Development and Characterization of Controlled Release Ketoprofen Microspheres
Marwa H. Abdallah, Omaima A. Sammour, Hanaa A. El-ghamry, Hanan M. El-nahas, Waleed Barakat
DOI: 10.7324/JAPS.2012.2310Pages: 60-67

Model-Based Bioequivalence assessment of a commercial Azithromycin Capsule against Pfizer Zithromax® Tablet marketed in Jamaica
Amusa S. Adebayo and Noel McFarlane
DOI: 10.7324/JAPS.2014.401012Pages: 062-068

Chromatogram profiles of andrographolide in A23187-induced New Zealand rabbit’s urine and faeces
Jutti Levita, Tanti Juwita, Selma Ramadhani, Nyi Mekar Saptarini, Mutakin Mutakin
DOI: 10.7324/JAPS.2017.70121Pages: 156-159

Pharmacokinetic and Pharmacodynamic evaluation of Camptothecin encapsulated Poly (methacylic acid-co-methyl methacrylate) nanoparticles
Mahalingam Manikandan, Krishnamoorthy Kannan
DOI: 10.7324/JAPS.2017.70303Pages: 009-016

Impact of sample storage conditions on gliclazide quantification in rat plasma by UHPLC/UV method: storage recommendation and pharmacokinetic application
Nesrin F. Taha, Ebtesam W. Elsayed, Ahmed A. El-Ashmawy, Aya R. Abdou, Laila H. Emara
DOI: 10.7324/JAPS.2021.110305Pages: 046-053

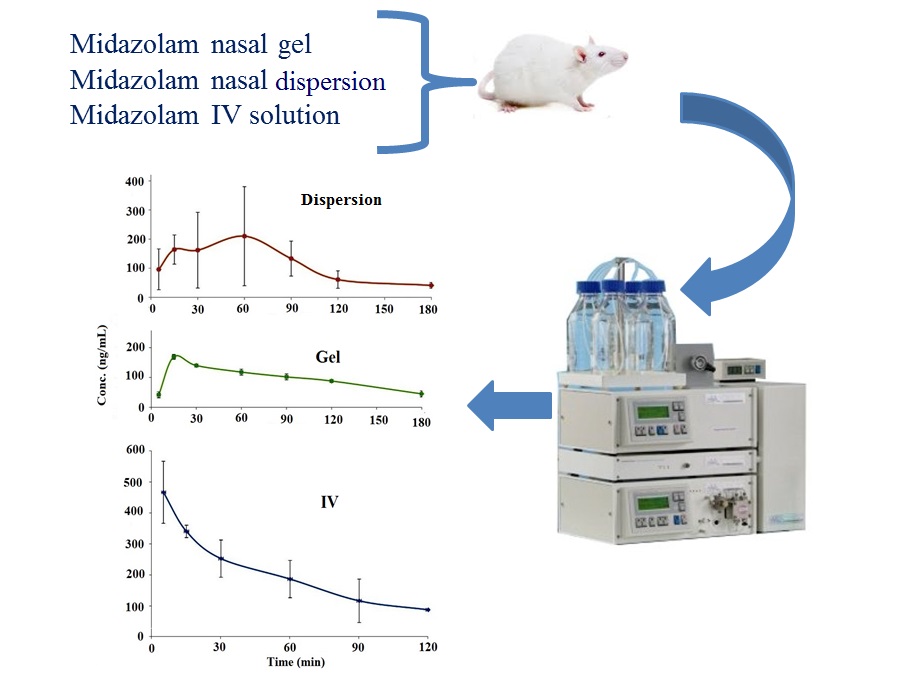
Pharmacokinetics and bioavailability of midazolam in rats following single dose administration of intravenous solution, intranasal dispersion, and in situ nasal gel
Elahehnaz Parhizkar, Saba Movaffagh, Shohreh Alipour
DOI: 10.7324/JAPS.2021.1101109Pages: 070–075