INTRODUCTION
Skin, an organ of the human body, functions as the first barrier against external environmental stress factors, such as dehydration, and chemical and biological damage [1]. Chemical and biological stress factors contribute to cell senescence and aging that may cause chronic wounds. Wounds disrupt the skin layer, contributing to the breaking and discontinuation of skin and soft tissue integrity [2]. The healing process begins immediately and comprises many overlapping phases, including coagulation, inflammation, proliferation, and remodeling [3]. Elastin and collagen are essential for maintaining skin flexibility, elasticity, and strength during skin repair. Collagen content represents an abundant protein structure in the dermis layer and elastin creates a fiber network in the connective tissue of the skin [4]. Reactive oxygen species (ROS) affect healing processes, leading to prolonged inflammation, tissue damage, and inhibition of elastin and collagen function, which may delay wound healing [5]. ROS generated after stress exposure can indirectly stimulate elastase and collagenase activity [6]. Antioxidant compounds are important for converting ROS into stable molecules and for enhancing wound healing [7]. Natural products, such as herbs and traditional medicinal plants, are frequently used to promote wound healing. Among them, Aloe L. species have been reported to have wound-healing properties owing to their various phytochemical compounds. Aloe gel contains several active ingredients such as polysaccharides, flavonoids, carbohydrates, anthraquinones, organic compounds, phytosterols, anthrones, sterols, terpenoids, hormones, vitamins, proteins, and minerals [8]. Moreover, studies have shown that extracts of A. vera, particularly when prepared using phosphate-buffered saline, possess an elastase-inhibitory effect [9]. The A. vera gel exhibits high antioxidant activity, inhibits matrix metalloproteinase 1 synthesis, and increases collagen production in human fibroblasts in vitro [10]. The ability of A. vera extracts to inhibit tyrosinase, collagenase, and elastase suggests their potential use as skin-care additives in natural remedies and cosmetics [11]. Molecular docking has significantly advanced our understanding of the interactions between bioactive compounds and target enzymes, such as elastase, collagenase, and hyaluronidase. This computational method provides valuable insights into binding mechanisms, including hydrogen bonding and hydrophobic contacts, within the active site of the enzyme [12]. For example, docking studies have demonstrated the strong binding affinity of phytochemicals, such as polyphenols and flavonoids, to elastase and collagenase, highlighting their potential to inhibit these enzymes [13]. To confirm these findings, experimental assays are often employed to verify the docking predictions, ensuring the accuracy of the interactions and affirming the inhibitory potential of these compounds. Although the wound-healing properties, antioxidant activity, and elastin- and collagen-producing activities of aloe gels have been reported, the anti-elastase, anti-collagenase, and anti-hyaluronidase properties have not been studied in relation to the wound-healing process. Therefore, we investigated the phytochemicals presented in Aloe barbadensis gel extract and determined their antioxidant, anti-elastase, anti-collagenase, anti-hyaluronidase, and wound-healing properties. In addition, the interactions between bioactive compounds of A. barbadensis gel extract and elastase, collagenase, and hyaluronidase were explored.
MATERIALS AND METHODS
Plant sample preparation and extraction
Aloe barbadensis was grown on the Homkajon farm located near Suan Dusit University, Suphanburi province, Thailand under Good Agricultural Practice principles and collected in December 2021. The plants were authenticated, and a voucher specimen (BK083620) was deposited in the plant variety protection, Department of Agriculture, Ministry of Agriculture and Cooperatives, Thailand. A. barbadensis leaves were washed, and the gel was separated from the leaf. Subsequently, the gel solution was squeezed and filtered through a nylon cloth and then freeze-dried using a freeze-dryer (12L, Labconco, USA). The A. barbadensis gel powder was extracted with 70% ethanol for 24 hour combined with agitation. Subsequently, ultrasound-assisted extraction was performed for 30 minutes for gel extraction. The gel extract solution was evaporated in an evaporator and dried via freeze-drying. The extract was yellowish in color, with a yield of 3.34% ± 0.31%. The A. barbadensis gel extract was stored at -20°C until analysis.
Determination of total phenolic content
The total phenolic content was measured using Folin–Ciocalteu reagent according to the method of Rungruang et al. [14], with some modifications. Briefly, 20 µl of the sample (1 mg/ml) was added to a 96-well plate and mixed with 10% Folin–Ciocalteu reagent (100 µl) for 5 minutes. Subsequently, 80 µl of 7.5% Na2CO3 was added to the mixture and the mixture was incubated at rest for 60 minutes. The absorbance was measured at 760 nm using a microplate reader (Biochrom EZ Read 2000, Cambridge, United Kingdom). The total phenolic content was calculated and compared to a gallic acid standard curve. The results were expressed as mg gallic acid equivalent/g extract.
Determination of the flavonoid content
The total flavonoid content was determined using the aluminum chloride method described by Rungruang et al. [15], with slight modifications. Aloe barbadensis gel extract (30 µl) was mixed with 100 µl of NaNO2 in a 96-well plate and agitated for 6 minutes. Subsequently, the 15 µl of AlCl3 was added to the mixture following incubation at 25°C ± 2°C for 6 minutes. Furthermore, the reaction solution was mixed with 1 M NaOH (55 µl) and incubated for 10 minutes. A microplate reader was utilized to measure absorbance at 510 nm. Total flavonoid content was calculated and compared to a quercetin standard curve. The results were expressed as quercetin equivalents (mg QE/g extract).
Antioxidant activity
2,2-Diphenyl-1-picrylhydrazyl radical-scavenging activity assay
2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical-scavenging activity of the aloe gel was determined as described by Rungruang et al. [15]. In a 96-well plate, 195 µl of DPPH radical solution in 95% ethanol (0.16 mM) was added to 5 µl to the extract and incubated for 30 minutes in the dark. The absorbance of the reaction mixture was measured at 515 nm using a microplate reader.
2,2-Azino-bis-(3-ethylbenzo-thiazoline-6-sulfonic acid) radical-scavenging activity assay
2,2-Azino-bis-(3-ethylbenzo-thiazoline-6-sulfonic acid) (ABTS) radical-scavenging activity assay was performed for the aloe gel extract as described by Rungruang et al. [15]. To generate the ABTS radicals, we combined 7.0 mM ABTS and 2.45 mM K2S2O8, followed by incubation, and incubated it in darkness for 16 hours. The radical solution was diluted with 95% ethanol, which gave an absorbance of 0.70 ± 0.05 units at 734 nm. Subsequently, 40 µl of the extract was mixed with 160 µl of ABTS•+ radical solution and allowed to stand for 6 minutes in the dark. Absorbance was recorded at 734 nm. The scavenging activity of the DPPH and ABTS radicals was determined using the following equation:
where A is the absorbance of blank;
B is the absorbance of sample.
