INTRODUCTION
Wound healing is a complex response to injury that involves regenerating or reconstructing damaged tissue. It follows a precise and highly regulated sequence of phases: hemostasis, inflammation, proliferation, and remodeling [1]. The healing process is initiated by platelets, which release coagulating chemicals, ultimately leading to the formation of a fibrin clot at the site of injury [2]. The inflammatory phase involves the vigorous mobilization of leukocytes to the site of the wound. During the initial stages of inflammation, interleukin (IL)-6 and IL-1, along with matrix metalloproteinases (MMPs), play a significant role [3]. The M2 macrophages, which have anti-inflammatory properties, secrete complement components and various growth factors, including vascular endothelial growth factor (VEGF), insulin-like growth factor 1, fibroblast growth factor (FGF), epidermal growth factor, and transforming growth factor (TGF). In addition, they release anti-inflammatory cytokines such as IL-10 during the later stage of inflammation [4,5].
The proliferative phase encompasses the primary regenerative mechanisms that commence on the third day following injury and persist for approximately 2 weeks. The process begins with a rapid increase in the growth and movement of fibroblasts, which then start producing new extracellular matrix (ECM) components such as fibrin, collagen I, collagen III, fibronectin, glycosaminoglycans, proteoglycans, and matrix protein hyaluronan [6]. An essential part of wound healing is the angiogenesis process, which entails creating and arranging new blood vessels. Numerous angiogenic stimulators, including platelet-derived growth factor (PDGF), FGF, VEGF, TGF-ß, tumor necrosis factor-alpha (TNF-α), angiogenin, and angiopoietin-1, are produced [7]. In the end, epithelialization takes place. Epithelial cells quickly migrate from the borders of the wound to the site of injury [8].
The final stage of wound healing, known as the remodeling phase, usually takes many months or even years to complete. In order to create thicker and more durable collagen fibers, type III collagen is gradually replaced with type I collagen during the progressive transition process. The result is a wholly formed scar with high tensile strength and fewer cells and blood vessels [5,6]. Although acute wounds typically heal naturally, chronic wounds can disrupt this process, leading to persistent inflammation characterized by excessive neutrophil infiltration and prolonged healing times. Managing chronic inflammation is a challenging medical issue that requires substantial health care resources [9].
Plants and plant-based compounds have long been used to treat and manage wounds. Herbal medicines have significantly advanced the treatment of skin wounds, especially in developing countries [3,10]. However, developing plant-derived drugs remains a considerable challenge [11]. Various biopolymers are being investigated to create cost-effective, environmentally friendly, durable, and efficient delivery systems for wound treatment. Natural herbal remedies have become crucial in managing skin disorders and treating infections because of their affordability and the adverse effects of modern medicine [12–14]. Indonesia has over 17,000 islands and a staggering 25,000–30,000 plant species, encompassing a diverse range of 50 natural ecosystems or vegetation types [15]. Indonesia is home to a diverse array of ethnic groups between 300 and 700. This rich ethnic diversity results in various cultures, traditions, and local knowledge systems that vary across different groups and regions. Among these practices, many ethnic Indonesians use natural resources to maintain their health. According to the 2018 Basic Medical Research (Riskesdas) data, 31.8% of the Indonesian population continues to rely on traditional medicine for their health care needs [16].
Given the high economic burden of treating and managing wound healing, exploring the scientific potential of medicinal plants from Indonesian regions is crucial. These plants possess properties that can positively impact the environment, quality of life, and the overall economy in both developed and developing countries. This review discusses medicinal plants commonly used to treat wounds in Indonesia, including their ethnopharmacology, active compounds, and molecular targets that support their bioactive properties.
METHODS
Study design
This investigation employed a comprehensive scoping review methodology, integrating the foundational York Framework principles with contemporary systematic review guidelines. The methodological framework synthesized the pioneering approach of Arskey and O'Malley [17], subsequent refinements by Levac et al. [18], and current PRISMA-ScR reporting standards [19]. This structured assessment examined Indonesian ethnomedicinal plants used in wound healing, encompassing both traditional knowledge and scientific evidence. The analytical process followed six sequential phases: research question formulation, literature identification, study selection based on predetermined criteria, systematic data extraction, evidence synthesis, and expert consultation for validation.
Research question
The investigative framework was developed to address the primary research question: “What is the current literature relating to the potential application of medicinal plants for wound healing in Indonesia, and to what extent has research explored the active compounds and their molecular targets?”
