INTRODUCTION
The most popular route of drug administration is oral, and its systemic distribution has been studied on the basis of several pharmacological yields in a range of dosage forms. Conventional preparations are often supplied often in numerous dose regimens as long-term remedies for the treatment of chronic illnesses and thereby have numerous negative impacts. They are cost-effective, frequently patients, have widespread regulatory acceptance, reduce the dose, maintain drug therapeutic concentrations, have side effects, and increase the efficacy of increased therapeutic agents [1].
The responsibilities of pharmaceutical development scientists encompass all the necessary tasks involved in transforming an active pharmaceutical ingredient (API) into a marketable product that is suitable for handling, distribution, and patient administration [2]. Hence, in contemporary pharmaceutical development, increasing the solubility and dissolution rate of drugs with poor water solubility is increasingly important to increase the bioavailability of potential drug candidates. The process of crystallization is useful for enhancing these pharmacological characteristics [3]. Crystals are described by the Food and Drug Administration as solid supramolecular complexes in crystalline form made up of two or more components that are in a neutral state and are held together by nonionic interactions [4]. Pharmaceutical cocrystals have complex crystalline structures and are made up of an API and a coformer approved by the pharmaceutical industry (Fig. 1). Molecular docking is a valuable technique in computer-aided structure-based drug discovery. This method involves predicting the binding possibility and orientation of one molecule API with another molecule (co-former) to form a new complex. The binding ability between the two molecules can be assessed through scoring features [5]. In the case of fenofibrate−atorvastatin calcium-para aminobenzoic acid multidrug cocrystals, five coformers were selected based on the existing literature [6].
 | Figure 1. Cocrystals formation. [Click here to view] |
Multidrug cocrystals (MDCs) are solid crystalline complexes with two or more therapeutically active substances present in a predetermined ratio inside a single crystal lattice [7]. These elements generally communicate with one another through nonionic interactions, while they occasionally engage in hybrid interactions that combine partial proton transfer and hydrogen bonding with ionic and nonionic interactions [8]. MDCs have the potential to provide advantages such as increased bioavailability, improved dissolution and solubility as a minimum of one constituent, and synergistic and/or additive effects [9].
In this study, novel drug?drug cocrystals composed of atorvastatin calcium (ATC) and fenofibrate (FE) were formulated and characterized.
Fenofibrate is a 2-{4-[4-chlorobenzoyl] phenoxy} (2-methyl propanoic acid 1-methyl ethyl ester). It is a Biopharmaceutical Classification System (BCS) class II medication with low water solubility but excellent permeability. Fenofibrate is effective in regulating blood cholesterol or lipid levels in individuals with normal lipid profiles as well as those with hyperlipoproteinaemia [10]. Its bioavailability ranges from 60% to 1%. Fenofibrate is classified as a prodrug of fenofibric acid, which becomes active after undergoing hydrolysis by plasma esterase enzymes. By altering the levels of very-low-density lipoprotein, low-density lipoprotein, and high-density lipoprotein, Fenofibrate effectively reduces cholesterol proportions [11]. This approach is particularly beneficial for patients with cardiovascular disease and metabolic disorders associated with insulin resistance. When used in conjunction with statins, it significantly decreases hypercholesterolemia and hypertriglyceridemia. The primary indications for fenofibrate use are lipid-related conditions such as severe hypercholesterolemia, hypertriglyceridemia, and dyslipidemia [12].
Atorvastatin, also known as (3R,5R)-7-[2-(4-fluorophenyl)-3-phenyl-4-(phenylcarbamoyl)-5-propan-2-ylpyrrol-1-yl]-3,5 dihydroxyheptanoic acid, is a substance whose method of action is known as an 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitor, and 3,5 dihydroxyheptanoic acid is one of those substances [13]. The hemi-calcium salt of atorvastatin, atorvastatin calcium, dissolves relatively poorly in water, phosphate buffers with a pH of 7.4, and acetonitrile, although it is very soluble in methanol [14]. A class II medication with high permeability and low water solubility, atorvastatin, is categorized according to the BCS [15]. Atorvastatin has high intestinal permeability at the normal pH of the intestines (6–6.5) [16]. It serves as a model for medications falling under Class II of the BCS that are poorly soluble. The medication inhibits HMG-CoA reductase, an enzyme that is essential for the production of cholesterol by converting HMG-CoA to mevalonate [17]. This inhibition is competitive and reversible [18]. Since it has a low water solubility and undergoes considerable hepatic degradation, its oral bioavailability is low (12%). This study presents the first successful synthesis and comprehensive characterization of fenofibrate–atorvastatin calcium cocrystals, aiming to enhance the physicochemical properties and therapeutic performance of the individual drugs. The novelty lies in the strategic combination of two lipid-lowering agents into a single multicomponent crystal system, offering improved solubility, dissolution rate, and potential bioavailability compared to their standalone forms. This work pioneers the application of pharmaceutical cocrystallization for dual-drug delivery in dyslipidemia management, offering a promising platform for fixed-dose combination therapies with enhanced patient compliance and therapeutic synergy.
