INTRODUCTION
Paraquat (PQ), a non-selective herbicide is widely employed particularly across developing countries, worldwide. The classification of pesticides in terms of hazard under the World Health Organization classifies PQ under Class II: moderately hazardous [1]. PQ poisoning is regarded as a global threat to public health, because of its high mortality rate, especially in case of suicidal or unintentional ingestion because of lack of specific treatment protocols [2]. Although, marketing of PQ is banned in one-fourth of the countries globally, reports of its intoxication continue to rise significantly [3]. PQ mediates its human toxicity via a redox cycling mechanism, producing reactive oxygen species (ROS), and diminishing the cell’s nicotinamide adenine dinucleotide phosphate hydrogen reserves, thus leading to lipid peroxidation in the cell membrane and ultimately resulting in cell death [4]. Upon absorption, PQ is primarily distributed in tissues with high perfusion, such as the lungs, liver and the kidneys, where it exerts organ toxicity. The major complications of PQ poisoning include acute respiratory distress syndrome (ARDS), toxic hepatitis and acute kidney injury (AKI), which can lead to multiple organ dysfunction syndrome (MODS) in severe cases [5]. Thus, several attempts have been made to develop an antidote or optimal strategies for the management of PQ poisoning, however, efforts have largely proven futile [6]. Other factors which are known to complicate patient prognosis include varying degrees of PQ exposure, heterogenous clinical presentation among patients, and delays in receiving emergency care [7].
Current management approaches for PQ poisoning, in addition to supportive and symptomatic treatment include antioxidants, N-acetyl cysteine (NAC), tocopherol (vitamin E), and ascorbic acid (vitamin C); immunosuppressive treatment (IST) such as corticosteroids, cyclophosphamide; and extra-corporeal removal (ECR) techniques, consisting of hemoperfusion (HP), conventional hemodialysis (HD), continuous venovenous hemofiltration, and continuous renal replacement therapy [8]. While ECR techniques have been employed to accelerate removal of certain poisonous substances in humans, their application as a therapeutic modality in PQ poisoning is a more recent introduction [9]. A few latest pharmacologic therapies have been introduced for PQ poisoning. Ambroxol, a mucolytic agent commonly used in respiratory conditions, has been applied in the management of lung damage caused by PQ poisoning. According to a meta-analysis by Wang et al. [10], adjuvant ambroxol therapy was found to reduce in-hospital mortality in PQ poisoning patients. Xuebijing, a traditional Chinese medicine, has been employed as an adjuvant antioxidant in combination with HP for PQ poisoning. A meta-analysis by Fu et al. [11] showed that this combination improved the 7-day survival rate in these patients. Other pharmacological therapies which are being researched for their therapeutic potential in PQ poisoning include rosiglitazone, doxycycline, febuxostat, rapamycin, fluorofenidone, tacrolimus, and octreotide [8].
Despite clinical advances in therapeutic approaches for PQ poisoning, observations regarding their effectiveness in improving patient prognosis remain inconsistent. While the study by Koh et al. [12] found that IST might potentially improve patient prognosis, the randomized controlled trial by Gawarammana et al. [13] found no significant survival benefit with IST administration. Ambiguities also exist concerning the effectiveness of the various ECR modalities in PQ poisoning. A meta-analysis by Eizadi-Mood et al. [14], found no association between HD and improved survival. Hsu et al. [15] found that HP administration early in the course of treatment was beneficial in decreasing the fatality rate in patients with PQ poisoning. Similarly, the meta-analysis by Nasr Isfahani et al. [16] found HP to be beneficial as an adjunct to traditional approaches in improving patient survival. Moreover, there is a lack of observational studies with a substantial sample size exploring treatment outcomes of PQ poisoning in the Indian clinical scenario. Thus, to address these ambiguities, and strengthen the existing evidence, we aimed to conduct this 10-year retrospective study to compare clinical outcomes between patients receiving ECR treatment and those who did not, in the management of PQ poisoning. In addition, we analyzed the risk factors associated with clinical non-improvement in patients with PQ poisoning.
MATERIALS AND METHODS
Study design and ethical approval
We conducted a medical record-based retrospective cohort study over a 10-year period from 1st January 2012 to 1st March 2023 at our tertiary care hospital in South India. We confirm that patient confidentiality was maintained as per the guidelines of the Institutional Ethics Committee, including anonymization of patient data. Since it was a retrospective study, the requirement of informed consent was waived by the Institutional Ethics Committee, and the study was approved vide approval no. 135/2023. The study has been reported in accordance with the Strengthening of the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [17]. The additional file 1 contains the STROBE checklist.
Inclusion and exclusion criteria
We included a total of 304 patients who were admitted to our tertiary care hospital with PQ poisoning in this study. Patients were included irrespective of their sex and age, regardless of time from exposure, route of exposure (oral, inhalational, or direct contact), and type of exposure (suicidal/accidental/homicidal). PQ poisoning was confirmed based on the qualitative sodium dithionite test of the serum, urine or stomach residues; or patient history; or circumstantial evidence as presented. Pregnant or lactating patients, patients with critical illness(es) such as cancer, history of organ transplantation, current use of immunosuppressants, mixed poisoning, and those with incomplete data were excluded.
