INTRODUCTION
Cancer remains one of the most significant global health challenges, with a rising incidence and a substantial burden on healthcare systems worldwide [1]. Despite the progress made in cancer treatment, the emergence of drug resistance and the complexity of cancer biology necessitate the continuous development of new, effective anticancer agents [2,3]. The discovery of novel compounds that can selectively target key molecular pathways involved in cancer progression is essential for improving therapeutic outcomes and reducing side effects [4].
Benzothiazole scaffolds have emerged as a promising class of compounds in drug discovery, largely due to their straightforward synthesis [5,6] and diverse therapeutic activities [7–9]. Among the 2-substituted benzothiazoles, derivatives such as amino benzothiazole [10–13], mercapto benzothiazole [14–18], and aryl benzothiazole [19–21] have demonstrated significant anticancer activity in various in vitro and in vivo cancer models. The potential of these compounds is further highlighted by the ongoing clinical trials of benzothiazole-based drugs like Riluzole [22,23] (Fig. 1) which showcase their promise as chemotherapeutic agents.
 | Figure 1. Structure of riluzole. [Click here to view] |
Recent literature has reported the efficacy of 2-substituted benzothiazoles in targeting the epidermal growth factor receptor (EGFR), a key protein involved in the regulation of cell proliferation and survival. Studies have demonstrated that 2-substituted benzothiazole derivatives can effectively inhibit EGFR signaling, which is crucial in many cancers where EGFR is overexpressed or mutated [24–27]. These findings underline the potential of 2-substituted benzothiazoles as effective EGFR inhibitors, highlighting their therapeutic promise in cancer treatment.
The promising results of benzothiazole-based compounds in targeting EGFR highlight the need for novel derivatives that offer enhanced efficacy and selectivity. This research is inspired by these results and seeks to advance the field by introducing novel benzothiazole–carboxamide hybrids. The study involves the synthesis of a series of benzothiazole–carboxamide hybrids and their systematic evaluation for anticancer potential against well-established cancer cell lines, including MCF-7 (breast cancer) and HCT-116 (colon cancer), as well as a normal human cell line (HEK-293). The novelty of this research lies in its integration of synthetic chemistry with molecular docking studies, specifically focusing on the binding interactions of these new hybrids with EGFR. Advanced docking techniques are employed using crystal structures of EGFR in complex with gefitinib (PDB ID: 4WKQ) and osimertinib (PDB ID: 6LUD). This comprehensive approach aims to identify new lead compounds with potentially superior anticancer activity and selectivity, providing valuable insights for the development of targeted therapies for breast and colon cancers.
MATERIALS AND METHODS
Synthetic-grade chemicals and solvents were sourced from Sigma-Aldrich, Bangalore, India, and used without further purification. Reactions were monitored using Merck-precoated aluminum TLC plates with silica gel 60 F254. Melting points were determined with a Remi electronic melting point apparatus. 1H and 13C NMR spectra were recorded using a BRUKER DRX spectrometer, with tetramethyl silane as the internal reference for chemical shift calibration in ppm. NMR splitting patterns were denoted as follows: singlet (s), doublet (d), triplet (t), quartet (q), and multiplet (m). High-resolution mass spectrometry (HRMS) spectra were obtained in positive ionization mode using a Waters Xevo Q-Tof mass spectrometer. A-549, PANC-1, and HEK-293 cell lines were acquired from ATCC and procured through Himedia Pvt Ltd, India.
Synthesis of benzothiazole–carboxamide hybrids
The Scheme of synthesis for the benzothiazole–carboxamide hybrids is depicted in Figure 2.
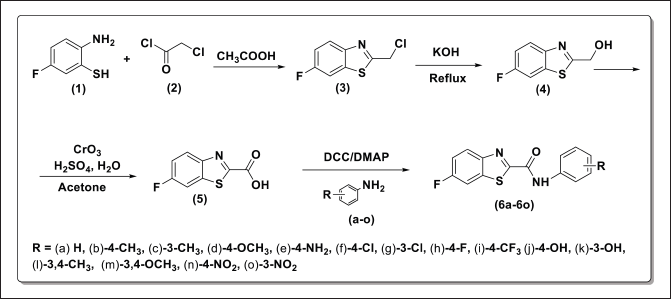 | Figure 2. Scheme of synthesis for benzothiazole–carboxamide hybrids (6a–6o). [Click here to view] |
Procedure for the synthesis of 2-Chloromethyl-benzothiazole (3)
To a solution of 2-amino-5-fluorobenzenethiol (1) (1 g, 7.93 mmol) in acetic acid (15 ml), and 2-chloroacetyl chloride (2) (1.35 g, 1.19 mmol) was added dropwise and refluxed for 3hrs. The reaction was monitored using thin-layer chromatography with a 10% ethyl acetate-hexane solvent system as the mobile phase. After cooling, the mixture was poured onto crushed ice (100 ml) and basified with 5 mol/l NaOH. The solution was extracted with chloroform (3 × 50 ml). The combined organic layers were dried over MgSO4 and concentrated under a vacuum. Purification of the residue by column chromatography on silica gel (petroleum ether/acetone, 10:1 v:v) gave compound 3 as a yellow solid [28].
Procedure for the synthesis of (6-fluorobenzo[d]thiazol-2-yl)methanol (4)
A round-bottom flask equipped with a reflux apparatus was charged with 10 ml of a 1M aqueous KOH solution. To this, a solution of 2-(chloromethyl)-6-fluorobenzo[d]thiazole (3) in ethanol was slowly added while maintaining constant stirring. The reaction mixture was then heated under reflux for 2 hours with continuous stirring to ensure complete reaction. After refluxing, the mixture was allowed to cool to room temperature. The cooled reaction mixture was transferred to a separation funnel, and 20 ml of distilled water and 20 ml of ethyl acetate were added to separate the organic and aqueous layers. The aqueous layer, containing potassium chloride (KCl), was discarded. The organic layer was washed three times with 20 ml portions of distilled water to remove residual salts and impurities. The washed organic layer was then dried over anhydrous sodium sulfate, and the drying agent was subsequently removed by filtration. Finally, the dried organic layer was subjected to vacuum evaporation to obtain the product (6-fluorobenzo[d]thiazol-2-yl) methanol (4) [29 30].