MATERIAL AND METHODS
Material
Atorvastatin calcium was obtained from Cadila Healthcare Ltd. (Ahmedabad, India), and fenofibrate was obtained from Emcure Pharmaceuticals (Pune, India) as a gift sample. Para-aminobenzoic acid was collected from Analab Fine Chemicals, Mumbai. Xanthan gum, sodium alginate, magnesium stearate, and microcrystalline cellulose were purchased from Analab Fine Chemicals. Ethanol and methanol (HPLC grade) were obtained from New Neeta Chemicals. All materials were used without further purification.
Methods
Molecular docking and selection of coformers
Molecular docking studies were conducted on these five coformers to evaluate their binding affinity. To visually compare the quality of the docking scores for the coformers, a radar chart was utilized, which presents multidimensional information without relying on statistical methods. Among the five coformers, para-aminobenzoic acid was confirmed as a suitable choice based on its potential for interaction, compatibility, and docking score in forming cocrystals with atorvastatin calcium and fenofibrate [19].
Solvent-assisted grid method of cocrystallization for fenofibrate and atorvastatin calcium
Liquid-assisted grinding is a technique that involves adding a small amount of solvent to dry solids before the milling process begins. The solvent is required throughout the grinding process since it catalyzes the production of cocrystals. Compared with neat methods, liquid-assisted methods are believed to result in more efficient cocrystal formation [20]. For the preparation of multidrug cocrystals, predetermined concentrations of each drug (FE and ATC) and conformer para-aminobenzoic acid (PABA) (in the molar ratio of 0.25:1:1) were combined and mixed for 30 minutes [21] with a mortar and pestle. During the grinding process, a few drops of ethanol were added to the powder mixture. After grinding, the mixture was left to dry naturally for 2 days to allow the solvent to evaporate. The crystals that resulted from this process were crushed in a mortar and pestle and kept at room temperature (Fig. 2).
 | Figure 2. Preparation of FE−ATC−PABA multidrug cocrystals by liquid-assisted grinding method. [Click here to view] |
Characterization of fenofibrate−atorvastatin calcium−para-aminob–enzoic acid multidrug Cocrystals
Thermal characterization
DSC was performed using the DSC7020 thermal analysis system (HITACHI) to evaluate the thermal properties of fenofibrate–atorvastatin calcium–para-aminobenzoic acid multidrug cocrystals. Approximately 2–5 mg of the sample was placed in an aluminum pan and heated from 30?°C to 300?°C under a nitrogen atmosphere at a flow rate of 40?ml/minute to observe melting, crystallization, and glass transition events [22].
Fourier transform infrared spectroscopy (FT-IR)
From 4,000 cm-1 to 400 cm-1, FT-IR spectra were recorded on a Nicolet iS10 spectrophotometer (Precisa XM60). A DTGS KBr detector was used to gather and examine the prepared multidrug cocrystals [23].
Powder X-ray diffraction (PXRD)
Fenofibrate–atorvastatin calcium–para-aminobenzoic acid multidrug cocrystals PXRD patterns were obtained via a silicon sample holder and an Ultima IV system operating at room temperature. Each sample was mounted on a motorized goniometer head that allowed it to spin while the data were being collected, and the instrument was fitted with a fine-focus X-ray tube [24]. At a voltage of 40.0 kV and a current of 40.0 mA, the samples were irradiated with Cu-K (α) radiation that had been Ni-filtered. At a diffraction angle of 2o/minute, the scanning rate was varied from 3º to 50º. After the values for 2 were obtained, a graph was drawn [25].
Scanning electron microscopy (SEM)
Scanning electron microscopy was used to inspect the surface appearance and structure of cocrystals of fenofibrate, atorvastatin calcium, para-aminobenzoic acid (JEOL FESEM FEI Nova NanoSEM 450). Samples of fenofibrate−atorvastatin calcium−para-aminobenzoic acid cocrystals were sheltered with a thin layer of gold−palladium via a sputter-coated unit, and mounted on double-faced adhesive tape, and the surface topography was studied. At 100X and 1,000X, different resolutions of images were acquired [26].