Data collection
A structured data collection form was prepared using Microsoft Excel to record patient data which was gathered from medical records accessed through the hospital’s medical record department. The following data was collected: Demographic details, including age and sex; medical and medication history, social history, exposure history (route, time, type, and amount of exposure), time from exposure to first aid, along with presenting symptoms. Baseline laboratory investigations at admission were recorded such as kidney function tests (serum urea, S.urea; serum creatinine, S.Cr), liver function tests (serum aspartate transaminase, AST; alanine transaminase, ALT), serum electrolytes (sodium, potassium), white blood cell count (WBC) and saturation of peripheral oxygen (SpO2). Additionally, the results of the qualitative toxicological screening of the urine, serum, or stomach residues with the sodium dithionite reagent were recorded. Details on emergency care interventions, if given, such as gastric lavage and activated charcoal were also collected. Details of the in-patient treatments administered were also collected, including NAC, antioxidants such as ascorbic acid (vitamin C), tocopherol (vitamin E); corticosteroids; antimicrobial agents such as antibiotics and antifungals; symptomatic therapy including antacids, antiemetics, antiplatelet agents; and ECR techniques administered, such as HD, HP or their combination (HP + HD). Data regarding patient outcomes, clinical improvement or non-improvement; complications such as AKI, toxic hepatitis, ARDS, sepsis/septic shock, gastrointestinal (GI) bleed and MODS; and hospital-related metrics such as duration of hospitalisation, intensive care unit (ICU), and mechanical ventilation (MV) were also collected.
Definitions
The severity of AKI was determined based on the RIFLE classification (class R, class I, or class F). Patients were diagnosed with AKI if they met any of the RIFLE criteria [18]. When the serum ALT level exceeded 70 IU/l (normal range: 0–35 IU/l), hepatitis was recognised in patients [19]. ARDS was diagnosed with bilateral lung infiltrates on chest radiography or computed tomography scan, and a PaO2/FiO2 ratio of less than 300 mm Hg [20]. Sepsis was defined as a life-threatening organ dysfunction because of a dysregulated host response to infection, with the onset recognised by organ dysfunction distant from infection site. Operationally, septic shock is described as necessitating vasopressor therapy to maintain an elevated plasma lactate level over 2 mmol/l and the mean arterial blood pressure above 65 mm Hg [21]. MODS was defined as the onset of a potentially reversible physiologic derangement involving two or more organ systems excluding the condition leading to ICU admission [22]. Hospitalisation days were defined as the duration of hospitalisation of the patient in the hospital from admission to discharge [23]. ICU days were defined as the duration of patient’s stay in the ICU during hospitalization [24]. MV days were defined as the duration for which the patient received MV (intubation) during hospitalization [25]. Patients who received any of the ECR methods (HP, HD, or a combination of both) were classified under the ECR group and those who were not administered ECR treatment were classified under the non-ECR group. We have considered clinical non-improvement in patients as worsening of clinical symptoms during discharge, morbidity, discharge against medical advice due to financial reasons, and mortality.
Outcome measures
We classified included patients under two groups on the basis of their clinical outcome: improvement and non-improvement. The primary outcome was to compare the clinical outcomes of patients in the ECR and non-ECR treatment groups. The secondary outcome was to analyze the risk factors associated with clinical non-improvement in the included patients.
Statistical analysis
We presented the categorical data as frequency (percentage). The continuous variables with a normal distribution were presented as mean (standard deviation, SD), and the variables not normally distributed were presented as median (interquartile range, IQR). To compare the outcomes of patients in the ECR and non-ECR treatment groups, we employed the chi-square test for categorical data and the Mann-Whitney U test for continuous data was performed. To evaluate risk factors of clinical non-improvement, all recorded variables were examined using univariate binary logistic regression to estimate the unadjusted odds ratio. Variables from the univariate analysis with a p value of < 0.2 were then subjected to a backward conditional stepwise multivariate logistic regression analysis to estimate the adjusted odds ratio [26–29]. The independent risk factors for clinical non-improvement were the variables with a p-value < 0.05 in the multivariate logistic regression analysis. Additionally, to reduce bias, we have conducted the logistic regression analysis by excluding patients who were discharged against medical advice due to financial constraints. We conducted the statistical analysis using the IBM SPSS software (version 22) [30].
RESULTS
Patient demographics and clinical characteristics
Out of 304 patients included in the study, the majority were male (n = 191, 62.8%). The median (IQR) age of the included patients was 25 (13.5) years. Mode of exposure was primarily intentional (n = 300, 98.7%). Most patients were employed (n = 166, 54.6%), and the majority of them were farmers/farm workers (n = 73, 24%). Most of the patients had exposure to an approximate amount of 10–50 ml of 24% PQ dichloride (n = 73, 24%). Almost 254 (83.5%) patients received pre-hospitalisation emergency treatment, with gastric lavage (stomach wash) being the most common modality (n = 166, 54.6%). Most of the patients received emergency treatment within 24 hours of PQ exposure (n = 220, 72.4%). Vomiting was the most frequently observed clinical presentation in 173 (57%) patients. Oropharyngeal lesions were the second most frequently observed clinical presentation in 108 (35.5%) patients. Qualitative toxicology screening with sodium dithionite reagent performed on serum, urine, or stomach residues was positive in 71 (23.3%) patients. There were 89 (29.3%) survivors, 159 (52.3%) non-survivors and 56 (18.4%) patients who were discharged against medical advice from the hospital due to financial constraints. Overall, majority of the patients did not show clinical improvement (n = 215, 70.7%), while 89 (29.3%) patients showed clinical improvement during their hospitalisation. Table 1 contains in detail the patient demographics and clinical characteristics.
