci, 2016; 19(4):430.
Mozaffarian V. A dictionary of Iranian plant names: Latin, English, Persian. Farhang Mo'aser, Tehran, Iran, 1996.
Mucciarelli M, Maffei M. Introduction to the genus. Taylor and Francis, London, UK, pp 1–50, 2002.
Nasseri S, Emami SA, Mojarrab M. Dicaffeoylquinic acids from the aerial parts of Artemisia ciniformis Krasch. & Popov ex Poljakov. Pharm Sci, 2019, in press.
Naghavi MR, Alaeimoghadam F, Ghafoori H. Artemisia species from Iran as valuable resources for medicinal uses. World Acad Sci Eng Technol Int J Biol Biomol Agric Food Biotechnol Eng, 2014; 8(11):1194–200.
Nobili S, Lippi D, Witort E, Donnini M, Bausi L, Mini E, Capaccioli S. Natural compounds for cancer treatment and prevention. Pharmacolo Res, 2009; 59(6):365–78. CrossRef
Park SB, Lee DS, Kang JY, Kim JM, Park SK, Kang JE, Kwon BS, Park SH, Lee CJ, Lee U, Heo HJ. Protective effect on neuronal cells of Orostachys japonicus A. Berger extract against reactive oxygen species-induced neuronal cytotoxicity and active compounds. Korean J Food Sci Technol, 2017; 49(5):524–31.
Porres-Martínez M, González-Burgos E, Carretero M, Gómez-Serranillos M. Major selected monoterpenes α-pinene and 1, 8-cineole found in Salvia lavandulifolia (Spanish sage) essential oil as regulators of cellular redox balance. Pharmaceut Biol, 2015; 53(6):921–9. CrossRef
Rustaiyan A, Masoudi S, Kazemi M. Volatile oils constituents from different parts of Artemisia ciniformis Krasch. et M. Pop. ex Poljak and Artemisia incana (L.) Druce. from Iran. J Essent Oil Res, 2007; 19(6):548–51. CrossRef
Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In: Methods in enzymology, Vol. 299, Academic Press, Cambridge, MA, pp 152–178, 1999. CrossRef
Taghizadeh Rabe SZ, Mahmoudi M, Ahi A, Emami SA. Antiproliferative effects of extracts from Iranian Artemisia species on cancer cell lines. Pharm Biol, 2011; 49(9):962–9. CrossRef
Taherkhani M, Rustaiyan A, Taherkhani T. Composition of the leaf essential oils of Artemisia ciniformis Krasch. et M. Pop. ex Poljak, Artemisia oliveriana J. Gay ex Bess. in DC. and Artemisia turanica Krasch., three Asteraceae herbs growing wild in Iran. J Essent Oil Bear Plant, 2012; 15(6):1006–12. CrossRef
Taherkhani M. Chemical investigation and protective effects of bioactive phytochemicals from Artemisia ciniformis. IJCCE, 2016; 35(2):15–26.
Tayarani-Najaran Z, Hajian Z, Mojarrab M, Emami SA. Cytotoxic and apoptotic effects of extracts of Artemisia ciniformis Krasch. & Popov ex Poljakov on K562 and HL-60 cell lines. Asian Pac J Cancer Prev, 2014; 15(17):7055–9. CrossRef
Tayarani-Najaran Z, Makki FS, Alamolhodaei NS, Mojarrab M, Emami SA. Cytotoxic and apoptotic effects of different extracts of Artemisia biennis Willd. on K562 and HL-60 cell lines. Iran J Basic Med Sci, 2017; 20(2):166.
Tian Y, Liimatainen J, Alanne A-L, Lindstedt A, Liu P, Sinkkonen J, Kallio H, Yang B. Phenolic compounds extracted by acidic aqueous ethanol from berries and leaves of different berry plants. Food Chem, 2017; 220:266–81. CrossRef
Uttara B, Singh AV, Zamboni P, Mahajan R. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Cur Neuropharmacol, 2009; 7(1):65–74. CrossRef
Wang Z, An L-J, Duan Y-L, Li Y-C, Jiang B. Catalpol protects rat pheochromocytoma cells against oxygen and glucose deprivation-induced injury. Neurol Res, 2008; 30(1):106–12. CrossRef
Xu P, Xu J, Liu S, Ren G, Yang Z. In vitro toxicity of nanosized copper particles in PC12 cells induced by oxidative stress. J Nanopart Res, 2012; 14(6):906. CrossRef
Yu J, Piao BK, Pei YX, Qi X, Hua BJ. Protective effects of tetrahydropalmatine against γ-radiation induced damage to human endothelial cells. Life Sci, 2010; 87(1–2):55–63. CrossRef
Zargari A. Medicinal plants. Tehrari University Publications, ISBN 1995.
Zhang L, Tu ZC, Wang H, Wen QH, Fu ZF, Xie X. Antioxidant activity and phenolic acids profiles of Artemisia selengensis Turcz extracted with various methods by HPLC-QTOF-MS/MS. J Food Biochem, 2016; 40(4):603–12. CrossRef
Zhu H, Wang W-J, Ding W-L, Li F, He J. Effect of panaxydol on hypoxia-induced cell death and expression and secretion of neurotrophic factors (NTFs) in hypoxic primary cultured Schwann cells. Chem Biol Interact, 2008; 174(1):44–50. CrossRef