INTRODUCTION
Amantadine is a tricyclic amine compound that has exhibited remarkable pharmacological versatility since its discovery in the early 1960s [1]. Amantadine possesses antiviral properties, specifically against the influenza A virus [2]. In 1966, it became the first FDA-approved antiviral medication for influenza A prophylaxis [3]. Amantadine acts by blocking the viral M2 proton channel, thereby preventing viral uncoating and replication [4,5].
The therapeutic applications of amantadine expanded unexpectedly in 1968 when Schwab et al. [6] reported motor symptom improvement in Parkinson’s disease patients. Subsequent studies revealed that amantadine exerts a multifaceted effect on the central nervous system through enhancement of dopamine release [7], inhibition of dopamine reuptake [8], N-methyl-D-aspartate (NMDA) receptor antagonism [9], and possible dopaminergic receptor agonism [10]. These properties contributed to its use in Parkinson’s disease [11].
During the 1980s and 1990s, clinical trials demonstrated amantadine’s utility in managing fatigue in multiple sclerosis and improving attention and arousal in patients with various neurological disorders [12,13]. By the early 2000s, controlled studies, such as those by Giacino et al. [14], confirmed its efficacy in accelerating recovery in patients with traumatic brain injury emerging from minimally conscious or vegetative states.
In recent years, attention has turned to amantadine’s anti-inflammatory and neuroprotective effects [15]. In vitro studies indicate it reduces pro-inflammatory cytokine production, such as TNF-α and IL-1β, and may modulate microglial activation [15,16]. These findings suggest potential applications in neurodegenerative and neuroinflammatory conditions.
In addition to its established clinical roles, amantadine has been the subject of numerous preclinical studies involving rodent models to investigate its effects on ion transport [17], hippocampal function, and dopaminergic signaling pathways [8–10]. These molecular insights have contributed to understanding its neuropharmacological mechanisms, particularly in relation to learning, memory, and synaptic plasticity. Moreover, like many central nervous system-active drugs, amantadine is associated with side effects such as dizziness, nervousness, and insomnia [18], which are important considerations in both therapeutic decisions.
This bibliometric analysis comprehensively maps the evolution of amantadine research from 1964 to April 2025, focusing on publication trends, international collaboration, therapeutic advancements, and emerging mechanistic insights that continue to shape its role in clinical pharmacology and neuroscience.
METHODS
Data source and search strategy
This bibliometric analysis draws on data sourced from the Scopus database, a comprehensive repository of academic literature. This bibliometric study was conducted and reported in accordance with the BIBLIO guidelines proposed by Montazeri et al. [19], with all key methodological decisions transparently described to ensure clarity and reproducibility.
The search process was conducted on April 12, 2025, targeting all published research on amantadine conducted globally, which retrieved data from 1964 to April 2025. The search strategy utilized the following terms and conditions: (((TITLE(Amantadine))) AND (EXCLUDE (SRCTYPE,”Undefined”)) AND (EXCLUDE (AFFILCOUNTRY,”Undefined”)) AND (EXCLUDE (PREFNAMEAUID,”Undefined”)) AND (EXCLUDE (SUBJAREA,”Undefined”)) AND (LIMIT-TO (DOCTYPE,”ar”)) AND (LIMIT-TO (LANGUAGE,”English”))). The scope was restricted to English-language publications to ensure consistency in analysis. To ensure high specificity, we restricted the search to records with “Amantadine” in the article title. This approach minimizes the inclusion of studies where amantadine is only mentioned peripherally in the abstract or keywords, thereby focusing the dataset on research in which amantadine is the principal subject. Review articles were excluded to avoid duplication of primary data and secondary citation clustering. This allowed the bibliometric analysis to reflect original research output rather than synthesized summaries.
Inclusion and exclusion criteria
This study focused on research publications that specifically addressed amantadine, retrieved from the Scopus database. Inclusion criteria were as follows: (i) articles published between 1964 and April 2025; (ii) English-language publications only; (iii) documents explicitly containing “Amantadine” in the title; and (iv) limited to original research articles (Scopus document type “ar”) to ensure scientific rigor. Exclusion criteria included (i) non-English articles to avoid inconsistencies in translation; (ii) non-research items such as reviews, editorials, letters, conference proceedings, and book chapters; and (iii) articles lacking metadata such as author names, affiliations, or publication year, or classified as “Undefined” under subject area or source type.