Solubility studies (saturation solubility)
To determine the saturation solubility’s of fenofibrate and atorvastatin calcium, powder equivalent to 10 mg of fenofibrate and atorvastatin calcium was added to a screw-capped bottle containing 10 ml of phosphate buffer (pH 7.4). The suspensions were stirred continuously for 72 hours at 750 rpm via a magnetic stirrer. After the stirring process was complete, 1ml of solution was taken and diluted to 10 ml with phosphate buffer at pH 7.4, and the aliquots were filtered with Whatman’s filter paper No. 41. The samples were then evaluated spectrometrically via a doubled beam UV spectrophotometer (Lab India 3200 UV Double Beam Spectrophotometer, Jasco) at 286 and 246 nm and the results were validated statistically [27].
Dissolution study
A dissolution rate study of fenofibrate−atorvastatin calcium−para-aminobenzoic acid multidrug cocrystals powder was performed via the USP apparatus II paddle type (LabIndia, model DS 8000, 2014). A total of 900 ml of phosphate buffer solution with a pH of 7.4 was used for the dissolution test. An equivalent of 10 mg of FE or ATC was transferred to each dissolving flask, and the stirring speed was adjusted to 75 rpm [28]. For 60 minutes, the temperature was maintained at 37°C ?±? 0.5°C. Samples from the dissolution medium were taken at predetermined intervals of 5, 10, 15, 30, 45, and 60 minutes. After each time interval, the dissolution media was changed to maintain a steady concentration gradient [29]. After the samples were passed through Whatman filter paper no. 41, the absorbance was determined via a UV spectrophotometer at wavelengths of 286 nm and 246 nm. A three-replicate study was conducted to ensure accuracy and reliability [30].
RESULT AND DISCUSSION
Evaluation of coformers
The goal of the current work was to improve the dissolution profile of two poorly soluble drugs, ATC and FE, through cocrystallization using coformers. This study emphasizes how important it is to choose the right coformer when creating a stable, improved cocrystal. To choose the best coformer, the binding energy and hydrogen bonding characteristics were taken into consideration. The coformer with the lowest binding energy was determined to be the most advantageous conformation via the docking search technique. Additionally, hydrogen bond interactions are estimated and described; the presence of H-bonds indicates a stable association between the ligand and receptor−ligand. The receptor−ligand and ligand interactions exhibit strong binding energies. Para-aminobenzoic acid, malonic acid, succinic acid, citric acid, and ascorbic acid were selected as coformers in this investigation. The coformer for creating cocrystals of fenofibrate and atorvastatin calcium was chosen according to the possibility of interaction, namely hydrogen bonding, docking score, and compatibility. The table given contains condensed information. Figure 3 shows a radar chart-based visual comparison of the docking scores. A radar chart has several axes that indicate several categories, and points are displayed along each axis to reflect the data. A data series can be produced by connecting the points. A point on an axis that is nearer the center represents a lower value, whereas a point that is farther from the center indicates a higher value.
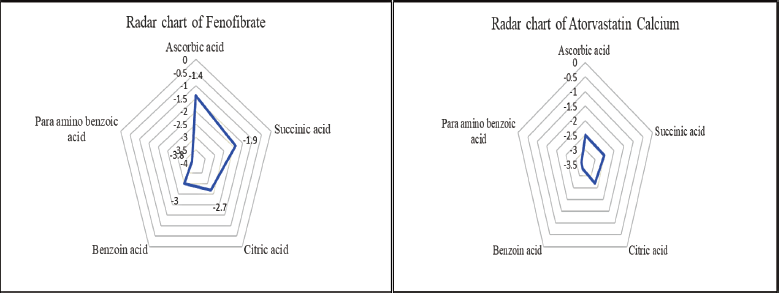 | Figure 3. Application of radar chart to evaluate the Docking Score of selected coformers for preparation of cocrystals. [Click here to view] |
An analysis of the radar charts and docking scores derived from molecular modeling revealed that para-aminobenzoic acid had the least favorable score (Fig. 4). The interaction between the API and coformer is determined by van der Waals forces and electrostatic energy. A higher docking score indicates a greater potential for repulsion, whereas a lower docking score signifies a reduced potential (Tables 1 and 2).
