INTRODUCTION
Metabolic syndrome is an assemblage of metabolic and systemic alterations, including obesity, hyperglycemia, hyperinsulinemia (due to insulin resistance), dyslipidemia, and hypertension [elevated systolic blood pressure (SBP) and diastolic blood pressure (DBP)] caused by nutrient overload, storage, and reduced energy expenditure. According to the NCEP ATP III guidelines, metabolic syndrome diagnosis requires the presence of at least three of the following characteristics: abdominal obesity, fasting sugars ≥ 110 mg/dl, dyslipidemia [increased triglycerides (TAGs), total cholesterol, decreased high-density lipoprotein (HDL) cholesterol], elevated SBP, or DBP. The current body of the literature suggests a global landscape change in the incidence rates of metabolic syndrome wherein 23.7% of type 1 diabetic patients have metabolic syndrome [1] and an alarming increased incidence of metabolic syndrome among children being 2.8% and 4.8% for adolescents, with higher incidence rates observed in low-income countries [2]. Emerging studies indicate a higher incidence rate among Asian Indians, with the majority of the affected individuals commonly exhibiting a reduction in HDL-cholesterol levels [3] with some studies indicating incidence rates in India to be 30% [4] with obesity or overweight individuals presenting a higher risk of developing metabolic syndrome [5].
One of the significant attributable risks for developing metabolic syndrome is overconsumption or nutrient excess conditions caused by increased consumption of energy-dense, high-fat, or high-fructose/sucrose-containing palatable foods and concomitant reduction in energy expenditure due to a sedentary lifestyle. One of the earliest changes observed due to the consumption of a high-calorie or high-fat diet is the phenotype of the gut microbial population, which effectively leads to reduced production of beneficial short-chain fatty acids (SCFAs) such as butyrate and propionate, altering the response of gut immune cells such as innate lymphoid cells, T- and B-lymphocytes, increased permeability of the gut epithelial barrier lining [6] increasing the transmigration of lipopolysaccharides from the gut lumen into systemic circulation causing the activation of the immune system. The overall consumption of fructose or sucrose-containing dietary items has increased in the last 10 years [7], which has had drastic effects on the overall metabolic profile since fructose consumption induces de novo lipogenesis in the liver. Fructose metabolism also aids in the production of advanced glycation end products such as methylglyoxal and N-(Carboxymethyl) lysine which have independent or cumulative effects on metabolic alterations, thus predisposing individuals to increased risk of developing obesity or metabolic syndrome [8]. The excess dietary carbohydrates and lipids absorbed are converted into fats and stored in adipose tissues, leading to hypertrophy and hyperplasia of adipose tissue, which alters the profile of multiple adipokines released from the adipose tissues, severely impacting the metabolic homeostasis, leading to a functional reduction in insulin action leading to insulin resistance further accentuated by activation of the immune system and release of pro-inflammatory cytokines. One of the significant adipokines altered in metabolic syndrome is adiponectin [9], which is reduced with adipose tissue expansion, causing an overall reduction in energy expenditure. Fructose consumption stimulates hepatic lipogenesis, producing TAGs which are transported to the adipose tissue via very low-density lipoproteins (VLDLs) [10,11]. The constant storage of lipids in adipocytes coupled with dysfunctional mobilization leads to saturation of both subcutaneous and visceral adipocytes, which initiates the spill-over mechanism leading to ectopic deposition of lipids in other tissue such as liver [12] and increasing the levels of circulating free fatty acids (FFAs), TAGs, cholesterol and associated lipoproteins such as low-density lipoprotein (LDL), VLDL but decreasing HDL. The over-expanding adipocytes start suffering from hypoxia, which initiates the hypoxic response leading to adipose tissue extracellular matrix (ECM) remodeling and anaerobic metabolism of glucose-inducing lactate production that, in turn, induces greater lipid uptake metabolic reprogramming of adipose tissue immune cells [13] such as macrophages toward the pro-inflammatory M1 profile further inducing the production of pro-inflammatory cytokines and a state of low-grade metainflammation. The elevated levels of circulating FFAs also induce the production of other lipid derivatives such as ceramides, which, along with FFAs and increased metabolic load, induce endoplasmic reticulum (ER) and mitochondrial stress in cells [14]. The existing pro-inflammatory cytokines, ceramide, FFA-induced ER, and mitochondrial stress inhibit the insulin signaling downstream pathway, which reduces insulin action, causing hyperglycemia and compensatory hyperinsulinemia [15]. The relative inefficiency of insulin and fructose-metabolism byproduct uric acid inhibits endothelial nitric oxide (NO) synthesis, which reduces vasodilation and high-fat, high-calorie-induced angiotensin II activation in adipocytes increasing the blood pressure (SBP and DBP) [16]. The increased load of lipids and pro-inflammatory immune cell activation stimulates the hepatocytes’ metabolic profile, reducing autophagy and increasing the activation of hepatic stellate cells and Kupffer cells, inducing hepatic ECM modification and causing steatohepatitis [17,18].
Flaxseed (Linum usitatissimum), the oldest cultivated crop, produces oil, provides food, and has appreciable amounts of soluble and insoluble fiber. It is a good source of the omega-3 fatty acid, linolenic acid [19], linoleic, oleic, palmitic, and stearic acids; tocopherols; sterols; sterol esters, phospholipids, waxes, FFAs, and carotenoids. Flaxseeds also contain cyanogenic glycosides, namely mono-glycosides such as linamarin and lotaustralin, and di-glycosides such as linustatin and neolinustatin and secoisolariciresinol diglucoside (SDG) [19], with SDG characterized as a flaxseed lignan polymer composed of phenylpropanoids and hydroxymethyl glutaric acid [20–24]. Some studies have also shown that including flaxseeds in the diet significantly helped reduce total plasma cholesterol, TAGs, and LDL-cholesterol. Still, the results are inconclusive since some studies have reported no effect on HDL-cholesterol levels or lipid levels in animal studies [25–29]. Studies also indicate that SDG may reduce DBP [30], and clinical studies involving peripheral arterial disease patients with high blood pressure reported that consuming flaxseeds for more than 3 months lowered blood pressure [31]. SDG also exhibits an inhibitory effect on lipogenic genes with concomitant stimulation of lipolytic genes [32]. Some studies have also indicated that flaxseed lignans regulate adipogenesis via modulation of peroxisome proliferator-activated receptor-gamma expression, which may also enhance GLUT-4 translocation in insulin-sensitive tissue and upregulation of adiponectin expression, which may help reduce insulin resistance [33]. Studies in animal models also indicate that flaxseed lignan (SDG) improves glycemic control in animal models (but not whole flaxseeds) [34] and ameliorates central obesity and inflammation by altering the gut microbiome in mouse models [35]. Flaxseed derived ω-3 fatty acids can promote the abundance of Bifidobacteria [36,37], flaxseed consumption increases Akkermansia muciniphila [22], Lactobacilli (Lactobacillus gasseri and reuteri) impairs lipid absorption [39–41], and Bifidobacterium, Clostridium, and Bacteroides [23] reduce this Firmicutes/Bacteroides ratio by promoting the comparative abundance of firmicutes [38,42,43]. Flaxseed consumption improves glycemic control in prediabetic obese men and women [24].
One of the significant lacunae identified from studies with flaxseed administration is the lack of induction or presence of metabolic syndrome parameters focusing on inspecting the effects of flaxseeds in hyperlipidemic or T2DM animals/humans with conflicting results [31,33,34,44–60]. In one study with metabolic syndrome induction in rodents [20], the dietary pattern was altered from a high-fat, high-calorie diet to normal diet, with studies demonstrating that dietary reversal from high-fat to normal diet by itself can induce metabolic alteration in rodents [61–63] and may even impact the epigenome thusaltering the overall energy expenditure via the hypothalamic network [64]. Therefore, our work focuses on identifying the metabolic alterations induced by different doses of flaxseed extract supplementation in the event of co-exposure or post-induction exposure to a high-fat, high-calorie diet and fructose-induced metabolic syndrome parameters in male Wistar rats without diet reversal.
MATERIALS AND METHODS
Diets
Normal Lab Diet 5L79 (Energy- 3.15 Kcal/g (13.18 KJ/g) obtained from Hylasco Biomedicals, Hyderabad, India.
High-calorie, high-fat diet (HCHF): 3.725 Kcal/g (15.585 KJ/g) was made by mixing Lab Diet 5L79 with cholesterol Sisco Research Laboratories Pvt. Ltd. (SRL), sucrose Loba Chemie (LOBA), sodium cholate SRL, casein (Vitamin-free casein, Himedia), and anhydrous milk fat in the compositional ratio of 48.5 % Lab Diet 5L79, 10% casein (vitamin-free casein), 30% sucrose, 10% anhydrous milk fat, 1% cholesterol, and 0.5% sodium cholate.
Fructose LOBA 60% (w/v): 2.4 Kcal/ml (10.04 KJ/ml).
Preparation of hydroethanolic extract of flaxseeds
Commonly (commercially) available flaxseeds were purchased, sun-dried, and microwave-dry roasted for about 15 minutes at 40°C followed by grinding to a fine powder. It was milled and passed through a sieve (mesh 300) and stored in a desiccator at 4°C. One hundred grams of powdered seeds were used for extraction using a warm maceration method with 80% (v/v) ethanol for 72 hours in the dark, with extract separation done by filtration every 24 hours, followed by fresh solvent replacement. The filtrate was concentrated under the rotary evaporator and dried in the oven at 40°C for 48 hours to obtain a dark-brown powder, which was stored (extraction ratio was 10:1) in a desiccator at 4°C until use [65]. The dark-brown powder was dissolved in distilled water with vortex mixing and left undisturbed for 24 hours with filtration to remove any suspended particles to produce a stock solution (1.0 g/ml).
Animals used
Fifty-six male Wistar rats, 3–4 months old, weighing 250 ± 25 g, utilized for the study were obtained from the Central Animal Research Facility (CARF), MAHE, Manipal, India. The rats were acclimatized for one week before the initiation of the study and maintained at a room temperature of 24°C ± 2°C, with a standard 12-hour light/dark cycle with free access to food and water throughout the entire study. Rats were individually housed, with all efforts made to minimize the suffering of rats.
Experimental design
Fifty-six male Wistar rats were divided into seven groups of eight animals (n = 8/group) with efforts to maintain constant body weight ranges across groups.
Group 1: Normal controls (NCs): Fed with a normal Lab Diet 5L79 for 16 weeks.
Group 2: Metabolic syndrome controls (MS): Fed with a high-calorie, high-fat diet with an additional administration of 1 ml/kg of 60% fructose solution (w/v) in distilled water daily via oral gavage for 16 weeks, with metabolic syndrome symptoms induction seen at 8 weeks.
Group 3: Low-dose post-exposure group (MS+F-G1): Put on a high-calorie, high-fat diet and 1 ml/kg of 60% fructose solution for 8 weeks, followed by the introduction of flaxseed extract at the dose of 0.5 g/kg body weight via oral gavage for the next 8 weeks with the continuation of high-calorie high-fat diet and 60% fructose with sufficient time intervals between doses.
Group 4: High-dose post-exposure group (MS+F-G2): Fed with a high-calorie, high-fat diet and 1 ml/kg of 60% fructose solution for 8 weeks, followed by the introduction of flaxseed extract at the dose of 1.0 g/kg body weight via oral gavage for the next 8 weeks with the continuation of high-calorie high-fat diet and 60% fructose with sufficient time intervals between doses.
Group 5: Low-dose co-exposure group (F+MS-G1): Put on a high-calorie, high-fat diet and 1 ml/kg of 60% fructose solution with flaxseed extract at the dose of 0.5 g/kg body weight via oral gavage for the entire 16 weeks, with sufficient time intervals between oral doses.