Data cleaning and preprocessing
The data retrieved from Scopus were exported in CSV format and underwent a stepwise cleaning process before analysis. Initially, the search of the Scopus database using “Amantadine” as the query yielded 2,417 articles. Within this initial set, there were 51 records with undefined authors, 367 records with undefined country information, and five records from undefined sources. These incomplete entries were excluded. Subsequently, we removed non-article document types (e.g., reviews, editorials, conference papers) and non-English publications, which resulted in a final dataset of 1,540 articles used for bibliometric analysis. The final search query, which yielded the final number of retrieved articles, is provided in the “Data source and search strategy” section. Standardization procedures were applied to harmonize author names, journal titles, and keywords by correcting for case sensitivity and typographical inconsistencies.
Bibliometric analysis
The analysis employed VOSviewer (v1.6.20) [20] and the Bibliometrix (R package) [21] to explore the intellectual structure of amantadine research. VOSviewer was employed to generate visual maps for co-authorship and keyword co-occurrence, enabling the identification of intellectual and social structures within the field. Bibliometrix facilitated quantitative assessments, including annual publication output, citation analysis, author productivity, and institutional contributions. Key bibliometric parameters analyzed included total number of publications, average citations per document, h-index, and thematic evolution based on keyword trends.
RESULTS
This study utilized the Scopus database to identify English-language publications related to the keyword “Amantadine,” covering the period from 1964 to April 12, 2025. Data was retrieved on April 12, 2025, yielding a total of 1,540 records.
Annual scientific production
The bibliometric analysis of amantadine research from 1964 to April 2025 reveals a dynamic publication history. Starting with just one article in 1964, output grew modestly, reaching five articles by 1965. The 1970s experienced a notable rise, with publications peaking at 36 in 1973, following 29 in 1974, and 28 in 1972. A slight decline followed, with 17 articles in 1976, and the 1980s maintained a steady output. The 2000s marked another rise, with 40 articles in 2004 and a high of 57 in 2022. The years 2021 and 2023 sustained strong activity with 52 and 41 articles, respectively, while 2025 recorded 16, indicating ongoing interest (Fig. 1).
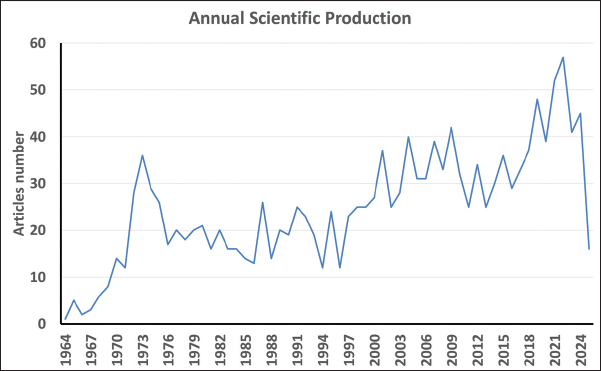 | Figure 1. The annual scientific production from 1964 to April 2025. [Click here to view] |
Most relevant sources
The Clinical Neuropharmacology, European Journal of Pharmacology, and Neurology emerged as the most active publication sources on amantadine. Journals focusing on both neurological and antiviral research, such as the Journal of Infectious Diseases and Movement Disorders, also showed strong contributions. Further details can be found in Table 1.
Table 1. Most relevant sources. Leading journals publishing amantadine-related research from 1964 to 2025.
| Sources | Articles |
|---|---|
| Clinical Neuropharmacology | 27 |
| European Journal of Pharmacology | 21 |
| Neurology | 21 |
| Journal of Infectious Diseases | 18 |
| Movement Disorders | 18 |
| Journal of Pharmacology and Experimental Therapeutics | 17 |
| Antimicrobial Agents and Chemotherapy | 16 |
| Journal of Neural Transmission | 15 |
| Brain Injury | 14 |
| Virology | 12 |
Author local impact
Key researchers have significantly shaped the research landscape surrounding amantadine. Among them, Z. Wang stands out with 18 publications since 2011, accumulating 567 citations and an h-index of 15, reflecting consistent scholarly impact. J. Wang, active since 2004, has authored 17 papers that have garnered 1,065 citations, with an h-index of 14, indicating a broad research reach. Another influential author, D.S. Sitar, has been publishing on the topic since 1978, contributing 31 papers and receiving 459 citations, with an h-index of 13. Other contributors can be found in Table 2.
