Generic Substitution in Malaysia: Recommendations from a Systematic Review
Mohamed Azmi Hassali, Jayabalan Thambyappa, Fahad Saleem, Noman ul Haq, Hisham Aljadhey
DOI: 10.7324/JAPS.2012.2827Pages: 159-164

Economic evaluation of antibiotic prescriptions: a cost minimization analysis
L. Ramesh
DOI: 10.7324/JAPS.2013.3627Pages: 160-163

Evaluation of the quality and stability of amoxicillin oral suspension
Blanca Elena Ortega Markman, Maria Regina Walter Koschtschak, Elizabeth Wu Meihuey, Paulo Cesar Pires Rosa
DOI: 10.7324/JAPS.2014.40706Pages: 038-040

A descriptive cross-sectional study to evaluate the Generic Drug User Fee Act: A boon or loss to the Indian generic pharmaceutical industry
Sarika Prasanna Pardhe
DOI: 10.7324/JAPS.2019.90206Pages: 044-051
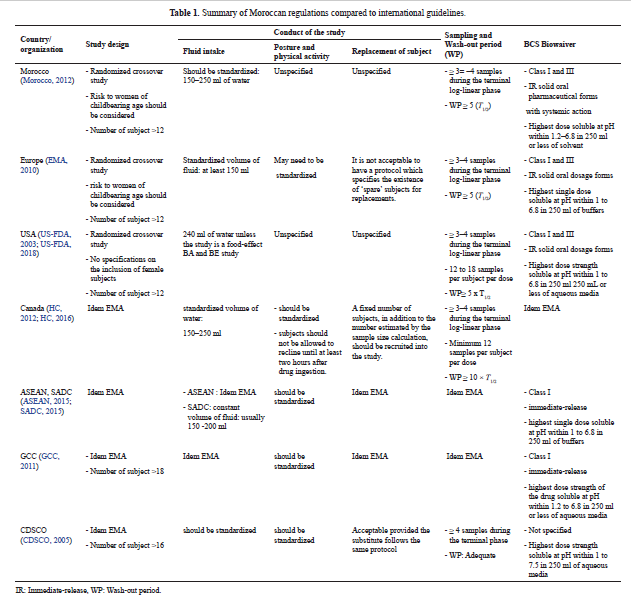
Bioequivalence regulation in emerging countries: Example of Moroccan regulations on immediate release formulations and comparison with international guidelines
Casimir Adade Adade, Amine Cheikh, Yahia Cherrah, Mustapha Bouatia, Jean Michel Cardot
DOI: 10.7324/JAPS.2019.91104Pages: 028-035
Factors affecting purchasing behaviors of generic drugs versus originator counterparts in Jordan
Maha N. Abu Hajleh, Ali AL-Samydai, Zahraa Aloosi, Raghad Abuhamdan, Sumaiah Al.Naimat, Lina Abdelfattah, Lidia Al-Halaseh
DOI: 10.7324/JAPS.2021.110902Pages: 009-017