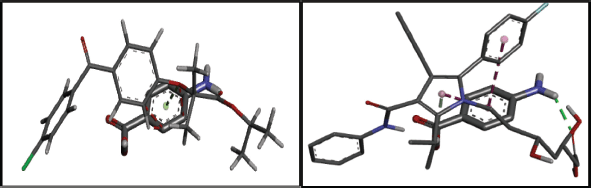 | Figure 4. Hydrogen bonding of fenofibrate and atorvastatin calcium with para-aminobenzoic acid. [Click here to view] |
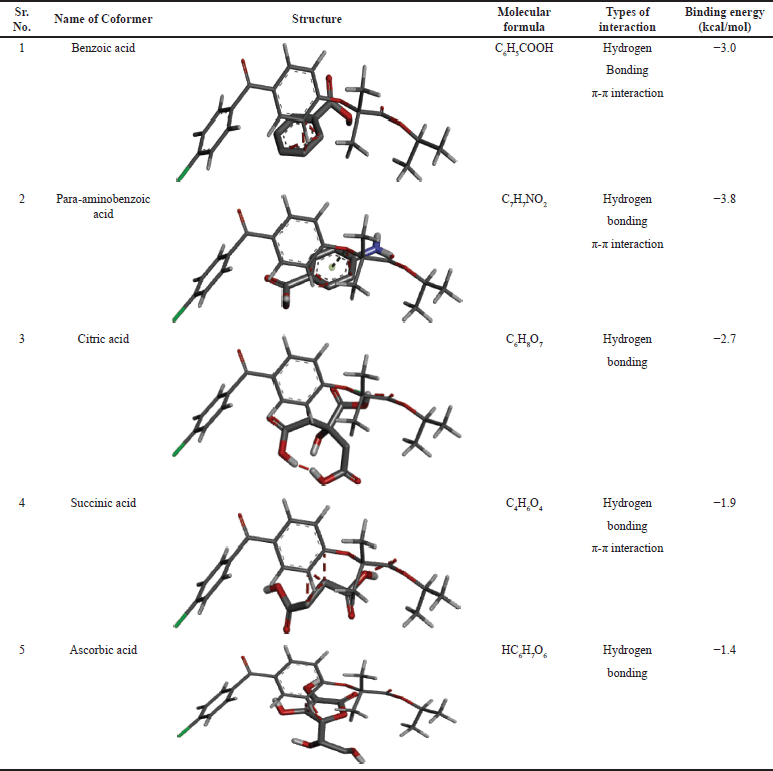 | Table 1. Virtual screening of coformers using molecular docking for fenofibrate. [Click here to view] |
 | Table 2. Virtual screening of coformers using molecular docking for atorvastatin calcium. [Click here to view] |
The utilization of ethanol as a solvent and PABA as a coformer in solvent-assisted grinding yielded the satisfactory formation of FE-ATC-PABA cocrystals. The initial evaluation of cocrystal formation involved comparing the properties of the pure drug with those of the cocrystals.
Thermal evaluation of cocrystals
The differential scanning calorimeter (DSC 7020 thermal analysis system HITACHI) is a method for thermal analysis that can be used to detect melting and crystallization events as well as the glass transition temperature. It could be used to identify melting and crystallization events. The DSC thermograms of fenofibrate and atorvastatin calcium showed sharp melting endotherms at 80.900C and 150.700C, respectively. The melting point of the cocrystals (77.60°C) differed noticeably from that of the pure drugs. As shown in the Figure, the cocrystals presented distinct acute endothermic peaks at lower temperatures, whereas the individual components did so at higher temperatures. DSC studies successfully confirmed the formation of fenofibrate–atorvastatin calcium–PABA cocrystals by showing the disappearance of the pure drug’s melting peaks and the appearance of new thermal events. The altered thermal properties of the cocrystals, coupled with their improved solubility and dissolution, suggest that the cocrystallization method effectively enhances the physicochemical properties of these drugs, making them a promising approach for improving their clinical efficacy. This finding suggests that cocrystal formation and component interactions may be possible (Fig. 5).