Table 2. Authors’ local impact by H-index.
| Author | h_index |
|---|---|
| Wang Z | 15 |
| Wang J | 14 |
| Sitar DS | 13 |
| Rogóz Z | 12 |
| JR | 11 |
| Li Y | 11 |
| Wang S | 11 |
| Danysz W | 10 |
| Saito R | 10 |
| Hauser RA | 9 |
Most relevant affiliations
Amantadine research is widely distributed across global institutions, with China Agricultural University leading the field with 121 published articles. The Institute of Pharmacology follows with 55 publications, emphasizing drug development, while Niigata University in Japan has contributed 54 articles. Another major contributor from China, Tianjin University of Science and Technology, produced 49 articles. Further details are shown in Table 3.
Table 3. Most relevant affiliations.
| Affiliation | Articles |
|---|---|
| China Agricultural University | 121 |
| Institute of Pharmacology | 55 |
| Niigata University | 54 |
| Tianjin University of Science and Technology | 49 |
| Ocean University of China | 39 |
| Liaoning University | 36 |
| Shandong Marine Resource and Environment Research Institute | 34 |
| University of Pittsburgh | 33 |
| Tehran University of Medical Sciences | 32 |
| Islamic Azad University | 31 |
Corresponding authors’ countries and the country’s scientific production
Based on the affiliations of corresponding authors, the USA leads amantadine research with 170 articles, with 145 single-country and 25 multi-country publications (Table 4). China follows with 140 articles, including 132 single-country and eight multi-country efforts. Japan contributed 57 articles, Germany 56 (3.64%), and Iran 44 (2.86%). Poland (34 articles), Italy (32), Canada (30), India (21), and the UK (20) also feature prominently.
Table 4. Corresponding authors’ countries. The top 10 countries.
| Country | Number of articles | Percentage % | SCP | MCP | MCP % |
|---|---|---|---|---|---|
| USA | 170 | 11.04% | 145 | 25 | 14.71% |
| China | 140 | 9.09% | 132 | 8 | 5.71% |
| Japan | 57 | 3.70% | 54 | 3 | 5.26% |
| Germany | 56 | 3.64% | 43 | 13 | 23.21% |
| Iran | 44 | 2.86% | 40 | 4 | 9.09% |
| Poland | 34 | 2.21% | 29 | 5 | 14.71% |
| Italy | 32 | 2.08% | 25 | 7 | 21.88% |
| Canada | 30 | 1.95% | 24 | 6 | 20.00% |
| India | 21 | 1.36% | 20 | 1 | 4.76% |
| United Kingdom | 20 | 1.30% | 14 | 6 | 30.00% |
Most globally cited papers
The most cited papers on amantadine highlight its transition from an antiviral to a neurological therapeutic, significantly influencing clinical practice (Table 5) [23]. Davies et al. [22] received 695 citations (11.21/year) for demonstrating amantadine’s inhibition of influenza A virus replication. Giacino et al. [14] received 656 citations (46.86/year) from a trial showing that amantadine accelerated functional recovery in 184 patients with severe traumatic brain injury in vegetative or minimally conscious states. Schwab et al. [6] gained 598 citations (10.49/year) for reporting that 66% of 163 Parkinson’s disease patients experienced improvements in akinesia, rigidity, and tremor. Cady et al. [4] achieved 557 citations (34.81/year) by explaining amantadine’s binding to the influenza M2 proton channel through nuclear magnetic resonance spectroscopy. Verhagen Metman et al. [12] received 530 citations (18.93/year) by confirming that amantadine reduced levodopa-induced dyskinesias in advanced Parkinson’s disease. Further details are shown in Table 6.
Table 5. Highly cited articles on amantadine, highlighting foundational studies in antiviral and neurotherapeutic applications.