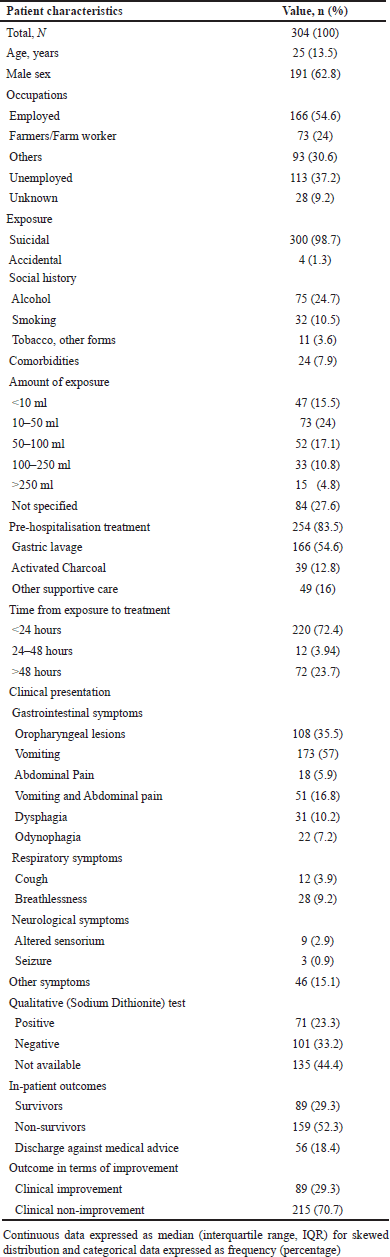 | Table 1. Demographic and clinical characteristics of the included patients (N = 304). [Click here to view] |
Clinical outcomes of the included patients
AKI was the most frequently observed complication (n = 212, 69.7%), followed by MODS (n = 147, 48.3%), and toxic hepatitis (n = 126, 41.4%). The median (IQR) hospitalization and ICU days were 4 (8) and 3 (5) days, respectively. Overall, 147 (48.3%) patients required MV, with a median (IQR) of 0 (1) days. The clinical non-improvement group was found to have higher levels of WBC, S.urea, AST, ALT and significantly lower SpO2 values. The frequency of the complications such as MODS, AKI, ARDS, toxic hepatitis, sepsis/septic shock were also higher in patients with clinical non-improvement. Table 2 depicts in detail the clinical characteristics between clinical non-improvement and improvement patient groups.
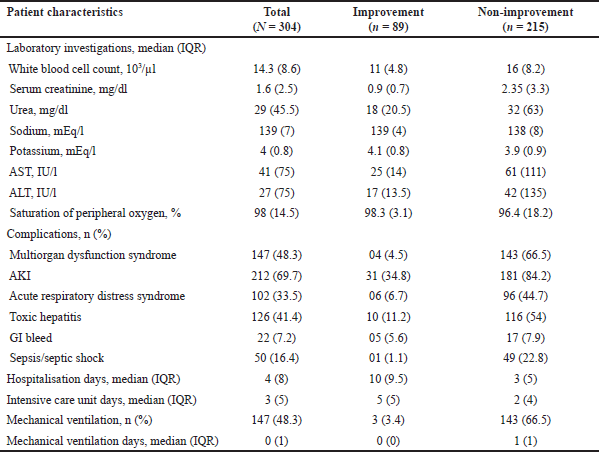 | Table 2. Clinical characteristics between improvement and non-improvement patient groups (N = 304). [Click here to view] |
Treatment outcomes in the included patients
A total of 205 (67.4%) patients received ECR treatment, with 45 (14.8%) patients receiving HD, 99 (32.6%) receiving HP, and 61 (20%) patients receiving a combination of HP and HD. A total of 218 (71.7%) patients received NAC, either alone or in combination with other antioxidants as shown in Table 3. IST including corticosteroid or cyclophosphamide was administered to 138 (45.4%) of patients. Antimicrobial agents including antibiotics and antifungal agents were prescribed to 196 (64.5%) and 5 (1.6%) of patients, respectively. Table 3 provides detailed treatment modalities administered to the inpatients.