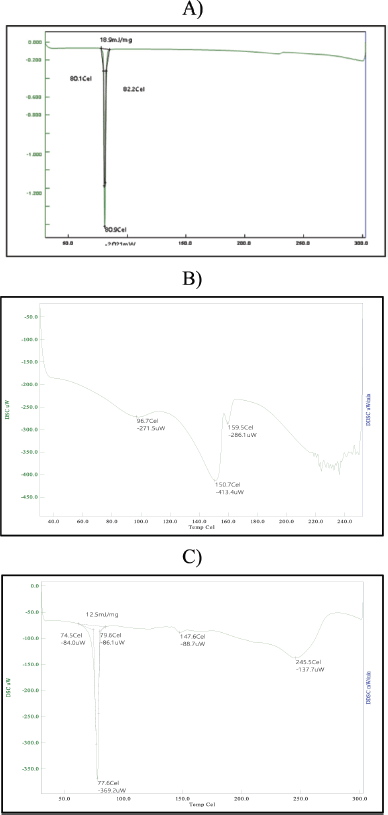 | Figure 5. Differential scanning colorimetry of A) fenofibrate, B) atorvastatin calcium, and C) fenofibrate−atorvastatin calcium cocrystals. [Click here to view] |
Fourier transform infrared spectroscopy
As shown in the image, the FTIR analysis revealed shifts and fluctuations in the peak intensities of both FE and ATC and associated cocrystals. The presence of hydrogen bonding in the cocrystals was shown by changes in the O-H peak strength, which decreased. In addition, a decrease in the frequency of N-H bond stretching revealed that hydrogen bonding was present in the cocrystals. The role of the degree and strength of the hydrogen bond is to reduce the frequency (Fig. 6).
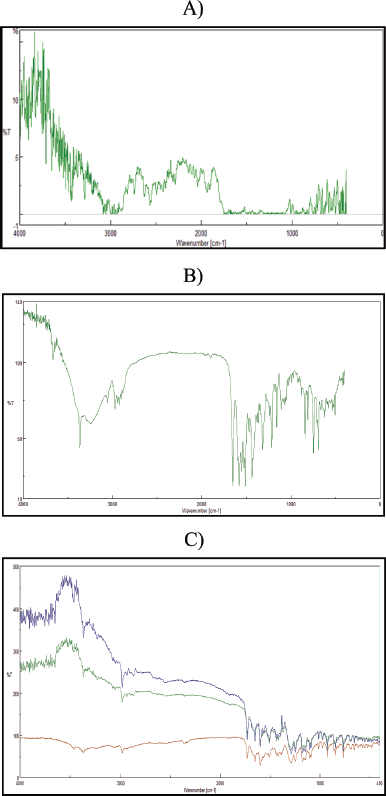 | Figure 6. FITR graph of A) fenofibrate, B) atorvastatin−calcium, and C) overlay of fenofibrate−atorvastatin calcium cocrystals. [Click here to view] |
Powder X-ray diffraction
The figure displays the X-ray diffraction patterns for fenofibrate and atorvastatin calcium. Both APIs exhibit distinct, sharp peaks with varying intensities, indicating their crystalline nature. The degree of crystallinity and the interactions between the materials in the prepared cocrystals were examined via PXRD. Compared with the individual components, such interactions can result in the emergence of new diffraction peaks. The PXRD spectra of the cocrystals differ from those of the individual components, confirming the creation of a novel phase. Furthermore, the reduced crystallinity of the APIs in the cocrystals may have contributed to the improved dissolution of the prepared samples (Fig. 7).
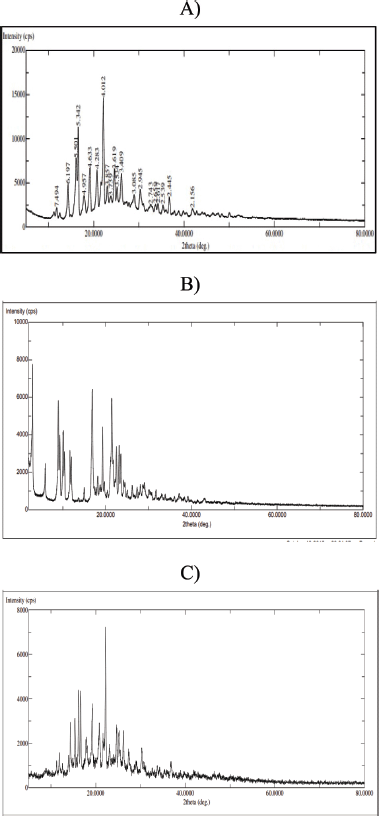 | Figure 7. PXRD spectra of A) fenofibrate, B) atorvastatin calcium, and C) fenofibrate−atorvastatin calcium cocrystals. [Click here to view] |
FTIR and PXRD analysis: peak values and interpretation
In the FTIR spectra, fenofibrate showed characteristic peaks at 1,735 cm?¹ (C=O stretching) and 1,242 cm?¹ (C–O stretching), while atorvastatin calcium displayed peaks at 3,360 cm?¹ (O–H stretching) and 1,650 cm?¹ (C=O stretching). Upon cocrystallization with PABA, notable shifts were observed—such as the C=O stretching peak shifting to 1,720 cm?¹ and broadening of the O–H band, indicating hydrogen bond formation and molecular interactions between drugs and the co-former.