| Paper | DOI | Total Citations |
|---|---|---|
| Davies et al., 1964 Science [22] | 10.1126/science.144.3620.862 | 695 |
| Giacino et al., 2012, New Engl J Med [14] | 10.1056/NEJMoa1102609 | 656 |
| Schwab et al., 1969, JAMA [6] | 10.1001/jama.1969.03160070046011 | 598 |
| Cady et al., 2010, Nature [4] | 10.1038/nature08722 | 557 |
| Verhagen Metman et al., 1998, Neurology [12] | 10.1212/wnl.50.5.1323 | 530 |
| Wang et al., 1993, J Virol [5] | 10.1128/JVI.67.9.5585-5594.1993 | 462 |
| Dolin et al., 1982, New Engl J Med [23] | 10.1056/NEJM198209023071002 | 400 |
Trend topics
Trending topics include “Parkinson’s disease” (66 occurrences, median year 2010) and “amantadine” (606, median 2011), with early terms like “catecholamines” (median 1975), “traumatic brain injury” (2019), “COVID-19” (2021), and “coma” (2023). Topics like “dopamine” (38, median 2003), “glutamate” (27, median 2007), “antiviral resistance” (2012), and “neuroprotection” (2015) reflect evolving pharmacological and clinical focus, showcasing amantadine’s transition from antiviral to neurological and emerging applications (Table 6).
Table 6. Key recurring and emerging research topics in amantadine literature based on keyword frequency and median appearance year.
| Term | Frequency | Year (Q1) | Year (median) | Year (Q3) |
|---|---|---|---|---|
| Catecholamines | 9 | 1972 | 1975 | 2000 |
| Catalepsy | 5 | 1972 | 1979 | 1994 |
| Apomorphine | 7 | 1975 | 1983 | 1989 |
| L-dopa | 9 | 1973 | 1987 | 1995 |
| Reserpine | 7 | 1980 | 1989 | 1994 |
| Cocaine | 7 | 1988 | 1996 | 2004 |
| Parkinsonism | 8 | 1975 | 1999 | 2019 |
| Amantadine sulfate | 6 | 1996 | 2002 | 2007 |
| NMDA antagonist | 5 | 1997 | 2002 | 2016 |
| Dopamine | 38 | 1995 | 2003 | 2012 |
| Interferon | 16 | 2003 | 2003 | 2004 |
| Chronic hepatitis C | 25 | 2001 | 2004 | 2005 |
| Ribavirin | 24 | 2003 | 2004 | 2007 |
| Serotonin | 8 | 2002 | 2005 | 2010 |
| Microdialysis | 7 | 1998 | 2005 | 2008 |
| Memantine | 21 | 1998 | 2006 | 2012 |
| Influenza | 16 | 2000 | 2006 | 2008 |
| Glutamate | 27 | 2004 | 2007 | 2015 |
| Antiviral resistance | 5 | 2006 | 2007 | 2016 |
| Amantadine hydrochloride | 29 | 1996 | 2008 | 2020 |
| Amantadine sulfate | 7 | 2004 | 2008 | 2015 |
| NMDA | 9 | 2005 | 2009 | 2015 |
| Oseltamivir | 7 | 2008 | 2009 | 2011 |
| Parkinson's disease | 66 | 2000 | 2010 | 2018 |
| Influenza A virus | 13 | 2000 | 2010 | 2016 |
| Amantadine | 606 | 2001 | 2011 | 2020 |
| Levodopa | 7 | 2004 | 2011 | 2020 |
| Treatment | 15 | 2006 | 2012 | 2021 |
| Amantadine resistance | 14 | 2008 | 2012 | 2017 |
| Dyskinesia | 16 | 2009 | 2013 | 2020 |
| Drug resistance | 5 | 2007 | 2013 | 2017 |
| Rimantadine | 17 | 2007 | 2014 | 2018 |
| Depression | 7 | 1998 | 2015 | 2022 |
| Neuroprotection | 7 | 2006 | 2015 | 2023 |
| Cognition | 11 | 2008 | 2016 | 2020 |
| Crystal structure | 11 | 2010 | 2016 | 2018 |
| Parkinson’s disease | 11 | 2012 | 2017 | 2022 |
| Synthesis | 9 | 2013 | 2017 | 2019 |
| Adamantane | 7 | 2016 | 2018 | 2022 |
| Apoptosis | 7 | 2012 | 2018 | 2020 |
| Traumatic brain injury | 19 | 2015 | 2019 | 2021 |
| Morris water maze | 5 | 2019 | 2019 | 2024 |
| Drug delivery | 6 | 2018 | 2020 | 2022 |
| Disorders of consciousness | 5 | 2020 | 2020 | 2022 |
| Covid-19 | 17 | 2021 | 2021 | 2022 |
| Multiple sclerosis | 14 | 2014 | 2021 | 2022 |
| Adsorption | 8 | 2018 | 2023 | 2023 |
| Coma | 6 | 2016 | 2023 | 2024 |
The global collaboration networks
The global collaboration network for amantadine research reveals a vibrant, interconnected scientific community (Fig. 2). The United States leads with strong ties, notably 13 collaborations each with Canada and Germany, 12 with the United Kingdom, and 10 with China, reflecting strong transatlantic and transpacific partnerships. Germany also emerges as a key hub, collaborating frequently with Greece (6), Canada (5), and the UK (4). European connections exist, with the UK and France sharing five collaborations, and Spain and Belgium linking four times. Emerging research hubs like Iran and Iraq (four collaborations) and India and Saudi Arabia (3) highlight growing contributions from the Middle East and South Asia.