The half-maximal inhibitory concentrations (IC50) were calculated using GraphPad Prism (Version 9.3, GraphPad Software, Inc., USA).
Liquid chromatography coupled with hybrid ion trap and time-of-flight mass spectrometry
To identify the phytochemical components found in A. barbadensis gel extracts, liquid chromatography was connected with a hybrid ion trap and time-of-flight-mass spectrometry (LC/MS-IT-TOF) [16]. The mass spectrometer (LCMS-IT-TOF; Shimadzu, Kyoto, Japan) had an ODS-3 column (4.6 × 150 mm, 5 μm) with a mobile phase gradient of 0.1% formic acid in distilled water (A) and methanol (B). A flow rate of 0.45 ml/minute was applied and the solution was eluted using a step gradient (5%–60% B for 40 minutes) with isocratic elution (60% B for 2 minutes and 5% B for 2 minutes). The column temperature was 40°C. MS was performed with a full scan over ranges of m/z 100–1,500 (MS1) and m/z 100–700 (MS2). Phytochemical compounds were interpreted using MS and MS/MS spectra and the profiles were compared with those reported in the literature.
Aloins A and B in gel extracts were quantified via high-performance liquid chromatography (HPLC) [17]. The Shimadzu HPLC system utilized an Agilent TC-C 18 column (250 mm × 4.6 mm, 5 µm) and a UV-visible diode-array detector. The injection volume was 15 μl (1 mg/ml), the flow rate was 1.8 ml/min, and the mobile phase gradient consisted of mobile phase A (0.1% (v/v) acetic acid in water) and mobile phase B (0.1% (v/v) acetic acid in acetonitrile). Quantification of aloin A and B content was performed based on the peak areas of chromatograms obtained using external standards (aloin A and B).
Inhibition of collagenase activity
DQTM collagen was used as a substrate to determine the collagenase inhibitory activity using an EnzChek® collagenase/gelatinase kit (Molecular Probe, Eugene, OR, USA) [15]. Collagenase derived from Clostridium hystolyticum and the aloe gel extract was mixed at various concentrations of 7.81–500 µg/ml and then dissolved in Tris-HCL buffer (pH 7.4). A diluted extract (80 µl) was mixed with collagen (20 µl) and collagenase (100 µl). After 90-minute incubation in the dark at room temperature, fluorescence was measured using a microplate reader (Infinite® 200 Pro; Tecan, Männedorf, Switzerland), with excitation and emission wavelengths of 485 and 538 nm, respectively. EGCG and 1,10-phenanthroline were used as positive controls, and solvent was used as a negative control. The collagenase inhibitory activity of the extract was expressed as the IC50 value (the concentration of extract capable of inhibiting 50% of collagenase activity). The equation for calculating collagenase inhibitory activity is as follows:
where A is the fluorescence intensity of enzyme and substrate;
B is fluorescence intensity of substrate;
C is fluorescence intensity of enzyme and substrate with extract;
D is fluorescence intensity of extract and substrate.
Inhibition of elastase activity
Porcine pancreatic elastase (PE-E.C.3.4.21.36), N-succinyl-Ala-Ala-Ala-p-nitroanilide (AAAPVN), and aloe gel extract were used to determine elastase inhibitory activity, as described by Rungruang et al. [15]. Porcine pancreatic elastase was added to aloe gel extract at different concentrations (7.81–500 µg/ml) in a 96-well plate and incubated at room temperature for 10 minutes. AAAPVN was added to the mixture make up to a final volume of 250 µl/well followed by incubation for 60 minutes at room temperature. Absorbance was recorded at 410 nm. Epigallocatechin gallate (EGCG) was used as a positive control, while the solvent used for the extraction was employed as a negative control. The elastase inhibitory activity of the extract was interpreted as the IC50 value.
Inhibition of hyaluronidase activity
The turbidimetric method was used to determine the hyaluronidase inhibitory activity [18]. The reaction mixture comprised A. barbadensis gel extract (10 µl), 30 U/ml hyaluronidase enzyme in acetate buffer (pH 7.0; 25 µl), sodium acetate buffer (50 mM; pH 7.0) in 77 mM NaCl, 1 mg/ml bovine albumin (25 µl), and sodium acetate buffer (50 mM, pH 4.5) (15 µl). The mixtures were incubated at 37°C for 10 minutes. Subsequently, 25 µl of hyaluronic acid solution [0.3 mg/ml in acetate buffer (pH 4.5)] was added and the mixture was incubated at 37°C for 45 minutes. Excess hyaluronidase was precipitated by adding 200 µl of 2.5% cetyltrimethylammonium bromide in 2% NaOH following storage at room temperature for 10 minutes. The absorbance of the turbid reaction mixture was determined at 600 nm. EGCG was used as the positive control, and the solvent used for the extract was employed as the negative control. The hyaluronidase inhibitory activity of the extract was interpreted as the IC50 value.
Molecular docking
Three-dimensional structures of collagenase (PDB CID: 1CGL), elastase (PDB CID: 1BRU), and hyaluronidase (PDB CID: 1FCV) were obtained from the RCSB Protein Data Bank. The ligand molecular structures of aloin A (Compound CID: 12305761) and aloin B (Compound CID: 14989) and the standard compound of this study, EGCG (Compound CID: CID 65064) were obtained from PubChem. AutoDock Vina v1.1.2 (The Scripps Research Institute, La Jolla, San Diego, CA, USA) was used to determine the binding modes of each ligand bound to the substrate-binding sites of collagenase, elastase, and hyaluronidase, which predicted the experimental binding poses and energies [19]. Polar hydrogens were introduced and Gasteiger and Marsili [20] partial charges were allocated. The search grid of the key site of all receptors is shown in Table 1. Docking accuracy was increased by adjusting the exhaustiveness value to 300. The thoroughness parameter was assigned using AutoDock Vina, which controls the thoroughness by which the software scans the lowest affinity energy. Each docking box’s size and center coordinates were established (Table 1). Following docking analysis, the protein-ligand interactions were visualized with BIOVIA Discovery Studio Visualizer v20.1.0.0 (Accelrys, San Diego, CA, USA). Validation was performed to verify the effectiveness of the docking parameters. This was achieved by re-docking the co-crystallized ligand into the active site. The objective was to confirm that the ligand binds precisely to the active site cleft with minimal deviation compared to the original co-crystallized complex. The native ligand of each original co-crystallized complex was removed and re-docked into the active site using the same grid parameters and protocol of each enzyme (Table 1). The re-docked complex was subsequently superimposed onto the reference co-crystalized structure, and the root mean square deviation (RMSD) between the co-crystallized and the docked poses for each enzyme was computed.