Information sources and search strategy
We systematically searched English-language publications from July 4, 2000 to April 30, 2023, across multiple electronic databases, including ScienceDirect, PubMed, Google Scholar, Scopus, and Web of Science. Our search was conducted in two stages: preliminary and primary. The preliminary search strategy used comprehensive keywords including: “wound healing” (“plant” or “herb” or “traditional medicine”), “Indonesia,” and “tribe”.
Study selection
Based on this preliminary research, the relevant and frequently used plants for wound healing are discussed. During this primary phase, specific keywords and phrases related to the study’s focus were scrutinized, such as “wound healing,” “medicinal plants,” “phytochemicals,” “compounds,” “chemical structures,” “receptors,” and “formulations” to capture the breadth of relevant research. We reviewed primary search results and included ethnopharmacy, phytochemicals, in vitro and in vivo on wound healing properties. We focused on original publications in English with abstracts available in the databases. The PRISMA flowchart showed the search results and selection process in the present scoping review study (Fig. 1).
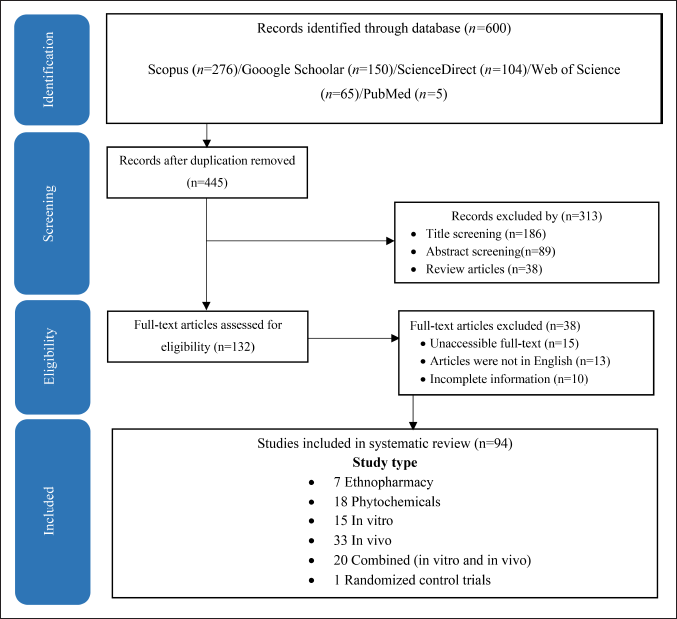 | Figure 1. PRISMA flowchart of the included studies. [Click here to view] |
Data extraction
Data extraction was performed systematically using a customized Microsoft Excel template to capture comprehensive information on medicinal plants used in Indonesia. The extracted parameters encompassed as follows: (1) demographic characteristics, including tribal affiliation, geographical location, and temporal context; (2) methodological classification (in vitro and/or in vivo investigations); (3) botanical specifications detailing plant taxonomy, used parts, and preparation methodologies; (4) phytochemical profiles with emphasis on bioactive constituents; (5) molecular mechanisms of action; and (6) therapeutic outcomes (detailed documentation available in Supplementary Tables S2 and S3).
Synthesis
Adhering to the PRISMA-ScR checklist, our rigorous data extraction process ensured a comprehensive and transparent analysis of the existing evidence landscape, providing a detailed understanding of medicinal plant research in wound healing (detailed documentation available in Supplementary Tables S1).
RESULTS
Ethnopharmacology
The PRISMA flowchart (Fig. 1) summarizes the search results and selection process of the present scoping review study. A total of 94 studies about medicinal plants used for wound healing in Indonesia were used in writing this review. This review assessed 238 plant species belonging to 60 families for wound-healing properties using research data from 11 islands and 25 ethnic groups in the Indonesian Archipelago, which can be accessed in Figure 2 [20]. Data from the Aceh Province of Sumatra were collected from three villages: Sebarjadi [21], Sekerak [22], and Labu [23]. In other areas in Sumatra, data were collected from North Sumatra [24,25], Riau’s Kampar Hilir [26], Jambi’s Serampas, and Lampung’s Way Kambass [27]. Data from Java were obtained from Subang in the western region [28] and Turgo and Mount Merapi in the central region [29,30]. In the eastern region of Java, data were obtained from Pacitan [31], Mount Tengger [32], and Mount Wilis [33].