Procedure for the synthesis of 6-fluorobenzo[d]thiazole-2-carboxylic acid (5)
To an orange, homogeneous solution of CrO3 (1.24 g, 0.0123 mol) in H2O (88.4 ml) at 0°C, H2SO4 (10.8 ml) was added dropwise via an addition funnel over 30 minutes with continuous stirring. The addition funnel was then rinsed with H2O (1 ml), resulting in a 1.23 M solution of Jones Reagent. To a solution of (6-fluorobenzo[d]thiazol-2-yl)methanol (2.16 mmol) in acetone (75 ml) at room temperature (immersed in a water bath), Jones Reagent (4.38 ml, 5.39 mmol) was added via an addition funnel over 30 minutes. The dark reaction mixture was stirred at room temperature overnight. Additional Jones Reagent (1.8 ml, 1.0 equivalent) was added, and the mixture was stirred for an additional 6.5 hours. Isopropanol (6 ml) was then added, and the mixture was stirred for 30 minutes, resulting in a dark green precipitate. The mixture was diluted with ether (60 ml) and washed with 2% aqueous NaHSO3 (3 x 20 ml). The layers were separated, and the aqueous layer was back-extracted with ether (2 x 20 ml). The combined organic layers were washed with H2O (20 ml) and brine (20 ml), then dried over Na2SO4. The aqueous layer was back-extracted with ether (30 ml), and the resulting organic layer was combined with the previous organic extracts. The organics were concentrated to yield the product as an off-white solid [31].
General procedure for the synthesis of benzothiazole–carboxamide hybrids (6a-6o)
To a magnetically stirred solution of carboxylic acid, 4 (1 mmol) in H2O was added 1 mmol of N, N’-diisopropylcarbodiimide, and the reaction mixture was stirred at room temperature for 1 hour. After this period, the amine (1 mmol) was added and the reaction mixture was stirred at room temperature for 12 hours until the starting materials were totally consumed as checked by TLC. Then, the solvent was separated by filtration, and the solid was washed several times with lukewarm water in order to remove the by-product diisopropyl urea (DIU) [32].
MTT assay
To assess the anticancer activity of Benzothiazole–carboxamide hybrids (6a-6r), an MTT assay was performed using MCF-7 (breast cancer), HCT-116 (colon cancer), and HEK-293 (normal human embryonic kidney) cell lines. Cells were cultured in RPMI-1640 or DMEM medium supplemented with 10% fetal bovine serum and 1% Penicillin–Streptomycin, maintained at 37°C in a 5% CO2 incubator. At 70%–80% confluence, adherent cells were trypsinized, while SET-2 cells were gently suspended. Approximately 5,000–10,000 cells per well were seeded in 96-well plates and stabilized for 24 hours.
Serial dilutions of compounds (0.1 µM to 100 µM) were prepared, and 100 µl of each dilution was applied to the wells, with DMSO as the vehicle control. After 48 hours of incubation, 10 µl of MTT reagent (5 mg/ml) was added and incubated for 4 hours. Formazan crystals were dissolved with 100 µl of DMSO, and absorbance was measured at 570 nm with a 630 nm reference. IC50 values were calculated by plotting cell viability against compound concentration. Experiments were conducted in triplicate to ensure reliability [33].
Molecular docking
The Protein Data Bank provided EGFR kinase domain with gefitinib (4WKQ) and EGFR in complex with Osimertinib (6LUD) X-ray crystal structures. Hydrogen atoms were added, and bond orders were assigned to the protein’s 3D structure using the Protein Preparation Wizard in Schrödinger. Chiral ligands were prepared and their 3D structures were optimized using the LigPrep module with the OPLS 2005 force field. Receptor sites for 5E1E, 7RN6, and 7SJ3 were analyzed with the SITEMAP tool in Maestro 11.8, followed by grid creation using Schrödinger’s grid generation tool. Molecular docking was conducted with Glide’s Standard Precision (SP) mode, where binding interaction energy, van der Waals energy, electrostatic potential, and strain energy were assessed to obtain the SP Glide score. Ligand binding to EGFR and CDK-4 active sites was studied using the Schrödinger Maestro interface [34].
RESULTS AND DISCUSSION
Chemistry
The synthesis of benzothiazole–carboxamide hybrids was successfully achieved through the outlined experimental procedures. Initially, 2-chloromethyl-benzothiazole (3) was synthesized by reacting 2-amino-5-fluorobenzenethiol with 2-chloroacetyl chloride, and was isolated as a yellow solid after column chromatography. Subsequently, (6-fluorobenzo[d]thiazol-2-yl)methanol (4) was prepared by reacting 2-chloromethyl-6-fluorobenzo[d]thiazole with aqueous KOH, with the product successfully isolated through extraction and drying. The oxidation of (6-fluorobenzo[d]thiazol-2-yl)methanol using Jones Reagent yielded 6-fluorobenzo[d]thiazole-2-carboxylic acid (5), which was obtained as an off-white solid following multiple extractions. Finally, 6a-6o were synthesized by coupling carboxylic acid 4 with various amines in the presence of N,N’-diisopropylcarbodiimide. These hybrids were successfully prepared and purified through filtration and washing, demonstrating the effectiveness of the synthesis process and setting the stage for further evaluation in anticancer studies.
Analytical characterization
6-fluoro-N-phenylbenzo[d]thiazole-2-carboxamide (6a)
Pale yellow solid, yield-82%; m.p-221-222 OC; 1H NMR (500 MHz, Chloroform-d) δ 10.26 (s, 1H), 8.05 (dd, J = 12.1, 2.1 Hz, 1H), 7.84 (dd, J = 7.6, 4.6 Hz, 1H), 7.65 – 7.60 (m, 2H), 7.32 (t, J = 6.9 Hz, 2H), 7.14 (ddd, J = 10.0, 7.5, 2.1 Hz, 1H), 7.10 – 7.03 (m, 1H). 13C NMR (125 MHz, Chloroform-d) δ 109.42, 109.61, 113.02, 113.20, 120.87, 123.25, 123.32, 123.79, 128.77, 138.04, 140.45, 140.55, 148.43, 148.46, 158.10, 158.13, 160.10, 162.21. HRMS: m/z: For C14H9FN2OS ([M + H]+): 273.0481, found 273.0479.