 | Table 1. Dimensions and center coordinates of docking boxes for each receptor. [Click here to view] |
Assessment of cytotoxicity in human skin fibroblasts
Cytotoxicity was determined based on the cleavage of (4-[3-(4-iodophenyl)-2-(4-nitro-phenyl)-2H-5-tetrazolio]-1,3-benzene sulfonate) (tetrazolium salt WST-1) into formazan, as described by Vichai and Kirtikara [21]. Primary dermal fibroblasts from healthy adults (ATCC PCS-201-012) were grown in DMEM with 10% fetal bovine serum and 1% penicillin/streptomycin. Briefly, 1 × 104 cells/well were added to a 96-well plate and incubated in a 5% CO2 atmosphere at 37°C for 24 hours. Subsequently, the cells were treated with aloe extract at 0.1–1,000 µg/ml and incubated in 5% CO2 at 37°C for 24 hours. The treated cells were washed with phosphate buffer, and 10 µl WST-1 was added to the washed cells. After incubation for 10 minutes, the absorbance was measured at 450 nm to determine the cell viability percentage.
Potential of wound healing
Primary dermal fibroblasts derived from normal neonatal humans (PCS-201-010 ™, ATCC) were seeded in a 24-well plate at a cell density of 2 × 105 cells/well; a wound was created using a cell scratcher [22]. Subsequently, the cells were treated with A. barbadensis gel extract at concentrations of 100, 200, and 500 µg/ml diluted in DMEM. Cell migration was observed under an inverted microscope (Carl Zeiss Microscopy, ZEISS Axio Vert.A1, USA) at 0, 24, and 48 hours. The wound area was determined using Zeiss Zen (ZEN 2.6, blue edition; Carl Zeiss, Oberkochen, Germany). The findings are shown as a percentage of the wound area relative to each condition at 0 hours. The results were expressed as the percentage of wound area relative to the baseline condition at 0 hours.
Statistical analysis
To statistically analyze the results, an analysis of variance was performed using SAS version 9 (SAS Institute Inc., Cary, NC, USA). Statistical analyses were conducted using a t-test for comparisons between two groups or a one-way analysis of variance followed by Fisher’s Least Significant Difference post hoc test for comparisons among more than two groups. A p < 0.05 was considered indicative of statistical significance.
RESULTS AND DISCUSSION
Total phenolic and total flavonoid contents along with the antioxidant activity of the aloe extract
Freeze-dried A. barbadensis gel was extracted with 70% ethanol while stirring for 24 hours, followed by ultrasound-assisted extraction for 30 minutes. The gel extract contained relatively high total phenolic and total flavonoid contents (Table 2). According to Jawade and Chavan [23], ultrasound-assisted ethanol extraction increases the aloin yield (30%–40%) and reduces the extraction time compared to that associated with non-sonicated extraction techniques. Plants generally produce phytochemical compounds via metabolism. These compounds are important in defense mechanisms that affect cell survival. The phytochemical content depends on many factors, including genetics and growth conditions [24]. In this study, A. barbadensis samples were obtained from plants grown via organic farming, which does not allow the use of chemical agents for pest, weed, and disease control. These plants are exposed to high levels of stress, leading to an increase in the total phenolic content and total flavonoid content. Huber et al. [25] found that organic-cultivated plants contain high vitamin C and phenolic compound contents. In addition, the contents of minerals, carotenoids, and antioxidants in pepper plants cultivated in organic farms are higher than those in pepper plants cultivated via conventional farming [26]. Moreover, the phytochemicals present in the aloe gel extract play a role in antioxidant activity, particularly in radical scavenging. In this study, the radical-scavenging activities were investigated using DPPH and ABTS assays. These assays measure radical-scavenging activity based on hydrogen and electron donation. The radical-scavenging assays showed that the gel extract treatment reduced the DPPH and ABTS radical levels (Table 2). The gel extract had better DPPH radical-scavenging activity than ABTS radical-scavenging activity. Previous studies have reported the antioxidant activity of A. barbadensis gel extract, which findings consistent with those of Hu et al. [27], who reported significant antioxidant properties of Aloe vera extracts, particularly in DPPH assays. In the current study, our gel extract exhibited higher antioxidant activity than previous reports in both DPPH and ABTS assays, though still less potent than L-ascorbic acid. The DPPH activity was found to be strongly correlated with the phenolic and flavonoid content of the extract, suggesting that these phytochemicals play a significant role in its antioxidant properties. Additionally, the radical scavenging effect observed in this study aligns with the findings of Quisep et al. [28] and Ozsoy et al. [29], who similarly attributed the highest antioxidant activity of Aloe vera to phenolic compounds such as cinnamic acids, flavonoids, and anthracene. These compounds, as highlighted by earlier studies, may also exhibit synergistic or antagonistic effects, influencing the overall antioxidant capacity of the extract [30]. However, different extraction methods or plant cultivations could contribute to variations in antioxidant capacity.
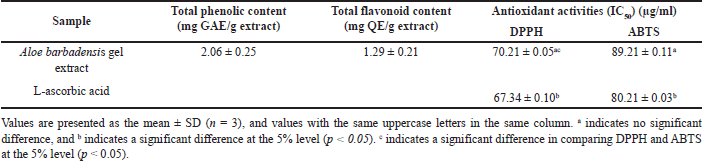 | Table 2. Total phenolic content, total flavonoid content, and antioxidant activity of Aloe barbadensis gel extract. [Click here to view] |
Chemical composition of aloe gel extract
The phytochemical profile of the A. barbadensis gel extract was determined via LC/MS-IT-TOF (Table 3) and HPLC (Fig. 1). The major bioactive compounds in the gel extract were anthraquinones (2′-O-feruloylaloesin, aloenin-2′-p-coumaroyl ester, trihydroxy octadecenoic acid, chrysoeriol-7-O-glycuronyl, 10-hydroxyaloin B, 10-hydroxyaloin A, 5-hydroxyaloin A, aloin B, and aloin A) followed by phenolic compounds (3-O-caffeoyl-4-O-feruloylquinic acid and 3,4-Di (E)-p-coumaroylquinic acid) and sterol (24-methylenecycloartanol). Aloin A (47.54 ± 0.22 mg/g extract) and Aloin B (35.85 ± 0.17 mg/g extract) quantified by HPLC were the main compounds. In general, the main phytochemical compounds in gel extracts are polysaccharides and anthraquinones [31]. Barbaloin (aloin A and aloin B), which is an active compound present in aloe gel extracts, has an anthraquinone C-glycoside structure [28]. Aloin B is preferentially formed in the aloe gel extract and converted into aloin A by non-enzymatic conversion [32]. However, several bioactive compounds present in aloe gel extract affect the bioactivities, including anti-inflammatory, anticancer, antioxidant, and wound-healing activities. Notably, various anthraquinones present in aloe gel are implicated in skin disorders such as skin inflammation, wound healing, and enhancement of skin regeneration [33].