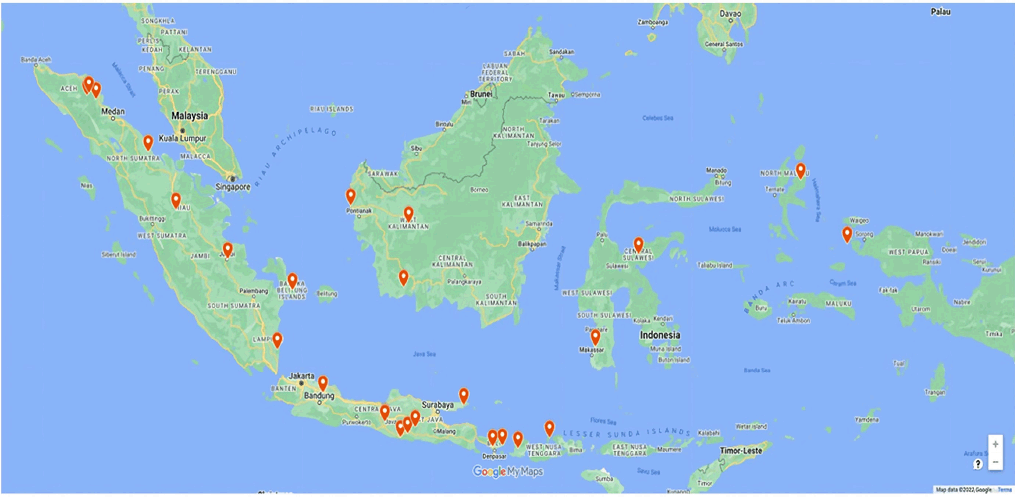 | Figure 2. The research distribution data on medicinal plants for wound healing in Indonesia [20] . [Click here to view] |
Data were collected from Dayak Sintang [34] and Mempawah [35] in West Kalimantan and Sukamara in Central Kalimantan [36]. In eastern Indonesia, data were collected from Minasatene in South Sulawesi [37] and Kalii Indra Triba in Central Sulawesi [38]. In the province of Irian Jaya, data were collected from Raja Ampat [39]. In addition, data were obtained from small islands such as South Bangka [40,41], Bali [42,43], Moyo Island in west Nusa Tenggara [44], Narmada and Belu in East Nusa Tenggara [45], Sumenep, Madura [46], and Halmahera in Maluku [47].
As shown in Figure 3a, plants from the Asteraceae family are frequently used in Indonesia for wound healing, including Acmella uliginosa (Sw.) Cass, Ageratum conyzoides L., Bidens pilosa L., Bidens biternata (Lour.), and Crassocephalum crepidioides (Benth.) S. moore; additional plant species mentioned were Chromolaena odorata (L.), Conyza sumatrensis (Retzius), Erechtites valerianifolius (Link ex Spreng.), Eupatorium inulifolium Kunth, Eupatorium odoratum, Gynura procumbens (Lour.) Merr, Synedrella nodiflora (L.) Gaerth, Mikania micrantha, and Sonchus arvensis [21,23,33,37,41]. Plants from this botanical family are frequently used for wound healing because they thrive in tropical habitats and have widespread occurrence and therapeutic properties. Consequently, these plants are primarily sought as an alternative to modern, expensive drugs and chemical-based treatments whose adverse effects can be harmful [48].
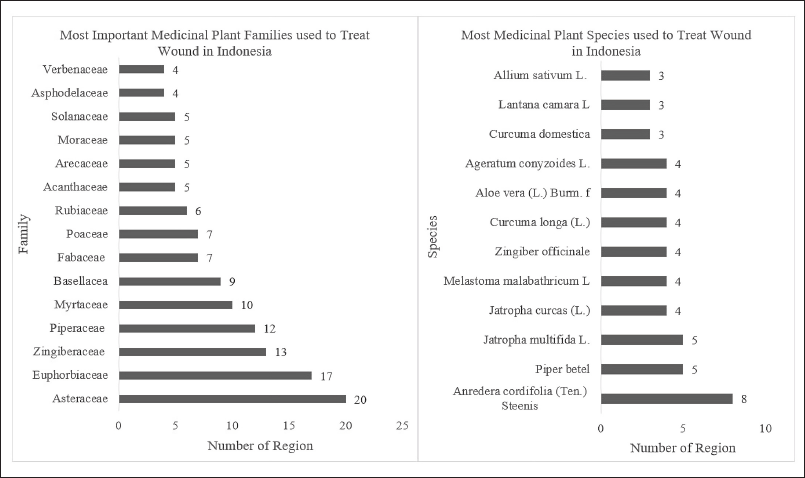 | Figure 3. Important medicinal plant families (a) and species used as wound healing in Indonesia (b). [Click here to view] |
Plants in the Euphorbia family were the second most used for wound healing. These plants are often found in cultivated and wild areas and are used for medicinal purposes. Most Indonesians have traditionally used Anredera cordifolia and Curcuma longa for their wound-healing properties (Fig. 3b). These plants could serve as reliable resources, and several studies have consequently been conducted on these plants to explore their biological activities and phytochemical compositions [49].
