INTRODUCTION
Drugs and inappropriate dietary habits are considered as key players in the induction and progression of liver injury. It was revealed that analgesics which are frequently prescribed as antipyretic drug produces dose-reliant hepatotoxicity and nephrotoxicity in rodents [1,2]. The non-diabetic liver injury was also discussed under the umbrella of drug-related liver injury. The prevalence of Paracetamol-induced hepatotoxicity was 15%–36% and 2%–7% in Western Caucasian patients and Asia, respectively [3–8]. On the other hand, inappropriate dietary habits like high fat and fructose-rich products are considered as major contributors to the advancement of liver pathology. Recent clinical reports revealed that there was a significant upsurge in de-novo lipogenesis, triglycerides (TG), free fatty acid (FFA), and hepatic-related insulin resistance due to the excessive-high fructose diet administration [9]. According to epidemiological research, the occurrence of MAFLD in people along with T2DM and obesity varies from 50% to 75% globally and 12.5%–87.5% in India [10]. It is sound recognized that a high-fructose, high-fat diet causes hyperglycemia and hyperlipidemia that causes the creation of proinflammatory mediators such as cytokines and interleukin [11,12]. In the same line, an increase in dietary carbohydrates such as fructose, induces a surge in glucose levels, which promotes the synthesis of fatty acids via glycogenolysis, consequential in higher circulation levels of TG and FFA, leading to hepatic steatosis inductions [13]. Similarly, long-term exposure and a higher dosage of paracetamol (PCM) leads to kidney and liver-related problems without altering the blood glucose levels [5]. It was also demonstrated that PCM instigated a substantial increase in TC, TG, and LDL, levels in mice as compared to normal rodents. Therefore, it is clearly stated that over usage of PCM also disturbs the metabolic factory which alter the physiological balance of the body [14]. The focus of the current study is also focused to find out the correlation of PCM and High fructose + high-fat diet (HFHF) induced diabetic and non-diabetic liver injury with respect to various biochemical parameters with reference to novel pharmacological interventions.
Adipokines, FFA, Glucagon-like peptidase, MMP, pro-inflammatory mediators, and specific genes are among the molecular pathways implicated in the progression of high fructose high fat-caused diabetic liver damage [15]. Our recently published research evidence of in-silico studies demonstrated that Pterostilbene (PTS), Arbutin (ARB), Purpurin (PUR), and Quercetin (QR) have significant interaction with two chief FAS domains, which produce an antiadipogenic effect, which was reinforced by in-vitro experimentation [16]. Similarly, another published research evidence revealed that PTS, PUR, QR, and ARB possess significant beneficial effects against various diabetes-associated liver complications in rodents [17]. The two references cited above provide a strong mechanistic justification for selecting PTS, ARB, and PUR as hepatoprotective agents.
In continuation, the present research design was centered on the exploration of anti adipogenic, hepatoprotective, and antidiabetic effects with reference to selected pharmacological compounds belonging to the class of stilbene, quionines, and flavonoid on diabetes and drug persuaded liver damage in rodents.
MATERIALS AND METHODS
Chemicals
Pterostilbene was received gift from Sami Labs Limited, Bangalore, India. Arbutin (A4256), Quercetin (Q4951), and Purpurin (229148) were purchased from Sigma–Aldrich. Elabscience in the United States provided the free fatty acids and IL-6 ELISA kit. Thermo Scientific provided the cDNA kit and Syber green, whereas Sigma–Aldrich provided, β-actin, FAS primers, and Trizol B.
Ethical approval
For the current study, Wistar male rats (120–150 g) were purchased from “LLRUVAS, Hisar, India, and Chitkara College of Pharmacy, Punjab, India,” and housed at the animal house of “Punjabi University, Patiala, Punjab (India), and Chitkara College of Pharmacy, Punjab, India”. In polyacrylic cages, all of the animals were kept in optimal animal house circumstances with a 12-hour light/dark cycle. All animal experiments took place at experimental laboratories of “Punjabi University, Patiala, Punjab (India), and Chitkara College of Pharmacy, Punjab, India”. The IAEC Committee of “Punjabi University, Patiala, and Chitkara University, Punjab,” authorized the research study protocol with approval numbers 107GOReBi/S/99/CPCSEA/2019-12 and IAEC/CCP/20/01/PR-09, respectively.
Treatment schedule for HFHF experimental rat model of diabetic liver injury
The high fructose and high-fat diet persuaded diabetes-associated liver damage rat model experimentation was successfully completed as per Lozano et al. [18] and as per D08040106 formula, (Research Diets) with minor amendments. After 6 months on the HFHF diet, hyperglycemia develops, followed by hyperinsulinemia and byzantine liver steatosis after 7–8 months [18].
All of the animals were separated into 13 different groups, vehicle normal diet control, HFHF disease control, standard Metformin (biguanides), and QR groups with 120 mg/Kg b.wt. dose and nine groups of three drugs, i.e., PTS, ARB, and PUR with different 30, 60, and 120 mg/Kg b.wt. The experiment was conducted as per the experimental schedule mentioned in Figure 1a for the initiation of diabetic liver injury (Fig. 1a). Except for the control group, all animals were kept singly in cages and fed an HFHF diet (20% Protein, 70% Carbohydrates, and 10% Fat Kcal). The HFHF diet was manufactured as per D08040106, (Research Diets) formula as represented in (Table 1). After 2–3 weeks on the HFHF diet, rodents with a level of blood glucose of more than 11.1 mmol/l were classified as diabetic and same were enrolled in the research schedule. Each experimental group except the control group were put on an HFHF diet for 28 weeks, whereas PTS, ARB, and PUR therapy was started from the 25th week. At the completion of the 28th week, biochemical and tissue tests were performed on the animals after they were euthanized by cervical dislocation method for rats having body weight less than 200 g whereas the above 200 gm rats were euthanized under high dose of sodium pentothol anesthesia (100–150 mg/Kg,i.p.). Body weight was measured at 1st, 8th, 20th, 24th, 26th, and 28th week in all groups, while blood glucose levels are measured (glucometer) by puncturing the tail vein and measured at 1st, 4th, 8th, 12th, 16th, 20th, 24th, 26th, and 28th week in all groups. In the last of the experiment blood was collected by retro orbital plexus of rats for biochemical assessment and liver tissues were isolated for histopathological evaluation.