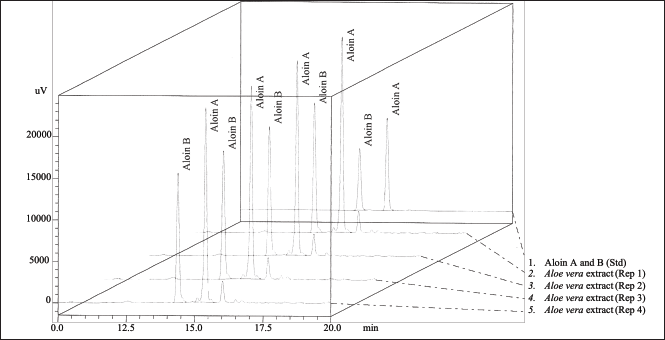 | Figure 1. Typical chromatograms of the standards aloin A and aloin B, and of the A. barbadensis gel extract. [Click here to view] |
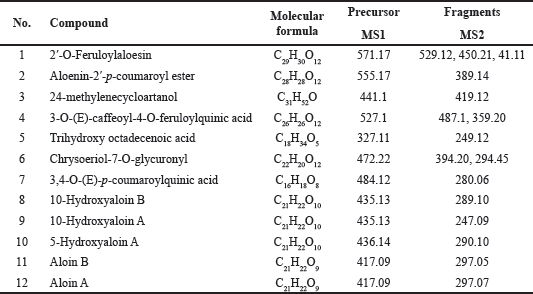 | Table 3. Chemical composition of Aloe barbadensis gel extract by LC/MS-IT-TOF. [Click here to view] |
Collagenase, elastase, and hyaluronidase activity inhibition
The inhibition of activities of Matrix metalloproteinases (MMPs), such as collagenase, elastase, and hyaluronidase, was investigated in vitro. These enzymes are responsible for the breakdown of elastin and collagen and are stimulated by transforming growth factors during wound repair. They regulate extracellular matrix (ECM) degradation and deposition, key processes for successful wound re-epithelialization. Hyaluronic acid (HA), the primary glycosaminoglycan in the skin, maintains moisture but is degraded by hyaluronidase. Inhibiting hyaluronidase activity prevents rapid HA breakdown, thereby preserving the ECM’s structural integrity and enhancing tissue permeability during the healing process [34]. The A. barbadensis gel extract showed inhibitory activity against collagenase, elastase, and hyaluronidase (Table 4). The half-maximal inhibitory concentrations (IC50) of A. barbadensis gel extract for anti-collagenase, anti-elastase, and anti-hyaluronidase activities were 79.01 ± 0.11, 78.23 ± 0.07, and 87.31 ± 0.13 µg/ml, respectively. Previous studies have reported the inhibitory effects of A. vera extracts on MMPs, particularly collagenase and elastase [35,36]. The present study extends these findings by demonstrating the potential of A. barbadensis gel extract to inhibit hyaluronidase activity as well. However, while the inhibition of hyaluronidase is critical for preventing the rapid degradation of HA in the EMCs and preserving structural integrity, the observed inhibitory effect of A. barbadensis gel extract was less than that of EGCG. In this study, EGCG was used as a positive control and showed strong inhibitory activities on collagenase, elastase, and hyaluronidase. Nevertheless, previous studies have highlighted the potential undesired effects of EGCG, including anemia, liver, and kidney failure [37,38]. Despite their strong inhibitory effects, EGCG is associated with severe side effects, emphasizing the need for further research to evaluate the safety and effectiveness of these compounds in vivo. Elastin and collagen are the major constituents of the extracellular matrix in the skin and play a key role in wound healing processes. The increase in collagen content in healing wounds treated with aloe gel provided evidence of the collagenase-inhibiting activity of aloins and aloe gel. This fact, combined with the ability of other aloe gel components, such as aloesin, glycoproteins, and polysaccharides, to stimulate cell growth, supports the use of aloe gel in the treatment of chronic ulcers, burns, and wounds [36]. Additionally, the inhibition of MMPs is desirable in the normal wound healing process. Our results indicate that the elastase and collagenase inhibitory activities of A. barbadensis gel extract may indirectly promote wound healing by inhibiting elastin and collagen degradation during the skin regeneration process. The interaction of aloins with Ca2+ represents another mechanism of inhibition. Low-molecular-weight aloins may bind to Ca2+, which is required for enzyme activity. Therefore, the interaction of Ca+ ions with aloin results in a decrease in intracellular Ca2+ availability [36]. Additionally, the structure of aloin is similar to that of doxycycline modified from tetracycline, which inhibits MMP-8 activity more potently than tetracycline does [36,39]. Furthermore, the bioactivity was most likely the result of a synergistic interaction between the components (Table 3). Considering the different groups of chemical compounds presented in Table 3, the bioactivity is most likely attributable to synergism between the components.