Leaves are the most commonly reported plant parts used in herbal medicine preparations in Indonesia (Fig. 4) [34] and are renowned for their suitability and durability in developing medicinal treatments [49,50]. Durability reflects the survival rate of therapeutic plants following harvesting, where taking a reasonable amount of leaf biomass does not harm the plant, in contrast to harvesting the stem, root, or entire plant, which can endanger the plant [51]. Moreover, many studies have demonstrated that leaves harbor many plant secondary metabolites, such as alkaloids, glycosides, and essential oils, which are responsible for their therapeutic qualities. In addition, leaves serve as the focal point for phytochemical reactions and as storage areas for organic matter [49]. Conversely, using stems or roots leads to the need for more sustainable use of plants, mainly when extracted from their natural habitats [51].
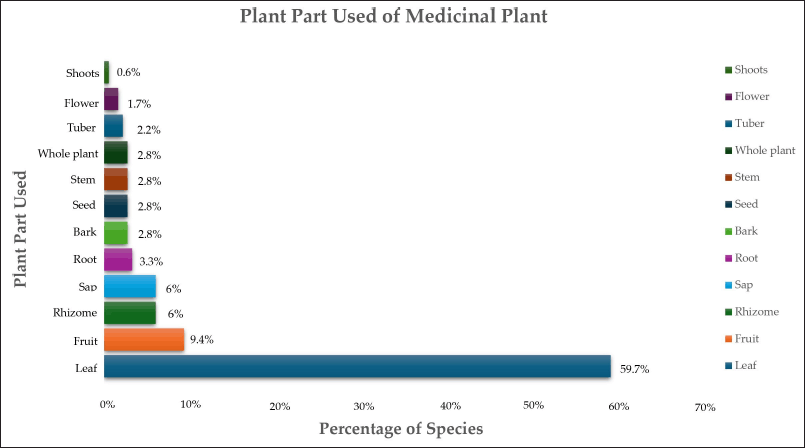 | Figure 4. Percentage of medicinal plant parts used for herbal preparation in Indonesia. [Click here to view] |
Plant components are subjected to various preparation methods before being used in herbal remedies. The predominant method of preparation involves crushing (49%), followed by drying (14%), powdering (14%), smearing (13%), and decoction (13%). Significant contributors used in the overall manner of preparation were found to be pasta (4%), raw material (4%), oils (2%), expressed juices (1%), and squeezed plant parts (1%) (Fig. 5). Several studies have yielded comparable findings, indicating that decoction is the most prevalent preparation technique [50,52] primarily because of its simplicity, ease of use, and cost-effectiveness. Moreover, additional research has demonstrated that the decoction process can enhance the effectiveness of extracting plant compounds, thereby increasing their bioactivity [53].