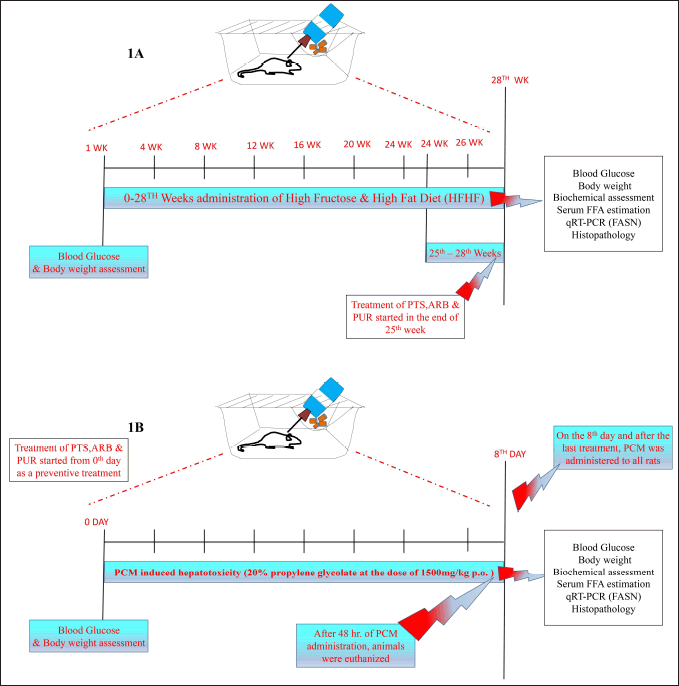 | Figure 1. Illustration of the treatment schedule and experimental design for HFHF (A) and PCM (B) induced preclinical experimentation of diabetic and non-diabetic liver injury. [Click here to view] |
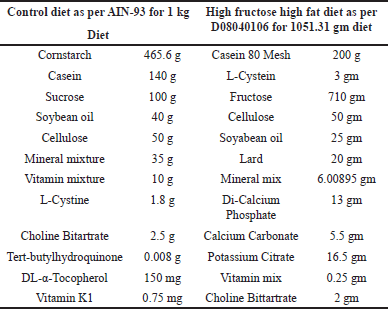 | Table 1. Composition of the HFHF diets based on D08040106 for 1051.31 gm diet [16]. [Click here to view] |
Treatment schedule for paracetamol-induced rat experimental model of non-diabetic liver injury
Rats were randomly divided into 7 sets (n = 6) that is Control, Disease control, PCM induced hepatotoxicity (20% propylene glycolate; 1,500 mg/kg p.o.), PCM induced hepatotoxicity and Standard Drug (Silymarin;120 mg/kg; p.o), PCM induced hepatotoxicity and Standard Drug (Quercetin;120 mg/kg; p.o) (FAS inhibitor), PCM induced liver injury + PTS (120 mg/kg; p.o.), PCM induced liver injury + ARB (120 mg/kg; p.o.), PCM induced liver injury + PUR (120 mg/kg; p.o.). The treatment was followed for 8 days and on the 8th day and after the last treatment, PCM (1,500 mg/kg p.o.) was given to all groups (Fig. 1b). The rats were euthanized by cervical dislocation after 48 hours following PCM administration, and serum samples were taken from the blood for the study of liver enzymes, biochemical parameters, and liver tissues were extracted for histological assessment [19].
Biochemical and clinical test parameters
At the end of the treatment plan, retro orbital plexus was used to collect the blood samples for biochemistry and hematological examination. Blood samples for blood biochemistry were collected in commercially available EDTA-coated vials. A commercially available kit was used to assess several biochemical metabolic parameters such as alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (AP), alkaline phosphate, total bilirubin (TB), total protein (TP), creatinine (Cr), glucose (GLU), albumin (A), and globulin (G).
Assessment of antioxidant enzyme, lipid peroxidation
To analyze the Thiobarbituric acid reactive substances (TBARSs) technique by Ohkawa et al. [20] was utilized; reduced glutathione levels were calculated using Boyne and Ellman’s [21] approach and protein content was calculated using the modified technique of Lowry. The liver tissue homogenate was prepared with cold phosphate buffer saline (pH 7.4; 4°C) for 10 minutes, and the topmost layer was collected for oxidative and antioxidant estimates [22,21,20].
Investigations of FASN gene expression by real-time polymerase chain reaction (qRT-PCR)
Total RNA was measured using a multimode reader (Teccan) and a concentration of μg/μl (260/280 = 1.8–2.0) RNA for cDNA synthesis using a revert aid first strand c-DNA synthesis kit by using Trizol Reagent. The FASN gene was amplified using a Real-Time PCR System utilizing USB VeriQuest; Roche LC96; SYBR Green master mix by using β-actin as an endogenous control (Table 2) [23]. The mean fold change of the target gene was calculated by Pfaffl method after normalizing with B-actin. The ΔΔCq was used to calculate the proportionate level of the target gene with reference to FASN gene as mean fold change in fold compared graph which is used to find relative quantities to estimate gene expression ratios The slope of the resulting line is used to calculate the PCR efficiency using the formula \(E=10(1/S)1\), where \(S\) is the slope of the standard curve.
 | Table 2. Illustration of forward and reverse primer with their annealing temperature used for qRT-PCR [21]. [Click here to view] |
Histopathological
The liver was extracted from the rats and stored in 10% formalin for histopathological examination. The liver was dehydrated in alcohol in graded concentrations, and then immersed into xylene before being fixed in paraffin. Light microscopy was used to assess pathological changes in sections of 3 m thickness stained with Hematoxylin and Eosin (H & E) (200X).
Statistical analysis
The statistical software (GraphPad prism) was selected to analyze the statistical differences between various experimental rodent groups using one-way and two-way ANOVA. The experimental information was provided as S.E.M. and analyzed using p values of less than 0.05.
Detail of Statistical analysis for graphical representation
For first HFHF disease condition
“*p < 0.05 versus control; @p < 0.05 versus Disease control HFHF; %p < 0.05 versus PTS LD (30 mg/Kg); $p < 0.05 versus PTS MD (60 mg/Kg); ξp < 0.05 versus ARB LD (30 mg/kg); ?p < 0.05 versus ARB MD (60 mg/Kg); σp < 0.05 versus ARB HD (120 mg/Kg); ηp < 0.05 versus PUR LD (30 mg/Kg); γp < 0.05 versus PUR MD (60 mg/Kg);πp < 0.05 versus PUR HD (120 mg/Kg).”
For first PCM disease condition
*p < 0.05 versus control; @p < 0.05 versus Disease control PCM (1,500 mg/kg); ? p < 0.05 versus Standard Silymarin (120 mg/kg); σp < 0.05 versus ARB HD (120 mg/Kg);πp < 0.05 versus PUR HD (120 mg/Kg).”
RESULTS
Body weight variations in both HFHF and PCM-induced rodent diabetic and non-diabetic liver injury model
The body weight of animals in the HFHF administrated group was markedly (p < 0.05) increased as compared to the normal control. In comparison to other groups, on the 28th week, there was a marked rise (p < 0.05) in rodent weight in the HFHF treated Disease group (Fig. 2a). The treatment groups exhibited a marked improvement (p < 0.05) in body weight in comparison to the HFHF treated set. The control group, on the other hand, experienced a typical and consistent growth in body weight from the 0th to the 28th week, as per the treatment plan (Fig. 2a).
