INTRODUCTION
Obesity is a disease condition associated with a significant disturbance in hormonal levels that can affect various systems leading to various diseases such as diabetes mellitus, hypertension, dyslipidemia, cardiovascular disease, lung diseases, osteoarthritis, some types of cancer, and certain reproductive and metabolic disorders (Hammoud et al., 2008). It can be caused by a combination of factors such as excessive food intake, lack of physical activity, medications, endocrine disorders, mental disorders, genes and genetic susceptibility (Guyenet and Schwartz, 2012). The prevalence of obesity has reached an alarming rate in many developing countries, including Malaysia, in which 29.1% were overweight while 14% were obese based on previous National Health and Morbidity Surveys (NHMSs) carried out in Malaysia (Chan et al., 2017; Mohamed, 2012; Nor et al., 2008). In men, the relationship between the male reproductive system and obesity is poorly understood (Fernandez et al., 2011; 2015). Some reports have shown that obesity in men is associated with a decrease in serum levels of total and free testosterone leading to a low sperm count (Du Plessis et al., 2010; Fernandez et al., 2011). On the other hand, there is a negative correlation between obesity and various semen parameters (Oliveira et al., 2017), while a recent study has suggested that there is no relationship between increased body mass index (BMI) and sperm DNA (Bandel et al., 2015). Natural products are chemical compounds or substances produced by living organisms which could be from plants, animal, microorganisms, and marine sources. For many years, natural products have been used in the prevention of diseases and have also played a very important role in health. The ancient civilizations of the North Africans, Indians, and Chinese provide written evidence for the use of natural sources for treating various diseases (Moudgil and Khalil, 2016). In those early times, mandrake was prescribed for pain relief, turmeric possessed blood clotting properties, roots of the endive plant were used for the treatment of gallbladder disorders, and raw garlic was prescribed for circulatory disorders. These natural products are still being used in several countries as alternative medicines (Arafat and Rahman, 2017). The role of these products in the treatment of obesity and fertility has received increased attention owing to the recent and rapid increase in the prevalence of obesity in the developed world (Hruby and Hu, 2015). In this review, information on obesity, natural products, pre-testicular, testicular, and post-testicular mechanisms of obesity, and male reproductive impairment were obtained through the following search databases: PubMed, Google Scholar, ScienceDirect, EBSCOhost, SCOPUS, and SpringerLink from 2000 to 2018. The keywords in single or in combination were also searched in these various databases based upon which the effects of natural products on obesity and male reproductive system were reviewed.
Classification of Obesity
Obesity can be generally classified into the following: Underweight (<18.5 kg/m2), normal (18.5–24.9 kg/m2), and overweight which is further divided into Class I Obesity (25.0–29.9 kg/m2), Class II Obesity (30.0–34.9 kg/m2), Class III obesity (35.0–39.9 kg/m2), and extreme obesity (40 kg/m2) (De Lorenzo et al., 2016). There are also several types of obesity, which include central/abdominal, android or apple peripheral/visceral, gynecoid or pear, diffuse, localized, formerly obese, childhood, morbid, and sarcopenic obesity (Mazidi and Kengne, 2017).
Effects of Obesity on Male Reproductive System and their Mechanisms
Obesity has been studied using different obesity models. These include monogenic obesity models (ob/ob mouse, obese Zucker rats, and s/s mouse), polygenic obesity models [high fat diet (HFD)-induced obese rats, diet-induced obese (DIO) rats, and New Zealand Obese (NZO) mouse], surgical models, seasonal models (Syrian and Siberian hamsters), and lipodystrophy model. Generally, obesity affects the male reproductive system at pre-testicular, testicular, and the post-testicular levels leading to impaired male reproductive and fertility potentials, which are summarized in Figure 1.
Pre-testicular Mechanisms of Obesity
Obesity has been recognized to interfere with the hypothalamic–pituitary–gonadal axis leading to secondary hypogonadism. Studies have also revealed that increased adipose tissue results in increased aromatase activity and a consequent elevation in estradiol levels, which inhibits gonadotropin follicle-stimulating hormone (FSH) and luteinizing hormone (LH) secretion from the anterior pituitary (Dimitriadis et al., 2017; Rosenblatt et al., 2017; Roth et al., 2008), Studies in experimental models in animals indicate that the most common cause of leptin insensitivity in the hypothalamus is obesity, which is responsible for the decreased KISS1 expression, and consecutively changes the release of gonadotropin-releasing hormone (GnRH) (Stefater et al., 2010). Pre-testicular mechanism involves two conditions, namely hypergonadotropic hypogonadism and hypogonadotropic hypogonadism. Hypogonadotropic hypogonadism/primary hypogonadism is caused by testicular deficit leading to reduced testosterone level and impaired spermatogenesis (Condorelli et al., 2015; Dimitriadis et al., 2017). This attenuates the attenuation of the testosterone-induced negative feedback loop to the secretory activities of the hypothalamus and pituitary, which in turn leads to increased amounts of GnRH and FSH/LH secretions. On the other hand, hypogonadotropic hypogonadism/secondary hypogonadism is caused by the deficit at the hypothalamus and/or pituitary. FSH and LH are secreted at reduced levels, which also lead to decreased stimulation of Leydig cells to secrete testosterone (Santi et al., 2017).