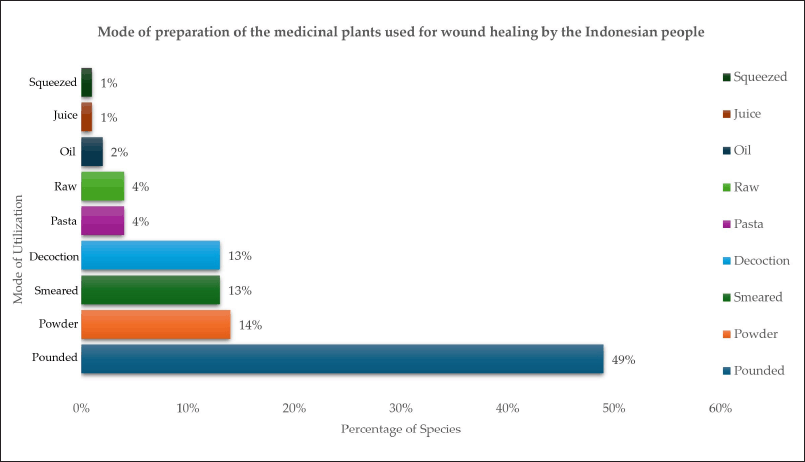 | Figure 5. Preparation of herbal drugs for wound healing by the Indonesians. [Click here to view] |
Bioactivity evidence
Figure 3b depicts the 11 most used Indonesian plants for wound treatment, some of these species, such as Allium sativum L., Melastoma malabathricum L., Piper betle L., C. longa L., and Zingiber officinale L., are indigenous to Indonesia and Southeast Asia [54–58]. The others are American natives, Jatropha multifida L. (Central and South America) [59], Jatropha curcas L. (Mexico to Central America) [60], A. cordifolia (Ten.) Steenis. (South America) [61], Lantana camara L. (Tropical America) [62], and A. conyzoides (Tropical America) [63]. Aloe vera (L.) Burm, on the other hand, is indigenous to the Mediterranean [64].
Ageratum conyzoides L.
Ageratum conyzoides L., often known as bandotan in Indonesia, is a herbaceous plant that thrives in many subtropical and tropical regions [65]. Ageratum conyzoides is an annual herb widely used as a traditional medicine in many countries worldwide. This specific plant has long been acknowledged for its medicinal qualities in treating minor injuries, burns, and infections through anti-inflammatory and hemostatic effects and numerous skin disorders [66]. Debbarma et al. [67] demonstrated that a leaf paste can treat cuts and wounds. The efficacy of petroleum ether, chloroform, methanol, and aqueous extracts of A. conyzoides was investigated using excision, incision, and dead space wound models, with methanol and aqueous extracts demonstrating significant wound-healing activity [68]. As shown in Figure 6, A. conyzoides extract can enhance cellular proliferation and collagen synthesis. Wounds treated with A. conyzoides extract healed faster as shown by higher rates of epithelialization, wound contraction, and histological results. Furthermore, this enhanced hexosamine- and uronic acid-induced levels of collagen synthesis after 8 days of treatment [69].
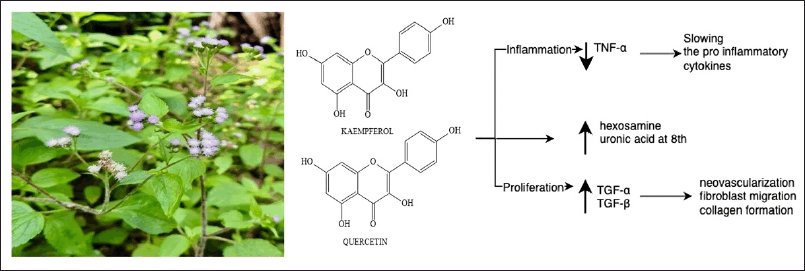 | Figure 6. Bioactive and pharmacological target of A. conyzoides L on wound healing. [Click here to view] |
Plant components such as saponins, phenolics, and flavonoids are believed to accelerate the wound-healing process [68]. The flavonoids in A. conyzoides L. leaves, including kaempferol and quercetin, have anti-inflammatory and antioxidant properties beneficial for wound healing. Furthermore, the alkaloid and saponin components of A. conyzoides are involved in wound-healing activity through fibroblast stimulation, anti-inflammatory effects, cell repair, and enhancing skin-cell strength [70]. During the inflammatory phase, A. conyzoides inhibits TNF-α production in wounded tissue while activating growth factors and cells to promote healing, slowing the release of proinflammatory cytokines, and increasing the production of anti-inflammatory cytokines. The aqueous extract of A. conyzoides enhances the synthesis of growth factors such as TGF-α and TGF-β at the wound site. TGF-α subsequently stimulates neovascularization, fibroblast migration, and collagen formation [71].
Allium sativum L.
Allium sativum L. (garlic) is a common edible and traditional plant used to prevent and treat various diseases and is referred to as bawang putih in Indonesia (Fig. 7). Allium sativum is a member of the Liliaceae family and contains a wide range of natural compounds, including organosulfur, amino acids, selenium derivatives, saponins, phenylpropanoids, flavonoids, alkaloids, fatty acids, and sterols [72,73]. Allium sativum L. has a substantial antibacterial effect on bacteria that cause skin infections and has broad antibacterial activity against gram-negative and gram-positive bacteria such as Staphylococcus aureus, Escherichia coli, Bacillus subtilis, and Mycobacterium, Pseudomonas, Klebsiella, Salmonella, Proteus, Micrococcus, and Clostridium species [73,74]. Allium extract had a lower minimum inhibitory concentration (MIC) than honey. Combined in treating burns, the allium and honey mixture displayed shorter epithelialization and wound contraction times than A. sativum extract alone [75].