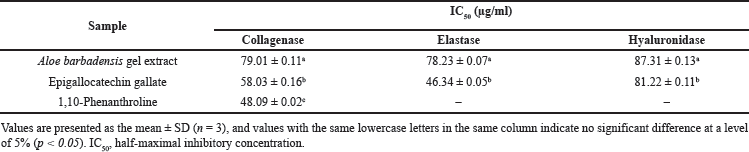 | Table 4. Anti-elastase, anti-collagenase, and anti-hyaluronidase activities of Aloe barbadensis gel extract, [Click here to view] |
Molecular docking
Molecular docking can be used to align biological activities following the chemical evaluation and identification of major compounds as well as to study the molecular mechanisms of action following purification and in vitro tests [40]. The docking parameters were examined to determine the optimal conditions by redocking the cocrystallized ligand into its respective active site. The validation analysis of the docking showed that the RMSD values for the native ligand were 1.92 Å for collagenase (PDB CID: 1CGL), 1.26 Å for elastase (PDB CID: 1BRU), and 1.56 Å for hyaluronidase (PDB CID: 1FCV), confirming the reliability of the docking method due to an RMSD value of less than 2 Å [41]. Figure 2 shows the overlay of the native ligand conformation before and after validation. Molecular docking of aloins A and B derived from the A. barbadensis gel extract with collagenase, elastase, and hyaluronidase active sites revealed strong binding energies ranging from -5.8 to -7.9 kcal/mol (Table 5). The analysis of H-bonds and residual interactions indicated that the binding affinities for these enzymes varied depending on the ligand structure. Aloins A and B, both anthraquinones, exhibited similar binding patterns due to their isoform structure [42], with minimal variation in the amino acids involved in binding (Table 5, Fig. 3). Aloin A showed a higher affinity for elastase with a binding energy of -7.9 Kcal/mol, followed by aloin B at -7.5 Kcal/mol. Both aloins exhibited a strong binding interaction with elastase via the common amino acid HIS57. The catalytic triad of elastase, comprising ASP102, HIS57, and SER195, is essential for its enzymatic activity, with GLY216 also identified as a key amino acid for inhibition [43,44]. Interestingly, aloin A and EGCG bind to HIS57, SER195, and GLY216, while aloin B interacts only with HIS57 and SER195. Despite this, EGCG showed a higher binding affinity (-84 Kcal/mol) due to its ability to form more binding interactions, resulting in stronger elastase inhibition compared to aloin A and B. For collagenase, all ligands formed H-bonds with basic amino acids, such as HIS218 and HIS228, and hydrophobic interactions with LEU181. Aloe A exhibited a higher binding affinity (-6.8 kcal/mol) than aloin B (-6.6 Kcal/mol) due to closer binding distances. EGCG showed the highest affinity (-8.2 Kcal/mol), as it formed more binding interactions. These findings suggest that EGCG exhibits stronger collagenase inhibition compared to aloin A and B. For hyaluronidase, the key binding amino acids identified were GLN200, CYS201, MET206, and LYS246. The binding affinity of the ligands varied with EGCG showing the strongest inhibition (-6.3 Kcal/mol), followed by aloin A (-6.0 Kcal/mol) and aloin B (-5.8 Kcal/mol). This pattern aligns with the results from in vitro enzyme inhibition tests (Table 4), confirming that A. barbadensis gel extract and EGCG most effectively inhibit elastase, followed by collagenase and hyaluronidase. These studies indicated that aloins A and B derived from A. barbadensis gel extract can be used as anti-elastase, anti-collagenase, and anti-hyaluronidase agents for developing cosmeceutical products.
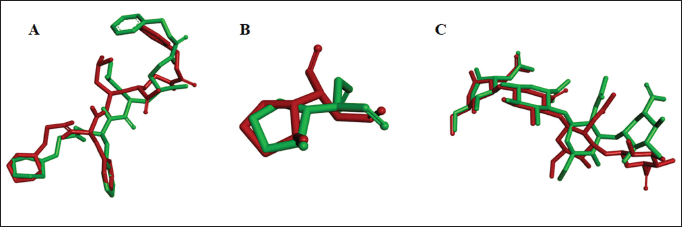 | Figure 2. Overlay native ligand conformation PubChem CID 57416166 (A); PubChem CID 5287452 (B); PubChem CID 156618357 (C) and GlyCosmos Entry G59412AX before validation (green) and after validation (red). [Click here to view] |
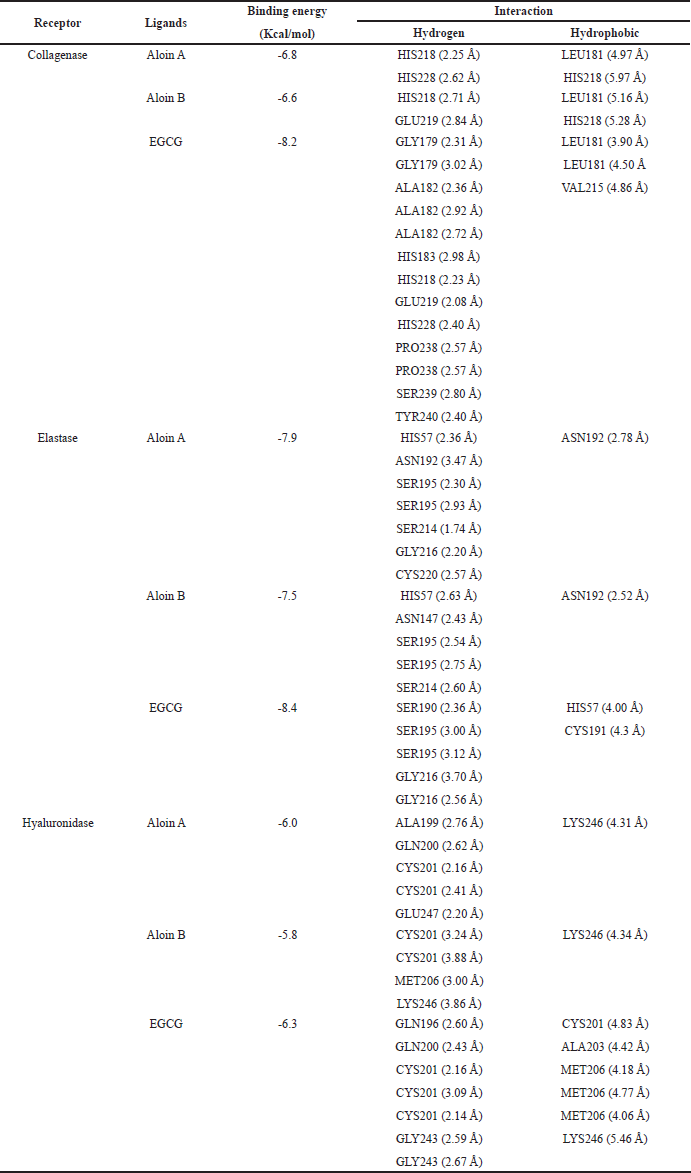 | Table 5. Docking score, binding sites, and bond length of different ligands with collagenase, elastase, and hyaluronidase. [Click here to view] |
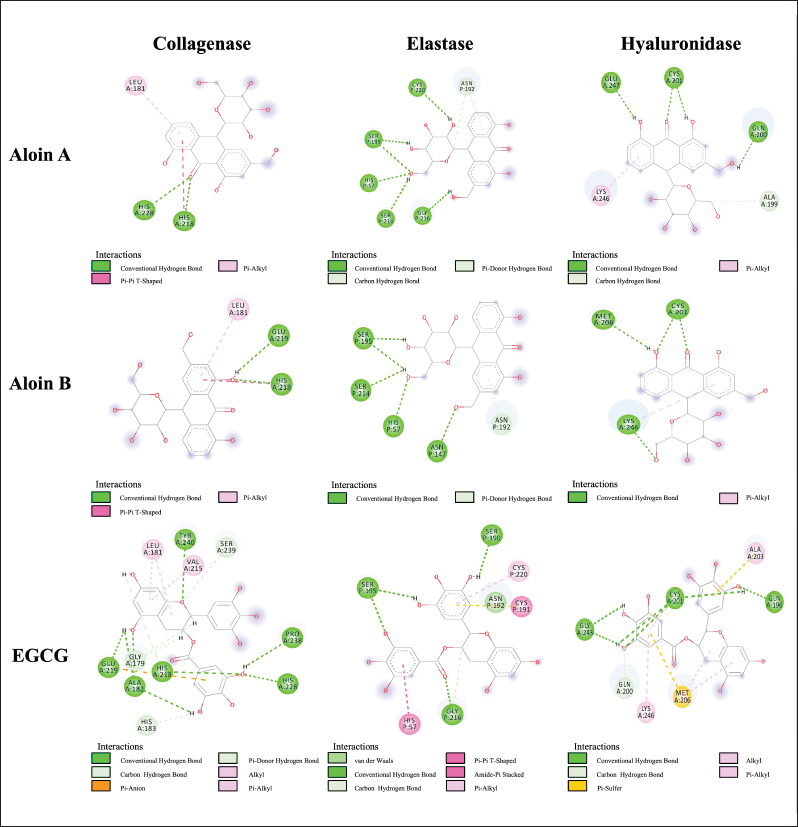 | Figure 3. Two-dimensional protein–ligand interactions at the collagenase, elastase, and hyaluronidase-related active sites of aloin A and aloin B. [Click here to view] |
Cell viability of human skin fibroblasts
The cytotoxicity of A. barbadensis gel extract in primary dermal fibroblasts (ATCC PCS-201-012TM) was evaluated in vitro. The cytotoxicity was calculated and expressed as a percentage of cell viability, which was compared between cells treated with A. barbadensis gel extract (0.1–1,000 µg/ml). Cell viability decreased in a dose-dependent manner. Gel extract treatment at concentrations ≤ 100 µg/ml was non-cytotoxic to cells when compared with untreated cells (Fig. 4). These concentrations of A. barbadensis gel extract were considered safe.