 | Figure 1. Summary of mechanisms of obesity on the male reproductive system [Click here to view] |
Testicular Mechanisms of Obesity
Testis is an important site of hormone production and metabolism, and accumulation of large amounts of body fat may interfere with the hormonal regulation of testicular function. Several studies on obesity suggest that high levels of plasma cholesterol and/or triglycerides have direct adverse effects on testicular function, leading to poor semen quality and infertility (Teerds et al., 2011). Kasturi et al. (2008) have reported the presence of 65% incidence of dyslipidemia as defined by isolated hypercholesterolemia, triglyceridemia, or both, in 106 male partners from infertile couples. Reactive oxygen species (ROS) resulting in lipid peroxidation, are extremely toxic to human spermatozoa, implicating a significant role of oxidative stress in causing of male infertility as spermatozoa from infertile men show signs of greater oxidative injury compared with normal fertile controls (Agarwal et al., 2003). Elevated DNA fragmentation index noted in obese men may reflect an abnormal oxidative state in the testicular microenvironment (Aitken et al., 2014). Similarly, in vitro study suggests that endogenously generated ROS in the adipocytes lead to an increase in sperm DNA fragmentation. This finding also suggests that oxidative stress may result in lipid peroxidation in the sperm plasma membrane. This may, in turn, lead to decreased motility, membrane dysfunction, and excessive oxidative stress in DNA of the affected sperm (Zhou et al., 2014).
Post-Testicular Mechanisms of Obesity
Over the years obesity has been linked to post-testicular etiology that may cause male infertility by affecting the male genital system after sperm production. The affected post-testicular structures include defects of the genital tract like vas deferens obstruction, congenital absence of vas deferens, prostatitis, ejaculatory duct obstruction, retrograde ejaculation, hypospadias, and impotence. In a study carried out by Ouvrier et al. (2011), 3-month-old male mice are fed with a lipid-enriched diet containing 1.25% cholesterol for 4 weeks. The result shows complete infertility in dyslipidemic male mice (the Liver X Receptor-deficient mouse model). The infertility results from post-testicular defects affecting the fertilizing potential of spermatozoa which are less viable and motile and highly susceptible to undergo a premature acrosome reaction. It suggests that obesogens may also cause erectile dysfunction (ED), apart from inflammatory responses, androgen deficiency, and endothelial dysfunction (Petrakis et al., 2017). Researchers suggest that visceral obesity contributes to ED via three interdependent (overlapping) pathophysiological mechanisms:
- i inflammatory cytokines that contribute to endothelial dysfunction and microvascular disease and reduced androgen levels,
- ii the insult on the endothelium resulting in endothelial injury and reduced nitrogen oxide (NO) synthase activity and NO production, leading to reduced tissue relaxation and poor hemodynamics, and
- iii disruption of the endocrine milieu, with a concomitant decrease in testosterone levels and increased E2 level, thus disrupting tissue homeostasis, tissue histo-architecture, and erectile tissue compliance (Siragusa and Fleming, 2016).
Natural Products and Obesity-induced Impairment in Male Reproductive Function
Natural products are used for the treatment of various diseases including to improve male reproductive health for several decades. They are shown to be effective, inexpensive, and available. The extraction and development of several drugs and chemotherapeutics from these natural products have been widely observed. Several researchers have suggested that two-thirds of the world’s plant species have medicinal value and many of them have great antioxidant potential. These products have shown to have significant effects at the pre-testicular, testicular, and post-testicular levels which are listed in Tables 1 and 2 (whole extracts and pure compounds isolated from plants, respectively).
Natural Products with Pre-Testicular hypothalamic-pituitary gonadal axis (HPG axis) Beneficial Effects in Obesity
The administration of Argyreia nervosa Bojer (Convolvulaceae) in DIO rats increased the synthesis and release of FSH (Galani et al., 2010). On the other hand, the messenger ribonucleic acid (mRNA) levels of GnRH mRNA and LH are significantly increased in HFD mice treated with Epimedium Herb (Zhang et al., 2011). In addition, administration of Nigella sativa increases testosterone and FSH in HFD-induced obese mice (Barakat and El-Masry, 2016) (Tables 1 and 2).