6-fluoro-N-(p-tolyl)benzo[d]thiazole-2-carboxamide (6b)
Pale yellow Solid, Yield-79%; m.p-234-236 OC; 1H NMR (500 MHz, Chloroform-d) δ 10.03 (s, 1H), 8.00 (dd, J = 7.6, 4.6 Hz, 1H), 7.87 (dd, J = 12.1, 2.1 Hz, 1H), 7.40 (d, J = 8.5 Hz, 2H), 7.24 (ddd, J = 9.8, 7.5, 2.2 Hz, 1H), 7.17 (d, J = 8.2 Hz, 2H), 2.35 (s, 3H). 13C NMR (125 MHz, Chloroform-d) δ 20.11, 106.85, 107.04, 112.51, 112.69, 120.43, 123.87, 123.94, 129.41, 132.17, 134.06, 139.44, 139.54, 146.07, 146.10, 158.00, 159.28, 159.98, 160.88. HRMS: m/z: For C15H11FN2OS ([M + H]+): 287.0628, found 287.0626.
6-fluoro-N-(m-tolyl)benzo[d]thiazole-2-carboxamide (6c)
Pale yellow solid, yield-80%; m.p-231-232 OC; 1H NMR (500 MHz, Chloroform-d) δ 10.23 (s, 1H), 7.92 (ddd, J = 22.6, 9.9, 3.4 Hz, 2H), 7.53 (dt, J = 7.5, 1.7 Hz, 1H), 7.34 (t, J = 2.2 Hz, 1H), 7.19 (ddd, J = 9.9, 7.7, 2.2 Hz, 1H), 7.13 (t, J = 7.7 Hz, 1H), 6.95 – 6.89 (m, 1H), 2.34 (s, 3H). 13C NMR (125 MHz, Chloroform-d) δ 20.62, 108.30, 108.49, 112.29, 112.47, 116.88, 120.81, 123.25, 123.32, 124.58, 128.84, 137.36, 138.75, 139.50, 139.60, 146.53, 146.55, 159.07, 160.40, 162.37, 162.54. HRMS: m/z: For C15H11FN2OS ([M + H]+): 287.0623, found 287.0623.
6-fluoro-N-(4-methoxyphenyl)benzo[d]thiazole-2-carboxamide (6d)
Pale yellow solid, yield-76%; m.p-205-206 OC; 1H NMR (500 MHz, Chloroform-d) δ 10.49 (s, 1H), 8.04 (dd, J = 12.1, 2.3 Hz, 1H), 7.88 (dd, J = 7.7, 4.6 Hz, 1H), 7.56 (d, J = 8.3 Hz, 2H), 7.21 (ddd, J = 9.8, 7.7, 2.2 Hz, 1H), 6.93 – 6.87 (m, 2H), 3.87 (s, 3H). 13C NMR (125 MHz, Chloroform-d) δ 55.19, 108.52, 108.71, 114.29, 115.12, 115.30, 122.61, 123.25, 123.32, 131.95, 139.15, 139.25, 147.49, 147.52, 156.34, 158.12, 159.07, 160.09, 160.34. HRMS: m/z: For C15H11FN2O2S ([M + H]+): 303.0615, found 303.0611.
N-(4-aminophenyl)-6-fluorobenzo[d]thiazole-2-carboxamide (6e)
Pale yellow solid, yield-78%; m.p-193-194 OC; 1H NMR (500 MHz, Chloroform-d) δ 10.74 (s, 1H), 8.01 (dd, J = 12.1, 2.2 Hz, 1H), 7.88 (dd, J = 7.7, 4.6 Hz, 1H), 7.63 (d, J = 8.4 Hz, 2H), 7.21 (ddd, J = 9.8, 7.7, 2.2 Hz, 1H), 6.78 (d, J = 8.5 Hz, 2H), 4.15 (s, 2H). 13C NMR (125 MHz, Chloroform-d) δ 109.36, 109.55, 114.26, 114.44, 115.48, 121.99, 123.87, 123.94, 131.45, 140.38, 140.49, 143.91, 148.61, 148.64, 158.12, 159.07, 160.09, 161.11. HRMS: m/z: For C14H10FN3OS ([M + H]+): 288.0598, found 288.0594.
N-(4-chlorophenyl)-6-fluorobenzo[d]thiazole-2-carboxamide (6f)
Pale yellow solid, yield-76%; m.p-239-240 OC; 1H NMR (500 MHz, Chloroform-d) δ 10.50 (s, 1H), 8.01 (dd, J = 12.1, 2.2 Hz, 1H), 7.92 (dd, J = 7.6, 4.6 Hz, 1H), 7.65 (d, J = 7.9 Hz, 2H), 7.38 (d, J = 8.1 Hz, 2H), 7.16 (ddd, J = 10.0, 7.5, 2.2 Hz, 1H). 13C NMR (125 MHz, Chloroform-d) δ 110.20, 110.39, 115.12, 115.30, 121.85, 123.25, 123.32, 127.46, 129.09, 136.88, 140.82, 140.92, 148.39, 148.42, 156.84, 158.34, 158.82, 162.04. HRMS: m/z: For C14H8ClFN2OS ([M + H]+): 308.0019, found 308.0017.