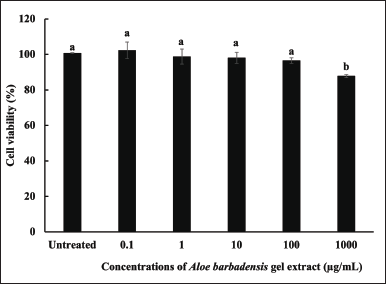 | Figure 4. Cell viability (%) of human skin fibroblasts treated with A. barbadensis gel extract at concentrations of 0.1–1,000 µg/ml compared with untreated cells (control). (a) indicates no significant difference, and (b) indicates a significant difference at the 5% level (p < 0.05). [Click here to view] |
Effect of aloe gel extract on wound healing
The wound healing potential of aloe gel extract was investigated based on the percentage of wound closure and observed cell migration. Primary dermal fibroblasts (ATCC, PCS-201-010™) were treated with A. barbadensis gel extract at concentrations of 0 (untreated), 100, 200, and 500 µg/ml. These concentrations were not toxic to the cells, as the survival rate exceeded 90% (data not shown). Crowded fibroblasts were observed after treatment with A. barbadensis gel extract at 100, 200, and 500 µg/ml for 24 and 48 hours (Fig. 5A), which was related to the wound area percentage (Fig. 5B). The wound areas were calculated at 0, 24, and 48 hours were found to decrease after 48 hours. The wound area of untreated cells was 11.81% ± 3.09%. Treatment with 100, 200, and 500 µg/ml of A. barbadensis gel extract significantly reduced the wound area by 9.19 ± 3.53, 9.69 ± 0.80, and 6.20% ± 2.72%, respectively, when compared with each condition at 0 h. Treatment with 500 µg/ml of A. barbadensis gel extract significantly promoted wound recovery compared with untreated cells. Therefore, A. barbadensis gel extract treatment resulted in fibroblast growth owing to mitochondrial activity [10]. Aloe gel is a traditional medicine used to promote skin wound healing, and its active compounds, such as polysaccharides, aloin, aloesin, and vitamins, play important roles in wound healing. In particular, aloins A and B in A. barbadensis gel extract play a major role in wound healing by promoting fibroblast growth factor (FGF) expression. FGF promotes fibroblast proliferation and regulates collagen fiber production during the wound-healing process [45]. In addition, the antioxidant properties of bioactive compounds can enhance skin regeneration [46,47]. However, while fibroblasts play a key role in tissue remodeling and collagen production, they may not fully replicate the role of keratinocytes in wound healing. Keratinocytes, which are crucial for epithelialization, close wounds and restore the skin barrier. As shown by Nowinski et al. [48], fibroblasts and keratinocytes interact through paracrine loops, which are vital for wound healing [49,50]. Thus, future studies using keratinocyte models are essential to better understand how A. barbadensis gel extract supports wound healing, particularly in skin regeneration and epithelial barrier repair.
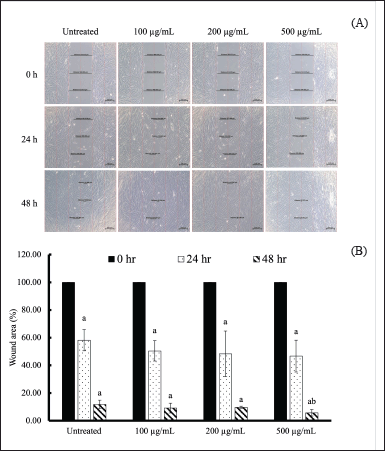 | Figure 5. Wound healing of human fibroblasts treated with A. barbadensis gel extract in vitro. (A) Morphology of the wound scratch treated with A. barbadensis gel extract at various concentrations (100, 200, and 500 µg/ml) compared with untreated cells determined via inverted light microscopy. (B) Percentage of wound area treated with A. barbadensis gel extract at various concentrations compared with untreated cells. The wound area was measured using Zeiss Zen (ZEN 2.6, blue edition). Superscript letter (a) indicates a significant difference when compared to cells at 0 h, whereas (b) indicates a significant difference when compared to untreated cells at p < 0.05. [Click here to view] |
CONCLUSION
This study demonstrated that the gel extract derived from A. barbadensis contained various bioactive compounds, including phenolic, flavonoid, anthraquinone, and sterol compounds. A. barbadensis exhibited antioxidant activity in both DPPH and ABTS assays, with stronger inhibition observed in the DPPH assay compared to ABTS. The wound closure rate was greater than 50% after treatment with the gel extract for 24 hour. Interestingly, A. barbadensis extract showed inhibitory activities against elastase, collagenase, and hyaluronidase. In particular, aloins A and B derived from A. barbadensis gel extract exhibited a strong affinity for elastase inhibition via the common amino acid His57. These properties indicate that the aloe gel extract of A. barbadensis can be used as an active ingredient in pharmaceutical and cosmetic products.