Natural Products with Testicular Beneficial Effects in Obesity
There are also studies showing the beneficial effects of natural products at the testicular level. A study on the seed of Achyranthes aspera Linn. (Amaranthaceae) has shown an increase in spermatogenesis in HFD-induced obese mice (Rani et al., 2012). In another study, the leaf of Aloe vera significantly increases the number of stem cells and primary spermatocytes in HFD-induced obese rats (Misawa et al., 2012). The rhizome of Alpinia galanga Linn. also increases the number of spermatozoa HFD mice (Ongwisespaiboon and Jiraungkoorskul, 2017). The root of Angelica gigas Nakai (Apiaceae) administered in HFD-induced obese mice increased sperm count, motility, and spermatogenic cell density (Bae et al., 2016). Leaf of Danae racemose (Khojasteh et al., 2016) and seeds of a combination of Cinnamomum zeylanicumon (Barakat and El-Masry, 2016; Fathiazad et al., 2013) and Citrullus vulgaris (Watermelon) (Khaki et al., 2013) administered in HFD-induced obese mice increase sperm concentration and sperm motility, respectively. Leaf of Murraya koenigii (L.) Spreng. (Rutaceae) in HFD-induced obese mice for 2 weeks (Birari et al., 2010) and roots of Panax ginseng C. A. Mey. (Araliaceae) (Park et al., 2013) in HFD C57BL6/J mice increase testicular function. Spirulina platensis (Esener et al., 2017) in HFD-induced obese rats increases spermatogenesis and testicular structure (Thounaojam et al., 2011). The treatment with Curcumin (Meydani and Hasan, 2010) in HFD-induced obese rats for 4 weeks has increased sperm concentration, normal sperm morphology, and semen volumes while treatments of ethyl caprylate, friedelin, and kaempferol glycoside in C57BL/6J mice, HFD-induced obese rats, and HFD-induced obese mice, respectively, also show an increase in testicular functions (Duraipandiyan et al., 2016; Hong and Lee, 2009; Zhang et al., 2011). However, leaf of Hibiscus sabdariffa L. (Malvaceae) (Hoper et al., 2013), fruits of Ligustrum lucidum (Oleaceae), and fruits of Garcinia cambogia (Saito et al., 2005) administered on Zucker rats cause potent testicular atrophy and toxicity (Chen et al., 2012; Höper et al., 2013). In various studies conducted on rhizome of Zingiber Officinale (Ginger), Alpinia officinarum Hance (Zingiberaceae), Artemisia iwayomogi (Compositae), Atractylodes lancea, Ligustrum lucidum (Oleaceae), Perilla frutescens (L.) Britton (Lamiaceae), Vaccinium corymbosum L. (Ericaceae), and isolated compounds like flavonoids, anthocyanins, and quercetin in the epididymal adipose fats are decreased (Ahn et al., 2008; Chen et al., 2012; Choi et al., 2013; Jung et al., 2012; Khaki, 2015; Khaki et al., 2009; Kim and Kim, 2009; Meydani and Hasan, 2010; Patra et al., 2015; Song et al., 2013). Nutshells of Arachis hypogaea and extracts of pumpkin oil (Galaly et al., 2014), stem bark of Bombax ceiba L. (Malvaceae) (Gupta et al., 2013), leaves, stems, and flower buds of Camellia sinensis (L.) Kuntze (Theaceae) (El-Sweedy et al., 2007), leaves of Cardiospermum halicacabum (Peiris et al., 2015) and Curcuma longa (Turmeric) (El-Sweedy et al., 2007) have increased sperm count, testicular histology, and functions in HFD-induced obese rats/mice. Leaves of Sida rhombifolia L. (Malvaceae) (Thounaojam et al., 2011) and fruits of Tamarindus indica L. (Leguminosae) (Azman et al., 2012; Esener et al., 2017) have increased testicular function in HFD-induced obese mice and DIO rats, respectively. Fruits of Morinda citrifolia L. (Rubiaceae) (Saminathan et al., 2013) and leaves of Ocimum basilicum (Umar et al., 2012) have increased sperm motility, viability, sperm count, and total antioxidant capacity but decrease malondialdehyde in HFD-induced obese mice. Letrozole (Loves et al., 2008) on the other hand, normalizes serum total testosterone while resveratrol (Aguirre et al., 2014) increases sperm concentration, normal sperm morphology, and semen volumes.