Group 6: High-dose co-exposure group (F+MS-G2): Administered a high-calorie, high-fat diet and 1 ml/kg of 60% fructose solution with flaxseed extract at the dose of 1.0 g/kg body weight via oral gavage for the entire 16 weeks with an adequate time gap between the oral gavages.
Group 7: Flaxseed control (FLXC): Fed with a normal Lab Diet 5L79 and flaxseed extract at a 1.0 g/kg body weight dose via oral gavage for 16 weeks.
The male rats were weighed weekly with measurement of abdominal circumference (AC) (measured with a non-expandable tape immediately anterior to the forefoot) and nose-anal length. For an anthropometric assessment, body mass index (BMI) was calculated using the formula BMI = body weight (g)/length2 (cm), body fat index (Lee index [66–68]) calculated using the formula (cube root of body weight (g)/nose-to-anus length (cm)). The flaxseed extract dosage of 0.5 g/kg body weight (low-dose) was decided based on its protective effects seen from a colitis model in rats [69]. The high-dose (1.0 g/kg body weight) was double the low dose (one-fifth of the limit dose of 5,000 mg/kg body weight) and did not have any acute toxicity effects, as indicated by other studies [70]. The total experimental duration was 16 weeks, with blood collection at baseline, 8, and 16 weeks in fluoridated tubes and non-fasting clotted blood samples. The choice of experimental duration was based on previous studies indicating hyperinsulinemia due to a high-fat and/or high-fructose diet will be induced by 8 weeks [71], which was seen in our study as indicated by the development of hyperglycemia, dyslipidemia, abdominal obesity, and BMI elevations. The overall unhealthy effects with structural and physiological abnormalities in organs such as the liver, pancreas, and adipose tissue may be observed by week 16, thus prompting the total experimental duration [71].
The animals were fasted (overnight) for 12 hours before blood collection, at baseline, after the completion of 8 weeks (on the first day of the ninth week), and at the end of 16 weeks (on the first day of the 17th week). Serum and plasma were collected to estimate the fasting glucose and lipid profile using standard biochemical kits (Agappe Diagnostics). Fasting insulin and adiponectin were also measured using standard ELISA kits (Elabscience Biotechnology Ltd). Serum uric acid levels (Agappe Diagnostic kits), NO metabolites [72,73], and total oxidant status (TOS) [74] were also measured. After 16 weeks, fasting plasma and serum were collected, followed by euthanasia and dissection of Wistar rats. The weight of fat pads (abdominal, epididymal, and subcutaneous) and liver was recorded. The visceral adiposity index (VAI) was calculated from the total weight of the adipose fat pads using the formula (total body fat/final body weight) × 100 [68,75,76], where total body fat was the sum of epididymal, retroperitoneal, and visceral fat.
Liver tissue was collected, weighed, and kept for histopathological assessment in neutral buffered formalin followed by treatment with sequential changes in ethanol solutions of increasing concentration followed by hematoxylin and eosin staining using standard protocols, after which non-alcoholic steatohepatitis (NASH) histopathological grading was done [77].
Statistical analysis
All the statistical tests were performed using Jamovi software version 2.3. Weekly changes in body weight and AC for 16 weeks were analyzed using repeated measure ANOVA after meeting the normality assumption using the Shapiro–Wilk normality assessment. BMI, Lee’s Fat Index, and biochemical parameters at baseline, 8 weeks, and 16 weeks were evaluated for Shapiro–Wilk normality. Study parameters that agreed with normality assumptions were analyzed using parametric one-way ANOVA with Tukey’s post hoc tests with statistical significance accepted at p ≤ 0.05 and results reported as mean ± SD. For parameters violating Shapiro–Wilk normality estimations, Kruskal–Wallis non-parametric tests were performed with p ≤ 0.05 considered statistically significant and results reported as median Inter-quartile range (IQR).
RESULTS
Anthropometric measurements
In rats receiving a high-calorie, high-fat diet and fructose, the body weight increased significantly compared to normal and FLXCs. Still, groups where flaxseed extract was introduced from day one had significantly lower body weights than groups without flaxseed extract (Table 1). Body weight and abdominal variations across groups in 16 weeks are indicated in Figures 1 and 2. An anthropometric indicator, such as BMI analysis, using Fisher’s one-way ANOVA at 8 weeks, shows a statistically significant difference between groups (F [6,49] = 16.516, p < 0.001), with post hoc comparisons using Tukey’s HSD (Honestly Significant Difference) revealing that groups receiving only HCHF, and fructose showed a significant increase in BMI when compared to NCs. In contrast, the groups that received flaxseed extract, HCHF, and fructose demonstrated no difference in BMI between them and NCs. At the end of 16 weeks, both flaxseeds extract post-exposure (8 weeks onwards), and co-exposure showed a lower BMI as compared to metabolic syndrome controls (F [6, 49] = 47.339, p < 0.001). Still, co-exposure groups exhibited a lower BMI than post-exposure groups (Fig. 3).
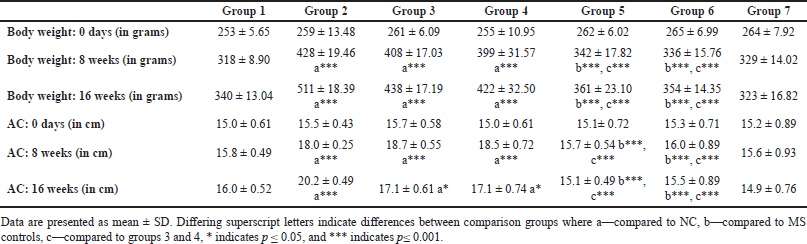 | Table 1. Body weight, AC at baseline, 8 and 16 weeks. [Click here to view] |
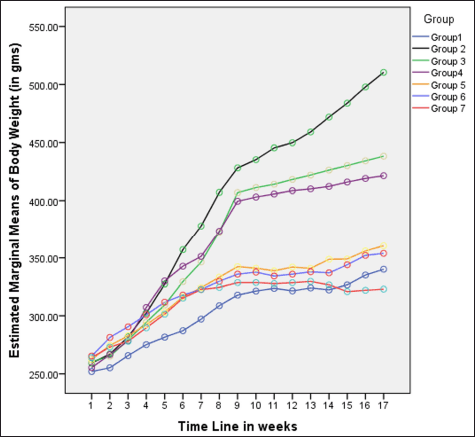 | Figure 1. Variations in body weight (in grams) among different groups in 16 weeks. [Click here to view] |
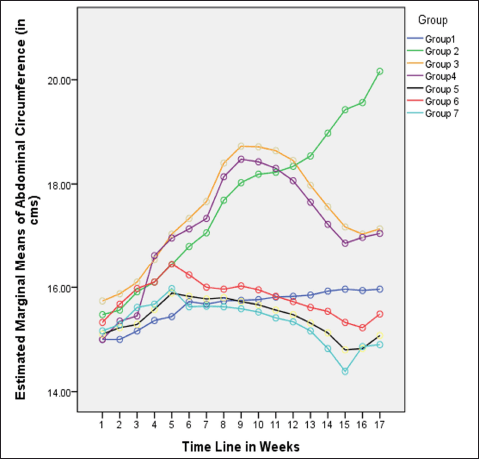 | Figure 2. Variations in AC (in cm) among different groups in 16 weeks. [Click here to view] |
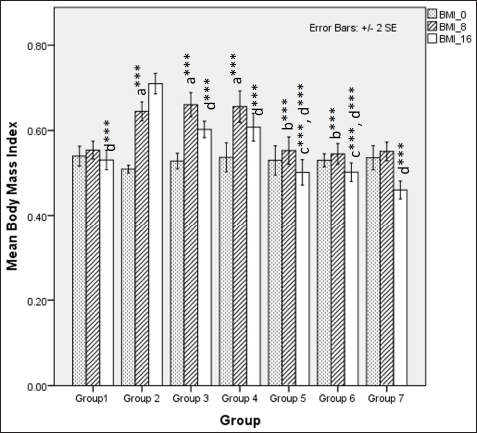 | Figure 3. Comparison of BMI at 0 days. 8 and 16 weeks a represents comparison to group 1, b represents comparison to group 3,4 and 5, c represents comparison to groups 3 and 4, d represents comparison to group 2 (Fisher’s one-way ANOVA with Tukey’s post hoc analysis) (*** p-value < 0.001, ** p <0.01, * p <0.05). [Click here to view] |
The body fat index at 8 weeks was significantly different among groups demonstrated by Fisher one-way ANOVA (F [6, 49] = 36.761, p < 0.001). Tukey’s post hoc analysis revealed that flaxseed extracts co-exposure groups showed a lower fat index than non-exposure groups when administered an HCHF and fructose dietary regime (Fig. 4). At 16 weeks, Fisher’s one-way ANOVA (F [6,49] = 78.769, p <0.001) test statistics indicated flaxseed extract lowered body fat index when compared to metabolic syndrome controls and Tukey’s post hoc comparison co-exposure groups showed a lowered body fat index as compared to post-exposure groups (Fig. 4).
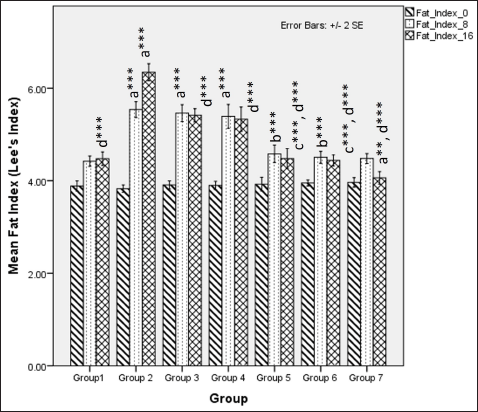 | Figure 4. Comparison of body fat index (Lee’s index) at 0 days. 8 and 16 weeks. a represents comparison to group 1, b represents comparison to groups 3, 4 and 5, c represents comparison to groups 3 and 4, d represents comparison to group 2 (Fisher’s one-way ANOVA with Tukey’s post hoc analysis) (*** p-value < 0.001, ** p <0.01, * p <0.05). [Click here to view] |
VAI analysis at 16 weeks, using a Kruskal–Wallis test, indicated a significant difference between groups [χ2 (6) = 29.0, p < 0.001, ?2 = 0.528], and pairwise comparison reveals a significant difference in visceral adiposity between metabolic syndrome controls versus NCs, high-dose flaxseed extract post-exposure, co-exposure, and control groups (Fig. 5).
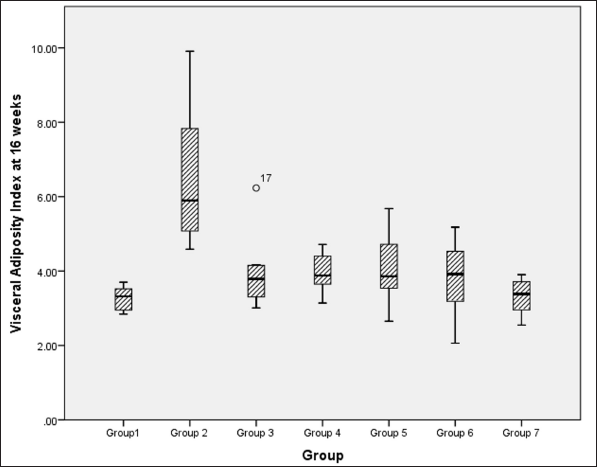 | Figure 5. Visceral adiposity index at 16 weeks among different groups. [Click here to view] |
At 16 weeks, subcutaneous fat accumulation also indicated a significantly lower fat content in flaxseed receiving groups when compared to metabolic syndrome controls using Kruskal–Wallis test statistics [χ2 (6) = 27.5, p < 0.001, ?2 = 0.500], with pairwise comparison indicating a statistically significant difference between metabolic syndrome controls and high and low-dose flaxseed extract post-exposure groups and high-dose co-exposure groups (Fig. 6).