N-(3-chlorophenyl)-6-fluorobenzo[d]thiazole-2-carboxamide (6g)
Pale yellow solid, yield-72%; m.p-236-237 OC; 1H NMR (500 MHz, Chloroform-d) δ 10.42 (s, 1H), 8.01 (dd, J = 12.1, 2.2 Hz, 1H), 7.94 (dd, J = 7.6, 4.6 Hz, 1H), 7.70 (t, J = 2.2 Hz, 1H), 7.54 – 7.48 (m, 1H), 7.28 (t, J = 7.9 Hz, 1H), 7.24 – 7.13 (m, 2H). 13C NMR (125 MHz, Chloroform-d) δ 108.74, 108.93, 113.24, 113.41, 119.53, 120.31, 123.25, 123.32, 123.53, 131.23, 134.78, 139.71, 140.60, 140.70, 148.39, 148.42, 158.12, 159.07, 160.09, 161.11. HRMS: m/z: For For C14H8ClFN2OS ([M + H]+): 308.0018, found 308.0015.
6-fluoro-N-(4-fluorophenyl)benzo[d]thiazole-2-carboxamide (6h)
Pale yellow solid, yield-81%; m.p-188-189 OC; 1H NMR (500 MHz, Chloroform-d) δ 10.34 (s, 1H), 8.01 (dd, J = 12.1, 2.2 Hz, 1H), 7.93 (dd, J = 7.5, 4.6 Hz, 1H), 7.40 (dd, J = 8.0, 3.4 Hz, 2H), 7.21 (ddd, J = 9.9, 7.7, 2.2 Hz, 1H), 7.12 (dd, J = 10.0, 7.9 Hz, 2H). 13C NMR (125 MHz, Chloroform-d) δ 110.92, 111.11, 114.26, 114.44, 117.01, 117.18, 123.65, 123.73, 124.38, 124.45, 134.45, 134.47, 139.50, 139.60, 147.49, 147.52, 157.02, 158.12, 158.99, 159.07, 160.09, 162.04. HRMS: m/z: For C14H8F2N2OS ([M + H]+): 291.0388, found 291.0383.
6-fluoro-N-(4-(trifluoromethyl)phenyl)benzo[d]thiazole-2-carboxamide (6i)
Pale yellow solid, yield-79%; m.p-181-182 OC; 1H NMR (500 MHz, Chloroform-d) δ 10.78 (s, 1H), 8.01 (dd, J = 12.1, 2.2 Hz, 1H), 7.93 (dd, J = 7.5, 4.6 Hz, 1H), 7.84 (dq, J = 7.6, 1.4 Hz, 2H), 7.77 (d, J = 7.3 Hz, 2H), 7.30 (ddd, J = 10.0, 7.5, 2.2 Hz, 1H). 13C NMR (125 MHz, Chloroform-d) δ 110.40, 110.59, 115.34, 115.52, 119.99, 120.02, 120.05, 120.08, 120.44, 122.61, 123.25, 123.32, 124.79, 124.97, 125.23, 125.48, 125.74, 126.66, 126.70, 126.74, 126.78, 126.96, 137.75, 139.50, 139.60, 147.49, 147.52, 158.12, 159.07, 160.09, 161.84. HRMS: m/z: For C15H8F4N2OS ([M + H]+): 341.0373, found 341.0372.
6-fluoro-N-(4-hydroxyphenyl)benzo[d]thiazole-2-carboxamide (6j)
Pale yellow solid, yield-70%; m.p-217-218 O C; 1H NMR (500 MHz, Chloroform-d) δ 10.77 (s, 1H), 8.07 (dd, J = 12.1, 2.3 Hz, 1H), 7.85 (dd, J = 7.7, 4.7 Hz, 1H), 7.64 (s, 1H), 7.31 (d, J = 8.3 Hz, 2H), 7.21 (ddd, J = 9.8, 7.6, 2.1 Hz, 1H), 6.78 (d, J = 8.5 Hz, 2H). 13C NMR (125 MHz, Chloroform-d) δ 108.53, 108.72, 114.26, 114.44, 115.55, 122.77, 123.25, 123.32, 130.50, 138.50, 138.60, 146.51, 146.53, 154.28, 158.12, 159.07, 160.09, 162.04. HRMS: m/z: For C14H9FN2O2S ([M + H]+): 289.0415, found 289.0415.
6-fluoro-N-(3-hydroxyphenyl)benzo[d]thiazole-2-carboxamide (6k)
Pale yellow solid, yield-71%; m.p-219-220 OC; 1H NMR (500 MHz, Chloroform-d) δ 10.69 (s, 1H), 8.01 (dd, J = 12.1, 2.2 Hz, 1H), 7.94 (dd, J = 7.6, 4.6 Hz, 1H), 7.36 (dt, J = 8.1, 1.6 Hz, 1H), 7.23 – 7.09 (m, 3H), 6.70 (s, 1H), 6.60 (dt, J = 8.3, 1.6 Hz, 1H). 13C NMR (125 MHz, Chloroform-d) δ 106.08, 109.46, 109.65, 110.72, 114.05, 115.12, 115.30, 122.50, 122.57, 129.85, 139.43, 139.50, 139.60, 148.41, 148.43, 157.93, 158.12, 159.09, 159.92, 160.09. HRMS: m/z: For C14H9FN2O2S ([M + H]+): 289.0417, found 289.0415.
N-(3,4-dimethylphenyl)-6-fluorobenzo[d]thiazole-2-carboxamide (6l)
Off white solid, yield-69%; m.p-251-252 OC; 1H NMR (500 MHz, Chloroform-d) δ 10.05 (s, 1H), 8.01 (dd, J = 12.1, 2.2 Hz, 1H), 7.93 (dd, J = 7.6, 4.6 Hz, 1H), 7.53 (dd, J = 7.9, 2.2 Hz, 1H), 7.45 (d, J = 2.2 Hz, 1H), 7.21 (ddd, J = 9.8, 7.7, 2.2 Hz, 1H), 7.07 – 7.02 (m, 1H), 2.34 (s, 3H), 2.28 (s, 3H). 13C NMR (125 MHz, Chloroform-d) δ 19.67, 20.33, 108.52, 108.71, 113.74, 113.92, 117.31, 120.43, 123.25, 123.32, 128.62, 134.16, 135.82, 136.65, 139.15, 139.25, 146.51, 146.53, 158.12, 159.07, 160.09, 161.09. HRMS: m/z: For C16H13FN2OS ([M + H]+): 301.0774, found 301.0771.