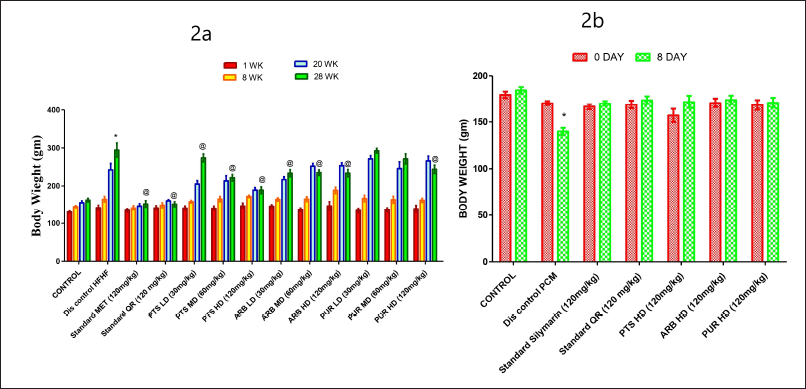 | Figure 2. The pharmacological interventions impact on body weight (2a) (0–28th week) variations in different preclinical rodent sets of HFHF and PCM (2b) (0–8th day) induced diabetic and non-diabetic liver injury respectively. [Click here to view] |
With reference to the 8th day of the PCM experiment, a marked drop (p < 0.05) in the body weight of rodents in the PCM-treated group was found in comparison to the normal control group. In comparison to other rodent sets, on the eighth day, the PCM treated Disease control group had a marked drop (p < 0.05) in body weight (Fig. 2b). Similarly, in comparison to the disease control group, the treatment groups exhibited a marked improvement (p < 0.05) in body weight. On the other hand, it was observed that there was a typical and consistent gain in body weight in the control group from zero to the eighth day, as per the experimental timetable (Fig. 2b).
Blood glucose variations in HFHF and PCM-induced rodent diabetic and non-diabetic liver injury model
On the 28th week, the experimental HFHF-treated disease group was found to be diabetic, as blood glucose levels were markedly higher (p < 0.05) than the normal group. Likewise, a marked difference was observed (p < 0.05) in the 1st, 4th, 8th, 12th, 16th, 20th, 24th, 26th, and 28th week of the HFHF-treated group in comparison to the normal control group (Fig. 3a). All of the therapeutic dosage ranges markedly reduced blood glucose levels in comparison to disease treated group. Pterostilbene and Arbutin (120 mg/Kg b.wt.) administered groups were exhibited to be nonsignificant when compared to Quercetine and Metformin administered groups.
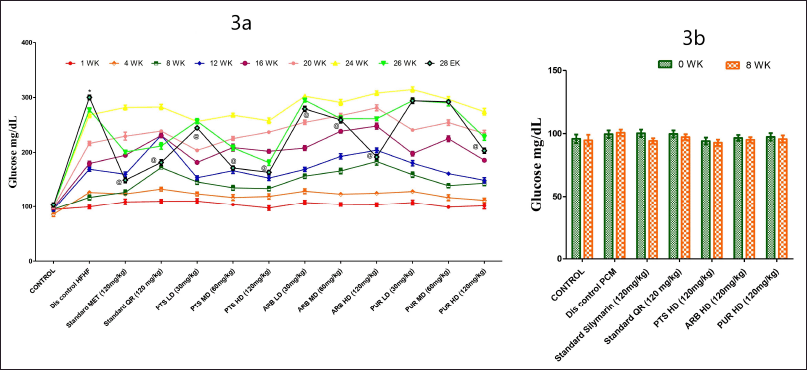 | Figure 3. The pharmacological interventions impact on blood glucose levels in various in different preclinical rodent sets of HFHF (3a) and PCM (3b) induced diabetic and non-diabetic liver injury. *statistical symbol details for comparison is mentioned in the statistical section. [Click here to view] |
Purpurin was found to have a hypoglycemic effect, although it was markedly less effective when compared to PTS and ARB dose ranges. When compared to other PTS doses (30, 60 mg/Kg. b.wt.), the larger dose of PTS (120 mg/Kg. b.wt.) markedly reduced glucose levels (Fig. 3a).
In PCM induced model of hepatotoxicity, there was no marked increase (p < 0.05) in blood glucose levels in the experimental PCM-treated disease group compared to the normal control group, indicating that they were not diabetic on the 8th day (Fig. 3b).
Liver profile variations in HFHF and PCM-induced rodent diabetic and non-diabetic liver injury model
It was observed that the levels of liver profile levels were raised markedly (p < 0.05) in HFHF and PCM-treated group in comparison to the control. When compared to the HFHF and PCM disease control group, all dosage ranges of PTS, ARB, and PUR (120 mg/kg) markedly reduced (p < 0.05) the levels of ALP, TB, and AST, ALT (Fig. 4a, 4b A–D). PUR has been found to have a hepatoprotective effect, though it is much less marked than PTS and ARB dose levels. The PTS (120 mg/Kg b.wt.) group markedly reduced (p < 0.05) the levels of liver profile as compared to different doses of PUR and ARB-treated groups. (Fig. 4a, 4b A–D). With regard to liver profile levels, the PTS high dosage (120 mg/Kg b.wt.) group was not found to be statistically different when compared to standard control groups (Fig. 4a,4b A–D).
