INTRODUCTION
Epithelial ovarian cancer (EOC) is a type of ovarian cancer that has more common prevalent rate when compared with non-EOC; it is about 90% of ovarian cancer cases (Berek and Hacker, 2005). EOC is the sixth most common cancer that causes death among women due to its gynecologic malignancies (McGuire et al., 2000). EOC in Indonesia is placed in the third rank among the other most prevalent gynecologic cancers; the first and second ranks are breast cancer and cervical cancer (Charles et al., 2016).
Treatment of EOC consists of the combination of cytoreductive surgery, combination of taxane chemotherapy, and platinum-based chemotherapeutic drugs (Kim et al., 2012). Paclitaxel belongs to taxane group that is derived from the bark of the Pacific yew tree, Taxus brevifolia Nutt (Kampan et al., 2015). Paclitaxel combined with cisplatin (platinum-containing agents) is now used as the first-line treatment for ovarian cancer. Paclitaxel-cisplatin chemotherapy is shown to improve therapeutic response rate, including complete or partial clinical response with the percentage of 38.7% and 42.7%, respectively (Bois et al., 2003). In Sanglah General Hospital (Bali, Indonesia), paclitaxel-cisplatin combined chemotherapy is a therapy used in the treatment of EOC patients (Medical Committe, 2004).
Paclitaxel-cisplatin shows efficacy in eradicating ovarian cancer cells; however, these chemotherapeutic drugs also cause side effects. Combination of paclitaxel-cisplatin causes myelosuppression through their mechanism of action in bone marrow cells (Kumar et al., 2010; Wilson et al., 2007). Myelosuppression leads to decreased hemoglobin, platelet level, and leukocyte level, so patients experience anemia, thrombocytopenia, and leukopenia (Bois et al., 2003). There have been various studies evaluating myelosuppression effects of paclitaxel-cisplatin chemotherapy on ovarian cancer patients carried out outside Bali-Indonesia. But until now, Bali has a lack of data about the side effect of paclitaxel-cisplatin chemotherapy on the hematologic status of ovarian cancer patients, especially on EOC patients. This study aims to evaluate the side effect of paclitaxel-cisplatin chemotherapy in the hematologic status of EOC patients at Sanglah General Hospital, Denpasar-Bali.
MATERIALS AND METHODS
Materials
The materials include collected data sheets of hemoglobin, platelet, leukocyte, and medical records of EOC patients who received paclitaxel-cisplatin chemotherapy from January 2015 to May 2018.
Methods
This study is observational retrospective research that has obtained a certificate of ethical clearance (Number: 159/UN.14.2/KEP/2018) and research permit (Number: LB.02.01/XIV.2.2.1/9396/2017) from Ethics Commission Research and Development of Faculty of Medicine, Udayana University/General Hospital Sanglah Denpasar. The study was conducted by collecting medical records of patients from January 2015 to May 2018. All subjects with EOC (stages I–IV) were recruited for this study. Patients were selected based on the inclusion criterion of patients with EOC at Sanglah Hospital who received six cycles of chemotherapy with paclitaxel-cisplatin from January 2015 to May 2018. The exclusion criterion was patients with EOC who have a history of bone marrow disorder. This study was conducted by recording hemoglobin, platelets, and leukocytes levels data from EOC patients that fulfilled inclusion criteria. Data were taken before the first and after the sixth chemotherapy. The data were analyzed using paired T-test. All data processing was conducted using STATA version 14 software. The value of hemoglobin, platelet, and leukocyte before and after paclitacxel-cisplatin chemotherapy was stated to have significant differences when it showed p < 0.05.
RESULTS AND DISCUSSION
Ovarian cancer stage classification (Table 1) showed two patients with phase III ovarian cancer (66.67%) and one patient with phase I ovarian cancer (33.33%). Ovarian cancer can hardly be detected at an early stage because of its unspecific symptoms, so 70% of its cases are diagnosed during advanced stages (Yurkovetsky et al., 2010). Survival of ovarian cancer patients depends on the stage of diagnosis; therefore, early detection is important to reduce mortality (Raul-Hain et al., 2011). According to McLemore et al. (2010), screening techniques using transvaginal ultrasound and CA 125 monitoring can be performed in early detection of ovarian cancer. However, a study conducted by Modugno (2004) showed that those screening techniques did not show reductions in mortality and morbidity of ovarian cancer patients.