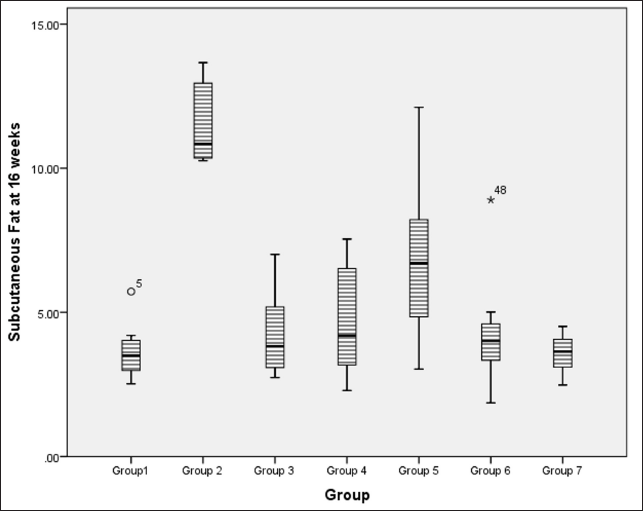 | Figure 6. Amount of subcutaneous fat accumulation (in grams) at 16 weeks in different groups. [Click here to view] |
Glucoregulatory estimations
Fasting plasma glucose at 8 weeks exhibited a statistically significant difference when compared between HCHF and fructose-consuming groups with and without flaxseed extract co-administration via one-way ANOVA (Fisher’s) F [6,49] = 23.0, p < 0.001. At 16 weeks, there was a difference in fasting plasma glucose levels among groups one-way ANOVA (Welch’s) F [6,21.5] = 22.7, p < 0.001 all the groups receiving HCHF and fructose had statistically significant elevated fasting blood glucose as compared to NCs and flaxseed extract administration both post and co-exposure exhibited a lower fasting plasma glucose when compared to metabolic syndrome controls (Welch’s one-way ANOVA with Games–Howell post hoc) (Table 2) which was statistically non-significant, but it may be clinically significant since the mainstay of any hyperglycemic conditions treatment actively targets reduction of plasma sugars.
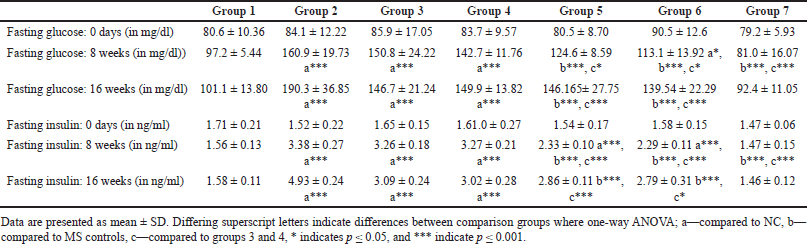 | Table 2. Comparisons of fasting blood glucose and insulin levels between groups at different time points. [Click here to view] |
Homeostatic assessment of insulin resistance (HOMA-IR) at different intervals also depicts a similar pattern as plasma sugars with initial increases in insulin resistance with HCHF and fructose consumption. Flaxseed extract co-administration reduces insulin resistance (Kruskal–Wallis χ2 (6) = 49.7, p < 0.001, ?2 = 0.903) with pairwise comparison revealing that values of HOMA-IR in flaxseed extract receiving groups were significantly lower than high-calorie high-fat and fructose consuming groups. At the end of 16 weeks, HOMA-IR values significantly differ among different groups (Kruskal–Wallis χ2 (6) = 45.2, p < 0.001, ?2 = 0.821), with significant reduction observed when compared between metabolic syndrome controls and flaxseed extract receiving groups (Table 3, Figs. 7 and 8).
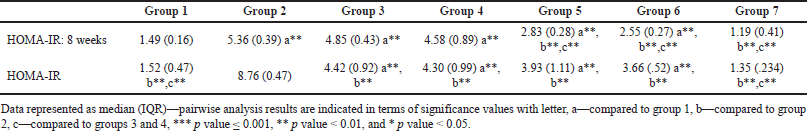 | Table 3. HOMA-IR at 8 and 16 weeks. [Click here to view] |
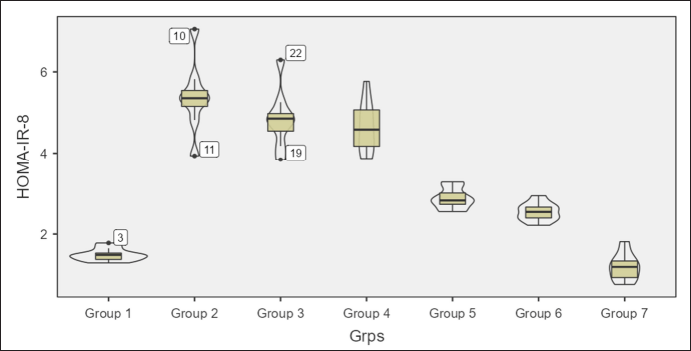 | Figure 7. HOMA-IR at 8 weeks in different groups. [Click here to view] |
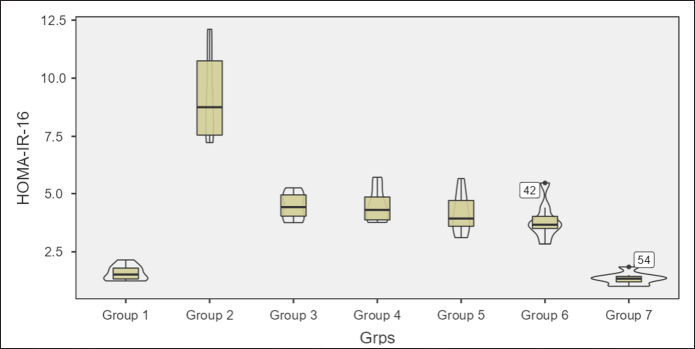 | Figure 8. HOMA-IR at 16 weeks in different groups. [Click here to view] |
Fasting serum lipids
In the first 8 weeks, the ingestion of HCHF and fructose causes a significant difference in serum fasting total cholesterol levels between groups (one-way ANOVA Welch’s F [6,20.6] = 155.7, p < 0.001) where Games–Howell post hoc analysis revealed that all groups receiving HCHF and fructose had higher total cholesterol compared to NCs (p < 0.001). Still, flaxseed extract exposure groups showed significantly lower serum cholesterol than non-exposure groups (p < 0.05). At 16 weeks, total cholesterol levels differed distinctly among groups (one-way ANOVA Welch’s F [6,20.4] = 20.9, p < 0.001) with Games–Howell post hoc analysis indicating a reduction in total cholesterol in all groups receiving flaxseed extract when compared to metabolic syndrome controls (p < 0.01) (Fig. 9).
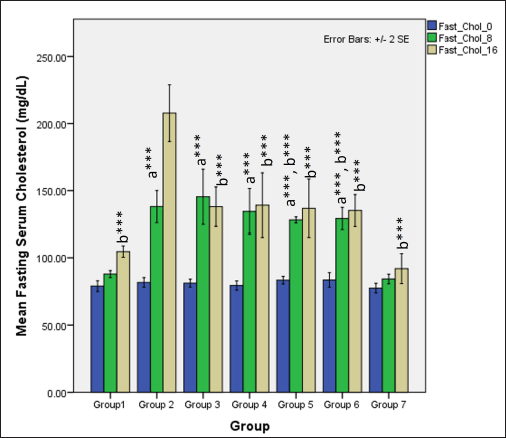 | Figure 9. Comparison of fasting total cholesterol at 0 days and 8 and 16 weeks a represents comparison to group 1, b represents comparison to 2, c represents comparison to groups 3 and 4 (one-way ANOVA with post hoc analysis) (*** p-value < 0.001, ** p < 0.01, * p < 0.05). [Click here to view] |
Serum fasting TAGs were significantly higher in groups receiving HCHF and fructose when compared to NCs (one-way ANOVA Fisher’s F [6,49] = 28, p < 0.001) and Tukey’s post hoc test indicated that high-dose flaxseed extract co-exposure groups demonstrated a significantly lower TAG values when compared to non-flaxseed extract exposure groups (p < 0.01). At 16 weeks, total TAG levels differed distinctly among groups (one-way ANOVA Welch’s F [6,20.7] = 20.8, p < 0.001) with Games–Howell post hoc analysis indicating reduction in total TAGs in all groups receiving flaxseed extract when compared to metabolic syndrome controls (p < 0.01) (Fig. 10).
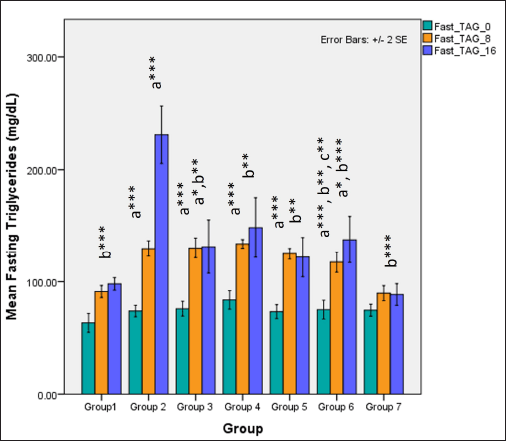 | Figure 10. Comparison of fasting triglyceride (TAG’s) at 0 days and 8 and 16 weeks a represents comparison to group 1, b represents comparison to 2, c represents comparison to groups 3 and 4 (one-way ANOVA with post hoc analysis) (*** p-value < 0.001, ** p < 0.01, * p < 0.05). [Click here to view] |
In the first phase of the study, serum fasting HDL-cholesterol levels significantly reduced in all groups receiving HCHF and fructose (one-way ANOVA Fisher’s F [6,49] = 24.1, p < 0.001) when compared to NCs (p < 0.001) but high-dose of flaxseed extract co-exposure prevented the drastic decline in HDL-cholesterol levels when compared to groups not receiving flaxseed extract (p < 0.01). At 16 weeks, total HDL-cholesterol levels differed distinctly among groups (one-way ANOVA Welch’s F [6,21.5] = 55.7, p < 0.001) with Games–Howell post hoc analysis indicating a reduction in total HDL-cholesterol in all groups receiving flaxseed extract when compared to metabolic syndrome controls (p < 0.001) and high-dose flaxseed extract co-exposure groups showing a higher HDL-cholesterol profile than the low-dose post-exposure group (p < 0.01) (Fig. 11).
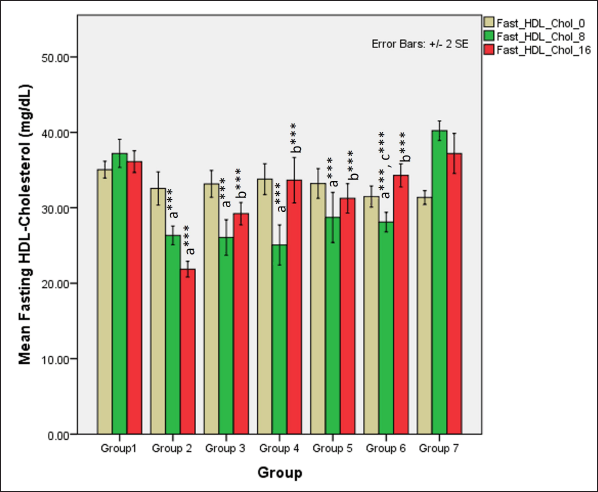 | Figure 11. Comparison of fasting HDL-cholesterol at 0 days and 8 and 16 weeks. a represents comparison to group 1, b represents comparison to 2, c represents comparison to groups 2, 3 and 4 (one-way ANOVA with post hoc analysis) (*** p value < 0.001, ** p < 0.01, * p < 0.05). [Click here to view] |
LDL-cholesterol levels during the first 8 weeks differed significantly among different groups (one-way ANOVA Welch’s F [6,20.6] = 88.6, p < 0.001) with Games–Howell post hoc tests revealing significant elevations in serum LDL-cholesterol receiving HCHF diet and fructose in comparison and among these groups receiving flaxseed extract has lower TAG when compared to metabolic syndrome controls (p < 0.05). At 16 weeks, total LDL-cholesterol levels differed significantly among groups (one-way ANOVA Welch’s F [6,20.4] = 16.8, p < 0.001) with Games–Howell post hoc analysis indicating a reduction in total LDL-cholesterol in all groups receiving flaxseed extract when compared to metabolic syndrome controls (p < 0.01) (Fig. 12).