In PXRD analysis, pure fenofibrate showed sharp peaks at 2θ = 4.7°, 12.3°, and 17.8°, and atorvastatin calcium showed distinct peaks at 2θ = 9.2°, 16.3°, and 22.7°. The cocrystals, however, exhibited new peaks at 2θ = 10.5°, 15.1°, and 20.3°, along with reduced intensity or disappearance of original peaks, confirming the formation of a new crystalline phase. The appearance of blended and less intense peaks also suggests partial amorphization.
Together, the shifts in FTIR peaks and the changes in PXRD patterns clearly indicate successful cocrystal formation, structural transformation, and strong intermolecular interactions between the drug molecules and PABA, contributing to improved solubility and dissolution. It is explained in Tables 3 and 4.
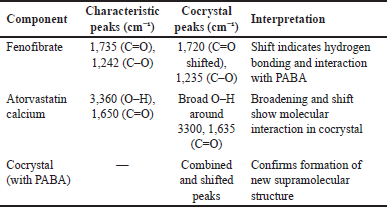 | Table 3. FTIR spectral data of pure drugs and cocrystals with interpretation. [Click here to view] |
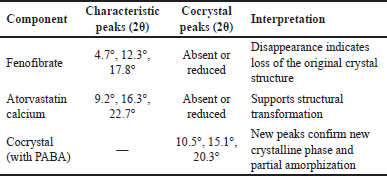 | Table 4. PXRD peak comparison of pure drugs and cocrystals with interpretation. [Click here to view] |
Scanning electron microscopy
Figures 8, 9, and 10 display SEM images of fenofibrate and atorvastatin calcium at various magnifications. The SEM images clearly reveal the morphological characteristics of the pure materials as well as their cocrystallization products. The morphology of the prepared cocrystals, observed through SEM analysis, showed irregular spherical shapes, which were significantly different from the well-defined crystalline structures of the pure drugs. This change in surface structure indicates a transformation during the cocrystallization process. The use of para-aminobenzoic acid as a coformer and the liquid-assisted grinding method led to partial amorphization, resulting in a more disordered, less crystalline surface. This is further supported by PXRD data, where the cocrystals exhibited blended and less intense peaks compared to the sharp peaks of pure drugs, confirming the formation of a new, partially amorphous solid phase with improved solubility characteristics.
 | Figure 8. SEM photographs of fenofibrate a) at ×500 magnification and b) at ×2,000 magnification. [Click here to view] |
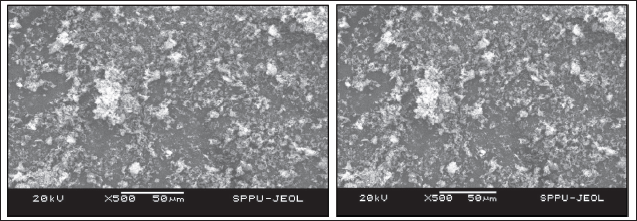 | Figure 9. SEM photographs of atorvastatin calcium a) at ×500 magnification and b) at ×1,000 magnification. [Click here to view] |
 | Figure 10. SEM photographs of fenofibrate−atorvastatin calcium cocrystals a) at ×1,000 magnification and b) at ×2,700 magnification. [Click here to view] |
In vitro dissolution study
An in vitro study was conducted to determine how the produced formulations behaved during dissolution in vitro. After taking medication into account for 60 minutes, the amount of medication released is shown in the Figure. According to the results, the ATC: FE: PABA (0.25:1:1) cocrystal batch showed the greatest drug release (Fig. 11).