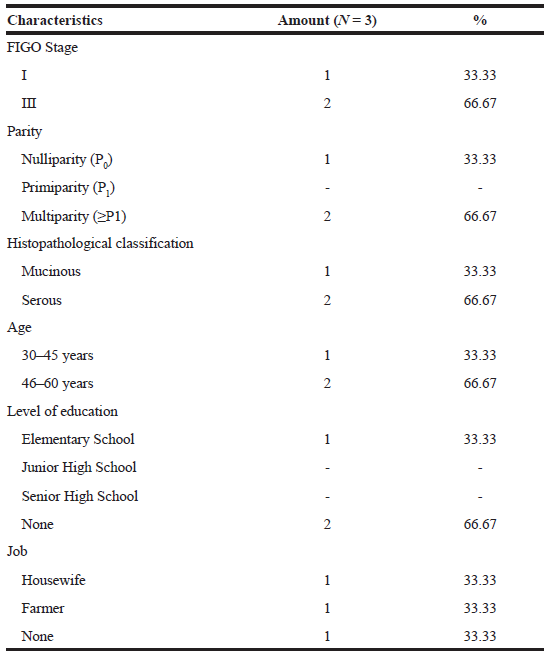 | Table 1. Patients characteristics. [Click here to view] |
Based on the parity parameter of the patients (Table 1), multiparity women (66.67%) were common than nulliparity women (33.33%). Another study by Gea et al. (2016) on Ovarian Cancer Overview at RSUP Prof Dr. RD Kandou Manado from January 2013 to December 2015, found that most ovarian cancer patients were multiparity (68.42%), followed by primiparity (24.21%) and nulliparity (7.37%) women. It was the same findings found by researchers. This was incompatible with the previous study showing that parous women had a lower risk of ovarian cancer than nulliparous women (Pike et al., 2004). Increased risk of ovarian cancer among parous women may be due to high ovulation cycle which caused the cumulative effect of any minor trauma to the ovarian epithelium that led to the malignant transformation of the cell (Mahdavi et al., 2006).
Histopathological classification (Table 1) shows that the most histopathologic type of patients was serous (66.67%) followed by mucinous (33.33%). This result was also found in the research conducted by Chang et al. (2018) that showed the most common types of EOC histopathology were (1) serous (43.3%), (2) clear cell (22.8%), (3) endometrioid (17.3%), and (4) mucinous (11.2%). Of all patients, the most common EOC patients are 46–60 years old (Table 1). Aging increased risk of ovarian cancer from 15.7 to 54 per 100,000 women with ages from 40 to 79 years (Partridge and Barnes, 1999). Table 1 showed that two patients never got formal education (66.67%) and one patient finished primary school program. Their jobs were farmer (33.33%), housewife (33.33%), and one of them did not work (33.33%).
 | Table 2. Hemoglobin levels before and after six cycles of paclitaxel-cisplatin chemotherapy. [Click here to view] |
 | Table 3. Platelet levels before and after six cycles of paclitaxel-cisplatin chemotherapy [Click here to view] |
 | Table 4. Leukocyte levels before and after six cycles of paclitaxel-cisplatin chemotherapy. [Click here to view] |
Differences in hemoglobin, platelets, and leukocytes levels before the first and after the sixth chemotherapy were because of how paclitaxel and cisplatin were cytotoxic drugs that induced myelosuppression by directly impairing hematopoiesis in the bone marrow (Kumar et al., 2010; Wilson et al., 2007). Paclitaxel causes myelosuppression by binding strongly with bone marrow cells’ microtubules, thus preventing depolymerization. This process later causes mitosis inhibition then apoptosis in cell division (Anderson et al., 2002). Whereas cisplatin binds covalently with bone marrow cells’ DNA to form intra and interstrand cross-links DNA that causes DNA damage during replication (Perry, 2008). Mechanism of those chemotherapeutic drugs resulted in a decrease of hemoglobin, platelets, and leukocytes levels (Wilson et al., 2007).
Grade 0 of thrombocytopenia and leukopenia was found in this study (Tables 3 and 4). The highest side effect observed was anemia grade 1 with a mean value of 10.69 g/dl after the sixth chemotherapy (Table 2). According to Gynecologic Oncology Group, anemia grade 1 referred to hemoglobin with a range between 10.0 and 12.0 g/dl. Cisplatin-induced nephrotoxicity that led to anemia through decreased renal production of erythropoietin. Erythropoietin is a cytokine produced in the kidneys that stimulate erythropoiesis in patients. Impaired erythropoiesis will decrease the production of red blood cells and hemoglobin levels (Rodgers et al., 2012).
CONCLUSIONS
There were differences of hemoglobin, platelet, and leukocyte levels before the first and after the sixth cycle of paclitaxel-cisplatin chemotherapy. Low side effects of paclitaxel-cisplatin (anemia grade 1) made these chemotherapeutic drugs highly recommended for EOC patients’ therapy.
ACKNOWLEDGMENTS
The authors would like to thank the Ministry of Research, Technology, and Higher Education of the Republic of Indonesia along with all of the obstetrics staffs at Sanglah Hospital Denpasar for their assistance and cooperation.