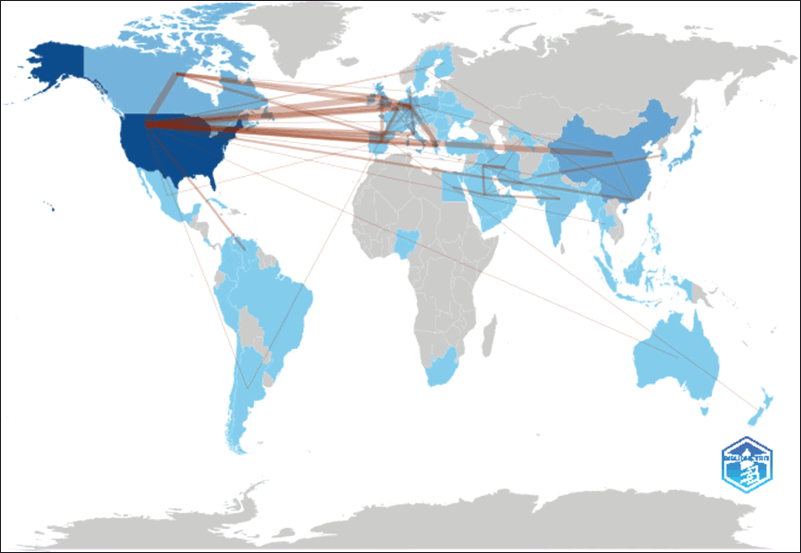 | Figure 2. The global collaboration networks. [Click here to view] |
Visualization of keyword co-occurrence in amantadine research
Figure 3 displays a co-occurrence network of keywords in amantadine research, organized into five distinct color-coded clusters. The graph is centered on “amantadine.” The blue cluster links to molecular and animal studies, encompassing “rat,” “ion transport,” “dopamine,” “hippocampus,” and “protein expression.” The red cluster focuses on neurological aspects, featuring “pain,” “hallucinations,” and “pathophysiology.” The green cluster highlights antiviral research, with “influenza,” “antiviral agent,” and “virus detection” as prominent terms. The yellow cluster focuses on clinical trials and effects, including “controlled clinical trial,” “vomiting,” and “drug effect.”
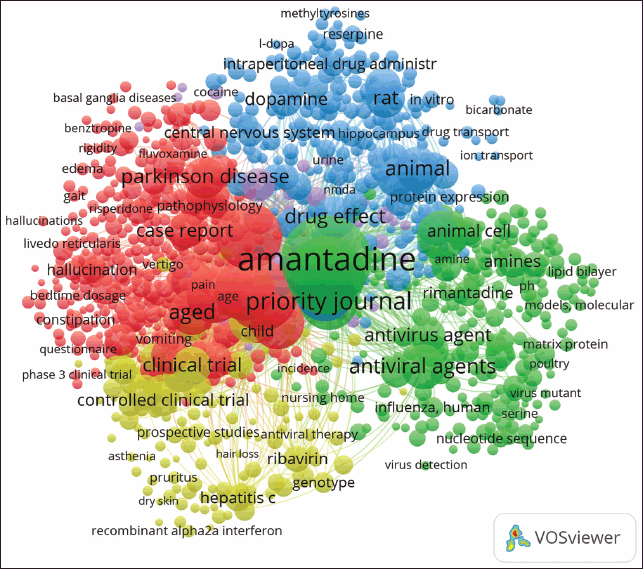 | Figure 3. Overlay visualization of keyword co-occurrence in amantadine research. [Click here to view] |
DISCUSSION
The bibliometric analysis of amantadine research spanning 1964 to April 2025 illuminates a dynamic and evolving field, marked by significant growth in publication output, international collaboration, and diversification of therapeutic applications. The study revealed a steady increase in research productivity, particularly since the early 2000s, with a peak in 2022, reflecting heightened global interest in amantadine’s multifaceted pharmacological properties. The increase in publications aligns with expanded clinical applications [24], from its initial role as an antiviral agent [25,26] to its established use in neurological disorders and emerging potential in neuroprotection and anti-inflammatory contexts [27]. A notable surge in amantadine-related publications began in 2004 and continued through the following two decades, peaking in 2022. Several factors may explain this rise. First, renewed clinical attention was directed toward amantadine’s efficacy in managing Parkinson’s disease [11,28]. Second, the emergence of viral epidemics—including SARS, H1N1, and COVID-19 [16,29,30]—revived interest in amantadine’s antiviral properties and repurposing potential.