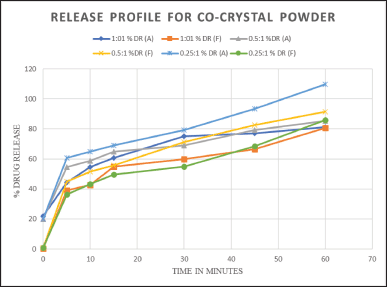 | Figure 11. In vitro dissolution profile for ATC: FE: PABA Cocrystals for batches (1:1:1, 0.5:1:1, 0.25:1:1) at pH 7.4. [Click here to view] |
CONCLUSION
In the present work, fenofibrate−atorvastatin calcium−para-aminobenzoic acid cocrystals presented outstanding physicochemical properties (solubility and dissolution rate) compared with those of pure medications. The excipients that have been used in the formulation are xanthan gum, sodium alginate, magnesium stearate, and microcrystalline cellulose. On the basis of the results of the present study, cocrystals with para-aminobenzoic acid prepared via liquid-assisted grinding had better solubility, pH-independent drug release, and dissolution rates than did pure drugs. Fenofibrate and atorvastatin calcium were evaluated by FTIR, DSC, PXRD, and SEM analyses. Compared with the pure drugs, cocrystals with para-aminobenzoic acid prepared via the technology of liquid-assisted grinding method showed improvement in the solubility as compared to pure drugs. The cocrystallization of atorvastatin calcium and fenofibrate improves solubility, potentially enhancing oral bioavailability and therapeutic effectiveness. It enables the development of fixed-dose combinations, reducing pill burden and improving patient compliance. This dual-drug delivery platform is scalable, commercially viable, and offers personalized treatment options for managing dyslipidemia. In future studies, the cocrystals can be tested in animals to check how well the drugs are absorbed and how long they stay in the body. Their stability over time can also be studied. Further work can focus on making these cocrystals on a larger scale and turning them into suitable tablets or capsules for real use.
ACKNOWLEDGMENTS
We would like to express our sincere gratitude to Dr. Vishwanath Karad, MIT World Peace University, Kothrud, Pune, for providing the necessary facilities and support to carry out this research. This work would not have been possible without the collaborative efforts and dedication of the entire research team.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The author reports no financial or any other conflicts of interest in this work.
ETHICAL APPROVAL
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
CONSENT FOR PUBLICATION
The study titled “Synthesis and Characterization of Fenofibrate-Atorvastatin Calcium Cocrystals: Design, Formulation, and Evaluation” is not published in any journal and is a unique work.
REFERENCES
1. Alam S, Naveed S, Dilshad H, Saleem S, Alam T, Qamar F, et al. Comparative formulation development of atorvastatin calcium tablets using two different super disintegrants. Lat Am J Pharm. 2018;1123–30.
2. Zhang L, Chai G, Zeng X, He H, Xu H, Tang X. Preparation of fenofibrate immediate-release tablets involving wet grinding for improved bioavailability. Drug Dev Ind Pharm. 2010;36(9):1054–63. CrossRef
3. Kumar R. Solubility and bioavailability of fenofibrate nanoformulations. ChemistrySelect. 2020;9123–30.
4. Wang L, Li S, Xu X, Xu X, Wang Q, Li D, et al. Drug-drug cocrystals of theophylline with quercetin. J Drug Deliv Sci Technol. 2022;70, 59–65. CrossRef
5. Verma N. Introduction to hyperlipidemia and its treatment: a review. Int J Curr Pharm Res. 2016;9(1):45–50. CrossRef
6. Gullapalli RP, Mazzitelli CL. Gelatin and non-gelatin capsule dosage forms. J Pharm Sci. 2017;106(6):1453–65. CrossRef
7. Mishra AK, Dubey V, Ghosh AR. Clinical and experimental; obesity: an overview of possible role(s) of gut hormones, lipid sensing and gut microbiota. Metabolism. 2016;65(1):48–65. CrossRef
8. Sastry SV, Nyshadham JR, Fix JA. Recent technological advances in oral drug delivery—a review. Pharm Sci Technol Today. 2000;3(4):138–45. CrossRef
9. Elacqua E, Bucar DK, Henry RF, Zhang GG, MacGillivray LR. Supramolecular complexes of sulfadiazine and pyridines: reconfigurable exteriors and chameleon-like behavior of tautomers at the co-crystal–salt boundary. Crystal Growth Design. 2013;13(1):393–403. CrossRef
10. Aitipamula S, Chow PS, Tan RBH. Trimorphs of a pharmaceutical cocrystal involving two active pharmaceutical ingredients: potential relevance to combination drugs. CrystEngComm. 2009;11:1823–7. CrossRef
11. Jacobs A, Amombo Noa FM. Hybrid salt-cocrystal solvate: P-Coumaric acid and quinine system. J Chem Crystallogr. 2014;44(2):521–9. CrossRef
12. Liu Y, Li B, Zhang K, Li J, Hou H. Novel hard capsule prepared by tilapia (Oreochromis niloticus) scale gelatin and konjac glucomannan: characterization, and in vitro dissolution. Carbohydr Polym. 2019;206:682–90. CrossRef
13. Taraka SKK, Pasala PK, Sahoo RK, Laddha UD, Khairnar SJ, Bendale AR, et al. Atorvastatin ascorbic acid cocrystal strategy to improve the safety and efficacy of atorvastatin. Pharmacia. 2022;69(2):295–302. CrossRef
14. Cheney ML, Weyna DR, Shan N, Hanna M, Wojtas L, Zaworotko MJ. Coformer selection in pharmaceutical cocrystal development: a case study of a meloxicam aspirin cocrystal that exhibits enhanced solubility and pharmacokinetics. J Pharm Sci. 2011;100(6):2172–81. CrossRef
15. Braga D, Grepioni F, Maini L, Capucci D, Nanna S, Wouters J, et al. Combining piracetam and lithium salts: ionic cocrystals and co-drugs?. Chem Commun. 2012;48(66):8219–21. CrossRef
16. Rowe RC, Shskey PJ, Quinn ME. Handbook of pharmaceuticals exipients 6th ed. London, Chicago: Pharm Press; 2009. pp 45–55.
