INTRODUCTION
Chitosan is a polysaccharide consisting of β-(1→4)- 2-acetamido-D-glucopyranose and β-(1→4)-2-amino-D-glucopyranose units, with the latter usually exceeding 80% (Zou et al., 2016). It has been proved to be nontoxic, biodegradable, and biocompatible compound manufactured by the deacetylation of chitin that exists in thshells of the crustaceans, the insect exoskeletons, and in the cell walls of fungi and algae (Hamed et al., 2016). It has three types of reactive functional groups, an amino/acetamido group, a primary and a secondary hydroxyl groups at the C-2, C-3, and C-6 positions, respectively, which are responsible for its various biological activities including antitumor activity, antimicrobial activity, antifungal activity, antioxidant, and immune-enhancing effects (Zou et al., 2016). The biological activities of chitosan have been suggested to be due to the activity of its hydrolysis products in the target tissues. So, hydrolysis of chitosan to chito-oligosaccharides (COS) is of a great interest due to the improved water solubility and lower viscosity that enhances its activity in many biological applications (Sanchez et al., 2017).
COS are the partial hydrolysis products of chitosan with an average molecular weight up to 3900 Da (Hamed et al., 2016). Several methods including depolymerization of chitosan under the action of reagents, enzymes, high-energy impact, and bioengineering are used for the preparation of COS. The enzymatic hydrolysis was the most specific, significant, safe, and environmental friendly method (Ismail, 2016; Pan et al., 2016). Similar to the parent chitosan, COS are positively charged in aqueous environments that allow them to bind strongly to the negatively charged surfaces result in various biological activities (Liaqat and Eltem, 2018; Zou et al., 2016).
Lipopolysaccharide (LPS) is the glycolipid constituent of the outer membrane of all pathogenic gram-negative bacteria that known as one of the most potent activators of the inflammatory responses. LPS activates the inflammatory signal transduction by the cell surface toll-like receptor-4 (TLR-4) that results in cellular activation and pro-inflammatory gene expression (Yang et al., 2016). During the inflammation process, macrophages actively participate in the inflammatory responses by releasing the pro-inflammatory cytokines, tumor necrotic factor-α (TNF-α), and interleukin-6 (IL- 6), as well as other inflammatory factors, such as the short-lived free radical nitric oxide (NO) and prostaglandins, that recruit additional immune cells to the sites of infection or tissue injury (Boscá et al., 2005). Nitric oxide is endogenously synthesized by nitric oxide synthases (NOSs) through the conversion of L-arginine to NO and L-citrulline. Inducible NOS (iNOS) are the most abundant type that expressed and released in macrophages activated by LPS (Kim et al., 2014).
Chitosan and COS are considered as immunomodulators since they stimulate the synthesis of some of the cytokines that cause either stimulation or inhibition of the immunity (Davydova et al., 2016). These discrepancies lead to the attraction of the research focus. The present study concerned with the enzymatic hydrolysis of chitosan using chitosanase to produce COS then investigated the effect of chitosan and the produced COS on the immune status of normal mice as well as evaluating their anti-inflammatory effect on LPS-inflamed mice.
MATERIALS AND METHODS
Chemical
Chitosan [low (50–190 kDa) and medium (190–310 kDa) molecular weight], standard glucosamine, standard N-acetyl glucosamine, yeast extract, RPMI (Roswell Park Memorial Institute) 1640 media with L-Glutamine, fetal calf serum (FCS), Micrococcus lysodeikticus bacteria, agarose, sulphanilamide, and N-(1-naphthyl)-alpha naphthyl amine were obtained from Sigma- Aldrich, Saint Louis. Dinitrosalicylic acid was obtained from Panreac, Barcelona, Spain. Thin-layer chromatography (TLC) Silica gel 60 plate was obtained from Merck, Darmstadt, Germany. Candida albicans kindly supplied by Department of Mycology (Animal Health Research Institute, Dokki, Cairo, Egypt). All other chemicals were of analytical or HPLC (High Performance liquid chromatography) grade.
Chitosanase production
A fungal strain Dothideomycetes sp. was used for the production of chitosanase using the fermentation medium consisted of (g/l): chitosan (medium molecular weight), 30; K2HPO4, 1.5; MgSO4, 0.4; KCl, 4.0; yeast extract, 18.5 and FeSO4, 0.01; at pH 5.5 (Ismail, 2016).
Partial purification of the enzyme
Partial purification of the produced chitosanase was done by fractional precipitation with ethanol (Thadathil et al., 2016). The fraction with the reasonable specific activity and stability was used in the preparation of COS.