N-(3,4-dimethoxyphenyl)-6-fluorobenzo[d]thiazole-2-carboxamide (6m)
Off white solid, yield-73%; m.p-237-239 OC; 1H NMR (500 MHz, Chloroform-d) δ 10.75 (s, 1H), 8.04 (dd, J = 12.1, 2.3 Hz, 1H), 7.92 (dd, J = 7.6, 4.6 Hz, 1H), 7.32 (dd, J = 8.6, 2.2 Hz, 1H), 7.21 (ddd, J = 9.8, 7.7, 2.2 Hz, 1H), 7.02 (d, J = 2.2 Hz, 1H), 6.89 (d, J = 8.5 Hz, 1H), 3.87 (s, 3H), 3.80 (s, 3H). 13C NMR (125 MHz, Chloroform-d) δ 55.63, 56.35, 106.51, 109.36, 109.55, 113.99, 114.26, 114.44, 117.09, 124.38, 124.45, 135.43, 139.50, 139.60, 142.61, 148.39, 148.42, 150.29, 158.12, 159.09, 160.09, 161.09. HRMS: m/z: For C16H13FN2O3S ([M + H]+): 333.0691, found 333.0691.
6-fluoro-N-(4-nitrophenyl)benzo[d]thiazole-2-carboxamide (6n)
Pale brown solid, yield-74%; m.p-174-175 OC; 1H NMR (500 MHz, Chloroform-d) δ 10.98 (s, 1H), 8.20 (d, J = 8.4 Hz, 2H), 8.04 (dd, J = 12.1, 2.3 Hz, 1H), 7.94 (dd, J = 7.6, 4.6 Hz, 1H), 7.83 (d, J = 8.5 Hz, 2H), 7.18 (ddd, J = 9.8, 7.7, 2.2 Hz, 1H). 13C NMR (125 MHz, Chloroform-d) δ 110.40, 110.59, 114.26, 114.44, 118.81, 123.25, 123.32, 125.47, 140.82, 140.92, 142.75, 144.64, 148.39, 148.42, 159.16, 160.15, 161.14, 161.84. HRMS: m/z: For C14H8FN3O3S ([M + H]+): 318.0347, found 318.0344.
6-fluoro-N-(3-nitrophenyl)benzo[d]thiazole-2-carboxamide (6o)
Pale brown solid, yield-76%; m.p-171-172 OC; 1H NMR (500 MHz, Chloroform-d) δ 11.03 (s, 1H), 8.47 (t, J = 2.2 Hz, 1H), 8.31 (dt, J = 7.9, 1.6 Hz, 1H), 7.99 (dd, J = 12.1, 2.3 Hz, 1H), 7.92 (dd, J = 7.6, 4.6 Hz, 1H), 7.72 (ddd, J = 8.0, 2.5, 1.2 Hz, 1H), 7.62 (t, J = 7.9 Hz, 1H), 7.30 (ddd, J = 10.0, 7.5, 2.1 Hz, 1H). 13C NMR (125 MHz, Chloroform-d) δ 110.91, 111.10, 114.26, 114.44, 114.90, 119.49, 124.60, 124.67, 127.97, 131.74, 139.30, 139.50, 139.60, 148.39, 148.42, 150.08, 159.10, 159.45, 161.43, 162.05. HRMS: m/z: For C14H8FN3O3S ([M + H]+): 318.0348, found 318.0348.
Molecular docking
Table 1 provides the docking scores of novel 6a-6o (compounds 6a-6r) against two protein targets, 4WKQ and 6LUD. The docking scores indicate the binding affinity of each compound to the target proteins, with lower (more negative) scores suggesting better binding affinities.
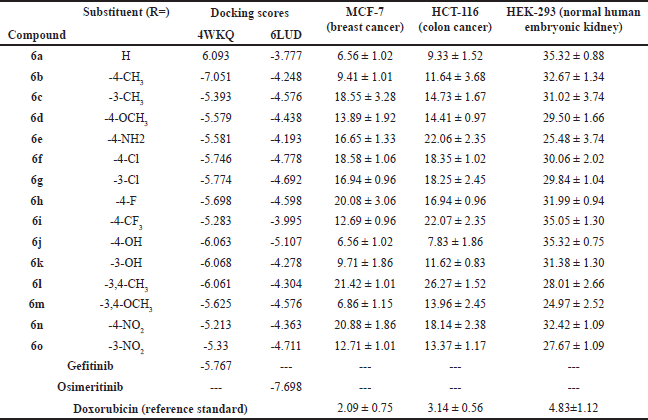 | Table 1. Summary of results of molecular docking and MTT assay of benzothiazole–carboxamide derivatives (6a–6o). [Click here to view] |
For 4WKQ, compound 6b (-7.051) shows the best binding affinity, outperforming even Gefitinib (-5.767). Other compounds such as 6a (-6.093), 6j (-6.063), 6k (-6.068), and 6l (-6.061) also show strong binding affinities, close to or better than Gefitinib. The interactions of these compounds are depicted in Figure 3. Moderate performers include compounds 6f (-5.746), 6g (-5.774), 6h (-5.698), and 6m (-5.625). The compounds with the weakest binding affinities are 6i (-5.283) and 6n (-5.213).