17. Thipparaboina R, Kumar D, Chavan RB, Shastri NR. Multidrug co-crystals: towards the development of effective therapeutic hybrids. Drug Discovery Today. 2016;21(3):481–90. CrossRef
18. Strickley RG, Iwata Q, Wu S, Dahl TC. Pediatric drugs--a review of commercially available oral formulations. J Pharm Sci. 2008;97(5):1731–74. CrossRef
19. Edwards AJ, Mackenzie CF, Spackman PR, Jayatilaka D, Spackman MA. Intermolecular interactions in molecular crystals: what’s in a name? Faraday Discuss. 2017;203:93–112. CrossRef
20. Sanphui P, Goud NR, Khandavilli UBR, Nangia A. Fast dissolving curcumin cocrystals. Cryst Growth Des. 2011;11(9):2932–40. CrossRef
21. Jones W, Motherwell WDS, Trask AV. Pharmaceutical cocrystals: an emerging approach to physical property enhancement. MRS Bull. 2006;31:875–9. CrossRef
22. Naqvi A, Ahmad M, Minhas MU, Khan KU, Batool F, Rizwan A. Preparation and evaluation of pharmaceutical cocrystals for solubility enhancement of atorvastatin calcium. Polym Bull. 2020;77:6191–211. CrossRef
23. Wilhelm LP, Wendling C, Védie B, Kobayashi T, Chenard M, Tomasetto C, et al. STARD 3 mediates endoplasmic reticulum-to-endosome cholesterol transport at membrane contact sites. EMBO J. 2017;36:1412–33. CrossRef
24. Wierzbicki AS, Mikhailidis DP, Wray R, Schachter M, Cramb R, Simpson WG, et al. Statin-fibrate combination therapy for hyperlipidaemia: a review. Curr Med Res Opin. 2003;19(3):155–68. CrossRef
25. Saikia S, Bordoloi M. Molecular docking: challenges, advances and its use in drug discovery perspective. Curr Drug Targets. 2019;20(5):501–21. CrossRef
26. Stanzione F, Giangreco I, Cole JC. Use of molecular docking computational tools in drug discovery. Prog Med Chem. 2021;60:273–343. CrossRef
27. Karimi-Jafari M, Padrela L, Walker GM, Croker DM. Creating cocrystals: a review of pharmaceutical cocrystal preparation routes and applications. Crystal Growth Design. 2018;18(10):4390–405. CrossRef
28. Guo M, Sun X, Chen J, Cai T. Pharmaceutical cocrystals: a review of preparations, physicochemical properties and applications. Acta Pharm Sin B. 2021;11(8):2537–64. CrossRef
29. Kelley SP, Narita A, Holbrey JD, Green KD, Reichert WM, Rogers RD. Understanding the effects of ionicity in salts, solvates, co-crystals, ionic co-crystals, and ionic liquids, rather than nomenclature, is critical to understanding their behavior. Cryst Growth Des. 2013;13(3):965–75. CrossRef
30. Schultheiss N, Newman A. Pharmaceutical cocrystals and their physicochemical properties. Crystal Growth and Design. 2009;9(6): 2676–87. CrossRef