Chitosanase assay
Chitosanase activity was measured by mixing 500 μl of the suitable diluted enzyme solution with 500 μl of 1% soluble chitosan (low molecular weight) prepared in 1 M acetic acid and the pH was adjusted to pH 4.5 by using 2 M sodium acetate. The reaction mixture was incubated in shaking water bath at 60°C for 30 minutes. At the end of the reaction time, 2.5 ml of dinitrosalicylic acid was added. The amount of the produced reducing sugars was estimated immediately using dinitrosalicylic acid method, with D-glucosamine as standard. One international unit of chitosanase was defined as the amount of enzyme that released 1 μmol of D-glucosamine per minute under the assay conditions (Ismail, 2016).
Preparation and analysis of COS
The hydrolysis of chitosan (low molecular weight) was performed using 2% soluble chitosan in 1 M acetic acid (pH 4.5) mixed with chitosanase in a reaction mixture containing enzyme/ substrate ratio 0.2 IU/mg incubated for different periods (30 minutes to 6 hours) at 45°C in a shaking water bath. At the end of the incubation period, the reaction was boiled for 10 minutes. The resulted hydrolysate was analyzed by TLC as follows: the chitosan hydrolysate was spotted on a silica gel TLC plate. The plate was run in a mixture of propanol:water:30% ammonia (70:15:15 v/v) as a mobile phase and the amino sugars were visualized with diphenyl amine-aniline reagent (Tanaka et al., 1999). Thin-layer chromatography plates were also used for the separation of COS from the resulted hydrolysate produced by the partial pure enzyme after suitable incubation period and extracted by distilled water after running the plate in the mobile phase then centrifuged for 15 minutes at 5,000 rpm followed by drying then kept until used. The produced mixture of COS was analyzed by HPLC using glucosamine as a standard.
Extraction of LPS from Escherichia coli
LPS was extracted from Escherichia coli (O157H7) according to Qureshi et al. (1982) by using extraction mixture containing liquid phenol (90 g phenol + 11 ml water), chloroform, and petroleum ether in a volume ratio 2:5:8, respectively.
Animal and experiment design
Two hundred and fifty Swiss albino mice (25–30 g each) were kept under observation for about 1 week for any signs of disease. Mice in all groups were received balanced ration and plain water ad libitum. All experimental procedures involving animals were performed in accordance with the ethical guidelines of the medical ethical committee of the National Research Centre in Egypt.
Experiment-1
To study the effect of chitosan (low molecular weight) and COS on the immune status of mice, 100 Swiss albino mice were allocated into five groups (20/each group). Mice in the control group (G1) were injected intraperitoneally (IP) with phosphate buffer saline (PBS), that of G2 and G3 were injected IP with chitosan (10 and 20 mg/kg body weight, respectively), and that G4 and G5 were injected IP with COS (10 and 20 mg/kg body weight, respectively). Chitosan, COS, and PBS were injected every 2 days for 10 days experimental period (five times). Ten mice from each group were kept under observation for 1 week to monitor the mortality rate.
Experiment-2
To study the anti-inflammatory effect of chitosan (low molecular weight) and COS on LPS-treated mice, 150 Swiss albino mice were allocated into six groups (25/each group) and treated as follows: mice in group 1(A1) were IP injected with PBS and kept as control. Mice in group 2 (A2) were IP injected with LPS (5 mg/kg) in addition to PBS. Mice in group 3 and 4 (A3 and A4) were IP injected with LPS in addition to chitosan in 10 or 20 mg/kg, respectively. While mice in group 5 and 6 (A5 and A6) were IP injected with LPS in addition to COS in 10 or 20 mg/kg, respectively. Chitosan, COS, as well as PBS, was injected three times (one every 2 days) before LPS injection, one time with LPS injection and one-time post-LPS injection by 1 hour. Ten mice from each group were kept under observation up to 1 week to investigate the mortality rate in each group.
Sampling
Peritoneal macrophages
They were collected from mice of all groups (seven samples/ group) to estimate the count of peritoneum cell infiltration (PCI) and phagocytic activities of peritoneal macrophages (PM).
Serum samples
They were collected from all groups (seven samples/ group) to estimate the levels of NO, TNF-α, IL-6, and lysozyme activity. The samples were collected once at 10th day (the end of the experiment-1) and twice at 4th hour and 24th hour post-LPS injection (experiment-2).
Collection, count, and culture of peritoneal macrophage monolayer
Peritoneal cells were obtained according to Victor et al. (2003), 10 ml of RPMI medium pH 7.4 was IP injected in each mouse and then peritoneal cells were collected via a needle inserted into inguinal region. After washing the collected cells two times, they were suspended in RPMI medium with 10% FCS, count and adjusted at a density of 4 × 106/ml. The monolayer of PM was obtained by seeding l ml of peritoneal cell per cell culture and staining chambers with a cover slip and incubated for 1 hour at 37°C in 5% CO2 and 90% humidity, after incubation non-adherent cells were removed by washing three times, then reincubated overnight in the same condition.