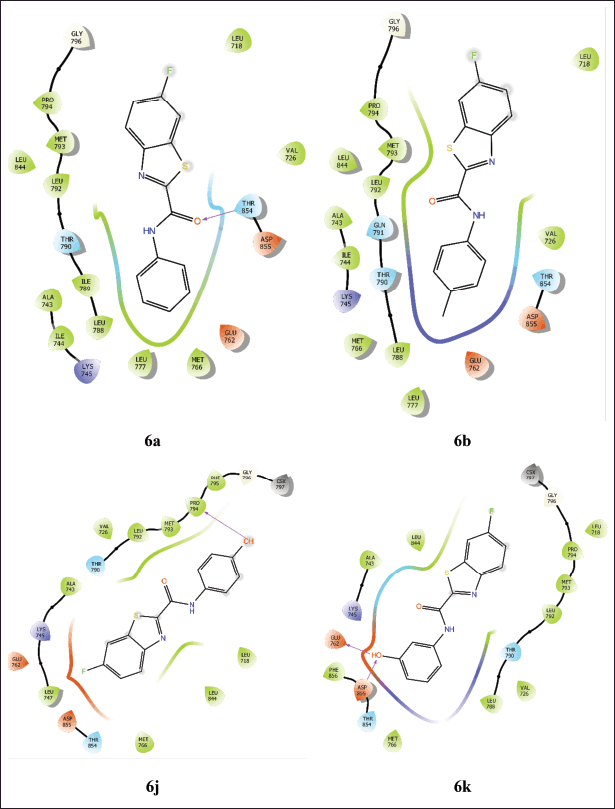 | Figure 3. Interactions of compounds with the active site of 4WkQ. [Click here to view] |
For 6LUD, Osimeritinib (-7.698) shows the best binding affinity, serving as a strong reference point. Among the benzothiazole–carboxamides, compound 6j (-5.107) performs the best. Other compounds with good binding affinities include 6f (-4.778), 6o (-4.711), and 6g (-4.692). Moderate performers in this category are 6c (-4.576), 6d (-4.438), 6m (-4.576), and 6n (-4.363). Compound 6a (-3.777) shows the weakest binding affinity among the tested derivatives. The interaction of compounds 6j and 6f at the active site of the 6LUD is disclosed in Figure 4.
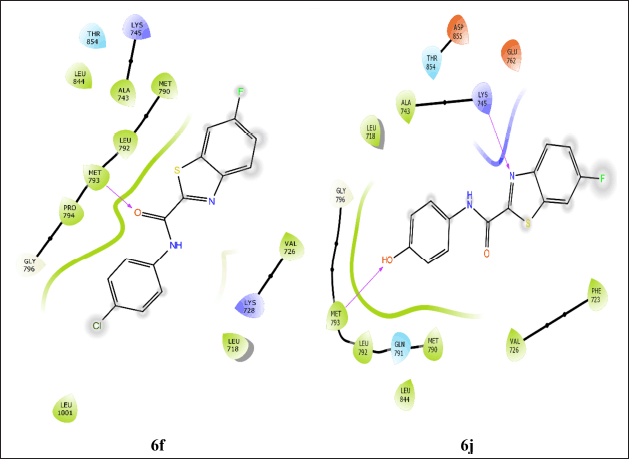 | Figure 4. Interactions of compounds 6f and 6j with the active site of 6LUD. [Click here to view] |
The influence of substituents is evident from the docking scores. Methyl groups at different positions exhibit varying effects; for instance, 4-CH3 (6b) shows significantly better binding affinity with 4WKQ (-7.051) compared to 3-CH3 (6c) (-5.393), indicating a favorable position-specific interaction. However, for 6LUD, 3-CH3 (6c) performs better than 4-CH3 (6b), suggesting different interaction dynamics with this target. Hydroxy groups also show notable effects; both 4-OH (6j) and 3-OH (6k) perform well, with 4-OH showing the best affinity for 6LUD (-5.107).
Halogen substituents such as chloro groups at both positions (6f and 6g) exhibit moderate to good affinities for both targets. Fluoro (6h) and trifluoromethyl (6i) substituents show variable results, with 4-F (6h) having better affinity than 4-CF3 (6i) for both targets. Nitro groups at positions 3 (6o) and 4 (6n) show moderate affinities, with 3-NO2 (6o) slightly outperforming 4-NO2 (6n).
In conclusion, the docking study reveals that specific substituents on the benzothiazole–carboxamide hybrids can significantly impact binding affinities. Methyl and hydroxy groups in particular positions show promise for further optimization. These results suggest that further experimental validation and optimization of these hybrids could lead to potent inhibitors targeting 4WKQ and 6LUD.
Anticancer activity
The detailed analysis and discussion of benzothiazole–carboxamide hybrids (compounds 6a-6r) against cancer cell lines provide valuable insights into their potency and toxicity profiles:
The IC50 values represent the concentration required to inhibit 50% of cell viability in three cell lines: MCF-7 (breast cancer), HCT-116 (colon cancer), and HEK-293 (normal human embryonic kidney cells). Lower IC50 values indicate greater potency against cancer cells, whereas higher values suggest lower toxicity towards normal cells.
Compound 6a (H) and 6j (4-OH) stand out as top performers with IC50 values of 6.56 µM, indicating strong potency against MCF-7 cells. Compound 6m (3,4-OCH3) also demonstrates good efficacy with an IC50 of 6.86 µM. In contrast, compounds 6l (3,4-CH3) and 6n (4-NO2) show the lowest potency, with IC50 values of 21.42 µM and 20.88 µM, respectively, suggesting reduced effectiveness against MCF-7.
Compound 6j (4-OH) exhibits the lowest IC50 value of 7.83 µM, indicating the highest potency against HCT-116 cells. Compound 6a (H) follows closely with an IC50 of 9.33 µM. Compounds 6e (4-NH2) and 6i (4-CF3) show lower potency, with IC50 values of 22.06 µM and 22.07 µM, respectively, suggesting diminished efficacy against HCT-116 cells.
Among the compounds tested, 6m (3,4-OCH3) exhibits the lowest IC50 value of 24.97 µM against HEK-293 cells, indicating relatively lower toxicity compared to others. Compounds 6a (H) and 6j (4-OH) show the highest IC50 values of 35.32 µM, suggesting lower toxicity towards normal cells.