ACKNOWLEDGMENTS
This work was supported by the National Research Council of Thailand and Suan Dusit University (FF-65-024). The authors would like to thank the Cosmeceutical Bioactivity Analysis and Testing Laboratory, Suan Dusit University for the provision of facilities and instruments used in the analysis.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Proksch E, Brandner JM, Jensen JM. The skin: an indispensable barrier. Exp Dermatol. 2008;17:1063–72. CrossRef
2. Enoch S, Leaper DJ. Basic science of wound healing. Surgery. 2008;26:31–7. CrossRef
3. Rosenbaum AJ, Banerjee S, Rezak KM, Uhl RL. Advances in wound management. JAAOS. 2018;26:833–43. CrossRef
4. Jiratchayamaethasakul C, Ding Y, Hwang O, Im ST, Jang Y, Myung SW, et al. In vitro screening of elastase, collagenase, hyaluronidase, and tyrosinase inhibitory and antioxidant activities of 22 halophyte plant extracts for novel cosmeceuticals. Fish Aquat Sci. 2020;23:1–9. CrossRef
5. Fitzmaurice SD, Sivamani RK, Isseroff RR. Antioxidant therapies for wound healing: a clinical guide to currently commercially available products. Skin Pharmacol Physiol. 2011;24:113–26. CrossRef
6. Chatatikun M, Chiabchalard A. Thai plants with high antioxidant levels, free radical scavenging activity, anti-tyrosinase and anti-collagenase activity. BMC Complement Altern Med. 2017;17:1–9. CrossRef
7. Dunnill C, Patton T, Brennan J, Barrett J, Dryden M, Cooke J, et al. Reactive oxygen species (ROS) and wound healing: the functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int Wound J. 2017;14:89–96. CrossRef
8. Nalimu F, Oloro J, Kahwa I, Ogwang PE. Review on the phytochemistry and toxicological profiles of Aloe vera and Aloe ferox. Future J. Pharm. Sci. 2021;7(1):1–21. CrossRef
9. Sacan O, Akev N, Yanardag R. In vitro inhibitory effect of Aloe vera (L.) Burm. f. leaf extracts on the activity of some enzymes and antioxidant activity. Indian J Biochem Biophys. 2017;54:82–9.
10. Ro HS, Jang HJ, Kim GR, Park SJ, Lee HY. Enhancement of the anti-skin wrinkling effects of Aloe arborescens miller extracts associated with lactic acid fermentation. J Evid Based Complement Altern Med. 2020;1:2743594. CrossRef
11. Sacan O, Akev N, Yanardag R. Inhibitory effects of Aloe vera extracts on anti-tyrosinase, anti-collagenase and anti-elastase potential. Bangladesh J. Bot. 2024;53(3):597–603. CrossRef
12. Mechqoq, H., Hourfane, S., El Yaagoubi, M., El Hamdaoui, A., da Silva Almeida, J. R. G., Rocha, J. M., et al. Molecular docking, tyrosinase, collagenase, and elastase inhibition activities of argan by-products. Cosmetics. 2022;9(1):24. CrossRef
13. Nutho B, Tungmunnithum D. Exploring major flavonoid phytochemicals from Nelumbo nucifera Gaertn. as potential skin anti-aging agents: in silico and in vitro evaluations. Int. J. Mol. Sci. 2023;24(23):16571. CrossRef
14. Rungruang R, Ratanathavorn W, Boohuad N, Selamassakul O, Kaisangsri N. Antioxidant and anti-aging enzyme activities of bioactive compounds isolated from selected Zingiberaceae plants. Agr Nat Res. 2021;55(1):153–60. CrossRef
15. Rungruang R, Panichakul T, Rattanathavorn W, Kaisangsri N, Kerdchoechuen O, Laohakunjit N, et al. Effects of extraction methods on the flavonoid and phenolic contents and anti-aging properties of Rhyncholaeliocattleya Haw Yuan Beauty extracts. Sci. Asia. 2021;47(6):698–706. CrossRef
16. Aldayel TS, Grace MH, Lila MA, Yahya MA, Omar UM, Alshammary G. LC-MS characterization of bioactive metabolites from two Yemeni Aloe spp. with antioxidant and antidiabetic properties. Arab J Chem. 2020;13:5040–49. CrossRef
17. Brown PN, Yu R, Kuan CH, Finley J, Mudge EM, Dentali S. Determination of Aloin A and Aloin B in Aloe vera raw materials and finished products by high-performance liquid chromatography: single-laboratory validation. J AOAC Int. 2014;97:1323–28. CrossRef
18. Studzi?ska-Sroka E, Dudek-Makuch M, Chanaj-Kaczmarek J, Czepulis N, Korybalska K, Rutkowski R, et al. Anti-inflammatory activity and phytochemical profile of Galinsoga Parviflora Cav. Molecules. 2018;23:2133. CrossRef
19. Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, et al. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem. 2009;30:2785–91. CrossRef
20. Gasteiger J, Marsili M. Iterative partial equalization of orbital electronegativity—a rapid access to atomic charges. Tetrahedron. 1980;36:3219–28. CrossRef
21. Vichai V, Kirtikara K. Sulforhodamine B. Colorimetric assay for cytotoxicity screening. Nat Protoc. 2006;1:1112–16. CrossRef
22. Panichakul T, Ponnikorn S, Tupchiangmai W, Haritakun, W, Srisanga K. Skin anti-aging potential of Ipomoea pes-caprae ethanolic extracts on promoting cell proliferation and collagen production in human fibroblasts (CCD-986sk Cells). Pharmaceuticals. 2022;15:969. CrossRef
23. Jawade NR, Chavan AR. Ultrasonic-assisted extraction of aloin from Aloe vera gel. Procedia Eng. 2013;51:487–93. CrossRef
24. Aida PUIA, Chedea VS, Levai AM, Bocsan IC, Buzoianu AD. Pot Aloe vera gel–a natural source of antioxidants. Not Bot Horti Agrobo. 2022;50:12732. CrossRef