Assay of phagocytosis
Phagocytic activity of PM was estimated using C. albicans following the method of De-La Fuente et al. (2000). The monolayer of macrophage was washed three times then incubated with 1 ml C. albicans (5 × 106/ml RPMI with 15% FCS) for 1 hour at 37°C, 5% CO2, and 90% humidity, and washed three time, air-dried, fixed, stained with Giemsa stain and finally, counted 100 PM cell under oil immersion to determine phagocytic percentage (number of macrophages engulf at least one candida spore/100 macrophage cell) and phagocytic index (number of macrophages engulf ≥ 3 candida spores/total number of macrophage).
Estimation of lysozyme activity
Lysozyme activity was measured by agarose gel plate lyses assay according to Peeters and Vantrapen (1977). Briefly, lysoplates were prepared by dissolving 1% agarose in PBS, 0.067 mol/L, pH 6.3, in which M. lysodeikticus (50 mg/100 ml agarose) had been dispersed. Nearly, 25 μl of serum samples, as well as the standard lysozyme, were added in each well. After 18 hours, the cleared zones diameter was measured. The concentration of the lysozyme was obtained from logarithmic curve prepared using standard lysozyme solution.
Estimation of nitric oxide
It carried out according to Yang et al. (2010). Briefly, 100 μl of serum sample was mixed with 80 μl of 375 mM ZnSO4 and 120 μl of 275 mM NaOH, and then centrifuged at 13,000 rpm for 20 minutes to precipitate serum proteins. Supernatant was obtained and added to 400 mg of Cu plated Cd, then shook for 2.5 hours at room temperature after adding 100 μl of 0.2 M glycine buffer. Nearly, 100 μl supernatant was added into 96 well ELISA plate with 100 μl of Griess reagent. The optical density was determined at 545 nm with an ELISA plate reader. Nitric oxide concentration was calculated from the standard curve using NaNO2.
Assay of Tumor necrotic factor-alpha and interleukin-6
Tumor necrotic factor-alpha (TNF-α) and IL-6 were assayed by using quantikine Elisa kits. (Ray Biotech, Inc) according to the manufacturer’s instructions.
Statistical analysis
Data were presented as means and standard errors. The significance of the results was evaluated using analysis of variance and LSD using Statistical Package for Social Science.
RESULTS
Analysis of the hydrolysis product of chitosan
The TLC plate shown in Figure 1 indicated that on the hydrolysis of low molecular weight chitosan by Dothideomycetes sp. chitosanase, a mixture of COS was produced after 1-hour incubation period. After that, no COS was observed and the monomers were the hydrolysis product. The produced COS mixture was analyzed by HPLC (Fig. 2) compared with the standard glucosamine that appeared at a retention time of 3.061.
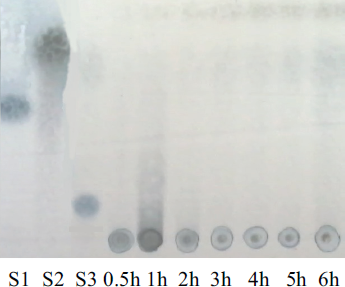 | Figure 1. TLC plate of chitooligosaccharides produced by the hydrolysis of soluble chitosan by Dothideomycetes sp. chitosanase at various incubation periods. S1: standard glucosamine, S2: standard N-acetyl glucosamine, S3: tetra oligosaccharides. [Click here to view] |
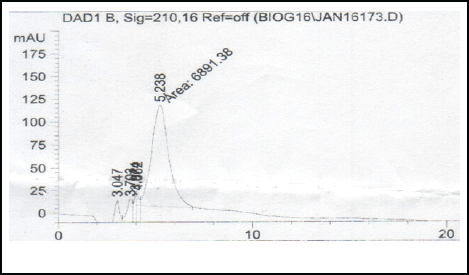 | Figure 2. HPLC of COS mixture. [Click here to view] |
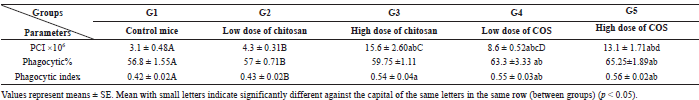 | Table 1. Effect of chitosan and COS injection on the count of peritoneum cell infiltration (×106), phagocytic %, and phagocytic index of peritoneum [Click here to view] |
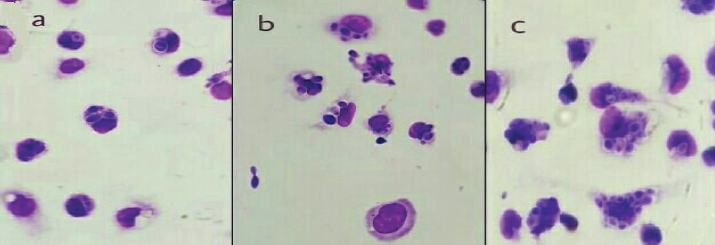 | Figure 3. Mice peritoneal macrophages engulfed or not C. albicans spores. (a) mouse peritoneal macrophages of the control group. (b) mouse peritoneal macrophages of group 3, treated with a high dose of Chitosan. (c) mouse peritoneal macrophages of group 5, treated with a high dose of COS. (Giemsa stain × 100). [Click here to view] |
Results of experiment-1
No deaths were recorded in all groups up to 1- week post-chitosan or COS injection.