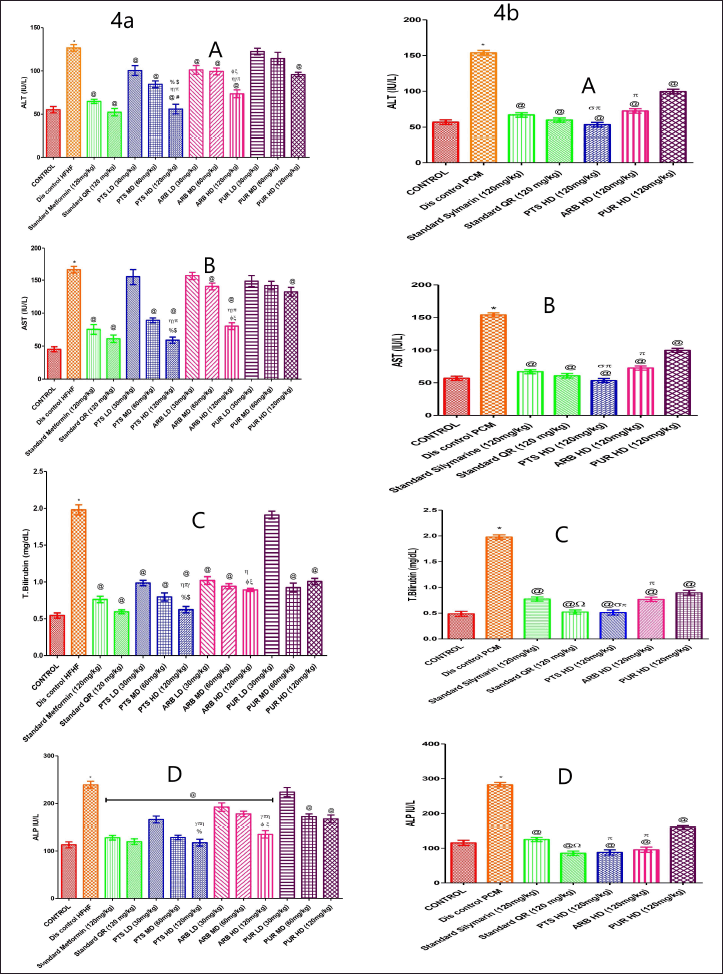 | Figure 4. The pharmacological interventions impact on liver function parameters levels in different preclinical rodent sets of HFHF (4a: A, B, C, and D) and PCM (4b: A, B, C, and D) induced diabetic and non-diabetic liver injury. *statistical symbol details for comparison is mentioned in the statistical section. [Click here to view] |
Serum BUN and creatinine variations in HFHF and PCM-induced rodent diabetic and non-diabetic liver injury model
HFHF diet and PCM-treated groups produce a marked upsurge (p < 0.05) in the BUN and creatinine levels in comparison to the vehicle-treated group (Fig. 5a, 5b A, B). The BUN and creatinine values in the Metformin standard treatment group were considerably less than those of the standard QR-treated group. Pterostilbene high dose showed a marked (p < 0.05) reduction in BUN in comparison to the QR-treated group. In the PTS, ARB (high dose) administered group, BUN and creatinine levels were markedly lower (p < 0.05) than that of the disease-treated group. Correspondingly, the PTS high dosage group was found to be effective in lowering the level of blood urea nitrogen and creatinine when compared to three doses of ARB and PUR administered groups (Fig. 5a, 5b A, B). The PTS high dosage (120 mg/Kg b.wt.) group was insignificant in comparison with standard groups in terms of creatinine levels. However, all dose concentrations of PUR, did not markedly lower BUN and creatinine levels as compared to HFHF-administered groups (Fig. 5a A, B).
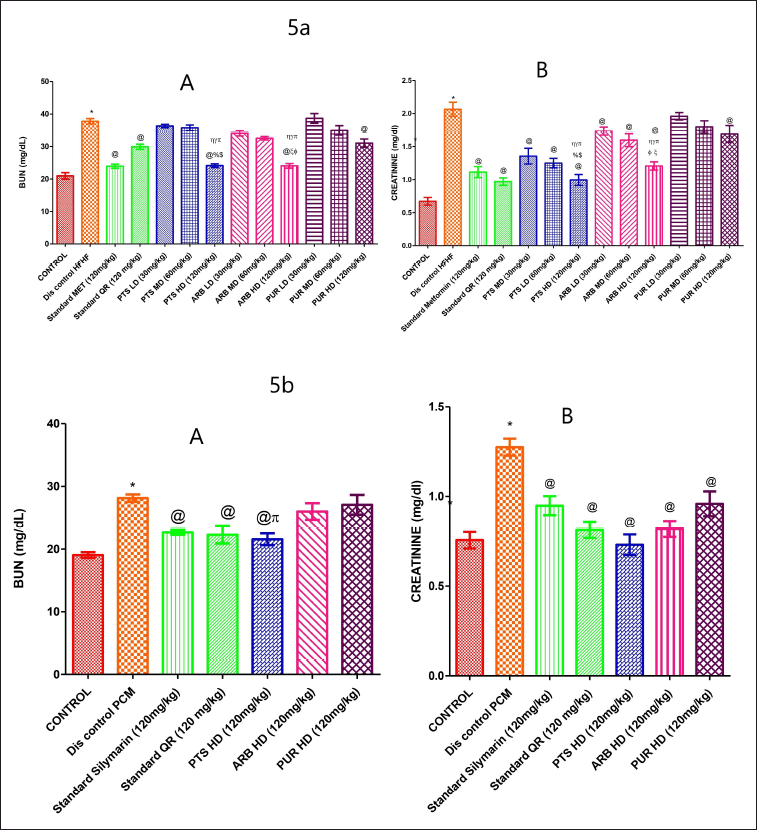 | Figure 5. The pharmacological interventions impact on BUN and creatinine levels in different preclinical rodent sets of HFHF (5a: A and B) and PCM (5b: A and B) induced diabetic and non-diabetic liver injury. *statistical symbol details for comparison is mentioned in the statistical section. [Click here to view] |
However, high-dose PUR did not markedly lower BUN levels when compared to PCM-induced hepatotoxicity disease control groups, although high-dose PUR considerably reduced creatinine levels when compared to PCM-induced hepatotoxicity disease control groups (Fig. 5b A, B).
Serum lipid levels variations in HFHF and PCM-induced rodent diabetic and non-diabetic liver injury model
The HFHF and PCM-treated disease control group had markedly lower HDL levels than that of the control group (Fig. 6a, 6b. D). Experimental groups of Pterostilbene, Arbutin, and Purpurin showed a marked increase (p < 0.05) in the values of HDL in comparison to the HFHF and PCM-treated experimental group. PUR has been demonstrated to have an antihyperlipidemic impact, but it has a less marked effect when compared to PTS and ARB dosage ranges (Fig. 6a, 6b. D).
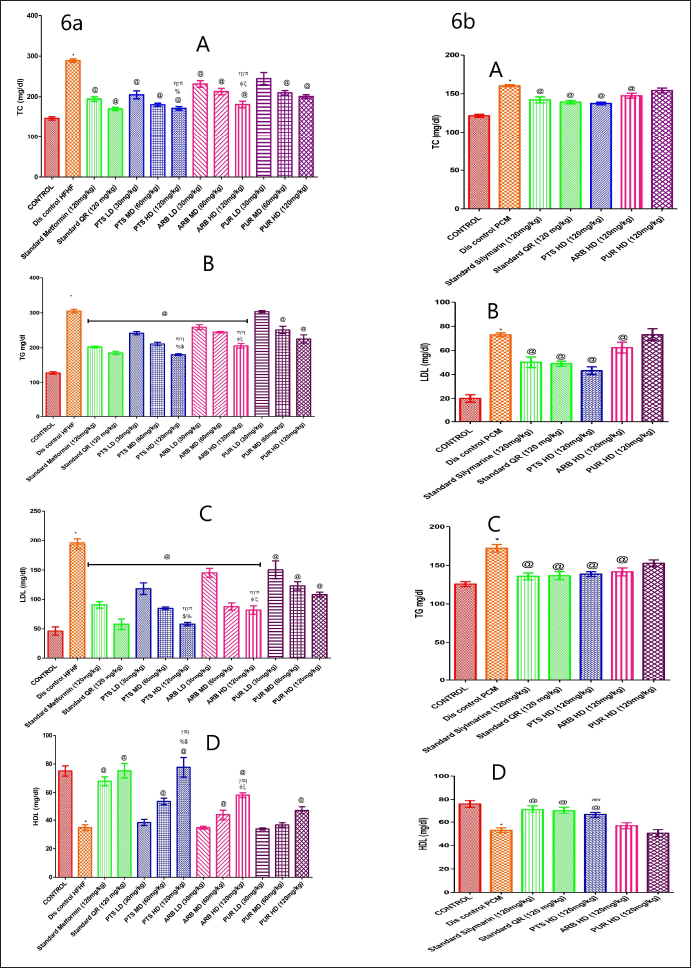 | Figure 6. The pharmacological interventions impact on lipid profile parameters levels in different preclinical rodent sets of HFHF (6a: A, B, C, and D) and PCM (6b: A, B, C, and D) induced diabetic and non-diabetic liver injury. *statistical symbol details for comparison is mentioned in the statistical section. [Click here to view] |
In the HFHF and PCM-treated disease control group, lipid profile levels were substantially higher (p < 0.05) than the normal diet group. In comparison to the disease control set, the entire drug-administered groups markedly reduced (p < 0.05) the lipid levels. In comparison to other doses of ARB and PUR administered groups, Pterostilbene (120 mg/Kg b.wt.) high dose showed marked (p < 0.05) reduction in lipid profile levels (Fig. 6a, 6b. A–C).