 | Table 1. Summary of some selected natural products and their effects on obesity and male reproductive system. [Click here to view] |
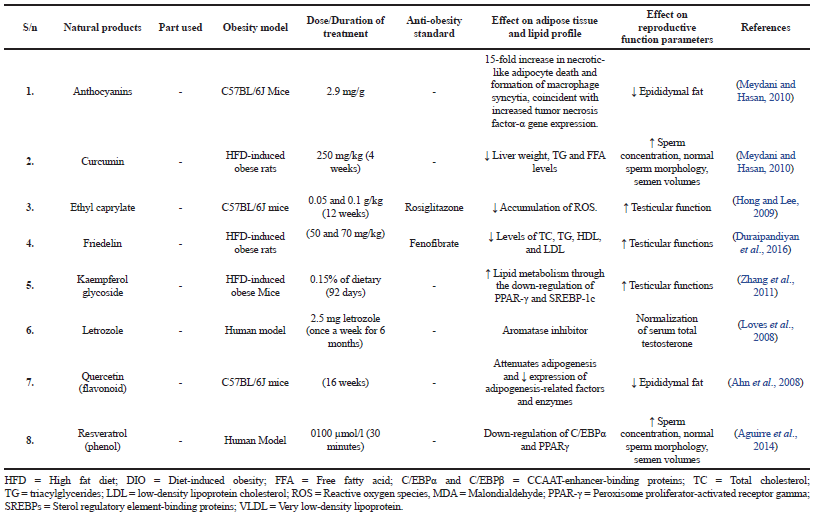 | Table 2. Summary of some selected isolated compounds from natural products and their effects on obesity and male reproductive system. [Click here to view] |
Natural Products with Post-Testicular Beneficial Effects in Obesity
Many natural products have also shown their potential beneficial effects in treating post-testicular impairment in male obesity as shown in Tables 1 and 2. A study carried out on HFD-induced obese mice for 30 days shows that Achyranthes bidentata Blume (Kamble et al., 2017) decreases spermatogenesis and inhibits testicular function without any side effects suggesting its potential contraceptive property (Rani et al., 2012). Another study carried out by Wu et al. (2009) on glucose challenged db/db mice treated with Acorus calamus Linn. (Araceae) for 3 weeks has shown an improved sexual performance, i.e., improved mount, intromission, and ejaculatory latencies, and their frequencies and inhibits prostaglandins E 5 (PDE-5) synthesis. Allium sativum Linn. administered on HFD-induced obese mice for 28 days also increases sexual behavior (Focho et al., 2009). Defo et al. (2017) have reported an improvement in sexual behavior and performance when Guibourtia tessmannii (Caesalpiniaceae) is administered in HFD-induced obese rats for 21 or 56 days. In addition, Icariside II (Epimedium) administered on Zuckers rat for 4 weeks also improves erectile function and pathologic changes through endogenous progenitor cell preservation and proliferation (Ruan et al., 2018).
Effects of Natural Products on Adipose Tissue and Lipid Profile
A large number of the natural products studied in this review demonstrated significant effects in reducing total cholesterol (TC), triacylglycerides (TG), high-density lipoprotein (HDL) (Jung et al., 2012), low-density lipoprotein (LDL) as well as fasting blood glucose (Table 1). Some pure isolated compounds also reduced the accumulation of ROS, attenuated adipogenesis (Zhang et al., 2011), and decreased expression of adipogenesis-related factors and enzymes (Table 2).
CONCLUSION AND FUTURE DIRECTION
There is enough evidence to show that male obesity has an impact on fertility through its effects on pre-testicular, testicular, and post-testicular mechanisms. Natural products, on the other hand, have been used over the years to improve obesity-induced male infertility at the aforementioned levels. This review identifies some selected natural products, with their effects and mechanisms on male reproductive functions in obesity. What does the future hold for the effect of natural products on the male reproductive system in obese men at these levels? With the exponential increase in the number of experiments in this area, it seems likely that many more will be conducted in the nearest future on natural plants, herbs, and other natural products emphasizing on new technologies which could help manage health and weight/energy balance more effectively and analyze the future impact of new technologies on lifestyle, dietary habits, thereby improving male fertility. However, the inclusion of studies on their phytochemical compounds and toxicity would further help appreciate their potentials to reduce obesity-induced impairment in the male reproductive system.