Effect of chitosan and COS injection on peritoneum cell infiltration and phagocytic activity of peritoneal macrophages of mice
As shown in Table 1 and Figure 3, mice treated with a high dose of chitosan (G3) showed a significant increase in PCI count, phagocytic index of PM comparing with the control group (G1). Also, mice treated with a low and high dose of COS (G4 and G5) recorded a significant increase in PCI count, phagocytic %, and phagocytic index of PM when comparing with mice in both G1 (control group) and G2 (mice treated with low dose of chitosan).
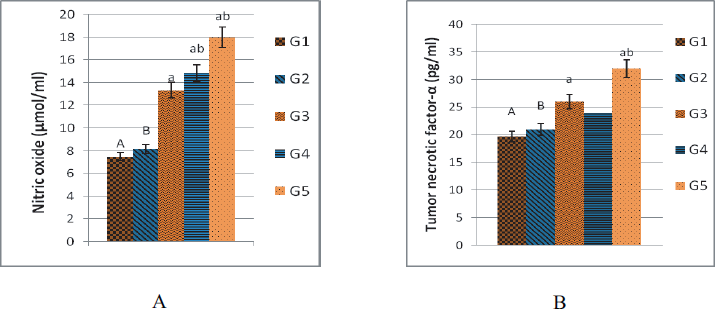 | Figure 4. Effect of chitosan and COS injection on the level of serum NO (μmol/ml) (A) and TNF-α (pg/ml) (B) in mice. G1: control group of mice injected IP with PBS. G2 and G3: groups injected IP with chitosan (10 and 20 mg/kg body weight, respectively). G4 and G5: groups injected IP with COS (10 and 20 mg/kg body weight, respectively). Data are represented as means ± SE (n = 7). Column with small litters indicates significantly different against the capital of the same litters on the other columns (p < 0.05). [Click here to view] |
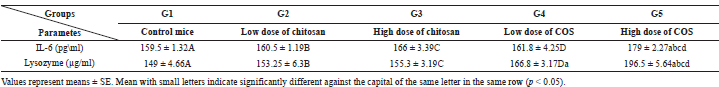 | Table 2. Effect of chitosan and COS injection on IL-6 and lysozyme activity of mice. [Click here to view] |
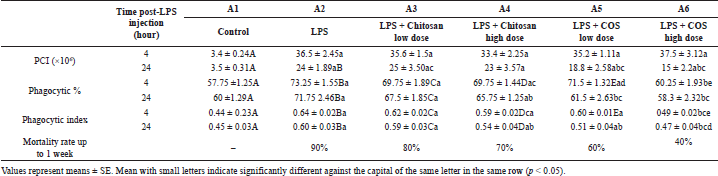 | Table 3. Effect of chitosan and COS injection on peritoneum cell infiltration (×106) count, Phagocytic% & index of peritoneum macrophage and mortality rate of LPS-treated mice. [Click here to view] |
Effect of chitosan and COS injection on NO and TNF-α in mice
Mice treated with high dose of chitosan or COS (G3 and G5) showed a significant increase in NO and TNF-α compared with the control group (G1). Also, mice treated with low dose of COS (G4) showed a significant increase in NO (Fig. 4).
Effect of chitosan and COS injection on interleukin-6 and lysozyme activity
Mice treated with high dose of COS (G5) showed a significant increase in IL-6 value and lysozyme activity comparing with control and all other treated groups. Also, mice treated with low dose of COS (G4) showed a significant increase in lysozyme activity comparing with the control group (Table 2).
Results of experiment-2
The mortality rate in LPS group was 90% of mice which died within 48 hours, while mice pretreated with chitosan and COS at low and high dose partially protect mice from LPS induced mortality (Table 3), the best protection recorded with a high dose of COS. The mice in the LPS group (A2) gave obvious significant increasing in all tested parameters.