The study highlights the considerable impact of different substituents on the activity of benzothiazole hybrids, significantly influencing their efficacy against cancer cells. The positioning of methyl groups plays a crucial role in determining potency, with the 4-CH3 substituent (6b) generally showing superior efficacy compared to the 3-CH3 substituent (6c) in both MCF-7 and HCT-116 cell lines. This suggests that the placement of the methyl group on the benzothiazole core affects the compound’s ability to interact with its target, potentially enhancing its anticancer activity. Hydroxy substituents, particularly 4-OH (6j), demonstrate strong anticancer activity, consistently outperforming 3-OH (6k). This indicates that the hydroxy group’s presence, especially when positioned at the 4-position, may enhance interaction with the target, leading to more effective inhibition of cancer cell growth. The effects of halogen substituents vary depending on their type and position. Chloro substituents (6f and 6g) and the fluoro substituent (6h) exhibit diverse impacts on potency, with some compounds showing moderate to high efficacy against either MCF-7 or HCT-116 cells. This variability highlights the importance of halogen type and positioning in modulating anticancer activity. Nitro groups exhibit moderate to low potency, with 4-NO2 (6n) being notably less effective against MCF-7 cells compared to 3-NO2 (6o), which shows better performance against HCT-116 cells. This suggests that the nitro group’s impact on potency depends on its position and the specific cancer cell type being targeted.
Compound 6j (4-OH) emerges as the most promising candidate, demonstrating strong potency against both MCF-7 and HCT-116 cells, suggesting its potential for further development. Doxorubicin, used as a reference, exhibits high potency against cancer cells but with greater toxicity toward normal cells when compared to the tested benzothiazole–carboxamides. The experiments conclude that optimizing 6a-6o, focusing on specific substituents like hydroxy and methyl groups, could lead to potent anticancer agents with improved safety profiles, warranting further experimental validation and optimization.
CONCLUSION
This study successfully synthesized and characterized a series of 6a-6o, demonstrating their potential as novel anticancer agents. The synthetic routes employed yielded high-purity compounds, with key intermediates isolated and characterized effectively. Molecular docking studies indicated that specific substituents significantly influence the binding affinities of these compounds to the protein targets 4WKQ and 6LUD. Notably, compound 6b exhibited the strongest binding affinity for 4WKQ, surpassing the reference compound Gefitinib, while compound 6j showed the best affinity for 6LUD, outperforming Osimertinib. The anticancer evaluation revealed that compound 6j (4-OH) displayed the highest potency against both MCF-7 and HCT-116 cancer cell lines, with IC50 values of 6.56 µM and 7.83 µM, respectively, and lower toxicity towards HEK-293 normal cells. The presence of hydroxy and methyl groups emerged as critical determinants of activity, with specific positional substitutions enhancing efficacy. Overall, these findings underscore the promising anticancer potential of 6a-6o, particularly those incorporating hydroxy and methyl substituents. Future research should focus on optimizing these compounds further and exploring their mechanisms of action to advance their development into effective targeted therapies.
ACKNOWLEDGMENT
Authors acknowledge the technical support provided by the Andhra University, Andhra Pradesh, India, and Sir C.R. Reddy College of Pharmaceutical Sciences, Eluru, Andhra Pradesh, India, in successful completion of this research.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. World Health Organization. Global cancer burden growing, amidst mounting need for services. Geneva: World Health Organization. [cited 2024 Feb 1]. Available from: https://www.who.int/news/item/01-02-2024-global-cancer-burden-growing--amidst-mounting-need-for-services
2. Piña-Sánchez P, Chávez-González A, Ruiz-Tachiquín M, Vadillo E, Monroy-García A, Montesinos JJ, et al. Cancer biology, epidemiology, and treatment in the 21st century: current status and future challenges from a biomedical perspective. Cancer Control. 2021 Jan-Dec;28:10732748211038735.
3. Pramesh CS, Badwe RA, Bhoo-Pathy N, Booth CM, Chinnaswamy G, Dare AJ, et al. Priorities for cancer research in low- and middle-income countries: a global perspective. Nat Med. 2022 Apr;28(4):649–57.
4. Puyol M, Seoane J, Aguilar E, Vozza LB, Orbe I, Crawford KH, et al. WORLD CANCER RESEARCH DAY: a call to action for a coordinated international research effort to prevent, diagnose, and treat cancer. Clin Cancer Res. 2021 Feb 15;27(4):963–6.
5. Zhilitskaya LV, Shainyan BA, Yarosh NO. Modern approaches to the synthesis and transformations of practically valuable benzothiazole derivatives. Molecules. 2021 Apr 10;26(8):2190.
6. Gao X, Liu J, Zuo X, Feng X, Gao Y. Recent advances in synthesis of benzothiazole compounds related to green chemistry. Molecules. 2020 Apr 5;25(7):1675. doi: 10.3390/molecules25071675
7. Yadav KP, Rahman MA, Nishad S, Maurya SK, Anas M, Mujahid M. Synthesis and biological activities of benzothiazole derivatives: a review. Int Pharm. 2023 Oct;1(3):122–132. CrossRef
8. Haider K, Shrivastava N, Pathak A, Dewangan RP, Yahya S, Yar MS. Recent advances and SAR study of 2-substituted benzothiazole scaffold based potent chemotherapeutic agents. Results Chem. 2022 Jan;4:100258. CrossRef