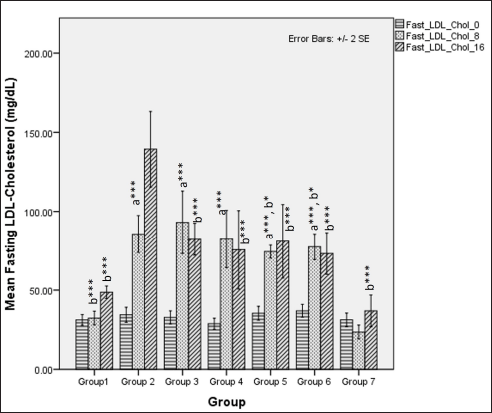 | Figure 12. Comparison of fasting LDL-cholesterol at 0 days and 8 and 16 weeks. a represents comparison to group 1, b represents comparison to 2, c represents comparison to groups 2, 3 and 4 (one-way ANOVA with post hoc analysis) (*** p-value < 0.001, ** p < 0.01, * p < 0.05). [Click here to view] |
Other metabolic parameters
Flaxseed extract administration significantly improved the plasma adiponectin levels at 16 weeks (one-way ANOVA Welch’s F [6, 21.7] = 40.1, p < 0.001) (Table 4).
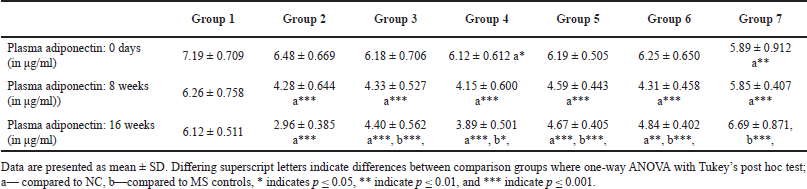 | Table 4. Plasma adiponectin levels at different time points [Click here to view] |
Serum uric acid levels were different during the first 8 weeks (one-way ANOVA Fisher’s F [6,49] = 236.8, p < 0.001), with higher values observed in groups receiving HCHF and fructose (p < 0.001). A high-dose flaxseed extract (group 6) exhibited a lower uric acid than other groups on HCHF and fructose (groups 2, 3, and 4). At 16 weeks, the serum uric acid levels were different among groups (one-way ANOVA Welch’s F [6, 21.3] = 90, p < 0.001). High-dose co-exposure groups (groups 5 and 6) exhibited a lower uric acid when compared to other groups receiving flaxseed extract for eight weeks and metabolic syndrome controls (p < 0.05) (Fig. 13).
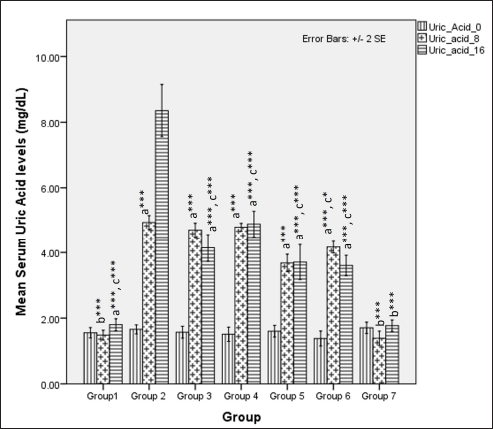 | Figure 13. Comparison of serum uric acid levels at 0 days and 8 and 16 weeks. a represents comparison to group 1, b represents comparison to 2, c represents comparison to Group 2, 3 and 4 (one-way ANOVA with post-hoc analysis) (*** p-value < 0.001, ** p < 0.01, * p < 0.05). [Click here to view] |
Serum TOS was significantly different among groups at 8 weeks (one-way ANOVA Fisher’s F [6,49] = 6.28, p < 0.001) with significantly higher TOS levels observed in groups only receiving HCHF and fructose (groups 2, 3, and 4) when compared to normal (p < 0.05) and flaxseed extract co-administration groups (groups 5 and 6) had significant lower TOS than HCHF and fructose receiving groups (groups 3 and 4) (p < 0.05). At 16 weeks, TOS levels were significantly different among groups (one-way ANOVA Fisher’s F [6, 49] = 63.93, p < 0.001). Among all groups, those receiving HCHF and fructose had higher TOS than NCs ( p < 0.001). However, high-dose flaxseed extract post-exposure (group 4) and both flaxseed extract co-exposure groups (groups 5 and 6) have lower TOS values than metabolic syndrome (group 2) and the low-dose post-exposure group (group 3) (p < 0.01) (Fig. 14).
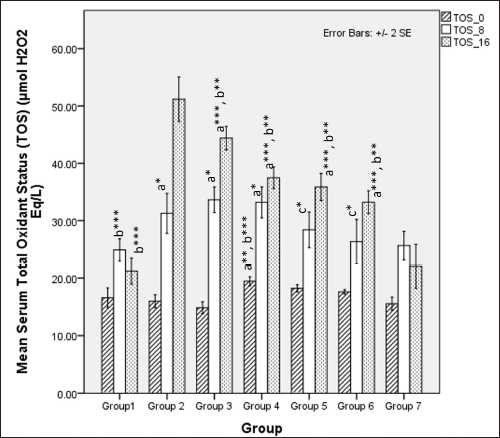 | Figure 14. Comparison of serum total oxidant status at 0 days. 8 and 16 weeks. a represents comparison to Group 1, b represents comparison to Group 2, c represents comparison to groups 3 and 4 (one-way ANOVA with post-hoc analysis) (*** p-value < 0.001, ** p < 0.01, * p < 0.05). [Click here to view] |
Serum NO metabolites (nitrate and nitrite) significantly differed among different groups at 8 weeks (one-way ANOVA Fisher’s F [6,49] =13.80, p < 0.001). Group comparison via Tukey’s post hoc test shows that groups receiving HCHF and fructose showed a significant reduction in serum NO metabolite levels as compared to NCs (p < 0.05), flaxseed extract administration significantly improved NO metabolites concentrations during the first 8 weeks as compared to groups where flaxseed was not administered (p ≤ 0.05). At 16 weeks, there was a difference in NO metabolite levels (one-way ANOVA Welch’s F [6, 21.5] = 75.4, p < 0.001 with Games–Howell post hoc tests) between the groups, indicating that receiving flaxseed extract at a high-dose for 16 weeks has more excellent positive effects on serum NO levels than 8 weeks (Fig. 15).
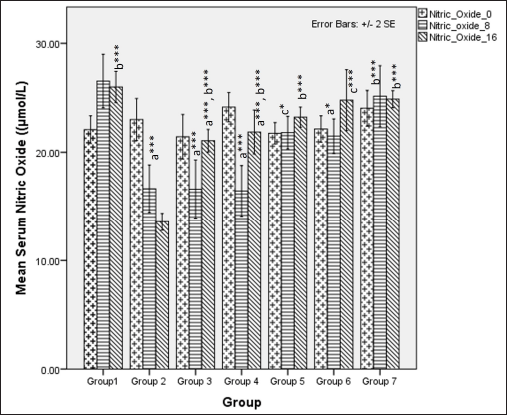 | Figure 15. Comparison of serum NO metabolite levels at 0 days and 8 and 16 weeks. a represents comparison to group 1, b represents comparison to 2, c represents comparison to groups 2, 3 and 4 (one-way ANOVA with post-hoc analysis) (*** p-value < 0.001, ** p < 0.01, * p < 0.05). [Click here to view] |
Histopathological assessment
Histopathological analysis of liver sections indicates the development of steatohepatitis in all the groups administered with HCHF and fructose due to steatotic vacuoles (caused by fat deposition), ballooned hepatocytes, and a few inflammatory foci present (Fig. 16).
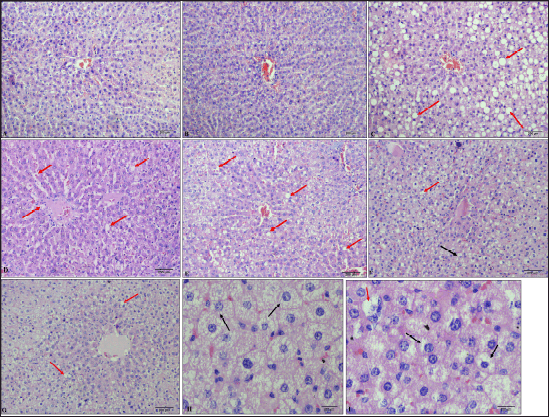 | Figure 16. Histological pictures of liver tissue (H & E staining). A: Group 1 (NCs at 100×), B: Group 7 (flaxseed extract controls at 100×), C: Group 2 (metabolic syndrome controls at 100×), D: Group 3 (low-dose flaxseed extract post-exposure at 100×), E: Group 4 (high-dose flaxseed extract post-exposure at 100×); F: Group 5 (low-dose flaxseed extract co-exposure at 100×), G: Group 6 (high-dose flaxseed extract co-exposure at 100×) (red arrows indicate steatotic vacuoles), H & I: Ballooned hepatocytes (at 400×) (Black arrows indicate ballooned hepatocytes where nucleus is pushed to the periphery). [Click here to view] |
Hepatic histopathological scoring of NASH indicated a statistically significant difference between groups (Kruskal–Wallis χ2 (6) = 36.5, p < 0.001, ?2 = 0.664). Pairwise analysis revealed that the difference observed was due to the difference in scores (data represented in Median IQR) between the control groups with normal and flaxseed extract control scores of 0 showing absence of steatotic features as compared to metabolic syndrome controls [group 2: 1.353 (0.714)]. All the groups receiving flaxseeds had an improved median NASH score when compared to metabolic syndrome controls, where the groups receiving flaxseed extract for 16 weeks [group 5: 0.980 (0.325)], [group 6: 0.750 (1.056)] showed a better steatohepatitis score than groups receiving flaxseed extract for 8 weeks only [group 3: 1.029 (0.390)], [group 4:1.442 (0.400)]. However, flaxseed extract administration did not inhibit the occurrence of steatohepatitis (Fig. 17).