A key finding is the pivotal role of international collaboration in driving amantadine research. The United States, China, and Japan emerge as leading contributors, with robust networks connecting North America, Europe, and Asia. These collaborations have facilitated knowledge exchange, as evidenced by the high proportion of multi-country publications. The prominence of institutions such as China Agricultural University in amantadine-related research may reflect multiple converging factors. As a leading agricultural and life sciences university, China Agricultural University likely engages in both experimental pharmacology and antiviral research related to animal health, where amantadine has applications in veterinary virology.
The thematic evolution of amantadine research highlights its transition from antiviral applications to neurological and neuroprotective roles. Early studies, such as Davies et al. [23], focused on influenza A prophylaxis, laying the foundation for amantadine’s antiviral legacy. Subsequent decades saw a shift toward neurological applications, with landmark studies like Schwab et al. [6] and Giacino et al. [14] establishing its efficacy in Parkinson’s disease and traumatic brain injury, respectively. More recent trends, particularly post-2010, emphasize neuroprotection and anti-inflammatory effects [16,24]. Emerging topics like COVID-19 and coma reflect amantadine’s adaptability to contemporary health challenges [16], though these areas remain underexplored compared to established themes like Parkinson’s disease and dopamine modulation.
The thematic clusters identified in the keyword co-occurrence analysis provide insight into the intellectual structure of the field. A cluster centered on “amantadine” and “Parkinson’s disease,” [6,12,28] represents the core of clinical research, while another underscores the enduring relevance of antiviral studies [22,24]. Additional clusters focusing on neurological mechanisms and clinical trial outcomes highlight the field’s interdisciplinary nature [29,30]. A separate cluster with terms like “rat” and “protein expression” points to a growing emphasis on preclinical and molecular studies, which could pave the way for novel derivatives with enhanced selectivity [31].
In a clinical context, the thematic clusters underscore amantadine’s dual role as both an antiviral and neurotherapeutic agent. The green cluster, centered on antiviral terms such as “influenza” and “antiviral agent,” reflects its well-documented action as an influenza A treatment. The reappearance of these terms in recent years, alongside “COVID-19,” suggests renewed interest in repurposing amantadine amid emerging viral threats. The red and blue clusters, rich in neurological and molecular terms such as “dopamine,” “NMDA,” “hallucinations,” and “ion transport,” mirror amantadine’s pharmacodynamic effects on dopaminergic transmission and NMDA receptor antagonism. These mechanisms underlie its clinical efficacy in managing motor symptoms and dyskinesia in Parkinson’s disease and cognitive dysfunction post-traumatic brain injury. The inclusion of “pain” and “pathophysiology” points to its expanding application in neuroinflammation and central sensitization, with growing preclinical evidence supporting its anti-inflammatory effects via microglial modulation and cytokine suppression. The yellow cluster, focusing on terms like “controlled clinical trial” and “drug effect,” aligns with efforts in assessing amantadine in structured clinical settings, including trials for fatigue in multiple sclerosis, disorders of consciousness, and post-viral fatigue syndromes.
Despite its strengths, this study has limitations. A notable limitation of this study is the exclusive reliance on the Scopus database. While Scopus offers comprehensive coverage of peer-reviewed literature, it may omit relevant articles indexed in other platforms or regional databases. This could result in an underrepresentation of publications from grey literature. The focus on English-language publications may also overlook valuable studies in other languages, such as Spanish, Chinese, or Japanese, which could offer unique perspectives. Future studies could complement this analysis with qualitative assessments to evaluate real-world applications.