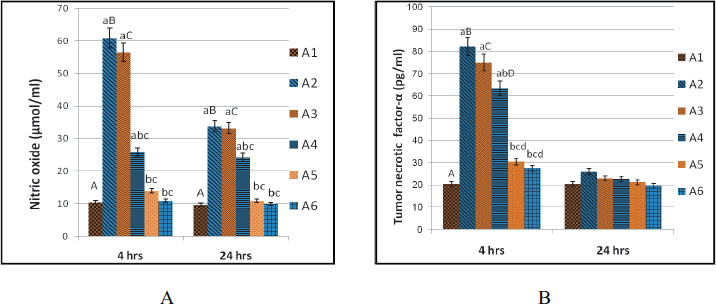 | Figure 5. The effect of chitosan and COS on the level of serum NO (μmol/ml) (A) and TNF-α (pg/ml) (B) of LPS-treated mice. A1: mice of the control group injected IP with PBS. A2: group injected IP with LPS (5 mg/kg). A3 and A4: groups injected IP with LPS in addition to chitosan (10 or 20 mg/kg, respectively). A5 and A6: groups injected IP with LPS in addition to COS (10 or 20 mg/kg, respectively). Data are represented as means ± SE (n = 7). Column with small litters indicates significantly different against the capital of the same litters on the other columns (p < 0.05) in each time. [Click here to view] |
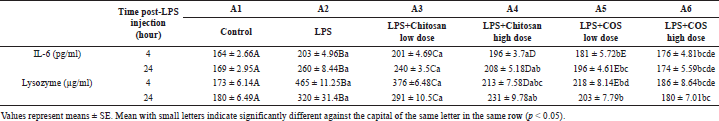 | Table 4. The effect of chitosan and COS on the levels of Interleukin-6 and lysozyme activity on LPS-treated mice. [Click here to view] |
Effect of chitosan and COS injection on peritoneum cell infiltration and phagocytic activity of peritoneal macrophage of LPS-treated mice
Treatment of mice with a high dose of chitosan (A4) revealed a significant decrease in phagocytic % and index after 24-hour post-LPS injection comparing with LPS group, but it still significantly increased compared to the control group. Mice treated with COS at low dose (A5) recorded a significant decrease in the count of PCI, phagocytic %, and index of PM at 24th hour post-LPS injection comparing with LPS group, however, COS at high dose (group A6) significantly decreased the count of PCI at 24th hour post-LPS injection and significantly decreased the phagocytic % and index at 4th hour and 24th hour post-LPS injection to approach the control values.
The effect of chitosan and COS injection on the levels of nitric oxide and TNF-α of LPS-treated mice
Treatment of mice with a high dose of chitosan (A4) revealed significant decrease in NO values at 4th and 24th hour post-LPS injection and in TNF-α at 4th hour post-LPS injection comparing to LPS group but it still significantly increased compared to the control group. However, COS at a low and high dose (A5 and A6) significantly decreased NO values at 4th and 24th hour post-LPS injection and TNF-α at 4th hour post-LPS injection to approach the control values. TNF-α retained to basal value in all groups at 24th hour post-LPS injection (Fig. 5).
The effect of chitosan and COS on the levels of interleukin-6 and lysozyme activity on LPS-treated mice
Treatment of mice with a high dose of chitosan (A4) revealed a significant decrease in IL-6 at 24 hours and in lysozyme at 4th and 24th hour post-LPS injection in comparing with LPS group. Also, treatment of mice with COS at a low and high dose (A5 and A6) showed significant decrease in the levels of IL-6 and lysozyme at 4th and 24th hour post-LPS injection to approach the control values (Table 4).
DISCUSSION
Natural products had received much attention to modulate the immune system since the current treatments induce severe side effects (Mei et al., 2013; Yang et al., 2016). Several natural compounds as polysaccharides and oligosaccharides reported to enhance the immune system (Petrovsky et al., 2011). Chitosan and COS have gained a great attention due to their significant in vitro immunomodulatory effects (Davydova et al., 2016; Zheng et al., 2018). The in vivo immunomodulatory effects of chitosan and COS were studied in two experiments as follows: Experiment-1: the immune-stimulatory effect of chitosan and COS on normal mice; Experiment-2: the anti-inflammatory effect of chitosan and COS in LPS-treated mice.
The obtained results of experiment-1 revealed that chitosan or COS in low and high doses (10 and 20 mg/kg) were non-toxic as no mortality was observed. This result agrees with Davydov et al. (2016), Kim et al. (2014), and Santos-Moriano et al. (2018). Intraperitoneal injection of a high dose of chitosan caused a significant increase of PCI, phagocytic index of PM and increase in the serum level of TNF-α and NO, while the immune-stimulatory effect of COS was more pronounced as COS in low and high doses showed a significant increase in nearly all tested parameters. The effect of chitosan is supported by previous studies which recorded that low or high molecular weight of Chitosan caused an increase of peritoneum cell count in the lavages (Brodaczewska and Doligalska, 2013) and significantly enhance the pinocytic activity and the production of TNF-α and NO in RAW264.7 macrophages (Wu et al., 2015). Also, Davydov et al. (2016) reported that chitosan (5200Da) tripled the synthesis of TNF-α in human blood cells. The immune-stimulatory effect of chitosan may be attributed to the similarity in their structure and the saccharide portion of lipid A in LPS and so it may similarly bind to the surface receptors of CD-14 or TLR-4 of the PM to activate the signal transduction system (Wu et al., 2007). In contrary, Guzman-Morales et al. (2011) found that chitosan-stimulated murine bone marrow-derived macrophages failed to produce NO. These discrepancies could be potentially explained by the difference in dosages, molecular weight, and the degree of acetylation of chitosan used in each study.