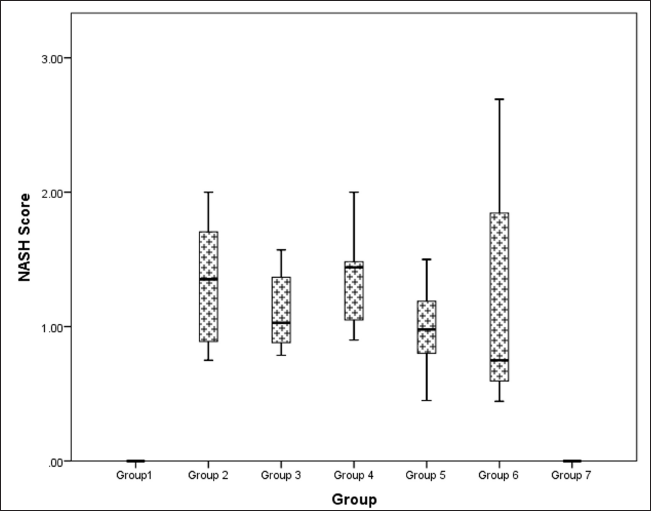 | Figure 17. Comparison of NASH (steatohepatosis damage) score at 16 weeks in different groups. [Click here to view] |
DISCUSSION
The usage of male rats for the study is to negate the effects of female hormones such as estrogen and their derivatives on the plasma lipids and, in turn, glucoregulatory mechanisms [78–81]. The reason for using whole flaxseeds rather than specific fractions like SDG (Secoisolariciresinol diglucoside) or alpha-linoleic acid fraction is that whole flaxseeds contain a diverse group of biomolecules that can provide a wide range of interactions. These interactions include altering the gut microbiome [35,38,82,83] due to microbial metabolism of indigestible flax lignans, increasing the availability of short-chain fatty acids like butyrate [82], and boosting the expression of adiponectin [34]. By improving gut health and metabolic profile, whole flaxseeds can reduce metainflammation. In groups fed with a high-calorie, high-fat diet and fructose only (group 2: 169 ± 12.,44; group 3: 146.4 ± 17.24, and group 4: 144.1 ± 27.72), change in body weight during the first eight weeks was more as compared to NCs (64.6 ± 4.44), whereas in groups that received flaxseed extracts along with a high-calorie high-fat diet and fructose, the change in body weight was much lesser (group 5: 80 ± 17.24; group 6: 70.5 ± 14.06). During the next 8 weeks in the two post-exposure groups, the gain in body weight was significantly reduced compared to metabolic syndrome controls due to flaxseed extract supplementation. The results indicate that flaxseed extract significantly inhibits body weight gain even when fed on a hypercaloric high-fat diet, consistent with earlier studies [84,85]. The effects of flaxseed extract on abdominal obesity were also one of the crucial observations where flaxseed extract administration significantly prevented the increase of AC when compared to animals without flaxseed extract when both were administered a high-calorie, high-fat diet, which is in opposition to previous studies [34]. The administration of flaxseed extract affects plasma adiponectin concentration, and this may be one of the mechanisms by which flaxseed extract regulates body weight and visceral obesity, as evident from reduced visceral adiposity index value among groups with flaxseed extract administration for 8 and 16 weeks [86,87]. Adiponectin, an adipokine released from adipocytes, significantly affects systemic homeostasis via its effects on different metabolic pathways in different organs, such as the activation of AMP-activated protein kinase (AMPK) in skeletal muscles and adipocytes, which may increase fatty acid oxidation pathways and induce beiging of white adipose tissue and brown adipocyte thermogenesis, all of which may promote energy expenditure and reduce adiposity [88–91]. Studies have also shown that circulating levels of adiponectin inversely correlate with body-fat mass [89], and flaxseed extract-induced elevations in adiponectin may account for the reduced adiposity and visceral fat index observed in the groups receiving flaxseed extract. In our study, introduction of a high-calorie, high-fat diet led to accumulation of lipids in tissues (increasing body fat index), which affects the serum adiponectin levels. However, in the groups that received flaxseed extract in conjunction with a high-calorie, high-fat diet, had a higher adiponectin level which is in line with the findings of previous studies [33,92,93]. Among the groups, receiving a high-calorie, high-fat diet and fructose in conjunction with flaxseed extract, the co-exposure group presented elevated levels of circulating adiponectin at 16 weeks, and the high-dose group presented the highest concentration, indicating that high-dose co-exposure may significantly increase adiponectin levels. Adiponectin also plays a major role in glucose metabolism and insulin sensitivity where the binding of adiponectin to its cognate receptors can induce the translocation of insulin-sensitive GLUT-4 receptors to the membrane, and increase the insulin signaling pathways via the activation of insulin receptor substrates [87,88]. Adiponectin can influence the transcription of insulin-synthesizing genes and exocytosis of insulin-containing granules from pancreatic beta-cells, all of which may play a role in improving insulin resistance [88,94,95]. In our study, the administration of a high-calorie, high-fat diet with fructose significantly increased plasma fasting glucose and insulin levels and increased insulin resistance. However, the administration of flaxseed extract reduced the plasma glucose levels with the most significant reductions in fasting glucose, insulin, and HOMA-IR observed in co-exposure groups when compared with those in the non-flaxseed and post-exposure groups. Among the co-exposure groups, the high-dose co-exposure group presented the greatest reduction in insulin resistance index (HOMA-IR), indicating its better efficacy. The effect of flaxseed extract on fasting plasma glucose levels also presents a promising area of interest since, in our study, we have seen that flaxseed extract lowers the fasting plasma glucose levels even in the event of continued high-calorie, high-fat, and fructose consumption. However, it fails to reduce the fasting glucose within normoglycemic limits (<7.2 mmol/L). Still, it lowers glucose levels compared to metabolic syndrome controls, which can be attributed to elevated insulin sensitivity and peripheral utilization [26]. The elevated insulin sensitivity in our study also explains the lower fasting insulin levels in the groups treated with the flaxseed extract than in the non-flaxseed extract groups, with maximum reductions in fasting insulin levels observed in the high-dose co-exposure group [96].
With regard to plasma lipids, during the first 8 weeks, the total cholesterol was lower in groups receiving a high-dose of flaxseed extract (1.0g/kg/bd.wt/day) when compared to groups receiving high-calorie, high-fat diet and fructose only, highlighting the hypolipidemic effects of flaxseed extract which is in line with some of the studies [97]. During the next 8 weeks, when flaxseed extracts were introduced into already hyperlipidemic hyperglycemic metabolic syndrome parametric groups, it reduced fasting total cholesterol. In those groups already on flaxseed extract, continuation of flaxseed extract maintains the total cholesterol levels with the continuation of a high-calorie, high-fat diet, which is in line with one of the other studies [84], which may be attributed to reduced hepatic cholesterol synthesis and inhibition of entero-hepatic bile acid absorption [98]. The levels of HDL-cholesterol in groups receiving high-calorie, high-fat, and fructose at the end of the first 8 weeks were reduced compared to NCs. However, groups co-receiving flaxseed extract showed a lesser reduction in HDL-cholesterol levels, with a higher dose of flaxseed extract providing a better HDL-cholesterol profile than those with a low dose. During the next phase of 8 weeks, when Wistar rats with lowered HDL-cholesterol were initiated on a diet of flaxseed extract, the levels of HDL-cholesterol were higher than levels at 8 weeks, indicating that flaxseed extract promotes the production of HDL-cholesterol via Paraoxonase-1 (PON-1) action in promoting a better reverse cholesterol transport [99]. The levels of LDL-cholesterol during the first 8 weeks of high-calorie, high-fat, and fructose feeding increased probably due to increased hepatic lipogenesis stimulation by fructose. However, in groups receiving flaxseed extract concurrently, the levels of LDL-cholesterol were lower. In the succeeding 8 weeks, when these hypercaloric high-fat fed rats were initiated on flaxseed extract, they showed a reduction in LDL-cholesterol level, which could be attributed to increased hepatic expression of LDL-receptor mediated by flaxseeds as indicated in rodents [98]. Recent evidence has indicated that adiponectin levels also play a key role in dyslipidemia, by reducing the levels of serum TAG, total cholesterol, and LDL-cholesterol [100–102]. Adiponectin and HDL-cholesterol share an intricate relationship in which adiponectin prevents a reduction in HDL-cholesterol levels, which in turn increases circulating adiponectin levels [101]. Therefore, the increase in HDL-cholesterol level and subsequent reduction in TAG, total cholesterol, and LDL-cholesterol levels can be attributed to increased serum adiponectin levels stimulated by flaxseed extract. Compared with the non-flaxseed groups, the groups that received the flaxseed extract presented an improved lipid profile, and compared with the low-dose groups, the high-dose flaxseed presented a greater increase in HDL-cholesterol.
The serum levels of uric acid were elevated upon ingestion of fructose diets due to the metabolic breakdown of fructose-generating uric acid [103,104], which positively correlates with metabolic syndrome in rodents and humans [105–107]. Our study found that uric acid levels at 8 weeks are elevated in all the groups, probably due to the combined effects of fructose feeding and hyperglycemia-induced kidney dysfunction. High-dose flaxseed extract supplementation lowered uric acid levels at the end of 16 weeks, similar to STZ models of T2DM [26]. This reduction in uric acid levels may be attributed to the improvement of hyperglycemia-induced renal function [108] and AMPK activation by flaxseed polysaccharides [35], which, in turn, may provide a protective function against AMPK-hyperuricemia interaction-mediated renal dysfunction [109]. The levels of TOS in our study also indicate the anti-oxidative properties of flaxseed extract wherein high-calorie, high-fat, fructose-induced oxidative stress is reduced only in the high-dose flaxseed extract administration group as compared to other groups, and at the end of 16 weeks, as compared to metabolic syndrome controls all other groups show a lower TOS level. However, the values at 16 weeks are higher than those at 8 weeks, indicating that flaxseed extract administration positively reduces oxidative stress by inducing the antioxidant genes [108] and due to the presence of ALA in flaxseeds. The importance of oxidative stress and antioxidants in the maintenance of metabolic homeostasis and prevention of diet-induced obesity has been elucidated in a recent study that revealed the reversal of high-fat diet-induced alterations in tyrosine phosphorylation by the antioxidant butylated hydroxyanisole [110].
The levels of NO metabolites were reduced upon administration of a high-calorie, high-fat diet-mediated obesity and hyperglycemia-induced oxidative stress [111,112], which initiates endothelial dysfunction resulting in hypertension. The reduction in NO levels may be due to a reduction in adiponectin levels, since it can promote NO via inducible NO synthase enzyme [88]. The levels of NO metabolites may also be affected by insulin resistance since there is an inverse relationship between NO metabolites and HOMA-IR [113–115]. Since flaxseed extract improves serum adiponectin levels and insulin sensitivity, it may be collectively responsible for increasing serum NO metabolites which may be protective since it can effectively reduce endothelial dysfunction and promote vasodilation both of which reduce the risk of hypertension, as observed in individuals with MetS [116]. Our results demonstrated that, among the groups that received the flaxseed extract, the high-dose co-exposure group showed the highest levels of serum NO metabolites as compared with the other groups that received flaxseed extract, suggesting a better endothelium-protective effect.
The liver histopathological analysis also reveals that flaxseed extract has a minor hepatoprotective effect and reduces the magnitude of steatohepatitis, which can be attributed to flaxseed-induced liver lipid metabolism changes leading to reduced hepatic lipid accumulation [117] and antioxidant properties of flaxseed extract (which include increasing liver reduced glutathione levels) [118]. However, they cannot prevent the occurrence of non-alcoholic fatty liver due to fructose insult, since the steatohepatitis scores (NASH scores) in the groups receiving flaxseed extract were not low, as observed in the normal or flaxseed extract control groups. However, the median NASH scores in the groups receiving flaxseed extract were lower than those in the non-flaxseed groups. Compared with those in the post-exposure groups, the median NASH scores in the flaxseed co-exposure groups improved, with the lowest scores observed in the high-dose co-exposure group. These observations indicate that flaxseed extract improves steatohepatosis scores and ameliorates or reduces the intensity of fructose-induced hepatic fat deposition but does not confer complete protection against fructose-induced steatohepatosis. The probable reason for this observation may be the flaxseed extract-induced increase in serum adiponectin which is known to downregulate hepatic gluconeogenic and de novo lipogenic genes and upregulate fatty acid oxidation [88], all of which might collectively regulate hepatic lipid accumulation. However, the continuation of the addition of fructose along with flaxseed extract which is known to promote hepatic de novo lipogenesis [119] may be responsible for the absence of complete protection.