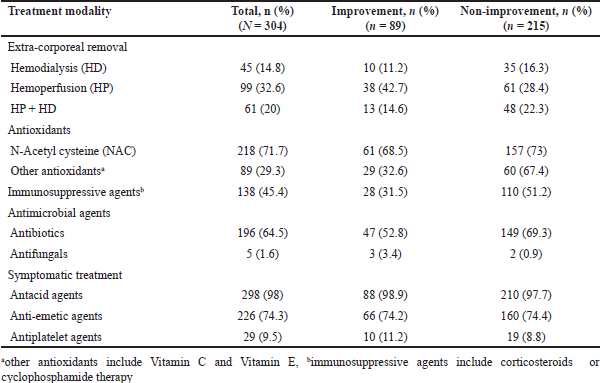 | Table 3. Treatment modalities administered to inpatients (N = 304). [Click here to view] |
Among the 205 patients who received ECR treatment (ECR group), 29.7% clinically improved. This was similar to the patients who did not receive ECR treatment (non-ECR group), depicting an improvement rate of 28.3%. We found no significant difference in the clinical improvement rate between the ECR and non-ECR treatment groups. AKI was found to be less frequent in the patients receiving ECR treatment (42.2%), compared to those who did not receive ECR treatment (64.6%), however, the difference was insignificant (p = 0.179). Similarly, the frequency of the complications ARDS, MODS, sepsis/septic shock, hepatitis, and GI bleed did not differ significantly in between ECR (36.1%, 49.8%, 17.6%, 42%, and 9.3%, respectively) and non-ECR treatment groups (28.3%, 45.5%, 14.1%, 40.4%, and 3%, respectively). MV days were similar in both groups (1%) (p = 0.114). Table 4 depicts the clinical outcomes of the patients with respect to treatment groups.
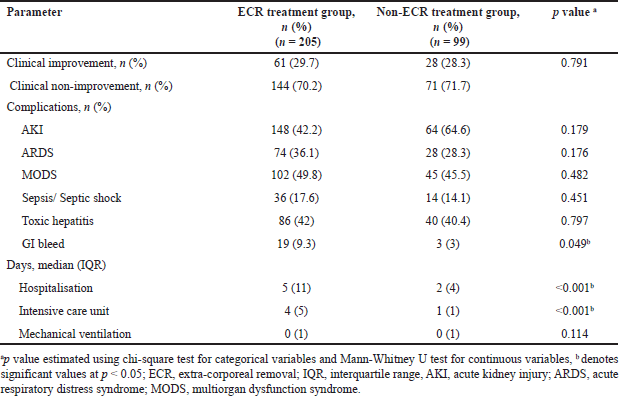 | Table 4. Clinical outcomes of patients between ECR and Non-ECR treatment groups. [Click here to view] |
Risk factors associated with clinical non-improvement
The risk factors for clinical non-improvement in the patients included were identified by conducting univariate analysis, which was then succeeded by multivariate logistic regression analysis. The significant variables (p < 0.2) in univariate logistic regression were found to be WBC (OR: 1.134, 95% CI: 1.078–1.194. p < 0.001); S.Cr (OR: 1.691, 95% CI: 1.367–2.091, p < 0.001); S. urea (OR: 1.021, 95% CI: 1.011–1.030, p < 0.001); AST (OR:1.038, 95% CI: 1.023–1.053, p < 0.001); ALT (OR: 1.036, 95% CI: 1.020–1.052, p < 0.001); SpO2 (OR: 0.991, 95% CI: 0.979–1.002, p = 0.117); AKI (OR: 9.960, 95% CI: 5.635–17.604, p < 0.001); ARDS (OR: 11.160, 95% CI: 4.670–26.667, p < 0.001); toxic hepatitis (OR: 9.25, 95% CI: 4.549–18.836, p < 0.001); sepsis/septic shock (OR: 25.976, 95% CI: 3.527–191.297, p = 0.001); HP (OR: 0.532, 95% CI: 0.318–0.889, p = 0.016); HP + HD (OR: 1.680, 95% CI: 0.860–3.284, p = 0.129); immunosuppressive agents (OR: 2.282, 95% CI: 1.355–3.844, p = 0.002); and antibiotics (OR: 2.017, 95% CI: 1.215–3.350, p = 0.007). The above significant variables were then subject to multivariate logistic regression to yield risk factors of clinical non-improvement. Multivariate logistic regression found that increased AST (OR: 1.027, 95% CI: 1.011–1.043, p < 0.001) and occurrence of sepsis/septic shock (OR: 14.556, 95% CI: 1.798–117.849, p = 0.012) were risk factors associated with clinical non-improvement (p < 0.05). Table 5 depicts the results of the univariate and multivariate logistic regression analysis.
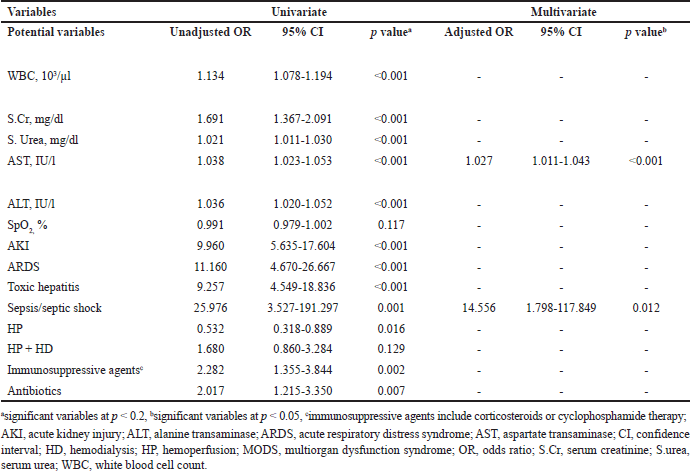 | Table 5. Univariate and multivariate logistic regression analysis for risk factors of clinical non-improvement. [Click here to view] |
The multivariate logistic regression analysis conducted by excluding patients who were discharged against medical advice due to financial constraints found that increased WBC (OR: 1.128, 95% CI: 1.055–1.205, p < 0.001), increased AST (OR: 1.027, 95% CI: 1.011–1.042, p < 0.001); occurrence of AKI (OR: 2.498, 95% CI: 1.144–5.456, p = 0.022), ARDS (OR: 5.796, 95% CI: 2.101–15.989 and sepsis/septic shock (OR: 15.030, 95% CI: 1.799–125.554, p = 0.012) were risk factors associated with clinical non-improvement (p < 0.05). The details are provided in the additional file 2 which contains the results of the univariate and multivariate logistic regression analysis conducted by excluding patients who were discharged against medical advice due to financial constraints.