The analysis identifies several research gaps and future directions. While neurological applications are well-developed, molecular and pharmacogenomic studies remain underrepresented. Further exploration of genetic factors influencing amantadine response could personalize treatment strategies, particularly for Parkinson’s disease and traumatic brain injury [32]. The potential of amantadine in neuroinflammatory and neurodegenerative conditions, supported by in vitro evidence of cytokine modulation, warrants larger clinical trials [31]. Additionally, the limited focus on amantadine’s role in emerging viral diseases, such as COVID-19, suggests an opportunity to reconsider its antiviral properties in the context of global health threats.
CONCLUSION
This bibliometric analysis of amantadine research from 1964 to 2025 reveals a significant and evolving scientific work spanning antiviral, neurological, and emerging therapeutic applications. The publication trend shows steady growth over six decades, with a marked increase in output during the 2000s and a peak in 2022, reflecting sustained global interest. The thematic analysis identified major clusters, including molecular/preclinical studies, neurological mechanisms, antiviral research, and clinical trials assessing drug effects. Highly cited papers and keyword trends demonstrate a progressive shift in research focus from amantadine’s traditional antiviral use toward neurotherapeutic indications, including its role in dopamine modulation, NMDA antagonism, and neuroprotection. The United States and China led in scientific productivity and international collaboration, while key institutions such as China Agricultural University and the University of Pittsburgh were prominent contributors. Despite extensive research into neurological disorders and antiviral efficacy, underexplored areas remain, particularly pharmacogenomic profiling, its role in COVID-19 treatment, and the development of novel derivatives.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This work was supported by the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia (Grant No. KFU251884).
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Martin WJ, Rabinowitz LG. Amantadine. In: Adam MP, Everman DB, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, et al., editors. GeneReviews® [Internet]. Seattle, WA: University of Washington, Seattle; 1993.
2. Zhirnov OP, Klenk HD, Wright PF. Aprotinin and similar protease inhibitors as drugs against influenza. Antiviral Res. 2011;92(1):27–36. CrossRef
3. De Clercq E, Li G. Approved antiviral drugs over the past 50 years. Clin Microbiol Rev. 2016;29(3):695–747. CrossRef
4. Cady SD, Schmidt-Rohr K, Wang J, Soto CS, Degrado WF, Hong M. Structure of the amantadine binding site of influenza M2 proton channels in lipid bilayers. Nature 2010;463(7281):689–92. CrossRef
5. Wang C, Takeuchi K, Pinto LH, Lamb RA. Ion channel activity of influenza A virus M2 protein: characterization of the amantadine block. J Virol. 1993;67(9):5585–94. CrossRef
6. Schwab RS, England AC Jr, Poskanzer DC, Young RR. Amantadine in the treatment of Parkinson’s disease. JAMA 1969;208(7):1168–70. CrossRef
7. Dekundy A, Pichler G, El Badry R, Scheschonka A, Danysz W. Amantadine for traumatic brain injury-supporting evidence and mode of action. Biomedicines 2024;12(7):1558. CrossRef
8. Heikkila RE, Cohen G. Evaluation of amantadine as a releasing agent or uptake blocker for H3-dopamine in rat brain slices. Eur J Pharmacol. 1972;20(2):156–60. CrossRef
9. Blanpied TA, Clarke RJ, Johnson JW. Amantadine inhibits NMDA receptors by accelerating channel closure during channel block. J Neurosci. 2005;25(13):3312–22. CrossRef
10. Peeters M, Page G, Maloteaux J-M, Hermans E. Hypersensitivity of dopamine transmission in the rat striatum after treatment with the NMDA receptor antagonist amantadine. Brain Res. 2002;949(1):32–41. CrossRef
11. Crosby NJ, Deane KH, Clarke CE. Amantadine for dyskinesia in Parkinson’s disease. Cochrane Database Syst Rev. 2003;2003(2):Cd003467. CrossRef