9. Singh RK. Key heterocyclic cores for smart anticancer drug–design part I. Sharjah: Bentham Science Publishers; 2022.
10. Videnovi? M, Mojsin M, Stevanovi? M, Opsenica I, Srdi?-Raji? T, Šolaja B. Benzothiazole carbamates and amides as antiproliferative species. Eur J Med Chem. 2018 Sep;157:1096–114. CrossRef
11. Corbo F, Carocci A, Armenise D, De Laurentis N, Laghezza A, Loiodice F, et al. Antiproliferative Activity Evaluation of a Series of N-1,3-Benzothiazol-2-ylbenzamides as Novel Apoptosis Inducers. J Chem. 2016 Jan;2016:1–5. CrossRef
12. Aouad MR, Almehmadi MA, Rezki N, Al-Blewi FF, Messali M, Ali I. Design, click synthesis, anticancer screening and docking studies of novel benzothiazole-1,2,3-triazoles appended with some bioactive benzofused heterocycles. J Mol Struct. 2019 Jul;1188:153–164. CrossRef
13. Fekri R, Salehi M, Asadi A, Kubicki M. Synthesis, characterization, anticancer and antibacterial evaluation of Schiff base ligands derived from hydrazone and their transition metal complexes. Inorg Chim Acta. 2019 Jan;484:245–254. CrossRef
14. Modi A, Singh M, Gutti G, Shanker OR, Singh VK, Singh S, et al. Benzothiazole derivative bearing amide moiety induces p53-mediated apoptosis in HPV16 positive cervical cancer cells. Investig New Drugs. 2019 Aug 20;38(4):934–945. CrossRef
15. Harisha S, Keshavayya J, Prasanna SM, Hoskeri HJ. Synthesis, characterization, pharmacological evaluation and molecular docking studies of benzothiazole azo derivatives. J Mol Struct. 2020 Oct;1218:128477. CrossRef
16. Racané L, Pti?ek L, Fajdeti? G, Trali?-Kulenovi? V, Klobu?ar M, Paveli? SK, et al. Green synthesis and biological evaluation of 6-substituted-2-(2-hydroxy/methoxy phenyl)benzothiazole derivatives as potential antioxidant, antibacterial and antitumor agents. Bioorg Chem. 2020 Jan;95:103537. CrossRef
17. Hebishy AMS, Abdelfattah MS, Elmorsy A, Elwahy AHM. Synthesis of novel bis-and poly(benzimidazoles) as well as bis- and poly(benzothiazoles) as anticancer agents. J Heterocycl Chem. 2020 Mar;57(5):2256–70. CrossRef
18. Narva S, Chitti S, Amaroju S, Goud S, Alvala M, Bhattacharjee D, et al. Design, synthesis, and biological evaluation of 2-(4-Aminophenyl)benzothiazole analogues as antiproliferative agents. J Heterocycl Chem. 2018 Dec;56(2):520–32. CrossRef
19. Song J, Gao QL, Wu BW, Zhu T, Cui XX, Jin CJ, et al. Discovery of tertiary amide derivatives incorporating benzothiazole moiety as anti-gastric cancer agents in vitro via inhibiting tubulin polymerization and activating the Hippo signaling pathway. Eur J Med Chem. 2020 Oct;203:112618. CrossRef
20. Rezki N, Almehmadi MA, Ihmaid S, Shehata AM, Omar AM, Ahmed HEA, Aouad MR. Novel scaffold hopping of potent benzothiazole and isatin analogues linked to 1,2,3-triazole fragment that mimic quinazoline epidermal growth factor receptor inhibitors: Synthesis, antitumor and mechanistic analyses. Bioorg Chem. 2020 Oct;103:104133. CrossRef
21. Osmaniye D, Levent S, Karaduman AB, Ilg?n S, Özkay Y, Kaplanc?kl? ZA. Synthesis of new benzothiazole acylhydrazones as anticancer agents. Molecules [Internet]. 2018 May 1;23(5):1054. CrossRef
22. Yip D, Le MN, Chan JL, Lee JH, Mehnert JA, Yudd A, et al. A phase 0 trial of riluzole in patients with resectable stage III and IV melanoma. Clin Cancer Res [Internet]. 2009 Jun 1;15(11):3896–902. CrossRef
23. Xu Q, Liu C, Zang J, Gao S, Chou CJ, Zhang Y. Discovery of a novel hybrid of vorinostat and riluzole as a potent antitumor agent. Front Cell Dev Biol. 2020 Jul 14;8:454.
24. El-Meguid EA, Moustafa GO, Awad HM, Zaki ER, Nossier ES. Novel benzothiazole hybrids targeting EGFR: design, synthesis, biological evaluation and molecular docking studies. J Mol Struct [Internet]. 2021 Sep 1;1240:130595. CrossRef
25. El-Meguid EA, Naglah AM, Moustafa GO, Awad HM, Kerdawy AME. Novel benzothiazole-based dual VEGFR-2/EGFR inhibitors targeting breast and liver cancers: synthesis, cytotoxic activity, QSAR and molecular docking studies. Bioorg Med Chem Lett [Internet]. 2022 Feb 1;58:128529. CrossRef
26. Al-Sanea MM, Hamdi A, Mohamed AAB, El-Shafey HW, Moustafa M, Elgazar AA, et al. New benzothiazole hybrids as potential VEGFR-2 inhibitors: design, synthesis, anticancer evaluation, and in silico study. J Enzyme Inhib Med Chem. 2023 Dec;38(1):2166036. CrossRef
27. Ewes WA, Tawfik SS, Almatary AM, Ahmad Bhat M, El-Shafey HW, Mohamed AAB, et al. Identification of benzothiazoles bearing 1,3,4-thiadiazole as antiproliferative hybrids targeting VEGFR-2 and BRAF kinase: design, synthesis, bio evaluation and in silico study. Molecules. 2024 Jul 4;29(13):3186. doi: 10.3390/molecules29133186
28. Sheng C, Che X, Wang W, Wang S, Cao Y, Yao J, et al. Design and synthesis of antifungal benzoheterocyclic derivatives by scaffold hopping. Eur J Chem. 2011;46:1706–12.
29. Smith MB, March J. March’s advanced organic chemistry: reactions, mechanisms, and structure. 6th ed. Hoboken, NJ: Wiley-Interscience; 2007.
30. Withers HW, Rose JL, LLC VC. US3557222A - Hydrolysis of benzyl chloride to benzyl alcohol [Internet]. Google Patents.
31. Gilmore JL, Sheppeck JE, Company BMS. WO2011017578A1¾Sphingosine-1-phosphate receptor agonists [Internet]. Google Patents.
32. Fattahi N, Ayubi M, Ramazani A. Amidation and esterification of carboxylic acids with amines and phenols by N,N′-diisopropylcarbodiimide: a new approach for amide and ester bond formation in water. Tetrahedron. 2018;74(32):4351–6. CrossRef
33. Carmichael J, DeGraff WG, Gazdar AF, Minna JD, Mitchell JB. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 1987;47(4):936–42
34. Bender BJ, Gahbauer S, Luttens A, Lyu J, Webb CM, Stein RM, et al. A practical guide to large-scale docking. Nat Protoc. 2021;16(10):4799–832. CrossRef