The probable reason for the delay in changes in some of the parameters seen only after eight weeks of flaxseed extract administration or only in cases of high-dose flaxseed extract may be due to the reason that adequate serum levels of beneficial metabolites like ALA from flaxseeds may occur only in high-dose or more-extended duration scenarios [120,121]. The high-dose co-exposure works best as seen in our study, still, a detailed pharmacokinetic or pharmacodynamic study would be essential. We have seen in our study that high-dose co-exposure provides the least reductions in adiponectin levels which could be one of the reasons, since high-fat high-calorie diets impair adiponectin gene transcription but components of flaxseeds induce adiponectin expression, and high concentration and exposure along with HCHF may prevent the HCHF and fructose-induced adiponectin repression.
Other natural approaches in metabolic syndrome treatment from different herbal or dietary components include nutraceuticals such as garlic (lowering blood pressure with no effects on adiposity) [122], blueberry, and polyphenols (improve glucose sensitivity but shows no mentioned effect on adiposity, serum lipids) [122], ginseng (improved fasting glucose and lipids, with no effect on blood pressure) [122], pomegranate (reducing blood pressure but no effects on adiposity, glucose and lipid levels) [122]. Some studies have included compounds such as quercetin (glucose sensitivity enhancers with no effect on blood pressure) [122], vitamins B3 and D (which only target metabolic syndrome indicators such as fasting lipids for B3 and HOMA-IR, SBP, and DBP for vitamin D) [122]. Compared to other nutraceuticals, green tea has shown the best results in targeting the majority of metabolic syndrome indicators, namely, reducing adiposity, blood pressure, fasting glucose, and lipids [122]. However, the effects of green tea on adiponectin and leptin are still not clearly elucidated.
Flaxseed supplementation improves glycemic control and insulin sensitivity among prediabetes and T2DM human subjects, but many studies have not been done in metabolic syndrome patients targeting glycemic control, dyslipidemia, or blood pressure [123,124]. Some studies conducted on metabolic syndrome subjects have different varied doses, different routes of administration, and usage of flaxseed oils [124] in some studies, yielding conflicting results with some studies indicating beneficial effects on glycemic control or lipid levels. Human clinical trials need to be more decisive about the maintenance of doses and administration routes and look at other parameters such as NO, PON-1, Apo-A1, and lipoprotein (a) levels in metabolic syndrome and how they are affected due to flaxseed administration. Our study has indicated a protective effect of flaxseeds in co-administration of flaxseeds and high-calorie, high-fat, and fructose diets. However, more studies need to be conducted to elucidate these protective effects in the co-consumption of flaxseeds and Western-based diets to decipher the mechanistic basis of the attenuative potential of flaxseeds.
CONCLUSION
Whole flaxseed extract co-administration at high doses improves blood glucose, lipid profiles, and anthropometric indices in high-calorie, high-fat fructose-fed rats mimicking metabolic syndrome symptoms and helps in mitigating the harmful effects of hypercaloric high-fat fructose diets.
LIMITATIONS OF THE STUDY
The limitations of the current study can be identified as the absence of a genomic approach like estimation of liver AMPK gene expression or the genomic expression of lipogenic genes or microRNAs in liver and adipose tissue to elucidate the probabilistic mechanistic action of flaxseeds in mitigating the effects of diet-induced obesity or fructose feeding. The future prospects of the study might include an epigenomic approach investigating the diet-metabolism interactions that may occur due to flaxseed consumption.
ACKNOWLEDGMENTS
We thank the Manipal Academy of Higher Education (MAHE) for the infrastructure and facilities provided. We also thank the Central Animal Research Facility (CARF), MAHE, and its personnel for supporting us with the animals and facilities required during the conduct of the study.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The study protocol was approved by the Institutional Animal Ethics Committee (IAEC) of Manipal Academy of Higher Education, Manipal, India (Approval No.: IAEC/KMC/103/2019).
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Belete R, Ataro Z, Abdu A, Sheleme M. Global prevalence of metabolic syndrome among patients with type I diabetes mellitus: a systematic review and meta-analysis. Diabetol Metab Syndr. 2021;13(1):25. CrossRef
2. Noubiap JJ, Nansseu JR, Lontchi-Yimagou E, Nkeck JR, Nyaga UF, Ngouo AT, et al. Global, regional, and country estimates of metabolic syndrome burden in children and adolescents in 2020: a systematic review and modelling analysis. Lancet Child Adolesc Health. 2022;6(3):158–70. CrossRef
3. Jayant SS, Gupta R, Rastogi A, Sachdeva N, Ram S, Dutta P, et al. Incidence and predictors of metabolic syndrome in Asian-Indians: a 10-year population-based prospective cohort study. Int J Diabetes Dev Ctries. 2023;43(6):1–7. CrossRef
4. Krishnamoorthy Y, Rajaa S, Murali S, Rehman T, Sahoo J, Kar SS. Prevalence of metabolic syndrome among adult population in India: a systematic review and meta-analysis. PLoS One. 2020;15(10):e0240971. CrossRef
5. Krishnamoorthy Y, Rajaa S, Murali S, Sahoo J, Kar SS. Association between anthropometric risk factors and metabolic syndrome among adults in India: a systematic review and meta-analysis of observational studies. Prev Chronic Dis. 2022;19:210231. CrossRef
6. Genser L, Aguanno D, Soula HA, Dong L, Trystram L, Assmann K, et al. Increased jejunal permeability in human obesity is revealed by a lipid challenge and is linked to inflammation and type 2 diabetes. J Pathol. 2018;246(2):217–30. CrossRef
7. Lancaster KJ. Current intake and demographic disparities in the association of fructose-rich foods and metabolic syndrome. JAMA Netw Open. 2020;3(7):e2010224. CrossRef
8. Giussani M, Lieti G, Orlando A, Parati G, Genovesi S. Fructose intake, hypertension and cardiometabolic risk factors in children and adolescents: from pathophysiology to clinical aspects. A narrative review. Front Med. 2022;9:792949. CrossRef
9. Ghadge AA, Khaire AA, Kuvalekar AA. Adiponectin: a potential therapeutic target for metabolic syndrome. Cytokine Growth Factor Rev. 2018;39:151–8. CrossRef
10. Herman MA, Birnbaum MJ. Molecular aspects of fructose metabolism and metabolic disease. Cell Metab. 2021;33(12):2329–54. CrossRef
11. Febbraio MA, Karin M. “Sweet death”: fructose as a metabolic toxin that targets the gut-liver axis. Cell Metab. 2021;33(12):2316–28. CrossRef
12. Levelt E, Pavlides M, Banerjee R, Mahmod M, Kelly C, Sellwood J, et al. Ectopic and visceral fat deposition in lean and obese patients with type 2 diabetes. J Am Coll Cardiol. 2016;68(1):53–63. CrossRef
13. Feng T, Zhao X, Gu P, Yang W, Wang C, Guo Q, et al. Adipocyte-derived lactate is a signalling metabolite that potentiates adipose macrophage inflammation via targeting PHD2. Nat Commun. 2022;13(1):5208. CrossRef
14. Hammerschmidt P, Brüning JC. Contribution of specific ceramides to obesity-associated metabolic diseases. Cell Mol Life Sci. 2022;79(8):395. CrossRef
15. James DE, Stöckli J, Birnbaum MJ. The aetiology and molecular landscape of insulin resistance. Nat Rev Mol Cell Biol. 2021;22(11):751–71. CrossRef
16. Menikdiwela KR, Ramalingam L, Allen L, Scoggin S, Kalupahana NS, Moustaid-Moussa N. Angiotensin II increases endoplasmic reticulum stress in adipose tissue and adipocytes. Sci Rep. 2019;9(1):8481. CrossRef
17. Rada P, González-Rodríguez Á, García-Monzón C, Valverde ÁM. Understanding lipotoxicity in NAFLD pathogenesis: is CD36 a key driver? Cell Death Dis. 2020;11(9):802. CrossRef
18. Kazankov K, Jørgensen SMD, Thomsen KL, Møller HJ, Vilstrup H, George J, et al. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat Rev Gastroenterol Hepatol. 2019;16(3):145–59. CrossRef
19. Shim YY, Gui B, Arnison PG, Wang Y, Reaney MJT. Flaxseed (Linum usitatissimum L.) bioactive compounds and peptide nomenclature: a review. Trends Food Sci Technol. 2014;38(1):5–20. CrossRef
20. Johnsson P, Kamal-Eldin A, Lundgren LN, Åman P. HPLC method for analysis of secoisolariciresinol diglucoside in flaxseeds. J Agric Food Chem. 2000;48(11):5216–9. CrossRef
21. Mueed A, Shibli S, Korma SA, Madjirebaye P, Esatbeyoglu T, Deng Z. Flaxseed bioactive compounds: chemical composition, functional properties, food applications and health benefits-related gut microbes. Foods. 2022;11(20):3307. CrossRef
22. Paynel F, Pavlov A, Ancelin G, Rihouey C, Picton L, Lebrun L, et al. Polysaccharide hydrolases are released with mucilages after water hydration of flax seeds. Plant Physiol Biochem. 2013;62:54–62. CrossRef
23. Matsumoto T, Shishido A, Morita H, Itokawa H, Takeya K. Conformational analysis of cyclolinopeptides A and B. Tetrahedron. 2002;58(25):5135–40. CrossRef
24. Cui W, Mazza G, Oomah BD, Biliaderis CG. Optimization of an aqueous extraction process for flaxseed gum by response surface methodology. LWT - Food Sci Technol. 1994;27(4):363–9. CrossRef
25. Shahidi S, Mahmoodi MS, Komaki A, Sadeghian R. The comparison of omega-3 and flaxseed oil on serum lipids and lipoproteins in hyperlipidemic male rats. Heliyon. 2022;8(6):e09662. CrossRef
26. Draganescu D, Andritoiu C, Hritcu D, Dodi G, Popa MI. Flaxseed lignans and polyphenols enhanced activity in streptozotocin-induced diabetic rats. Biology (Basel). 2021;10(1):43. CrossRef