Liver tissue oxidative stress and antioxidant levels in HFHF and PCM-induced rodent diabetic and non-diabetic liver injury model
There was marked up surge of TBARS levels (p < 0.05) in the HFHF and PCM-treated experimental group than in the control group. All of the treatment groups markedly decreased (p < 0.05) the ranges of TBARS when correlated to the diseased rodents (Fig. 7a. A). PUR has been found to have an antioxidant effect, but it is markedly less effective than other PTS and ARB dose levels (Fig. 7a, 7b A). The higher dose of PTS considerably (p < 0.05) reduced TBARS levels in comparison to the low, medium, and higher doses of ARB and PUR-administered rats. Furthermore, PTS was shown to be substantial in comparison to standard administered rodent sets (Fig. 7a, 7b A). The HFHF and PCM disease treatment group showed substantially lower glutathione levels, in comparison to the experimental control sets (Fig. 7a, 7b B). The entire drug-treated rodent sets (PUR, ARB, and PTS) markedly (p < 0.05) increased the reduced glutathione levels in comparison to the disease groups (Fig. 7a, 7b B).
 | Figure 7. The pharmacological interventions impact on TBARS, antioxidant enzyme levels in different preclinical rodent sets of HFHF (7a: A and B) and PCM (7b: A and B)induced diabetic and non-diabetic liver injury. *statistical symbol details for comparison is mentioned in the statistical section. [Click here to view] |
Serum IL-6 and free fatty acid levels in HFHF and PCM-induced rodent diabetic and non-diabetic liver injury model
On comparing the HFHF and PCM treated to the control group, it was discovered that the IL-6 level was markedly (p < 0.05) higher in the HFHF and PCM-treated group (Fig. 8a). In comparison to the different doses of Arbutin and Purpurin groups, Pterostilbene (120 mg/Kg b.wt.) markedly (p < 0.05) reduced the IL-6 (Figure 8a).
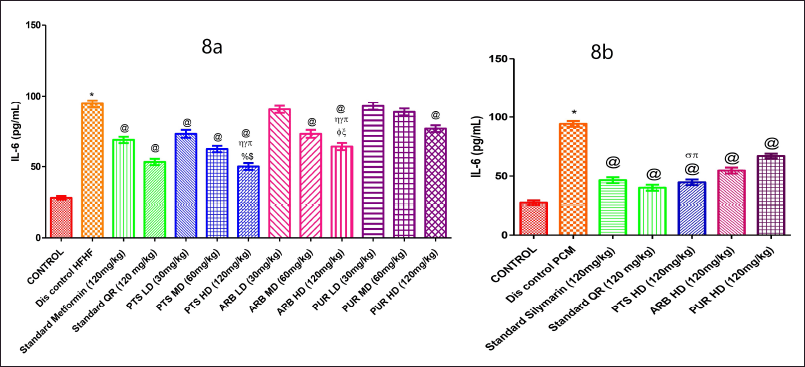 | Figure 8. The pharmacological interventions impact on serum IL-6 levels in different preclinical rodent sets of HFHF (8a) and PCM (8b) induced diabetic and non-diabetic liver injury. *statistical symbol details for comparison is mentioned in the statistical section. [Click here to view] |
The free fatty acid level in serum was markedly increased in HFHF-administered group in comparison to the normal diet administered group (Fig. 9a). The Pterostilbene 60 and 120 mg/kg and Arbutin 120 mg/kg dose treatment groups reduced serum free fatty acid levels markedly (p < 0.05) as compared to the HFHF treated group (Fig. 9a). In PCM-treated disease group, the FFA levels were increased markedly when compared to control group (Fig. 9b). In comparing ARB and PUR high doses administered rodents sets, it was discovered that higher dose of PTS (120 mg/Kg b.wt.) markedly reduced blood free fatty acid levels (p < 0.05) (Fig. 9b).
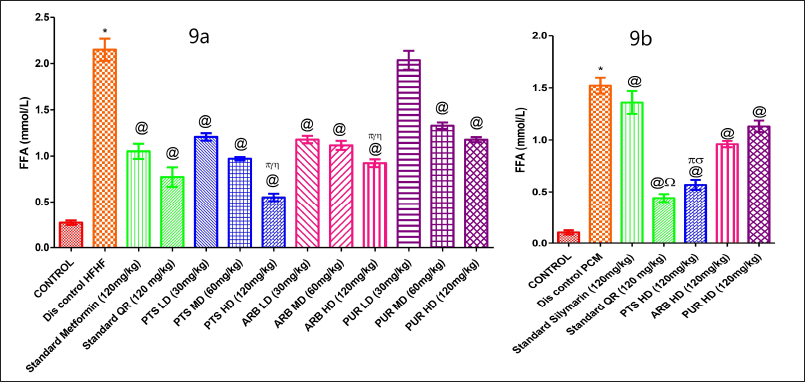 | Figure 9. The pharmacological interventions impact on serum free fatty acid levels in different preclinical rodent sets of HFHF (9a) and PCM (9b) induced diabetic and non-diabetic liver injury. *statistical symbol details for comparison is mentioned in the statistical section. [Click here to view] |
Transcriptional analysis of FASN levels of liver tissue in HFHF and PCM-induced rodent diabetic and non-diabetic liver injury model
The FASN gene expression alteration (Table 2) was checked experimentally in all rodent groups using qRT-PCR. In comparison to the normal control-treated group, the HFHF-treated group showed a marked rise in the mean fold of the FASN gene (p < 0.05). Except for the lowest dose of all selected treatments (p < 0.05), the entire selected pharmacological dose range treatments (PTS, ARB, and PUR) markedly decreased FASN expression (Fig. 10a A, B, C). Amongst the low dose ranges of all the chosen treatments and the disease control group, there was no statistically marked difference (p < 0.05). When compared to standard treated groups, higher dose ranges of chosen drugs had not shown any marked difference (Fig. 10a A, B, C).