DISCUSSION
PQ poisoning has the highest fatality rates among pesticide toxicities worldwide, making it a major global concern [31]. Despite advances in pharmacological management, there is still no antidote or a specified treatment guideline for managing patients with PQ poisoning [8]. Furthermore, introduction of ECR techniques for PQ elimination has yielded largely conflicting results on its effectiveness [12,13,16,28,32]. Moreover, observational studies with an adequate sample size analysing treatment outcomes in PQ poisoning have been lacking, especially in the Indian context [33–36]. In addition, studies analysing factors contributing to clinical non-improvement in patients are scarce. Thus, we conducted this decade-long retrospective study to analyse treatment outcomes and risk factors of clinical non-improvement in patients admitted with PQ poisoning at a tertiary care teaching hospital in South India.
The median (IQR) age reported in our study, 25 (13.5) years, was similar to the study by Rao et al. [33], which observed the mean (SD) age of PQ poisoning patients as 27 (7.7) years. However, the prospective study conducted by Lin et al. [37] reported a significantly higher median age of 37 years among the included patients. The proportion of non-survivors in our study (52.3%) was significantly higher than the survivors (29%). We found similar results in the studies performed by Lin et al. [37] (78%); Rao et al. [33] (61.4%); Rao et al. [38] (63%); and Goyal et al. [39] 2024 (88%). However, some studies reported higher survival rates. This may be explained by variable treatment protocols in their studies [40,41]. Regarding complications, AKI (69.7%) and MODS (48.3%) were the most frequently observed complications in our study. This observation was in accordance to the research study performed by Goyal et al. [39]; however, it was in contrast with the studies of Wu et al. [42], reporting highest frequency of respiratory failure; and the studies by Rao et al. [33], Tajai et al. [41], and Lin et al. [37] which reports MODS as the most common cause for mortality. The need for MV in our study (48.3%) was slightly higher than the study by Goyal et al. [39] (34.9%) and lower than the study by Rao et al. [38] (63%).
HP was administered to a higher population of patients in the study by Rao et al. [38], i.e., 53.5%, and to a comparatively lower number of patients in the study by Tajai et al. [41] (18.9%). However, IST administration was reported in a similar number of patients in the study of Rao et al. [38] compared to our study. The lone study by Goyal et al. [39] observed a higher frequency of AKI among patients who did not receive HP, which was consistent with our study findings. Our study found a higher frequency of AKI in the non-ECR treatment group (64.6%), with an absolute difference of 22.4% between the groups, suggesting potential clinical relevance despite statistical insignificance. This lack of statistical significance is likely due to the smaller sample size, data variability, or the influence of associated covariates on specific outcomes.
PQ elimination is primarily through the renal pathway, because of which PQ poisoning accounts for the severe renal damage, often progressing to MODS in later stages [40]. This is consistent with our study finding showing AKI as a significant risk factor for poor clinical outcomes, as evidenced by its association with clinical non-improvement in patients (OR: 2.498, 95% CI: 1.144–5.456, p = 0.022). This necessitates the timely clearance of PQ using ECR techniques in enhancing PQ elimination, which further protects multiple organ damage and reduces the need for intensive care. ECR is a critical intervention that should be implemented in emergency settings to improve patient outcomes in PQ intoxication. PQ-induced AKI is primarily mediated by ROS generation in renal proximal tubule cells, leading to lipid peroxidation and cell death [43]. ECR accelerates PQ removal from circulation, reducing ROS-mediated toxicity and alleviating systemic organ damage [14,44]. This was reflected in the lower AKI frequency observed in the ECR treatment group in our study (42.2%). Additionally, ECR has been recognized as an effective strategy for protecting renal function in critically ill patients, further supporting its role in PQ poisoning management [45].
The clinical relevance of our findings lies in the potential effectiveness of ECR in managing PQ-induced toxicity. Even if statistical significance is not achieved, the clinical benefits of reducing AKI are substantial, as a lower incidence of AKI leads to better patient outcomes, fewer complications, and reduced economic burden. Ultimately, clinical improvement and patient recovery should take precedence over statistical significance in evaluating the impact of ECR in PQ poisoning management.
None of the studies investigated the difference in outcomes between the patients receiving ECR and non-ECR modalities. Our study found no significant difference in the clinical improvement rate between patients receiving ECR and non-ECR modalities; however, we could not compare the results with other studies due to limited research focusing on the improvement status of patients. This highlights the need for further research using standard treatment protocols for ECR modalities, for a better understanding of its role in the management of PQ poisoning and to inform clinical decision-making.