ABBREVIATIONS
aP2 activating protein 2
ABCA1 Adenosine triphosphate binding cassette transporters A1
AMPK 5’ AMP-activated protein kinase
BMI body mass index
C/EBPα and C/EBPβ CCAAT-enhancer-binding proteins
C57BL/6J C57 black 6
CD 36 cluster of differentiation 36
DIO diet-induced obese; diet-induced obesity
FAS fatty acid synthase
GnRH Gonadotropin-releasing hormone
HDL high-density lipoprotein
HFD high fat diet
HPT hypothalamic–pituitary–testicular axis
IFNα Interferon-alpha
IL-6 interleukin-6
LDL low-density lipoprotein
LH Luteinizing hormone
LXRα Liver X receptor α
MCP1 monocyte chemotactic protein-1
MDA Malondialdehyde
mRNA messenger ribonucleic acid
NHMSs National Health and Morbidity Surveys
NO nitrogen oxide
NZO New Zealand Obese mouse
ob/ob mouse obesity mouse
PPAR-γ Peroxisome proliferator-activated receptor gamma
ROS reactive oxygen species
SREBPs Sterol regulatory element-binding proteins
TAC total antioxidant activity
TBARS Thiobarbituric acid reactive substances
TC total cholesterol
TG Triacylglycerides
TNFα tissue necrotic factor
VLDL very low-density lipoprotein
ACKNOWLEDGMENTS
The authors hereby acknowledge the Ministry of Higher Education (Fundamental Research Grant Scheme: 203.PPSP.6171195), Malaysia for funding this review.
CONFLICT OF INTEREST
No conflict of interest.
FUNDING
Ministry of Higher Education, Malaysia (Fundamental Research Grant Scheme: 203.PPSP.6171195).
ETHICS APPROVAL
Not applicable.
REFERENCES
Agarwal A, Saleh RA, Bedaiwy MA. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril, 2003; 79:829–43.
Aguirre L, Fernández-Quintela A, Arias N, Portillo MP. Resveratrol: anti-obesity mechanisms of action. Molecules, 2014; 19:18632–55.
Ahn J, Lee H, Kim S, Park J, Ha T. The anti-obesity effect of quercetin is mediated by the AMPK and MAPK signaling pathways. Biochem Biophys Res Commun, 2008; 373:545–9.
Aitken RJ, Smith TB, Jobling MS, Baker MA, De Iuliis GN. Oxidative stress and male reproductive health. Asian J Androl, 2014; 16:31.
Arafat M, Rahman N. Evaluation of antioxidant, anti-microbial and cytotoxic activity of methanolic extract of phyllanthus acidus leaves. Degree thesis, East West University, Dhaka, Bangladesh, 2017.
Azman KF, Amom Z, Azlan A, Esa NM, Ali RM, Shah ZM, Kadir KKA. Antiobesity effect of Tamarindus indica L. pulp aqueous extract in high-fat diet-induced obese rats. J Nat Med, 2012; 66:333–42.
Bae WJ, Ha U, Choi JB, Kim KS, Kim SJ, Cho HJ, Hong SH, Lee JY, Wang Z, Hwang SY. Protective effect of decursin extracted from angelica gigas in male infertility via Nrf2/HO-1 signaling pathway. Oxid Med Cell Longev, 2016; 2016:1–9; doi:10.1155/2016/5901098
Bandel I, Bungum M, Richtoff J, Malm J, Axelsson J, Pedersen H, Ludwicki J, Czaja K, Hernik A, Toft G. No association between body mass index and sperm DNA integrity. Hum Reprod, 2015; 30:1704–13.
Barakat H, El-Masry S. Oils of Nigella sativa L. and Cinnamon zeylanicum inhibit the testicular cytotoxicity and genotoxicity induced by mancozeb in rats. Int J Biochem Res Rev, 2016; 14:1–11.
Birari R, Javia V, Bhutani KK. Antiobesity and lipid lowering effects of Murraya koenigii (L.) Spreng leaves extracts and mahanimbine on high fat diet induced obese rats. Fitoterapia, 2010; 81:1129–33.
Chan YY, Lim KK, Lim KH, Teh CH, Kee CC, Cheong SM, Khoo YY, Baharudin A, Ling MY, Omar MA. Physical activity and overweight/obesity among Malaysian adults: findings from the 2015 National Health and morbidity survey (NHMS). BMC Public Health, 2017; 17:733.
Chen T, Xiong S, Wang M, Wu Q, Wei H. Effects of traditional Chinese medicines on intestinal bacteria: a review. Indian J Trad Knowl, 2012; 11:401–7.
Choi Y, Yanagawa Y, Kim S, Whang WK, Park T. Artemisia iwayomogi extract attenuates high-fat diet-induced obesity by decreasing the expression of genes associated with adipogenesis in mice. Evid Based Complement Alternat Med, 2013; 2013:1–11.
Condorelli R, Calogero A, Vicari E, La Vignera S. Chronic consumption of alcohol and sperm parameters: our experience and the main evidences. Andrologia, 2015; 47:368–79.