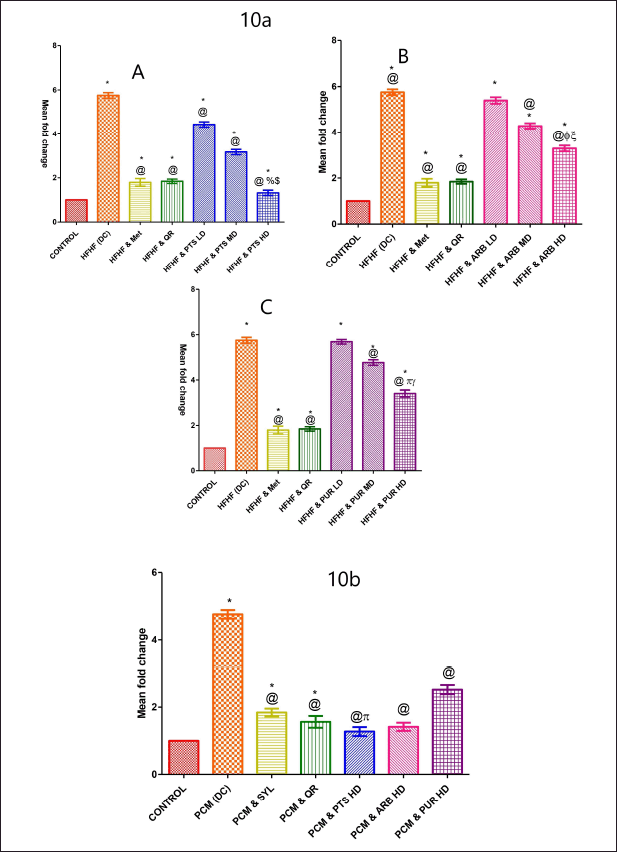 | Figure 10. The pharmacological interventions impact on liver tissue transcriptional level analysis of fatty acid synthase (FASN) in HFHF (10a: A, B, and C) and PCM (10b) induced diabetic and non-diabetic liver injury in rodents. *statistical symbol details for comparison is mentioned in the statistical section. [Click here to view] |
When comparing the PCM-treated group to the normal-treated group, a marked upsurge in the mean fold of the FASN gene was reported (p < 0.05). The expression of FASN was considerably reduced in the different dosages of chosen pharmacological drugs (PTS, ARB, and PUR) treated group (p < 0.05) (Fig. 10b). Higher dose levels of PTS had not shown any marked difference in comparison to the standard administered rodent groups (Fig. 10b).
Histopathology findings in HFHF and PCM-induced rodent diabetic and non-diabetic liver injury model
H & E staining showed hepatocyte nuclei displaced by one or more fat vacuoles (depicted with black arrows) and also distributed in the cytoplasm which shown the macrovesicular and microvesicular steatosis which leads to hepatocyte degeneration in the HFHF disease group (B) in comparison to normal rodents (A) (Fig. 11). The marked reduction in hepatocyte fat vacuoles and fat accumulation in the standard treatment groups, Quercetine (D) and Metformin (C). Pterostilbene (E-G), Arbutin (H-J), and Purpurin (K-M) at different dosages (30, 60, and 120 mg/kg) effectively reduce the development of fat and degeneration of hepatocyte caused by HFHF-associated liver damage.
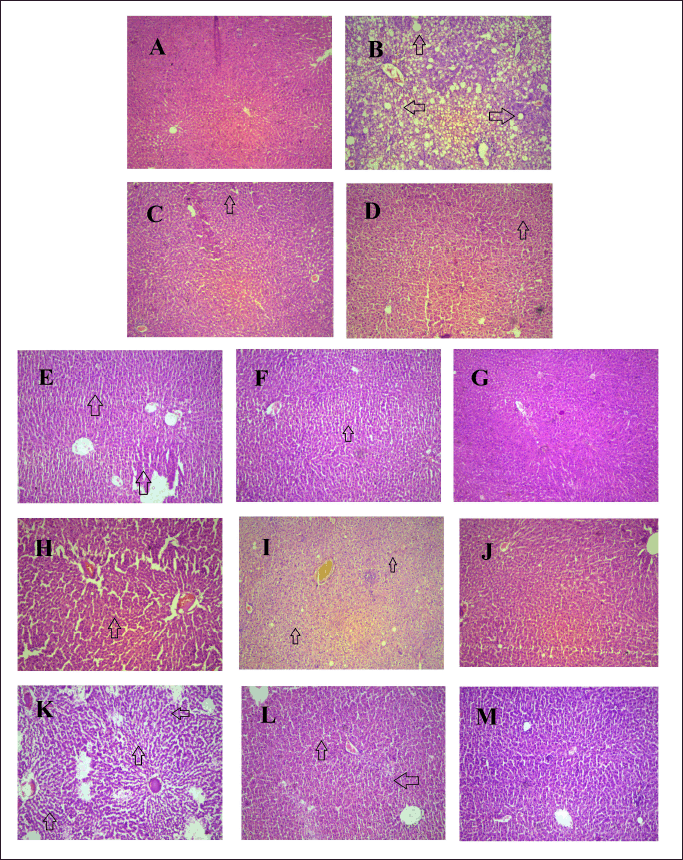 | Figure 11. The pharmacological interventions impact on H & E histopathology staining appearance on liver tissue of HFHF induced diabetic liver injury. Photomicrographs of transverse section of rat liver tissues included A Normal control; B HFHF treated Disease control; C MET treated group; D QR treated group E-G, PTS (30,60,120 mg/kg) treated group; H-J, ARB (30,60,120 mg/kg) treated group, K-M, PUR (30,60,120 mg/kg) treated group (magnification, × 200). [Click here to view] |
Another report of H & E staining (Fig. 12) revealed that there was hepatocyte deterioration (depicted with red arrows)in the PCM-treated group (B) in comparison to vehicle-treated control (A). Within the same context, it was found that the standard treated group, MET (C) and QR (D), exhibited markedly less hepatocyte deterioration.