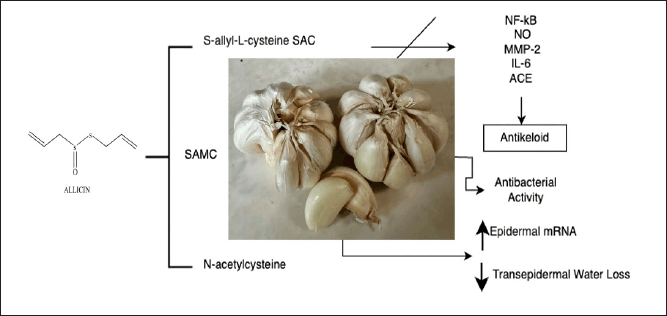 | Figure 7. Bioactive and pharmacological target of A. sativum L on wound healing. [Click here to view] |
Organic sulfurs such as S-allyl-L-cysteine (SAC), S-allylmercaptocysteine (SAMC), and N-acetylcysteine are significant bioactive compounds derived from the alliin compound. SAC has anti-inflammatory, antiapoptotic, and antioxidant properties, whereas SAMC has anticancer properties [76]. Reducing the size of keloids is an aim of wound healing. Allium sativum extract has been shown to inhibit the production of nuclear factor-kB (NF-kB), nitric oxide (NO), MMP-2, IL-6, and angiotensin-converting enzyme and, therefore, is a potentially effective keloid treatment [76,77]. In treating full-thickness excisional wounds, an allium ointment was used to reduce wounds twice as effectively as the vehicle, leaving more visually appealing scars [78]. In another study, thiosulfinate-enriched A. sativum extract upregulated the expression of epidermal mRNA, 3 days after wounding and reduced transepidermal water loss on day 8 [79].
Aloe vera (L.) Burm.f
Aloe vera (L.) Burm. f, also called lidah buaya, is a long-established medicinal plant belonging to the Liliaceae family. Both leaves and gel are frequently employed to treat wounds and are particularly effective in treating skin conditions such as burns, infections, and diabetic dermal wounds by accelerating wound healing due to their antibacterial and antioxidant characteristics. Aloe vera is more effective better than regular antibiotics in killing gram-positive bacteria such as S. aureus, S. epidermidis, and S. pyogenes and gram-negative bacteria P. aeruginosa associated with skin wounds, burns, and acne [50,52]. Aloe vera extracts contain ascorbate, vitamins A, C, and E, phenolic compounds, anthraquinones, and other chemical components that have potent antioxidant properties that effectively decrease lipid oxidation due to their synergistic effect [80].
Moreover, the most prevalent components in A. vera gel are aloin, emodin, and ß-sitosterol, which are considered to have significant bioactivities in treating wound healing (Fig. 8) [81]. In addition, A. vera effects different phases of the wound-healing process [82]. Aloe vera gel enhances the proliferation and migration of fibroblast cells during inflammation and proliferation and the production of ECM components, such as fibronectin, proteoglycans, and collagen, leading to an accelerated proliferative phase and expedited wound healing. During the remodeling and maturation phase of wound healing, A. vera treatment was shown to significantly increase the number of fibrocytes and enhance the density of collagen mass in a more structured manner, which can reduce the size of scar tissue and optimize biomechanical function [83].