De Lorenzo A, Soldati L, Sarlo F, Calvani M, Di Lorenzo N, Di Renzo L. New obesity classification criteria as a tool for bariatric surgery indication. World J Gastroenterol, 2016; 22:681–703.
Defo PBD, Wankeu-Nya M, Ngadjui E, Fozin GRB, Kemka FX, Kamanyi A, Kamtchouing P, Watcho P. The methanolic extract of Guibourtia tessmannii (Caesalpiniaceae) improves sexual parameters in high fat diet-induced obese sexually sluggish rats. Asian Pac J Reprod, 2017; 6:202–11.
Dimitriadis F, Adonakis G, Kaponis A, Mamoulakis C, Takenaka A, Sofikitis N. Pre-testicular, testicular, and post-testicular causes of male infertility. Endocrinol Testis Male Reprod, 2017; 1:981.
Du Plessis SS, Cabler S, McAlister DA, Sabanegh E, Agarwal A. The effect of obesity on sperm disorders and male infertility. Nat Rev Urol, 2010; 7:153–61.
Duraipandiyan V, Al-Dhabi NA, Irudayaraj SS, Sunil C. Hypolipidemic activity of friedelin isolated from Azima tetracantha in hyperlipidemic rats. Rev Brasil Farmacogn, 2016; 26:89–93.
El-Sweedy M, Abdel-Hamid N, El-Moselhy M. The role of a mixture of green tea, turmeric and chitosan in the treatment of obesity-related testicular disorders. J Appl Biomed (De Gruyter Open), 2007; 5:131–8.
Esener OBB, Gurel-Gurevin E, Isbilen-Basok B, Yigit F, Bilal T, Altiner A, Yilmazer N, Armutak EI. Spirulina platensis affects factors involved in spermatogenesis and increases ghrelin receptors in testis tissue of rats fed a high-fat diet. Pol J Vet Sci, 2017; 20:467–75.
Fathiazad F, Khaki A, Nouri M, Afshin KA. Effect of Cinnamon Zeylanicum on serum Testosterone and anti-oxidants levels in rats. Int J Women’s Health Reprod Sci, 2013; 1:29–35.
Fernandez A, Ordóñez R, Reiter RJ, González-Gallego J, Mauriz JL. Melatonin and endoplasmic reticulum stress: relation to autophagy and apoptosis. J Pineal Res, 2015; 59:292–307.
Fernandez CD, Bellentani FF, Fernandes GS, Perobelli JE, Favareto APA, Nascimento AF, Cicogna AC, Kempinas WD. Diet-induced obesity in rats leads to a decrease in sperm motility. Reprod Biol Endocrinol, 2011; 9:32.
Focho D, Ndam W, Fonge B. Medicinal plants of Aguambu-Bamumbu in the Lebialem highlands, southwest province of Cameroon. Afr J Pharm Pharmacol, 2009; 3:001–13.
Galaly SR, Hozayen WG, Amin KA, Ramadan SM. Effects of Orlistat and herbal mixture extract on brain, testes functions and oxidative stress biomarkers in a rat model of high fat diet. Beni-Suef Univ J Basic Appl Sci, 2014; 3:93–105.
Galani V, Patel B, Patel N. Argyreia speciosa (Linn. f.) sweet: a comprehensive review. Pharmacogn Rev, 2010; 4:172.
Gupta P, Goyal R, Chauhan Y, Sharma PL. Possible modulation of FAS and PTP-1B signaling in ameliorative potential of Bombax ceiba against high fat diet induced obesity. BMC Complement Altern Med, 2013; 13:281.
Guyenet SJ, Schwartz MW. Regulation of food intake, energy balance, and body fat mass: implications for the pathogenesis and treatment of obesity. J Clin Endocrinol Metab, 2012; 97:745–55.
Hammoud AO, Wilde N, Gibson M, Parks A, Carrell DT, Meikle AW. Male obesity and alteration in sperm parameters. Fertil Steril, 2008; 90:2222–5.
Hong JH, Lee IS. Effects of Artemisia capillaris ethyl acetate fraction on oxidative stress and antioxidant enzyme in high-fat diet induced obese mice. Chem Biol Interact, 2009; 179:88–93.
Höper AC, Salma W, Sollie SJ, Hafstad AD, Lund J, Khalid AM, Raa J, Aasum E, Larsen TS. Wax esters from the marine copepod calanus finmarchicus reduce diet-induced obesity and obesity-related metabolic disorders in mice–3. J Nutr, 2013; 144:164–9.
Hruby A, Hu FB. The epidemiology of obesity: a big picture. Pharmacoeconomics, 2015; 33:673–89.