The multivariate regression analysis found that the complication of sepsis/septic shock was an independent determinant of clinical non-improvement. This can be due to the role of sepsis in the development of MODS. MODS in sepsis is triggered by a combination of factors including extensive inflammation, oxidative stress, endothelial damage, and microvascular clotting, all of which disrupt blood flow and oxygenation to organs, resulting in cell damage and organ failure. The process is further intensified due to the generation of pro-inflammatory cytokines and ROS, which aggravate tissue injury and contribute to the advancement of MODS [46]. In addition, we found that increased AST levels were an important risk factor for clinical non-improvement, likely due to the hepatic toxicity of PQ [47]. This finding was similar to the research conducted by Gheshlaghi et al. [5], wherein, elevated AST levels on day 3 had a 100% specificity in predicting mortality and morbidity in PQ poisoning patients. Altogether, these findings point out the necessity for early identification and timely management of sepsis and hepatotoxicity in PQ poisoning, which may lead to better patient clinical outcomes. The multivariate logistic regression analysis was also conducted by excluding patients who were discharged against medical advice due to financial constraints. Additionally, we observed that increased WBC, occurrence of AKI and ARDS were the additional risk factors for clinical non-improvement.
Our study demonstrated numerous strengths. Our study was the longest (10-year) retrospective study including a large sample of PQ poisoning patients in South India. To the best of our knowledge, it was the most comprehensive Indian study analyzing demographics, clinical presentations, exposure history, and treatment patterns in PQ poisoning patients. In addition, this was the first study highlighting outcomes in terms of clinical improvement and non-improvement, ensuring that the significant number of patients discharged against medical advice in PQ poisoning cases were not excluded.
However, our study had a few limitations. Being a retrospective study, our information was derived from medical records of patients, which restricted us from verifying the accuracy of exposure history and prehospitalisation duration/treatment. Since the data on ingestion volume was not consistently available in all the included patients, it could not be included as a covariate in the statistical analysis. Additionally, the lack of uniform data on the timing of PQ ingestion limited our ability to analyze outcomes based on early vs late ECR administration. Even though the study includes an appreciable sample size of 304 PQ poisoning patients, the number of patients receiving ECR treatment was limited, indicating potential sample size limitations. Furthermore, being the first study to analyze outcomes in terms of clinical non-improvement, there was a significant lack of research to compare our results, since most studies focused on mortality as the clinical outcome. Future prospective studies with well-defined treatment protocols for ECR regimens are necessary to provide clarity on the effectiveness of ECR approaches in managing PQ poisoning.
CONCLUSION
ECR treatment modalities did not significantly improve clinical outcomes in patients with PQ poisoning. Sepsis/septic shock and high baseline AST levels were identified as risk factors for clinical non-improvement. To strengthen the existing evidence, additional prospective studies are necessary to evaluate the comparative effectiveness of the various ECR modalities considering the timing and duration of their administration.
LIST OF ABBREVIATIONS
AKI, acute kidney injury; ALT, alanine transaminase; ARDS, acute respiratory distress syndrome; AST, aspartate transaminase; ECR, extra-corporeal removal; GI, gastrointestinal; HD, hemodialysis; HP, hemoperfusion; ICU, intensive care unit; IQR, interquartile range; IST, immunosuppressive treatment; MODS, multi-organ dysfunction syndrome; MV, mechanical ventilation; NAC, N-acetyl cysteine; PQ, paraquat; S.Cr, serum creatinine; SpO2, saturation of peripheral oxygen; STROBE, strengthening of the reporting of observational studies in Epidemiology; WBC, white blood cell count.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report for the study.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
Ethical approval details is given in the ‘Materials and Methods section’.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Eddleston M, Buckley NA, Gunnell D, Dawson AH, Konradsen F. Identification of strategies to prevent death after pesticide self-poisoning using a Haddon matrix. Inj Prev. 2006;12(5):333–7. CrossRef