The obvious immune-stimulatory effect of COS recorded in this study was supported by several studies which showed that COS cause a significant increase in the levels of TNF-α (Feng et al., 2004) and NO (Wu et al., 2007). Also, it improved the immunity of broilers by the release of IL-1, IL-6, TNF-α, and NO (Deng et al., 2008). The immune-stimulatory effect of COS may be due to its internalization to macrophage through a macrophage lectin receptor with mannose specificity (Feng et al., 2004) or due to binding with CD14 receptor and TLR-4 of macrophage which lead to activation of nuclear factor kappa beta (NF- κβ) and activation of genes expression (Wu et al., 2007).
The anti-inflammatory effects of chitosan and COS in LPS-elicited inflammatory response in mice were determined. Intraperitoneal injection of LPS alone (5 mg/Kg) induces a potent inflammatory response in mice, manifested by a marked increase in all tested parameters. These results come in accordance with Kim et al. (2014) and Yang et al. (2016). It was known that LPS is recognized by TLR-4 on immune cells which activate (NF- κβ) through stimulation of IκB kinase which phosphorylates IκB and thereby induces its degradation resulting in dissociation and translocation of NF-κB into the nucleus and activation of the target genes of inflammatory cytokines (Li et al., 2014).
Both chitosan and COS had different protecting effects against LPS-induced mortality in mice. The less mortality rate (40%) was recorded in the group injected with a high dose of COS (A6), while that of LPS group was 90%. IP injection with a high dose of Chitosan and both low and high dose of COS significantly overcome LPS-induced sever activation of PM and modulate the overproduction of NO, lysozyme, TNF-α, and IL-6, which approach the control value as in COS groups or not as in chitosan groups. The down-regulation of LPS-induced inflammation by chitosan come in accordance with (Ji et al., 2013; Yermak et al., 2006) which attributed to the formation of a complex with LPS by ionic binding between negatively charged groups on LPS (anionic compound) and positively charged amino groups on chitosan (cationic polymer) (Wilkinson et al., 1996). These LPS-chitosan complexes are accompanied by the essential modification of several immunological properties of LPS and possess 10 to 20 times less toxicity than LPS alone (Yermak et al., 2006), which may be attributed to the blocking of the toxophoric center of endotoxin or the alterations in the molecular charge and/or the structure of LPS (Ji et al., 2013).
The down-regulation of LPS-induced inflammation by COS agreed with many in vitro studies which reported that COS exposure led to dose-dependent attenuation of LPS induced secretion of NO, TNF-α, and IL-6 in RAW264.7 cells (Kim et al., 2014; Xu et al., 2017; Yoon et al., 2007) and in microglia cell (Pangestuti et al., 2011; Wei et al., 2012). This anti-inflammatory feature of COS may be attributed to its reduction of gene expression of inflammatory cytokines (Deng et al., 2008) and suppression of the translocation of a subunit of NF-κB into the nucleus by inhibiting degradation of IκB-α p65 (Kim et al., 2014). Also, Liu et al. (2010) suggested that COS inhibit LPS induced up-regulation of IL-6 by at least two parallel signaling pathways: one via p38 mitogen-activated protein kinases pathway and one via the extracellular signal-regulated kinase 1⁄2 pathway. From the above data, it was recorded that both chitosan and COS have dual activities, both immune-stimulatory activity in normal mice model and anti-inflammatory activity in LPS-inflamed mice model. It was explained by the fact that once the inflammatory cascade is induced, other mechanisms operate to down-regulate the process of inflammation. In the same regard, Porporatto et al. (2003) recorded that high molecular weight chitosan interacting with resident macrophages by stimulated NO production and with inflammatory macrophages by inhibition of NO production through enhanced the arginase expression.
The more pronounced activities of COS present in this study may be attributed to their highly water solubility and lower viscosity compared with the parent chitosan that was characterized by its highly viscous nature with lower water solubility that lead to a less efficient interaction with the cell (Liaqat and Eltem, 2018; Sanchez et al., 2017).