Jung CH, Jang SJ, Ahn J, Gwon SY, Jeon T-I, Kim TW, Ha TY. Alpinia officinarum inhibits adipocyte differentiation and high-fat diet–induced obesity in mice through regulation of adipogenesis and lipogenesis. J Med Food, 2012; 15:959–67.
Kamble A, Reddy C, Patil S. Testicular activity of mice treated with MeOH extract of Achyranthes aspera leaves. J Adv Med Sci Appl Technol, 2017; 3:93–100.
Kasturi SS, Tannir J, Brannigan RE. The metabolic syndrome and male infertility. J Androl, 2008; 29:251–9.
Khaki A. Effect of Cinnamomum zeylanicumon on Spermatogenesis. Iran Red Cresc Med J, 2015; 17.
Khaki A, Fathiazad F, Nouri M. Effects of watermelon seed extract (citrullus vulgaris) on spermatogenesis in rat. Int J Women’s Health Reprod Sci, 2013; 1:99–104.
Khaki A, Fathiazad F, Nouri M, Afshin Khaki A, Ozanci CC, Ghafari-Novin M, Hamadeh M. The effects of Ginger on spermatogenesis and sperm parameters of rat. Int J Reprod BioMed, 2009; 7:7–12.
Khojasteh SMB, Khameneh RJ, Houresfsnd M, Yaldagard E. A review on medicinal plants used for improvement of spermatogenesis. Biol Med, 2016; 8:1.
Kim MJ, Kim HK. Perilla leaf extract ameliorates obesity and dyslipidemia induced by high-fat diet. Phytother Res, 2009; 23:1685–90.
Loves S, Ruinemans-Koerts J, de Boer H. Letrozole once a week normalizes serum testosterone in obesity-related male hypogonadism. Eur J Endocrinol, 2008; 158:741–7.
Mazidi M, Kengne AP. Nutrient patterns and their relationship with general and central obesity in US adults. Eur J Clin Invest, 2017; 2017:1–13.
Meydani M, Hasan ST. Dietary polyphenols and obesity. Nutrients, 2010; 2:737–51.
Misawa E, Tanaka M, Nabeshima K, Nomaguchi K, Yamada M, Toida T, Iwatsuki K. Administration of dried Aloe vera gel powder reduced body fat mass in diet-induced obesity (DIO) rats. J Nutr Sci Vitaminol (Tokyo), 2012; 58:195–201.
Mohamed AB. National Health and Mobidity Survey 2011 (NHMS 2011). Methodol General Find, 2012; 1:258.
Moudgil KD, Khalil AA. The 1st Euro-Mediterranean workshop: natural products in health and diseases: Cairo, Egypt, March 2, 2015. Asian J Pharm Sci, 2016; 11:292–6.
Nor M, Safiza N, Khor GL, Shahar S, Kee CC, Haniff J, Appannah G, Rasat R, Wong N, Zainuddin AA. The Third National Health and Morbidity Survey (NHMS III) 2006: nutritional status of adults aged 18 years and above. Malays J Nutr, 2008; 14:1–87.
Oliveira J, Petersen C, Mauri A, Vagnini L, Renzi A, Petersen B, Mattila M, Dieamant F, Baruffi R, Franco J. Association between body mass index and sperm quality and sperm DNA integrity. A large population study. Andrologia, 2017; 1–10; doi:10.1111/and.12889
Ongwisespaiboon O, Jiraungkoorskul W. Fingerroot, Boesenbergia rotunda and its aphrodisiac activity. Pharmacogn Rev, 2017; 11:27.
Ouvrier A, Alves G, Damon-Soubeyrand C, Marceau G, Cadet R, Janny L, Brugnon F, Kocer A, Pommier A, Lobaccaro J-MA. Dietary cholesterol-induced post-testicular infertility. PLoS One, 2011; 6:e26966.
Park J, Jeon Y-D, Kim H-L, Lim H, Jung Y, Youn D-H, Jeong M-Y, Kim H-J, Kim S-H, Kim S-J. Interaction of veratrum nigrum with panax ginseng against obesity: a Sang-ban relationship. Evid Based Complement Alternat Med, 2013; 2013:1–13.
Patra S, Nithya S, Srinithya B, Meenakshi S. Review of medicinal plants for anti-obesity activity. Transl Biomed, 2015; 6.
Peiris LD, Dhanushka M, Jayathilake T. Evaluation of aqueous leaf extract of Cardiospermum halicacabum (L.) on fertility of male rats. BioMed Res Int, 2015; 2015:1–6; doi:10.1155/2015/175726.