27. Naik HS, Srilatha Ch, Sujatha K, Sreedevi B, Prasad TNVK V. Supplementation of whole grain flaxseeds (Linum usitatissimum) along with high cholesterol diet and its effect on hyperlipidemia and initiated atherosclerosis in Wistar albino male rats. Vet World. 2018;11(10):1433–9. CrossRef
28. Prasad K, Khan AS, Shoker M. Flaxseed and its components in treatment of hyperlipidemia and cardiovascular disease. Int J Angiol. 2020;29(4):216–22. CrossRef
29. Toulabi T, Yarahmadi M, Goudarzi F, Ebrahimzadeh F, Momenizadeh A, Yarahmadi S. Effects of flaxseed on blood pressure, body mass index, and total cholesterol in hypertensive patients: a randomized clinical trial. Explore. 2022;18(4):438–45. CrossRef
30. Prasad K. Secoisolariciresinol diglucoside (SDG) isolated from flaxseed, an alternative to ACE inhibitors in the treatment of hypertension. Int J Angiol. 2013;22(4):235–8. CrossRef
31. Rodriguez-Leyva D, Weighell W, Edel AL, LaVallee R, Dibrov E, Pinneker R, et al. Potent antihypertensive action of dietary flaxseed in hypertensive patients. Hypertension. 2013;62(6):1081–9. CrossRef
32. Dong S, Bai W, Chen J, Zhang L, Sheng W, Feng R. Secoisolariciresinol diglucoside regulates adipose tissue metabolic disorder in obese mice induced by a western diet. J Food Qual. 2021;2021:1–10. CrossRef
33. Fukumitsu S, Aida K, Ueno N, Ozawa S, Takahashi Y, Kobori M. Flaxseed lignan attenuates high-fat diet-induced fat accumulation and induces adiponectin expression in mice. Br J Nutr. 2008;100(3):669–76. CrossRef
34. Shafie SR, Wanyonyi S, Panchal SK, Brown L. Linseed components are more effective than whole linseed in reversing diet-induced metabolic syndrome in rats. Nutrients. 2019;11(7):1677. CrossRef
35. Luo J, Li Y, Mai Y, Gao L, Ou S, Wang Y, et al. Flaxseed gum reduces body weight by regulating gut microbiota. J Funct Foods. 2018;47:136–42. CrossRef
36. Pusceddu MM, El Aidy S, Crispie F, O’Sullivan O, Cotter P, Stanton C, et al. N-3 polyunsaturated fatty acids (PUFAs) reverse the impact of early-life stress on the gut microbiota. PLoS One. 2015;10(10):e0139721. CrossRef
37. Wall R, Ross RP, Shanahan F, O’Mahony L, Kiely B, Quigley E, et al. Impact of administered bifidobacterium on murine host fatty acid composition. Lipids. 2010;45(5):429–36. CrossRef
38. Yang C, Xu Z, Deng Q, Huang Q, Wang X, Huang F. Beneficial effects of flaxseed polysaccharides on metabolic syndrome via gut microbiota in high-fat diet fed mice. Food Res Int. 2020;131:108994. CrossRef
39. Ogawa A, Kadooka Y, Kato K, Shirouchi B, Sato M. Lactobacillus gasseri SBT2055 reduces postprandial and fasting serum non-esterified fatty acid levels in Japanese hypertriacylglycerolemic subjects. Lipids Health Dis. 2014;13:1–8. CrossRef
40. Drissi F, Raoult D, Merhej V. Metabolic role of lactobacilli in weight modification in humans and animals. Microb Pathog. 2017;106:182–94. CrossRef
41. Chung H, Yu JG, Lee I, Liu M, Shen Y, Sharma SP, et al. Intestinal removal of free fatty acids from hosts by Lactobacilli for the treatment of obesity. FEBS Open Bio. 2016;6(1):64–76. CrossRef
42. Pittler MH, Ernst E. Complementary therapies for reducing body weight: a systematic review. Int J Obes. 2005;29(9):1030–8. CrossRef
43. Gao R, Zhu C, Li H, Yin M, Pan C, Huang L, et al. Dysbiosis signatures of gut microbiota along the sequence from healthy, young patients to those with overweight and obesity. Obesity. 2018;26(2):351–61. CrossRef
44. Hutchins AM, Brown BD, Cunnane SC, Domitrovich SG, Adams ER, Bobowiec CE. Daily flaxseed consumption improves glycemic control in obese men and women with pre-diabetes: a randomized study. Nutr Res. 2013;33(5):367–75. CrossRef
45. Edel AL, Rodriguez-Leyva D, Maddaford TG, Caligiuri SPB, Austria JA, Weighell W, et al. Dietary flaxseed independently lowers circulating cholesterol and lowers it beyond the effects of cholesterol-lowering medications alone in patients with peripheral artery disease. J Nutr. 2015;145(4):749–57. CrossRef
46. Rhee Y, Brunt A. Flaxseed supplementation improved insulin resistance in obese glucose intolerant people: a randomized crossover design. Nutr J. 2011;10:1–7. CrossRef
47. Machado AM, de Paula H, Cardoso LD, Costa NMB. Effects of brown and golden flaxseed on the lipid profile, glycemia, inflammatory biomarkers, blood pressure and body composition in overweight adolescents. Nutrition. 2015;31(1):90–6. CrossRef
48. Yari Z, Rahimlou M, Eslamparast T, Ebrahimi-Daryani N, Poustchi H, Hekmatdoost A. Flaxseed supplementation in non-alcoholic fatty liver disease: a pilot randomized, open labeled, controlled study. Int J Food Sci Nutr. 2016;67(4):461–9. CrossRef
49. Torkan M, Hassan Entezari M, Siavash M. Effect of flaxseed on blood lipid level in hyperlipidemic patients. Rev Recent Clin Trials. 2015;10(1):61–7. CrossRef
50. Saxena S, Katare C. Evaluation of flaxseed formulation as a potential therapeutic agent in mitigation of dyslipidemia. Biomed J. 2014;37(6):386–90. CrossRef
51. Wu H, Pan A, Yu Z, Qi Q, Lu L, Zhang G, et al. Lifestyle counseling and supplementation with flaxseed or walnuts influence the management of metabolic syndrome. J Nutr. 2010;140(11):1937–42. CrossRef
52. Wong H, Chahal N, Manlhiot C, Niedra E, McCrindle BW. Flaxseed in pediatric hyperlipidemia: a placebo-controlled, blinded, randomized clinical trial of dietary flaxseed supplementation for children and adolescents with hypercholesterolemia. JAMA Pediatr. 2013;167(8):708–13. CrossRef
53. Zhang W, Wang X, Liu Y, Tian H, Flickinger B, Empie MW, et al. Dietary flaxseed lignan extract lowers plasma cholesterol and glucose concentrations in hypercholesterolaemic subjects. Br J Nutr. 2008;99(6):1301–9. CrossRef
54. Abdelkarem HM, Fadda LH. Flaxseed and quercetin improve anti-inflammatory cytokine level and insulin sensitivity in animal model of metabolic syndrome, the fructose-fed rats. Arab J Chem. 2017;10:S3015–20. CrossRef
55. Kapuriya PB, Sadariya KA, Bhavsar SK, Thaker AM. Antidiabetic activity of aqueous extracts of Linum usitatissimum in streptozotocin induced diabetic rats. Pharma Innov J. 2018;7(7):149–54.
56. Tharwat S, Shaheen D, El-Megeid AA, Salam R, Rashed L, El-Hamid S, et al. Effectiveness of adding flaxseed to type 2 diabetic patient’s regimen. Endocrinol Metab Syndr. 2017;6(3):267–71. CrossRef
57. Kanikowska D, Mali?ska A, Mickiewicz A, Zawada A, Rutkowski R, Pawlaczyk K, et al. Effect of flaxseed (Linum usitatissimum L.) supplementation on vascular endothelial cell morphology and function in patients with dyslipidaemia—a preliminary observation. Nutrients. 2022;14(14):2879. CrossRef
58. Ahmed DH, Fateh HL. Impact of flaxseed supplementation on lipid profile and liver enzymes in patients with non-alcoholic fatty liver disease: systematic review and meta-analysis of randomized controlled trials. Prostaglandins Other Lipid Mediat. 2024;173:106838. CrossRef
59. Jalili C, Pezeshki M, Askarpour M, Marx W, Hassani B, Hadi A, et al. The effect of flaxseed supplementation on circulating adiponectin and leptin concentration in adults: a systematic review and meta-analysis of randomized controlled trials. Phytother Res. 2020;34(7):1578–86. CrossRef
60. Hadi A, Askarpour M, Salamat S, Ghaedi E, Symonds ME, Miraghajani M. Effect of flaxseed supplementation on lipid profile: an updated systematic review and dose-response meta-analysis of sixty-two randomized controlled trials. Pharmacol Res. 2020;152:104622. CrossRef
61. Akowuah P, Rumbaut R, Burns A. Diet-reversal strategy for attenuating high fat diet-induced corneal dysregulation. Invest Ophthalmol Vis Sci. 2022;63(7):3256–A0291.
62. Braga SP, Delanogare E, Machado AE, Prediger RD, Moreira ELG. Switching from high-fat feeding (HFD) to regular diet improves metabolic and behavioral impairments in middle-aged female mice. Behav Brain Res. 2021;398:112969. CrossRef
63. Adedeji TG, Olapade-Olaopa EO. Dietary reversal reverts diet-induced alterations in obstructed bladders of Wistar rats. Nutrition. 2021;89:111346. CrossRef
64. Rapps K, Kisliouk T, Marco A, Weller A, Meiri N. Dieting reverses histone methylation and hypothalamic AgRP regulation in obese rats. Front Endocrinol. 2023;14:1121829. CrossRef
65. Pourjafari F, Haghpanah T, Sharififar F, Nematollahi-Mahani SN, Afgar A, Asadi Karam G, et al. Protective effects of hydro-alcoholic extract of foeniculum vulgare and linum usitatissimum on ovarian follicle reserve in the first-generation mouse pups. Heliyon. 2019;5(10):e02540. CrossRef
66. Dobrian AD, Davies MJ, Prewitt RL, Lauterio TJ. Development of hypertension in a rat model of diet-induced obesity. Hypertension. 2000;35(4):1009–15. CrossRef
67. Diniz YS, Burneiko RM, Seiva FRF, Almeida FQA, Galhardi CM, Filho JLVBN, et al. Diet compounds, glycemic index and obesity-related cardiac effects. Int J Cardiol. 2008;124(1):92–9. CrossRef
68. Bastías-Pérez M, Serra D, Herrero L. Dietary options for rodents in the study of obesity. Nutrients. 2020;12(11):3234. CrossRef
69. Hanif Palla A, Talat Iqbal N, Minhas K, Gilani A-H. Flaxseed extract exhibits mucosal protective effect in acetic acid induced colitis in mice by modulating cytokines, antioxidant and antiinflammatory mechanisms. Int Immunopharmacol. 2016;38:153–66. CrossRef
70. Es-Said S, Lafhal K, Elkhiat A, Hammoud M, Regbaoui N, Ezoubeiri A, et al. Flaxseed extract reduces tissue accumulation and enhances urinary excretion of chondroitin sulphate in the rat: a possible new path in substrate reduction therapy for mucopolysaccharidosis. Pharm Biol. 2022;60:879–88. CrossRef
71. Rodríguez-Correa E, González-Pérez I, Clavel-Pérez PI, Contreras-Vargas Y, Carvajal K. Biochemical and nutritional overview of diet-induced metabolic syndrome models in rats: what is the best choice? Nutr Diabetes 2020;10:24. CrossRef
72. Ghasemi A, Hedayati M, Biabani H. Protein precipitation methods evaluated for determination of serum nitric oxide end products by the Griess assay. Jmsr. 2007;2(15):29–32.