In conclusion, the produced mixture of COS possessed an immunostimulatory activity in normal mice as that of the parent chitosan; therefore, both might be used as functional food components and additives. Moreover, they have an anti-inflammatory effect on LPS-treated mice that potentially enable their use as therapeutic agents for disorders where inflammation is one of the pathological features. Chito-oligosaccharide is clearly much better than chitosan as an immunomodulatory agent.
CONFLICT OF INTEREST
Natural product and their biological applications.
REFERENCES
Bosca L, Zeini M, Traves PG, Hortelano S. Nitric oxide and cell viability in inflammatory cells: a role for NO in macrophage function and fate. Toxicology, 2005; 208(2):249–58.
Brodaczewska K, Doligalska M. Differential effects of low and high molecular weight chitosan administrated intraperitoneally to mice infected with heligmosomoides polygyrus. Prog Chem Appl Chitin Derivatives, 2013; 18(18):77–83.
Davydova VN, Kalitnik AA, Markov PA, Volod’ko AV, Popov SV, Ermak IM. Cytokine-inducing and anti-inflamatory activity of chitosan and its low-molecular derivative. Appl Biochem Microbiol, 2016; 52(5):476–82.
De La Fuente M, Carazo MS, Correa R, Del Rio M. Changes in macrophage and lymphocyte functions in guinea-pigs after different amounts of vitamin E ingestion. Br J Nutr, 2000; 84(1):25–9.
Deng X, Li X, Liu P, Yuan S, Zang J, Li S, Piao X. Effect of chito-oligosaccharide supplementation on immunity in broiler chickens. Asian-Australas J Anim Sci, 2008; 21(11):1651–8.
Feng J, Zhao L, Yu Q. Receptor-mediated stimulatory effect of oligochitosan in macrophages. Biochem Biophys Res Commun, 2004; 317(2):414–20.
Guzman-Morales J, Ariganello MB, Hammami I, Thibault M, Jolicoeur M, Hoemann CD. Biodegradable chitosan particles induce chemokine release and negligible arginase-1 activity compared to IL-4 in murine bone marrow-derived macrophages. Biochem Biophys Res Commun, 2011; 405(4):538–44.
Hamed H, Ozogul F, Regenstein JM. Industrial applications of crustacean by-products (chitin, chitosan and COS): a review. Trends Food Sci Technol, 2016; 48:40–50.
Ismail SA. Physiological and biochemical studies of microbiological production of chitosanase. PhD thesis, Faculty of Pharmacy, Cairo University, Egypt, 2016.
Ji Q, Deng J, Yu X, Xu Q, Wu H, Pan J. Modulation of pro-inflammatory mediators in LPS-stimulated human periodontal ligament cells by chitosan and quaternized chitosan. Carbohydr Polymer, 2013; 92(1):824–9.
Kim JH, Kim YS, Hwang JW, Han YK, Lee JS, Kim SK, Jeon YJ, Moon SH, Jeon BT, Bahk YY, Park PJ. Sulfated chitosan oligosaccharides suppress LPS-induced NO production via JNK and NF-kB inactivation. Molecules, 2014; 19(11):18232–47.
Li Y, Liu H, Xu QS, Du YG, Xu J. Chitosan oligosaccharides block LPS-induced O-GlcNAcylation of NF-κB and endothelial inflammatory response. Carbohydr Polymers, 2014; 99:568–78.
Liaqat F, Eltem R. Chitooligosaccharides and their biological activities: a comprehensive review. Carbohydr Polymers, 2018; 184:243–59.
Liu HT, Li WM, Li XY, Xu QS, Liu QS, Bai XF, Yu C, Du YG. Chitosan oligosaccharides inhibit the expression of interleukin-6 in lipopolysaccharide-induced human umbilical vein endothelial cells through p38 and ERK1⁄2 protein kinases. Basic Clin Pharmacol Toxicol, 2010; 106(5):362–71.
Mei YX, Chen HX, Zhang J, Zhang XD, Liang YX. Protective effect of chitooligosaccharides against cyclophosphamide-induced immunosuppression in mice. Int J Biol Macromol, 2013; 62:330–5.
Pan AD, Zeng HY, Foua GB, Alain C, Li YQ. Enzymolysis of chitosan by papain and its kinetics. Carbohydr Polymers, 2016; 135:199–206.
Pangestuti R, Bak SS, Kim SK. Attenuation of pro-inflammatory mediators in LPS-stimulated BV2 microglia by chitooligosaccharides via the MAPK signaling pathway. Int J Biol Macromol, 2011; 49(4):599–606.
Peeters TL, Vantrapen GR. Factors influencing lysozyme determination by lysoplate method. Clin Chim Acta, 1977; 74(3):217–55.
Petrovsky N, Cooper PD. Carbohydrate-based immune adjuvants. Expert Rev Vaccines, 2011; 10(4):523–37.