2. Gawarammana IB, Buckley NA. Medical management of paraquat ingestion. Br J Clin Pharmacol. 2011;72(5):745–57. CrossRef
3. It’s time to ban paraquat | Environmental Working Group [Internet]. 2024 [cited 2024 Apr 30]. Available from: https://www.ewg.org/news-insights/news/2024/02/its-time-ban-paraquat
4. Dinis-Oliveira RJ, Duarte JA, Sánchez-Navarro A, Remião F, Bastos ML, Carvalho F. Paraquat poisonings: mechanisms of lung toxicity, clinical features, and treatment. Crit Rev Toxicol. 2008;38(1):13–71. CrossRef
5. Gheshlaghi F, Haghirzavareh J, Wong A, Golshiri P, Gheshlaghi S, Eizadi-Mood N. Prediction of mortality and morbidity following paraquat poisoning based on trend of liver and kidney injury. BMC Pharmacol Toxicol. 2022;23(1):67. CrossRef
6. Dinis-Oliveira RJ, de Pinho PG, Santos L, Teixeira H, Magalhães T, Santos A, et al. Postmortem analyses unveil the poor efficacy of decontamination, anti-inflammatory and immunosuppressive therapies in paraquat human intoxications. PLoS One. 2009;4(9):e7149. CrossRef
7. Kaur H, Chandran VP, Rashid M, Kunhikatta V, Poojari PG, Bakkannavar SM, et al. The significance of APACHE II as a predictor of mortality in paraquat poisoning: a systematic review and meta-analysis. Journal of Forensic and Legal Medicine. 2023;97:102548. CrossRef
8. Shahsavarinia K, Balafar M, Tahmasbi F, Gharekhani A, Milanchian N, Hajipoor Kashgsaray N, et al. Evidence of efficacy the various management methods in paraquat poisoning: an umbrella review. Toxin Reviews. 2024;43(3):437–51. CrossRef
9. Isfahani SN, Farajzadegan Z, Sabzghabaee AM, Rahimi A, Samasamshariat S, Eizadi-Mood N. Does hemoperfusion in combination with other treatments reduce the mortality of patients with paraquat poisoning more than hemoperfusion alone: a systematic review with meta-analysis. J Res Med Sci. 2019 Jan 1;24(1):2. CrossRef
10. Wang J, Yu W, Wu N, Gitonga EN, Shen H. Efficacy of high-dose ambroxol for paraquat poisoning: a meta-analysis of randomized controlled trials. J Res Med Sci. 2020;25:67. CrossRef
11. Fu Y, Yan M, Zeng X, Xie C, Xu W, Feng J, et al. Meta-analysis of the efficacy of Xuebijing combined with hemoperfusion in treating paraquat poisoning. Ann Palliat Med. 2020;9(4):2152162. CrossRef
12. Koh KH, Tan CHH, Hii LWS, Lee J, Ngu LLS, Chai AJM, et al. Survival predictors in paraquat intoxification and role of immunosuppression. Toxicol Rep. 2014;1:490. CrossRef
13. Gawarammana I, Buckley NA, Mohamed F, Naser K, Jeganathan K, Ariyananada PL, et al. High-dose immunosuppression to prevent death after paraquat self-poisoning - a randomised controlled trial. Clin Toxicol (Phila). 2018;56(7):633–9. CrossRef
14. Eizadi-Mood N, Jaberi D, Barouti Z, Rahimi A, Mansourian M, Dorooshi G, et al. The efficacy of hemodialysis on paraquat poisoning mortality: a systematic review and meta-analysis. J Res Med Sci. 2022;27:74. CrossRef
15. Hsu CW, Lin JL, Lin-Tan DT, Chen KH, Yen TH, Wu MS, et al. Early hemoperfusion may improve survival of severely paraquat-poisoned patients. PLoS One. 2012;7(10):e48397. CrossRef
16. Nasr Isfahani S, Farajzadegan Z, Sabzghabaee AM, Rahimi A, Samasamshariat S, Eizadi-Mood N. Does hemoperfusion in combination with other treatments reduce the mortality of patients with paraquat poisoning more than hemoperfusion alone: a systematic review with meta-analysis. J Res Med Sci. 2019;24:2. CrossRef
17. Cuschieri S. The STROBE guidelines. Saudi J Anaesth. 2019;13(Suppl 1):S31–4. CrossRef
18. Cartin-Ceba R, Haugen EN, Iscimen R, Trillo-Alvarez C, Juncos L, Gajic O. Evaluation of “Loss” and “End stage renal disease” after acute kidney injury defined by the Risk, Injury, Failure, Loss and ESRD classification in critically ill patients. Intensive Care Med. 2009;35(12):2087–95. CrossRef
19. Aithal GP, Watkins PB, Andrade RJ, Larrey D, Molokhia M, Takikawa H, et al. Case definition and phenotype standardization in drug-induced liver injury. Clin Pharmacol Ther. 2011;89(6):806–15. CrossRef
20. Diamond M, Peniston HL, Sanghavi DK, Mahapatra S. Acute respiratory distress syndrome. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2024. Available from: http://www.ncbi.nlm.nih.gov/books/NBK436002/
21. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801–10. CrossRef
22. Deitch EA. Multiple organ failure. Pathophysiology and potential future therapy. Ann Surg. 1992;216(2):117–34. CrossRef
23. Eskandari M, Alizadeh Bahmani AH, Mardani-Fard HA, Karimzadeh I, Omidifar N, Peymani P. Evaluation of factors that influenced the length of hospital stay using data mining techniques. BMC Med Inform Decis Mak. 2022;22(1):280. CrossRef
24. Moitra VK, Guerra C, Linde-Zwirble WT, Wunsch H. Relationship between ICU length of stay and long-term mortality for elderly ICU survivors. Crit Care Med. 2016;44(4):655–62. CrossRef