73. Miranda KM, Espey MG, Wink DA. A Rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 2001;5(1):62–71. CrossRef
74. Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38(12):1103–11. CrossRef
75. Leopoldo AS, Lima-Leopoldo AP, Nascimento AF, Luvizotto RAM, Sugizaki MM, Campos DHS, et al. Classification of different degrees of adiposity in sedentary rats. Braz J Med Biol Res. 2016;49(4):e5028. CrossRef
76. Taylor BA, Phillips SJ. Detection of obesity QTLs on mouse chromosomes 1 and 7 by selective DNA pooling. Genomics. 1996;34(3):389–98. CrossRef
77. Carreres L, Jílková ZM, Vial G, Marche PN, Decaens T, Lerat H. Modeling diet-induced NAFLD and NASH in rats: a comprehensive review. Biomedicines. 2021;9(4):378. CrossRef
78. do Nascimento AL, Furtado G da C, Vilhena VM, Carvalho H de O, Sales PF, Barcellos AON, et al. Osteoprotective effect of the phytonutraceutical Ormona® on ovariectomy-induced osteoporosis in Wistar rats. Nutraceuticals. 2024;4(2):147–64. CrossRef
79. Folahan JT, Olorundare OE, Ajayi AM, Oyewopo AO, Soyemi SS, Adeneye AA, et al. Oxidized dietary lipids induce vascular inflammation and atherogenesis in post-menopausal rats: estradiol and selected antihyperlipidemic drugs restore vascular health in vivo. Lipids Health Dis. 2023;22(1):107. CrossRef
80. Oliva L, Alemany M, Fernández-López JA, Remesar X. Circulating oestradiol determines liver lipid deposition in rats fed standard diets partially unbalanced with higher lipid or protein proportions. Br J Nutr. 2022;128(8):1499–508. CrossRef
81. Quirós Cognuck S, Reis WL, Silva MS, Zorro SV, Almeida-Pereira G, Debarba LK, et al. Age and sex influence the response in lipid metabolism of dehydrated Wistar rats. Sci Rep. 2022;12(1):9164. CrossRef
82. Plissonneau C, Sivignon A, Chassaing B, Capel F, Martin V, Etienne M, et al. Beneficial effects of linseed supplementation on gut mucosa-associated microbiota in a physically active mouse model of Crohn’s disease. Int J Mol Sci. 2022;23(11):5891. CrossRef
83. Ren Y, Xu Z, Qiao Z, Wang X, Yang C. Flaxseed lignan alleviates the paracetamol-induced hepatotoxicity associated with regulation of gut microbiota and serum metabolome. Nutrients. 2024;16(2):295. CrossRef
84. Afzal U, Butt MS, Ashfaq F, Bilal A, Suleria HAR. Bioassessment of flaxseed powder and extract against hyperglycemia and hypercholesterolemia using Sprague Dawley rats. Clin Phytosci. 2020;6(1):5. CrossRef
85. Pereira JPC, Oliveira EA, Pereira FAC, Seixas JN, Guimaraes CS de O, Del Bianco Borges B. Beneficial effects of flaxseed and/or mulberry extracts supplementation in ovariectomized Wistar rats. Nutrients. 2022;14(15):3238. CrossRef
86. Seike M, Ashida H, Yamashita Y. Dietary flaxseed oil induces production of adiponectin in visceral fat and prevents obesity in mice. Nutr Res. 2024;121:16–27. CrossRef
87. Paschos GK, Zampelas A, Panagiotakos DB, Katsiougiannis S, Griffin BA, Votteas V, et al. Effects of flaxseed oil supplementation on plasma adiponectin levels in dyslipidemic men. Eur J Nutr. 2007;46(6):315–20. CrossRef
88. Roy B, Palaniyandi SS. Tissue-specific role and associated downstream signaling pathways of adiponectin. Cell Biosci. 2021;11:77. CrossRef
89. Nguyen TMD. Adiponectin: role in physiology and pathophysiology. Int J Prev Med. 2020;11:136. CrossRef
90. Maeda N, Funahashi T, Matsuzawa Y, Shimomura I. Adiponectin, a unique adipocyte-derived factor beyond hormones. Atherosclerosis. 2020;292:1–9. CrossRef
91. Bauzá-Thorbrügge M, Vuji?i? M, Chanclón B, Palsdottir V, Pillon NJ, Benrick A, Wernstedt Asterholm I. Adiponectin stimulates Sca1+CD34--adipocyte precursor cells associated with hyperplastic expansion and beiging of brown and white adipose tissue. Metabolism. 2024;151:155716. CrossRef
92. Abbasi S, Karimi K, Hossein Moridpour A, Musazadeh V, Faghfouri AH, Jozi H. Can flaxseed supplementation affect circulating adipokines in adults? An updated systematic review and meta-analysis of randomized controlled trials. Front Nutr. 2023;10:1179089. CrossRef
93. Wang M, Zhang XJ, Feng K, He C, Li P, Hu YJ, et al. Dietary α-linolenic acid-rich flaxseed oil prevents against alcoholic hepatic steatosis via ameliorating lipid homeostasis at adipose tissue-liver axis in mice. Sci Rep. 2016;6(1):26826. CrossRef
94. Guan Y, Zuo F, Zhao J, Nian X, Shi L, Xu Y, et al. Relationships of adiponectin to regional adiposity, insulin sensitivity, serum lipids, and inflammatory markers in sedentary and endurance-trained Japanese young women. Front Endocrinol. 2023;14:1097034. CrossRef
95. Guo Q, Chang B, Yu QL, Xu ST, Yi XJ, Cao SC. Adiponectin treatment improves insulin resistance in mice by regulating the expression of the mitochondrial-derived peptide MOTS-c and its response to exercise via APPL1–SIRT1–PGC-1α. Diabetologia. 2020;63(12):2675–88. CrossRef
96. Yu X, Deng Q, Tang Y, Xiao L, Liu L, Yao P, et al. Flaxseed oil attenuates hepatic steatosis and insulin resistance in mice by rescuing the adaption to ER stress. J Agric Food Chem. 2018;66(41):10729–40. CrossRef
97. Morshedzadeh N, Rahimlou M, Shahrokh S, Karimi S, Mirmiran P, Zali MR. The effects of flaxseed supplementation on metabolic syndrome parameters, insulin resistance and inflammation in ulcerative colitis patients: an open-labeled randomized controlled trial. Phytother Res. 2021;35(7):3781–91. CrossRef
98. Yuan X, Bao X, Liu X, Li X. Flaxseed-derived peptides ameliorate hepatic cholesterol metabolism in Sprague-Dawley rats fed a high-cholesterol and high-fat diet. J Sci Food Agric. 2022;102(12):5348–57. CrossRef
99. Youness ER, Hussein JS, Ibrahim AMM, Agha FE. Flaxseed oil attenuates monosodium glutamate-induced brain injury via improvement of fatty acids contents. Biomed Pharmacol J. 2019;12(2):527–32. CrossRef
100. Dias GD, Cartolano FC, Freitas MCP, Santa-Helena E, Markus MRP, Santos RD, et al. Adiponectin predicts the antioxidant capacity and size of high-density lipoprotein (HDL) in individuals with diabetes mellitus. J Diabetes Complications. 2021;35:107856. CrossRef
101. Zocchi M, Della Porta M, Lombardoni F, Scrimieri R, Zuccotti GV, Maier JA, et al. A potential interplay between HDLs and adiponectin in promoting endothelial dysfunction in obesity. Biomedicines. 2022;10:1344. CrossRef
102. Wang G, Wang Y, Luo Z. Effect of adiponectin variant on lipid profile and plasma adiponectin levels: a multicenter systematic review and meta-analysis. Cardiovasc Ther. 2022;2022:4395266. CrossRef
103. Zhang P, Sun H, Cheng X, Li Y, Zhao Y, Mei W, et al. Dietary intake of fructose increases purine de novo synthesis: a crucial mechanism for hyperuricemia. Front Nutr. 2022;9:1045805. CrossRef
104. Zhang C, Li L, Zhang Y, Zeng C. Recent advances in fructose intake and risk of hyperuricemia. Biomed Pharmacother. 2020;131:110795. CrossRef
105. Lin CR, Tsai PA, Wang C, Chen JY. The association between uric acid and metabolic syndrome in a middle-aged and elderly Taiwanese population: a community-based cross-sectional study. Healthcare. 2024;12(1):113. CrossRef
106. Bowden RG, Richardson KA, Richardson LT. Uric acid and metabolic syndrome: findings from national health and nutrition examination survey. Front Med. 2022;9:1039230. CrossRef
107. Raya-Cano E, Vaquero-Abellán M, Molina-Luque R, De Pedro-Jiménez D, Molina-Recio G, Romero-Saldaña M. Association between metabolic syndrome and uric acid: a systematic review and meta-analysis. Sci Rep. 2022;12(1):18412. CrossRef
108. Al Za’abi M, Ali H, Ali BH. Effect of flaxseed on systemic inflammation and oxidative stress in diabetic rats with or without chronic kidney disease. PLoS One. 2021;16(10):e0258800. CrossRef
109. Xiao J, Zhu S, Guan H, Zheng Y, Li F, Zhang X, et al. AMPK alleviates high uric acid-induced Na+-K+-ATPase signaling impairment and cell injury in renal tubules. Exp Mol Med. 2019;51(5):1–14. CrossRef
110. Tamir TY, Chaudhary S, Li AX, Trojan SE, Flower CT, Vo P, et al. Structural and systems characterization of phosphorylation on metabolic enzymes identifies sex-specific metabolic reprogramming in obesity. Mol Cell. 2025;85:2211–29.e8. CrossRef
111. Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87(1):315–424. CrossRef
112. Mangge H. Antioxidants, inflammation and cardiovascular disease. World J Cardiol. 2014;6(6):462. CrossRef
113. Salvatore T, Galiero R, Caturano A, Vetrano E, Loffredo G, Rinaldi L, et al. Coronary microvascular dysfunction in diabetes mellitus: pathogenetic mechanisms and potential therapeutic options. Biomedicines. 2022;10(9):2274. CrossRef
114. Reijrink M, De Boer SA, Van Roon AM, Slart RHJA, Fernandez BO, Feelisch M, et al. Plasma nitrate levels are related to metabolic syndrome and are not altered by treatment with DPP-4 inhibitor linagliptin: a randomised, placebo-controlled trial in patients with early type 2 diabetes mellitus. Antioxidants. 2021;10(10):1548. CrossRef
115. Oishi JC, Castro CA, Silva KA, Fabricio V, Cárnio EC, Phillips SA, et al. Endothelial dysfunction and inflammation precedes elevations in blood pressure induced by a high-fat diet. Arq Bras Cardiol. 2018;110(6):558–67. CrossRef
116. Ren G, Hwang PTJ, Millican R, Shin J, Brott BC, van Groen T, et al. Subcutaneous administration of a nitric oxide-releasing nanomatrix gel ameliorates obesity and insulin resistance in high-fat diet-induced obese mice. ACS Appl Mater Interfaces. 2022;14(17):19104–15. CrossRef
117. Eslami Z, Moghanlou AE, Kandi YMNP, Arabi MS, Norouzi A, Joshaghani H. Atorvastatin and flaxseed effects on biochemical indices and hepatic fat of NAFLD model in rats. Adv Biomed Res. 2023;12(1):98. CrossRef
118. AlRamadneh TN, AlQurashi N, Khan MSA, Hashimi SM, Javaraiah R, Al-Ostoot FH, et al. Flaxseed oil ameliorates mercuric chloride-induced liver damage in rats. J Trace Elem Med Biol. 2022;71:126965. CrossRef
119. Geidl-Flueck B, Gerber PA. Fructose drives de novo lipogenesis affecting metabolic health. J Endocrinol. 2023;257:e220270. CrossRef
120. Greupner T, Kutzner L, Nolte F, Strangmann A, Kohrs H, Hahn A, et al. Effects of a 12-week high-α-linolenic acid intervention on EPA and DHA concentrations in red blood cells and plasma oxylipin pattern in subjects with a low EPA and DHA status. Food Funct. 2018;9(3):1587–600. CrossRef
121. Meuronen T, Lankinen MA, Fauland A, Shimizu BI, de Mello VD, Laaksonen DE, et al. Intake of camelina sativa oil and fatty fish alter the plasma lipid mediator profile in subjects with impaired glucose metabolism – a randomized controlled trial. Prostaglandins Leukot Essent Fatty Acids. 2020;159:102143. CrossRef
122. Patti AM, Al-Rasadi K, Giglio RV, Nikolic D, Mannina C, Castellino G, et al. Natural approaches in metabolic syndrome management. Arch Med Sci. 2018;14:422–41. CrossRef
123. Kavyani Z, Pourfarziani P, Mohamad Jafari Kakhki A, Sedgh Ahrabi S, Hossein Moridpour A, Mollaghasemi N, et al. The effect of flaxseed supplementation on glycemic control in adults: an updated systematic review and meta-analysis of randomized controlled trials. J Funct Foods. 2023;110:105816. CrossRef
124. Yang C, Xia H, Wan M, Lu Y, Xu D, Yang X, et al. Comparisons of the effects of different flaxseed products consumption on lipid profiles, inflammatory cytokines and anthropometric indices in patients with dyslipidemia related diseases: systematic review and a dose-response meta-analysis of randomized controlled trials. Nutr Metab. 2021;18:91. CrossRef