Porporatto C, Bianco ID, Riera CM, Correa SG. Chitosan induces different l-arginine metabolic pathways in resting and inflammatory macrophages. Biochem Biophys Res Commun, 2003; 304(2):266–72.
Qureshi N, Takayama K, Ribi E. Purification and structural determination of non toxic lipid A obtained from the lipoplysaccharide of Slamonella typhimurium. J Biol Chem, 1982; 257(19):11808–15.
Sanchez A, Mengibar M, Rivera-Rodriguez G, Moerchbacher B, Acosta N. The effect of preparation process on the physicochemical characteristics and antibacterial activity of chitooligosaccharides. Carbohydr Polymers, 2017; 157:251–7.
Santos-Moriano P, Fernandez-Arrojo L, Mengibar M, Belmonte- Reche E, Penalver P, Acosta FN, Ballesteros AO, Morales JC, Kidibule P, Fernandez-Lobato M, Plou FJ. Enzymatic production of fully deacetylated chitooligosaccharides and their neuroprotective and anti-inflammatory properties. Biocatalysis Biotransformation, 2018; 36(1):57–67.
Tanaka T, Fujiwara S, Nishikori S, Fukui T, Takagi M, Imanaka TA. Unique chitinase with dual active sites and triple substrate binding sites from the hyperthermophilic Archaeon Pyrococcus Kodakaraenis KOD1. Appl Environ Microbiol, 1999; 65(12):5338–44.
Thadathil N, Velappan SP. Recent developments in chitosanase research and its biotechnological applications: a review. Food Chem, 2014; 150:392–99.
Victor VM, Rocha M, De La Fuenle M. Regulation of macrophage function by the antioxidant N-acetyle cysteine in mouse-oxidative stress by endotoxin. Int Immunopharmacol, 2003; 3(1):97–106.
Wei P, Ma P, Xu QS, Bai QH, Gu JG, Xi H, Du YG, Yu C. Chitosan oligosaccharides suppress production of nitric oxide in lipopolysaccharide-induced N9 murine microglial cells in vitro. Glycoconj J, 2012; 29(5–6):285–95.
Wilkinson SG. Bacterial lipopolysaccharides-themes and variations. Prog Lipid Res, 1996; 35(3):283–343.
Wu GJ, Tsai GJ. Chitooligosaccharides in combination with interferon- gamma increase nitric oxide production via nuclear factor kappa B activation in murine RAW264.7 macrophages. Food Chem Toxicol, 2007; 45(2):250–8.
Wu N, Wen ZS, Xiang XW, Huang YN, Gao Y, Qu YL. Immunostimulative activity of low molecular weight chitosans in RAW264.7 macrophages. Mar Drugs, 2015; 13(10):6210–25.
Xu Q, Liu M, Liu Q, Wang W, Du Y, Yin H. The inhibition of LPS-induced inflammation in RAW264.7 macrophages via the PI3K/Akt pathway by highly N-acetylated chitooligosaccharide. Carbohydr Polymers, 2017; 174:1138–43.
Yang Y, Tong Q, Luo H, Huang R, Li Z. Chitooligosaccharides attenuate lipopolysaccharide-induced inflammation and apoptosis of intestinal epithelial cells: Possible involvement of TLR4/NF-kB pathway. Ind J Pharm Educ Res, 2016; 50(1):109–15.
Yang X, Sun Q, Raza Asim A, Jiang X, Zhong B, Shahzad M, Zhang F, Han Y, Lu S. Nitric oxide in both bronchoalveolar lavage fluid and serum is associated with pathogenesis and severity of antigen-induced pulmonary inflammation in rats. J Asthma, 2010; 47(2):135–44.
Yermak IM, Davidova VN, Gorbach VI, Luk’yanov PA, Solov’eva TF, Ulmer AJ, Buwitt-Beckmann U, Rietschel ET, Ovodov YS. Forming and immunological properties of some lipopolysaccharide-chitosan complexes. Biochimie, 2006; 88(1):23–30.
Yoon HJ, Moon ME, Park HS, Im SY, Kim YH. Chitosan oligosaccharide (COS) inhibits LPS-induced inflammatory effects in RAW 264.7 macrophage cells. Biochem Biophys Res Commun, 2007; 358(3):954–9.
Zheng B, Wen ZS, Huang YJ, Xia MS, Xiang XW, Qu YL, Molecular weight-dependent immunostimulative activity of low molecular weight chitosan via regulating NF-KB and AP-1 signaling pathways in RAW264. 7 macrophages. Mar Polysaccharides, 2018; 2(2):1.
Zou P, Yang X, Wang J, Li Y, Yu H, Zhang Y, Liu G. Advances in characterization and biological activities of chitosan and chitosan oligosaccharides. Food Chem, 2016; 190:1174–81.