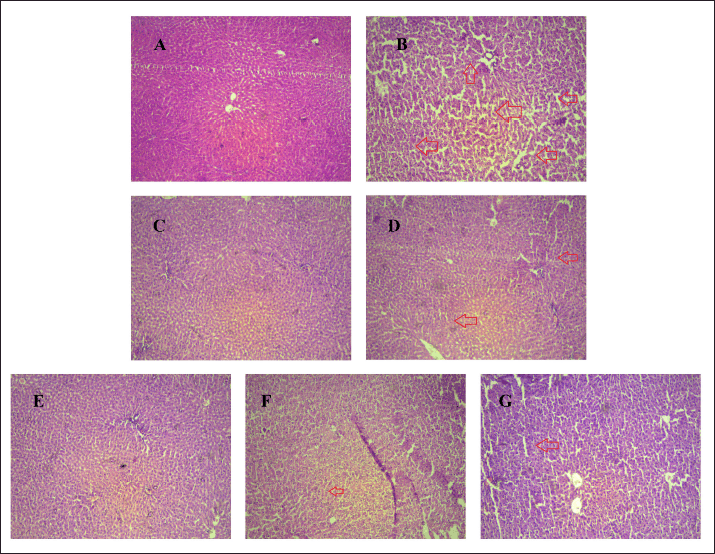 | Figure 12. The pharmacological interventions impact on H & E histopathology staining appearance on liver tissue of PCM induced non-diabetic liver injury. Photomicrographs of transverse section of rat liver tissues included A Normal control; B PCM treated Disease control; C SYL treated group; D QR treated group E-G, PTS, ARB and PUR (120 mg/kg) treated group (magnification, × 200). [Click here to view] |
However, hepatocyte deterioration in the PCM disease vehicle group (B) was observed in comparison to the normal control group (A). Standard administered group Quercetine (QR) (D) and Metformin (MET) (C), exhibited markedly reduced hepatocyte deterioration. However, PCM-induced liver damage resulted in the production of fat and hepatic degeneration, which was markedly reversed with PTS, ARB, and PUR administration (E-G).
DISCUSSION
HFHF diet raises oxidative burden, leptin, and resistance concerned with insulin, all collectively contributing to diabetic liver damage. The high-calorie diet raises FFA levels in the blood, causing hepatocytes to accumulate long-chain acyl-coenzyme A, Triglycerides, and Diacylglycerols, resulting in the development of an obesity, NAFLD, and T2DM [15,24,25]. It was revealed that HFHF-treated rodents are shown to possess diabetes-related liver complications as there was a substantial upsurge in the glucose and liver profile levels in comparison to the normal control [1,18,26]. The current study demonstrated that there was a substantial upsurge in the lipid levels, with respect to total cholesterol, low-density lipoprotein, and triglycerides in HFHF-treated rodents, which were reversed after selected pharmacological treatment. Renal indicators such as BUN (mg/dl) and serum creatinine (mg/dl) were markedly (p < 0.05) higher in the diseased set as compared to the normal control, suggesting oxidative damage to liver tissues, which might be the reason of HFHF-induced diabetic liver injury. The same results were demonstrated in 2020 that there was a marked increase in liver enzyme and lipid levels in the HFHF administered rodents which creates a burden on the liver [9,27,28]. The HFHF-induced diabetic-associated liver damage causes a significant increase in the cytokine, chemokine, and TBARS burden on the liver as depicted in the present study. A similar report was published in 2020 that there was a substantial upsurge in the oxidative burden and inflammation in HFHF-administered rodents [29]. In the current research design, there was an upsurge in fatty acid synthase enzyme gene expression leading to significantly increased serum level free fatty acid in the HFHF-treated diabetic liver injury experimental rodents as compared to the normal diet administered group, which was supported by qRT-PCR analysis. The above data provides evidence that there is an interrelationship between fatty acid synthase enzyme and the pathogenesis of HFHF-persuaded diabetic liver injury which is also well supported by the other published research evidence that inappropriate dietary incorporation causes the progression of the dual disease model of diabetic liver injury (DLI) [30–32]. Moreover, the three particular pharmacological drugs significantly lower the LDL, TC, TG, FFA, and IL-6 levels in HFHF-induced diabetic liver injury rodents. The current evidence from the rodent model of diabetic liver injury scientifically proved that the selected pharmacological evidence possesses antiadipogenic, antihyperlipidemic, antihyperglycemic, and hepatoprotective effects.
The non-diabetic liver injury model also had the goal of correlating and determining the influence of fat on incorrect food consumption and non-dietary alterations in rats with paracetamol-induced hepatotoxicity. Although PCM-induced hepatotoxicity is unrelated to diabetes, but it may obstruct the metabolic machinery, interfering with lipid biotransformation and altering liver function [19,33]. The current evidence depicted that there was a substantial upsurge in the various liver enzymes; inflammatory responses and oxidative stress in the hepatocytes of PCM-treated rodents. The PCM-treated rodent group proved to have no significant rise in blood glucose levels, indicating that it is a non-diabetic injury model that causes a fat burden due to a disruption in the metabolic machinery. Similar results were revealed by Gopi et al. [2] that PCM causes kidney and liver-related problems without altering the blood glucose levels. PTS, ARB, and PUR are pharmacological agents that have strong hepatoprotective effects by reversing the pathological conditions caused by PCM-induced non-diabetic liver damage. Therefore, based on the preceding explanation, it appears that all agents work as fatty acid synthase enzyme inhibitors, nonetheless, further data and biochemical parameters are needed to confirm the present findings.
CONCLUSION
It was clearly demonstrated that fatty acid synthase inhibition via selected pharmacological intervention improved hyperglycemia condition and is shown to be effective for the management of diabetic and non-DLI. Therefore, from the above discussion, it was evidently proposed that PTS, PUR, and ARB act as fatty acid synthase enzyme inhibitors but still more supportive evidence and biochemical parameters will be required to authenticate the current results and to move towards the industrial translational approach. While this study does not include a time-course analysis, we have addressed this limitation in our future research to better assess the progression and reversal of liver injury. However, the qRT-PCR studies authenticate the present results that three selected pharmacological agents significantly decrease the expression of FASN in PTS, PUR, and ARB-treated groups when compared with diabetic and non-diabetic liver injury disease control groups.
ACKNOWLEDGMENT
We extend our thanks to the Chitkara University for support in the flourishing of this manuscript.
LIST OF ABBREVIATIONS
ALT, Alanine transaminase; ARB, Arbutin; AST, Aspartate transaminase; FFA, free fatty acid; GLP-1, Glucagon-like peptidase; HFHF, High fructose high fat; LDL, low density lipoprotein; MMP, Matrix metalloproteinase; NAFLD, Nonalcoholic fatty liver disorders; PCM, Paracetamol; PTS, Pterostilbene; PUR, Purpurin; QR, Quercetin; RG, Reduced Glutathione; SYL, Silymarin.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVAL
Ethical Approvals details are given in the ‘Material and Method’ section.
DATA AVAILABILITY
Data are available upon request to the corresponding author.
PUBLISHER’S NOTE:
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
CONSENT TO PARTICIPATE
No requirement of clinical patient participation consent.
REFERENCES
1. Siegers CP, Möller-Hartmann W. Cholestyramine as an antidote against paracetamol-induced hepato-and nephrotoxicity in the rat. Toxicol Lett. 1989;47:179–84. doi: CrossRef
2. Gopi KS, Reddy AG, Jyothi, K, Kumar BA. Acetaminophen-induced Hepato-and Nephrotoxicity and Amelioration by silymarin and Terminalia chebula in Rats. Toxicol Int. 2010;17:64–6. doi: CrossRef
3. Ayonrinde OT, Phelps GJ, Hurley JC, Ayonrinde OA. Paracetamol overdose and hepatotoxicity at a regional Australian hospital: a 4-year experience. Intern Med J. 2005;35:655–60. doi: CrossRef
4. Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42(6):1364–72. doi: CrossRef
5. Mohd Zain ZF, Fathelrahman AI, Ab Rahman AF. Characteristics and outcomes of paracetamol poisoning cases at a general hospital in Northern Malaysia. Singapore Med J. 2006;47:134–7.