Petrakis D, Vassilopoulou L, Mamoulakis C, Psycharakis C, Anifantaki A, Sifakis S, Docea AO, Tsiaoussis J, Makrigiannakis A, Tsatsakis AM. Endocrine disruptors leading to obesity and related diseases. Int J Environ Res Public Health, 2017; 14:1282.
Rani N, Sharma SK, Vasudeva N. Assessment of antiobesity potential of Achyranthes aspera Linn. seed. Evid Based Complement Alternat Med, 2012; 2012:1–7; doi:10.1155/2012/715912.
Rosenblatt A, Faintuch J, Cecconello I. Androgen and estrogen shifts in men before and after bariatric surgery and links to vitamins and trace elements. Int J Vitam Nutr Res, 2017; 1:1–17.
Roth MY, Amory JK, Page ST. Treatment of male infertility secondary to morbid obesity. Nat Rev Endocrinol, 2008; 4:415.
Ruan Y, Lin G, Kang N, Tamaddon A, Zhou J, Wang B, Wang HS, Wang G, Banie L, Xin Z. In situ activation and preservation of penile progenitor cells using Icariside II in an obesity-associated erectile dysfunction rat model. Stem Cells Develop, 2018; 27:207–15.
Saito M, Ueno M, Ogino S, Kubo K, Nagata J, Takeuchi M. High dose of Garcinia cambogia is effective in suppressing fat accumulation in developing male Zucker obese rats, but highly toxic to the testis. Food Chem Toxicol, 2005; 43:411–9.
Saminathan M, Rai RB, Dhama K, Tiwari R, Chakraborty S, Amarpal, Ranganath GJ, Kannan K. Systematic review on anticancer potential and other health beneficial pharmacological activities of novel medicinal plant Morinda citrifolia (Noni). Int J Pharmacol, 2013; 9:462–92.
Santi D, Spaggiari G, Casarini L, Fanelli F, Mezzullo M, Pagotto U, Granata ARM, Carani C, Simoni M. Central hypogonadism due to a giant,“silent” FSH-secreting, atypical pituitary adenoma: effects of adenoma dissection and short-term Leydig cell stimulation by luteinizing hormone (LH) and human chorionic gonadotropin (hCG). Aging Male, 2017; 20:96–101.
Siragusa M, Fleming I. The eNOS signalosome and its link to endothelial dysfunction. Pflügers Arch-Eur J Physiol, 2016; 468:1125–37.
Song Y, Park HJ, Kang SN, Jang S-H, Lee S-J, Ko Y-G, Kim G-S, Cho J-H. Blueberry peel extracts inhibit adipogenesis in 3T3-L1 cells and reduce high-fat diet-induced obesity. PLoS One, 2013; 8:e69925.
Stefater MA, Pérez-Tilve D, Chambers AP, Wilson-Pérez HE, Sandoval DA, Berger J, Toure M, Tschöp M, Woods SC, Seeley RJ. Sleeve gastrectomy induces loss of weight and fat mass in obese rats, but does not affect leptin sensitivity. Gastroenterology, 2010; 138:2426–36. 2436.e1–3.
Teerds K, De Rooij D, Keijer J. Functional relationship between obesity and male reproduction: from humans to animal models. Hum Reprod Update, 2011; 17:667–83.
Thounaojam MC, Jadeja RN, Ramani UV, Devkar RV, Ramachandran A. Sida rhomboidea. Roxb leaf extract down-regulates expression of PPARγ2 and leptin genes in high fat diet fed C57BL/6J mice and retards in vitro 3T3L1 pre-adipocyte differentiation. Int J Mol Sci, 2011; 12:4661–77.
Umar I, Mohammed A, Dawud F, Kabir A, Sai J, Muhammad F, Okalor M. The hypolipidemic and antioxidant actions of aqueous extracts of Ocimum basilicum and Ocimum suave in high fat fed Rats. J Med Plants Res, 2012; 6:3501–5.
Wu H-S, Zhu D-F, Zhou C-X, Feng C-R, Lou Y-J, Yang B, He Q-J. Insulin sensitizing activity of ethyl acetate fraction of Acorus calamus L. in vitro and in vivo. J Ethnopharmacol, 2009; 123:288–92.
Zhang D, Li K, Zhou M. Epimedium herb extract intervening in hypothalamic-pituitary-testicular axis of male rats in delayed puberty caused by diet-induced obesity. J Med Plants Res, 2011; 5:364–70.
Zhou Q, Guan W, Qiao H, Cheng Y, Li Z, Zhai X, Zhou Y. GATA binding protein 2 mediates leptin inhibition of PPARγ1 expression in hepatic stellate cells and contributes to hepatic stellate cell activation. Biochim Biophys Acta, 2014; 1842:2367–77.