25. Esteban A, Anzueto A, Frutos F, Alía I, Brochard L, Stewart TE, et al. Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28-day international study. JAMA. 2002;287(3):345–55. CrossRef
26. Cecatto SB, Monteiro-Soares M, Henriques T, Monteiro E, Moura CIFP. Derivation of a clinical decision rule for predictive factors for the development of pharyngocutaneous fistula postlaryngectomy. Braz J Otorhinolaryngol. 2015;81(4):394–401. CrossRef
27. Wilson W, Bhat R, Angadi B, Lekha N, Balaji B, Balakrishnan JM. Predictors of mortality in paraquat poisoning: a two-year retrospective analysis from a tertiary care teaching hospital in South India. Indian J Forensic Med Toxicol 2021;15(3):4435–43. CrossRef
28. Yeh YT, Chen CK, Lin CC, Chang CM, Lan KP, How CK, et al. Does hemoperfusion increase survival in acute paraquat poisoning? a retrospective multicenter study. Toxics. 2020;8(4):84. CrossRef
29. Lin XH, Pan HY, Cheng FJ, Huang KC, Li CJ, Chen CC, et al. Association between liberal oxygen therapy and mortality in patients with paraquat poisoning: a multi-center retrospective cohort study. PLoS One. 2021;16(1):e0245363. CrossRef
30. IBM SPSS Statistics [Internet]. [cited 2023 Oct 16]. Available from: https://www.ibm.com/products/spss-statistics
31. Wesseling C, van Wendel de Joode B, Ruepert C, León C, Monge P, Hermosillo H, et al. Paraquat in developing countries. Int J Occup Environ Health. 2001;7(4):275–86. CrossRef
32. Kavousi-Gharbi S, Jalli R, Rasekhi-Kazerouni A, Habibagahi Z, Marashi SM. Discernment scheme for paraquat poisoning: a five-year experience in Shiraz, Iran. World J Exp Med. 2017;7(1):31–9. CrossRef
33. Rao R, Bhat R, Pathadka S, Chenji SK, Dsouza S. Golden hours in severe paraquat poisoning-the role of early haemoperfusion therapy. J Clin Diagn Res. 2017;11(2):OC06–8. CrossRef
34. Ravichandran R, Amalnath D, Shaha KK, Srinivas BH. Paraquat poisoning: a retrospective study of 55 patients from a tertiary Care Center in Southern India. Indian J Crit Care Med. 2020;24(3):155–9. CrossRef
35. Paraquat – boon or bane? A retrospective study of paraquat poisoning and outcomes in a tertiary care center in South India. 2001 [cited 2023 Jan 18]; Available from: https://www.mjdrdypv.org/preprintarticle.asp?id=339952
36. Kanchan T, Bakkannavar SM, Acharya PR. Paraquat poisoning: analysis of an uncommon cause of fatal poisoning from Manipal, South India. Toxicol Int. 2015;22(1):30–4. CrossRef
37. Lin JL, Lin-Tan DT, Chen KH, Huang WH, Hsu CW, Hsu HH, et al. Improved survival in severe paraquat poisoning with repeated pulse therapy of cyclophosphamide and steroids. Intensive Care Med. 2011;37(6):1006–13. CrossRef
38. Rao S, Maddani SS, Chaudhuri S, Bhatt MT, Karanth S, Damani A, et al. Utility of clinical variables for deciding palliative care in paraquat poisoning: a retrospective study. Indian J Crit Care Med. 2024;28(5):453–60. CrossRef
39. Goyal P, Sharma S, Taneja V. A Study of paraquat poisoning presentation, severity, management and outcome in a tertiary care hospital: is there a silver lining in the dark clouds? Indian J Crit Care Med. 2024;28(8):741–7. CrossRef
40. Jamshidi F, Fathi G, Davoodzadeh H. Investigation paraquat poisoning in Southwest of Iran – from sign to mortality and morbidity. Arch Med S?d Kryminol. 2017;67(1):35–45. CrossRef
41. Tajai P, Kornjirakasemsan A. Predicting mortality in paraquat poisoning through clinical findings, with a focus on pulmonary and cardiovascular system disorders. J Pharm Policy Pract. 2023;16(1):123. CrossRef
42. Wu WP, Lai MN, Lin CH, Li YF, Lin CY, Wu MJ. Addition of immunosuppressive treatment to hemoperfusion is associated with improved survival after paraquat poisoning: a nationwide study. PLoS One. 2014;9(1):e87568. CrossRef
43. Kim SJ, Gil HW, Yang JO, Lee EY, Hong SY. The clinical features of acute kidney injury in patients with acute paraquat intoxication. Nephrol Dial Transplant. 2009;24(4):1226–32. CrossRef
44. Guo H, Yuan Y, Ma Y, Shi J, Gu H. Effects of early repeated hemoperfusion combined with hemodialysis on the prognosis of patients with paraquat poisoning. Am J Transl Res. 2023;15(9):5613–23.
45. Tamargo C, Hanouneh M, Cervantes CE. Treatment of acute kidney injury: a review of current approaches and emerging innovations. J Clin Med. 2024;13(9):2455. CrossRef
46. Srdi? T, ?uraševi? S, Laki? I, Ruži?i? A, Vujovi? P, Jev?ovi? T, et al. From molecular mechanisms to clinical therapy: understanding sepsis-induced multiple organ dysfunction. Int J Mol Sci. 2024;25(14):7770. CrossRef
47. Yang CJ, Lin JL, Lin-Tan DT, Weng CH, Hsu CW, Lee SY, et al. Spectrum of toxic hepatitis following intentional paraquat ingestion: analysis of 187 cases. Liver Int. 2012;32(9):1400–6. CrossRef