6. Larson AM, Acetaminophen hepatotoxicity. Clin Liver Dis. 2007;11:525–48. doi: CrossRef
7. Ostapowicz G., Lee WM. Acute hepatic failure: a Western perspective. J Gastroenterol Hepatol. 2000;15:480–8. doi: CrossRef
8. Marzilawati AR, Ngau YY, Mahadeva S. Low rates of hepatotoxicity among Asian patients with paracetamol overdose: a review of 1024 cases. BMC Pharmacol Toxicol. 2012;13:8. doi: CrossRef
9. Geidl-Flueck B, Hochuli M, Németh Á, Eberl A, Derron N, Köfeler HC. Fructose- and sucrose-but not glucose-sweetened beverages promote hepatic de novo lipogenesis: a randomized controlled trial. J Hepatol. 2021;75:46–54. doi: CrossRef
10. Kalra S, Vithalani M, Gulati G, Kulkarni CM, Kadam Y, Pallivathukkal J. Study of prevalence of nonalcoholic fatty liver disease (NAFLD) in type 2 diabetes patients in India (Sprint). J Assoc Physicians India. 2013;61:448–53.
11. Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr. 2004;79:537–43. doi: CrossRef
12. Bedi O, Aggarwal S, Trehanpati N, Ramakrishna G, Krishan P. Molecular and pathological events involved in the pathogenesis of diabetes-associated nonalcoholic fatty liver disease. J Clin Exp Hepatol. 2019;9:607–18. doi: CrossRef
13. Jürgens H, Haass W, Castañeda TR, Schürmann A, Koebnick C, Dombrowski F. Consuming fructose-sweetened beverages increases body adiposity in mice. Obes Res. 2005;13:1146–56. doi: CrossRef
14. Almajwal AM, Elsadek MF. Lipid-lowering and hepatoprotective effects of Vitis vinifera dried seeds on paracetamol-induced hepatotoxicity in rats. NRP. 2015;9:37–42.
15. Ekta, Gupta M, Kaur A, Singh TG, Bedi O. Pathobiological and molecular connections involved in the high fructose and high fat diet induced diabetes associated nonalcoholic fatty liver disease. Inflamm Res. 2020;69:851–67. doi: CrossRef
16. Bedi O, Aggarwal S, Trehanpati N, Ramakrishna G, Grewal AS, Krishan P. In vitro targeted screening and molecular docking of stilbene, quinones, and flavonoid on 3T3-L1 pre-adipocytes for anti-adipogenic actions. Naunyn Schmiedebergs Arch Pharmacol. 2020;393:2093–106. doi: CrossRef
17. Bedi O, Srivastava N, Parsad D, Krishan P. Fatty acid synthase inhibition ameliorates diabetes induced liver injury in rodent experimental model. Eur J Pharmacol. 2021;901:174078. doi: CrossRef
18. Lozano I, Van der Werf R, Bietiger W, Seyfritz E, Peronet C, Pinget M. High-fructose and high-fat diet-induced disorders in rats: impact on diabetes risk, hepatic and vascular complications. Nutr Metab (Lond). 2016;13:15. doi: CrossRef
19. Salem GA, Shaban A, Diab HA, Elsaghayer WA, Mjedib MD, Hnesh AM. Phoenix dactylifera protects against oxidative stress and hepatic injury induced by paracetamol intoxication in rats. Biomed Pharmacother. 2018;104:366–74. doi: CrossRef
20. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: CrossRef
21. Boyne AF, Ellman G.L. A methodology for analysis of tissue sulfhydryl components. Anal Biochem. 1972;46:639–53. doi: CrossRef
22. Lowry OH, Rosebrough NJ, Farr AL, Randall R.J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. doi: CrossRef
23. Sawano T, Shimizu T, Yamada T, Nanashima N, Miura T, Morohashi S. Fatty acid synthase-positive hepatocytes and subsequent steatosis in rat livers by irinotecan. Oncol Rep. 2015;33:2151–60. doi: CrossRef
24. Lim JS, Mietus-Snyder M, Valente A, Schwarz JM, Lustig R.H. The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat Rev Gastroenterol Hepatol. 2010;7:251–64. doi: CrossRef
25. Arora A, Behl T, Sehgal A, Singh S, Sharma N, Chigurupati S. Free fatty acid receptor 1: a ray of hope in the therapy of type 2 diabetes mellitus. Inflammopharmacology. 2021;29:1625–39. doi: CrossRef
26. Van Dam RM, Willett WC, Rimm EB, Stampfer MJ, Hu F.B. Dietary fat and meat intake in relation to risk of type 2 diabetes in men. Diabetes Care. 2002;25:417–24. doi: CrossRef
27. Stanhope KL. Sugar consumption, metabolic disease and obesity: the state of the controversy. Crit Rev Clin Lab Sci. 2016;53:52–67. doi: CrossRef
28. Chyau CC, Wang HF, Zhang WJ, Chen CC, Huang SH, Chang C.C. Antrodan alleviates high-fat and high-fructose diet-induced fatty liver disease in C57BL/6 mice model via AMPK/Sirt1/SREBP-1c/PPARγ pathway. Int J Mol Sci. Mol. 2020;21:360. doi: CrossRef
29. Gómez-Zorita S, González-Arceo M, Trepiana J, Aguirre L, Crujeiras AB, Irles E. Comparative effects of Pterostilbene and its parent compound resveratrol on oxidative stress and inflammation in steatohepatitis induced by high-fat high-fructose feeding. Antioxidants (Basel). 2020;9:1042. doi: CrossRef
30. Aeberli I, Gerber PA, Hochuli M, Kohler S, Haile SR, Gouni-Berthold I. Low to moderate sugar-sweetened beverage consumption impairs glucose and lipid metabolism and promotes inflammation in healthy young men, a randomized controlled trial. Am J Clin Nutr. 2011;94:479–85. doi: CrossRef
31. Ahmad MM, Rezk NA, Fawzy A, Sabry M. Protective effects of curcumin and silymarin against paracetamol induced hepatotoxicity in adult male albino rats. Gene. 2019;712:143966. doi: CrossRef
32. Dusilová T, Ková? J, Drobný M, Šedivý P, Dezortová M, Poledne R. Different acute effects of fructose and glucose administration on hepatic fat content. Am J Clin Nutr. 2019;109:1519–26. doi: CrossRef
33. Sharma VK, Singh TG. Chronic stress and diabetes mellitus: interwoven pathologies. Curr Diabetes Rev. 2020;16:546–56. doi: CrossRef