CONFLICT OF INTEREST
The authors declare no conflict of interests.
FUNDING
This research is funded by Ministry of Research, Technology, and Higher Education of the Republic of Indonesia through PDUPT Grant with contract number: No. 171.74/UN 14.4.A/LT/2018.
REFERENCES
Anderson PO, Knoben JE, Troutman WG. Handbook of clinical drug data. 10th edition, McGraw-Hill, San Diego, CA, 2002.
Berek JS, Hacker NF. Epithelial ovarian cancer: Piver Editor. Handbook of gynecologic oncology. 2nd edition, Lipponcott Williams & Wilkins, Philadelphia, PA, 2005.
Bois AD, Luck HJ, Meier W, Adams HP, Mobus V, Costa S, Bauknecht T, Richter B, Warm M, Schroöder W, Olbricht S. A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/ paclitaxel as first-line treatment of ovarian cancer. JNCI, 2003; 95(17):1320–30.
Chang LC, Huang CF, Lai MS, Shen LJ, Wu FLL, Cheng WF. Prognostic factors in epithelial ovarian cancer: a population-based study. Plos One, 2018; 13(3): 1–11
Charles A, Dewayani BM, Sahiratmadja E, Winarno GNA, Susanto H. Paclitaxel-carboplatin chemotherapy induced hematologic toxicities among epithelial ovarian cancer patients. Univ Med, 2016; 35(3):165–70.
Gea IT, Loho MF, Wagey FW. Gambaran jenis kanker ovarium di RSUP Prof. Dr. R.D. Kandou Manado periode Januari 2013–Desember 2015. J e-Clinic, 2016; 4(2):1–5.
Kampan NC, Madondo MT, McNally OM, Quinn M, Plebanski M. Paclitaxel and its evolving role in the management of ovarian cancer. BioMed Res Int, 2015; 2015:1–21.
Kim A, Ueda Y, Naka T, Enomoto T. Therapeutic strategies in epithelial ovarian cancer. JECCR, 2012; 31(14):1–8.
Kumar S, Mahdi H, Bryant C, Shah JP, Garg G, Munkarah A. Clinical trials and progress with paclitaxel in ovarian cancer. Int J Womens Health, 2010; 2:411–27.
Mahdavi A, Pejovic T, Nezhat F. Induction of ovulation and ovarian cancer: a critical review of the literature. Fertil Steril, 2006; 85(4):819–26.
McGuire V, Whittemore AS, Norris R, Oakley-Girvan I. Survival in epithelial ovarian cancer patients with prior breast cancer. Am J Epidemiol, 2000; 152(6):528–32.
Medical Committee. Standard operating procedures of ovarian cancer chemotherapy. Sanglah General Hospital, Denpasar, Indonesia, 2004.
Modugno F. Ovarian cancer and high-risk women-implications for prevention, screening, and early detection. Gynecol Oncol, 2003; 91(1):15–31.
McLemore MR, Miaskowski C, Aouizerat BE, Chen LM, Dodd MJ. Epidemiologic and genetic factors associated with ovarian cancer. Cancer Nurs, 2009; 32(4):281–90.
Partridge EE, Barnes MN. Epithelial ovarian cancer: prevention, diagnosis, and treatment. CA Cancer J Clin, 1999; 49(5):297–320.
Perry MC. The chemotherapy source book. Lippincott William and Wilkins, Colombia, 2008.
Pike MC, Pearce CL, Peters R, Cozen W, Wan P, Wu AH. Hormonal factors and the risk of invasive ovarian cancer: a population-based case-control study. Fertil Steril, 2004; 82(1):186–95.
Raul-Hain JA, Krivak TC, Carmen MGD, Olawaiye AB. Ovarian cancer screening and early detection in the general population. Rev Obstet Gynecol, 2011; 4(1):15–21.
Rodgers GM, Becker PS, Blinder M, Cella D, Chanan-Khan A. Cancer- and chemotherapy induced anemia. NCCN, 2012; 10(5):628–53.
Wilson J, Yao GL, Raftery J, Bohlius J, Brunskill S, Sandercock J, Bayliss S, Moss P, Stanworth S, Hyde C. A systematic review and economic evaluation of epoetin alfa, epoetin beta and darbepoetin alfa in anaemia associated with cancer, especially that attributable to cancer treatment. Health Technol Assess, 2007; 11(13):1–202.
Yurkovetsky Z, Skates S, Lomakin A, Nolen B, Pulsipher T, Modugno F, Marks J, Godwin A, Gorelik E, Jacobs I, Menon U. Development of a multimarker assay for early detection of ovarian cancer. J Clin Oncol, 2010; 28:2159–166.