12. Verhagen Metman L, Del Dotto P, van den Munckhof P, Fang J, Mouradian MM, Chase TN. Amantadine as treatment for dyskinesias and motor fluctuations in Parkinson’s disease. Neurology 1998;50(5):1323–6. CrossRef
13. Rosenberg GA, Appenzeller O. Amantadine, fatigue, and multiple sclerosis. Arch Neurol. 1988;45(10):1104–6. CrossRef
14. Giacino JT, Whyte J, Bagiella E, Kalmar K, Childs N, Khademi A, et al. Placebo-controlled trial of amantadine for severe traumatic brain injury. N Engl J Med. 2012;366(9):819–26. CrossRef
15. Unluer C, Bektasoglu PK, Erguder BI, Arikok AT, Ermutlu I, Gurer B, et al. Amantadine’s neuroprotective effects in rabbit spinal cord ischemia/reperfusion model. Turk Neurosurg. 2024;34(6):1133. CrossRef
16. Jiménez-Jiménez FJ, Alonso-Navarro H, García-Martín E, Agúndez JAG. Anti-inflammatory effects of amantadine and memantine: possible therapeutics for the treatment of Covid-19? J Pers Med. 2020;10(4):217. CrossRef
17. Takeda M, Pekosz A, Shuck K, Pinto LH, Lamb RA. Influenza A Virus M2 ion channel activity is essential for efficient replication in tissue culture. J Virol. 2002;76(3):1391–9. CrossRef
18. Bryson YJ, Monahan C, Pollack M, Shields WD. A prospective double-blind study of side effects associated with the administration of amantadine for influenza A virus prophylaxis. J Infect Dis. 1980;141(5):543–7. CrossRef
19. Montazeri A, Mohammadi S, Hesari PM, Ghaemi M, Riazi H, Sheikhi-Mobarakeh Z. Preliminary guideline for reporting bibliometric reviews of the biomedical literature (BIBLIO): a minimum requirements. Syst Rev. 2023;12(1):239. CrossRef
20. Van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010;84(2):523–38. CrossRef
21. Darvish H. Bibliometric analysis using bibliometrix an R package. J Scientometric Res. 2020;8:156–60. CrossRef
22. Davies WL, Grunert RR, Haff RF, McGahen JW, Neumayer EM, Paulshock M, et al. Antiviral activity of 1-adamantanamine (amantadine). Science 1964;144(3620):862–3. CrossRef
23. Dolin R, Reichman RC, Madore HP, Maynard R, Linton PN, Webber-Jones J. A controlled trial of amantadine and rimantadine in the prophylaxis of influenza A infection. N Engl J Med. 1982;307(10):580–4.
24. Danysz W, Dekundy A, Scheschonka A, Riederer P. Amantadine: reappraisal of the timeless diamond-target updates and novel therapeutic potentials. J Neural Transm (Vienna). 2021;128(2):127–69. CrossRef
25. Torre F, Campo N, Giusto R, Ansaldi F, Icardi GC, Picciotto A. Antiviral activity of amantadine in elderly patients with chronic hepatitis C. Gerontology 2001;47(6):330–3. CrossRef
26. Sasaki-Tanaka R, Shibata T, Moriyama M, Okamoto H, Kogure H, Kanda T. Amantadine and rimantadine inhibit hepatitis A virus replication through the induction of autophagy. J Virol. 2022;96(18):e0064622. CrossRef
27. Hofmann A, Blum C, Single C, Adeyemi K, Schwarz P, Siokas V, et al. Amantadine for Neuroenhancement in Acute Patients Study- a protocol for a prospective pilot proof of concept phase IIb study in intensive and intermediate care unit patients (ANNES). BMC Neurol. 2023;23(1):308. CrossRef
28. Sharma VD, Lyons KE, Pahwa R. Amantadine extended-release capsules for levodopa-induced dyskinesia in patients with Parkinson’s disease. Ther Clin Risk Manag. 2018;14:665–73. CrossRef
29. Harandi AA, Pakdaman H, Medghalchi A, Kimia N, Kazemian A, Siavoshi F, et al. A randomized open-label clinical trial on the effect of Amantadine on post Covid 19 fatigue. Sci Rep. 2024;14(1):1343. CrossRef
30. Weis N, Bollerup S, Sund JD, Glamann JB, Vinten C, Jensen LR, et al. Amantadine for COVID-19 treatment (ACT) study: a randomized, double-blinded, placebo-controlled clinical trial. Clin Microbiol Infect. 2023;29(10):1313–9. CrossRef
31. Pi?tkowska-Chmiel I, Gawro?ska-Grzywacz M, Popio?ek ?, Herbet M, Dudka J. The novel adamantane derivatives as potential mediators of inflammation and neural plasticity in diabetes mice with cognitive impairment. Sci Rep. 2022;12(1):6708. CrossRef
32. Liu JS, Chen Y, Shi DD, Zhang BR, Pu JL. Pharmacogenomics—a new frontier for individualized treatment of Parkinson’s disease. Curr Neuropharmacol. 2023;21(3):536–46.