25. Huber M, Rembia?kowska E, ?rednicka D, Bügel S, Van De Vijver LPL. Organic food and impact on human health: assessing the status quo and prospects of research. NJAS Wageningen J. Life Sci. 2011;58:103–9. CrossRef
26. Bicikliski O, Trajkova F, Mihajlov L, Jordanovska S, Tashev K. Vitamin C and total antioxidant content in pepper fruits (Capsicum annuum L.): Comparative analysis of peppers grown in conventional and organic agricultural systems. Annu. Res Rev Biol. 2018;27(5):1–11. CrossRef
27. Hu Y, Xu J, Hu Q. Evaluation of antioxidant potential of Aloe vera (Aloe barbadensis Miller) extracts. J. Agric. Food Chem. 2003;51(26):7788–91. CrossRef
28. Quispe C, Villalobos M, Bórquez J, Simirgiotis M. Chemical composition and antioxidant activity of Aloe vera from the Pica Oasis (Tarapacá, Chile) by UHPLC-Q/Orbitrap/MS/MS. J Chem. 2018;6123850. CrossRef
29. Ozsoy N, Candoken E, Akev N. Implications for degenerative disorders: antioxidative activity, total phenols, flavonoids, ascorbic acid, β-carotene and β-tocopherol in Aloe vera. Oxid Med Cell Longev. 2009;2(2):99–106. CrossRef
30. Tomsone L, Kruma Z, Galoburda R. Comparison of different solvents and extraction methods for isolation of phenolic compounds from horseradish roots (Armoracia rusticana). Int. J. Agric. Eng. 2012;6:236–41. CrossRef
31. Martínez-Burgos WJ, Serra JL, MarsigliaF RM, Montoya P, Sarmiento-Vásquez Z, Marin O, et al. Aloe vera: from ancient knowledge to the patent and innovation landscape–a review. S Afr J Bot. 2022;147:993–1006. CrossRef
32. Patel K, Patel DK. Medicinal importance, pharmacological activities, and analytical aspects of aloin: a concise report. J Acute Dis. 2013;2:262–69. CrossRef
33. Curto EM, Labelle A, Chandler HL. Aloe vera: an in vitro study of effects on corneal wound closure and collagenase activity. Vet. Ophthalmol. 2014;17:403–10. CrossRef
34. Ac?kara OB, Ilhan M, Kurtul E, Šmejkal K, Akkol EK. Inhibitory activity of Podospermum canum and its active components on collagenase, elastase and hyaluronidase enzymes. Bioorg. Chem. 2019;93:103330. CrossRef
35. Kudalkar MD, Nayak A, Bhat KS, Nayak RN. Effect of Azadirachta indica (Neem) and Aloe vera as compared to subantimicrobial dose doxycycline on matrix metalloproteinases (MMP)-2 and MMP-9: an in-vitro study. Ayu. 2014;35(1):85–9. CrossRef
36. Barrantes E, Guinea M. Inhibition of collagenase and metalloproteinases by aloins and aloe gel. Life Sci. 2003;72:843–50. CrossRef
37. Thring TS, Hili P, Naughton DP. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement Altern Med. 2009; 9:1–11. CrossRef
38. Mereles D, Hunstein W. Epigallocatechin-3-gallate (EGCG) for clinical trials: more pitfalls than promises?. Int. J. Mol. Sci. 2011;12(9):5592–603. CrossRef
39. Greenwald RA, Golub LM, Ramamurthy NS, Chowdhury M, Moak SA, Sorsa T. In vitro sensitivity of the three mammalian collagenases to tetracycline inhibition: relationship to bone and cartilage degradation. Bone. 1998;22:33–8. CrossRef
40. Yuriev E, Holien J, Ramsland PA. Improvements, trends, and new ideas in molecular docking: 2012–2013 in review. JMR. 2015;28:581–604. CrossRef
41. Nursamsiar, Siregar M, Awaluddin A, Nurnahari N, Nur S, Febrina E, Asnawi A. Molecular docking and molecular dynamic simulation of the aglycone of curculigoside A and its derivatives as alpha glucosidase inhibitors. Rasayan J Chem. 2020;13(1):690–98. CrossRef
42. H?? M, Dziedzic K, Górecka D, J?drusek-Goli?ska A, Gujska E. Aloe vera (L.) Web: natural sources of antioxidants—a review. Plant Foods Hum Nutr. 2019;74(3):255–65. CrossRef
43. Eun Lee K, Bharadwaj S, Yadava U, Gu Kang S. Evaluation of caffeine as inhibitor against collagenase, elastase and tyrosinase using in silico and in vitro approach. J Enzyme Inhib Med Chem. 2019;34:927–36. CrossRef
44. Donarska B, Z ??czkowski K. Recent advances in the development of elastase inhibitors. Future Med Chem. 2020;12:1809–13. CrossRef
45. Atiba A, Nishimura M, Kakinuma S, Hiraoka T, Goryo M, Shimada Y, et al. Aloe vera oral administration accelerates acute radiation-delayed wound healing by stimulating transforming growth factor-β and fibroblast growth factor production. Am J Surg. 2011;201:809–18. CrossRef
46. Oliveira, ACL, Tabrez S, Shakil S, Khan MI, Asghar MN, Matias BD, et al. Mutagenic, antioxidant and wound healing properties of Aloe vera. J. Ethnopharmacol. 2018;227:191–97. CrossRef
47. Dat AD, Poon F, Pham KB, Doust J. Aloe vera for treating acute and chronic wounds. CDSR. 2012;(2):1–24. CrossRef
48. Nowinski D, Lysheden AS, Gardner H, Rubin K, Gerdin B, Ivarsson M. Analysis of gene expression in fibroblasts in response to keratinocyte derived factors in vitro: potential implications for the wound healing process. J. Invest. Dermatol. 2004;122:216–21. CrossRef
49. Menon SN, Flegg JA, McCue SW, Schugart RC, Dawson RA, McElwain DS. Modelling the interaction of keratinocytes and fibroblasts during normal and abnormal wound healing processes. Proc Biol Sci. 2012;279(1741):3329–38. CrossRef
50. Spiekstra SW, Breetveld M, Rustemeyer T, Scheper RJ, Gibbs S. Wound-healing factors secreted by epidermal keratinocytes and dermal fibroblasts in skin substitutes. Wound Repair Regen. 2007;15(5):708–17. CrossRef