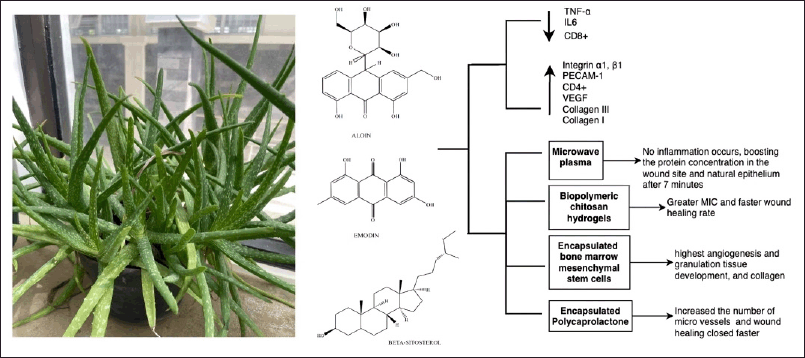 | Figure 8. Bioactive and pharmacological target of A. vera (L.) Burm. f on wound healing. [Click here to view] |
In vivo investigations show that A. vera cream decreases TNF-α and IL6 levels while increasing the ratio of CD4+ and CD8+ lymphocytes in the wound region [84]. Numerous studies have created drug-delivery systems (DDSs) for A. vera to treat wound healing. Activating A. vera with microwave plasma produced a noninflammatory treatment that increased the protein concentration in the wound site and natural epithelium after 7 minutes of exposure in an incision albino mice wound [85]. The combination of A. vera and biopolymers chitosan hydrogels produced a high MIC and a rapid rate of wound healing at 3, 7, and 14 days [81,86]. The highest levels of angiogenesis and granulation tissue growth were observed when A. vera-encapsulated mesenchymal stem cells from bone marrow were administered to patients with burns. In addition, an increased expression of collagen III and I and VEGF peaked on day 14 of treatment [81]. Furthermore, microvessels increased when a full-thickness excisional wound was treated with A. vera–encapsulated polycaprolactone (PCL) gel, producing faster wound healing than that observed with PCL or A. vera alone [87].
An in vitro study revealed that A. vera enhanced TGF-β1 and b-fibroblast growth factor (bFGF) expression in mouse embryonic fibroblasts [88]. Adding A. vera gel to fibroblasts and endothelial cells also increased the expression of integrin α1, β1, and platelet endothelial cell adhesion molecule (PECAM-1) genes [89].
Anredera cordifolia (Ten.) Steenis
Anredera cordifolia (Ten.) Steenis belongs to the genus Anredera juss. The plant is commonly referred to as the Madeira vine and, in Indonesia, is known as binahong or pinahong. Anredera cordifolia is commonly and extensively used in traditional wound treatment and healing practices and is readily available due to its cultivation in Indonesia [90]. The flavonoids in A. cordifolia leaves exhibit broad-spectrum antibacterial properties, and the plant possesses antioxidant and anti-inflammatory characteristics (Fig. 9) [91].
 | Figure 9. Bioactive and pharmacological target of A. cordifolia (Ten.) Steenis on wound healing. [Click here to view] |
The leaves of A. cordifolia are effective against black-pigmented bacteria, specifically Porphyromonas gingivalis and Prevotella intermedia, which cause chronic periodontitis [92]. Anredera cordifolia leaf-based soap exhibits antibacterial properties against S. aureus and E. coli. An extensive study conducted in Indonesia on the therapeutic properties of A. cordifolia for treating wounds showed that A. cordifolia extract and fraction facilitates wound healing by diminishing inflammation, enhancing tissue regeneration, and stimulating collagen and hydroxyproline production in incisions, excisions, burns, and chronic wounds in diabetes patients [93–95]. Binahong leaf extract has also been found to prevent the activation of inflammatory mediators, such as TNF-α, IL-1, and IL-6 [96,97].
Anredera cordifolia contains saponin, tannin, triterpenoid, alkaloid, flavonoid, phenolic, steroid, glycoside, oleanolic acid, protein, β-sitosterol, ursolic acid, and ascorbic acid [93,96,98]. Ursolic acid, used to standardize the ethanolic extract of A. cordifolia, is most abundant in the leaves and interferes in wound healing by inhibiting phospholipase in the prostaglandin synthase cascade. Ursolic acid also increases the expression of peroxisome proliferator-activated receptor-α, which drives epidermis development in the second step of the wound-healing process [99].
Curcuma longa L.
Turmeric, in Indonesia, known as kunyit (C. longa L.), is a rhizomatous perennial herb in the Zingiberaceae family [100,101]. Turmeric has been clinically confirmed to have pharmacological qualities, including antioxidant, anti-inflammatory, and chemoprotective effects (Fig. 10). Turmeric rhizomes contain various bioactive compounds, such as curcumin, turmerone, zingiberene, phellandrene, sabinene, borneol, and cineol, but curcuminoids have received the most attention [102]. The anti-inflammatory, protective, and antioxidant qualities of turmeric offer excellent potential for research on wound healing [103].
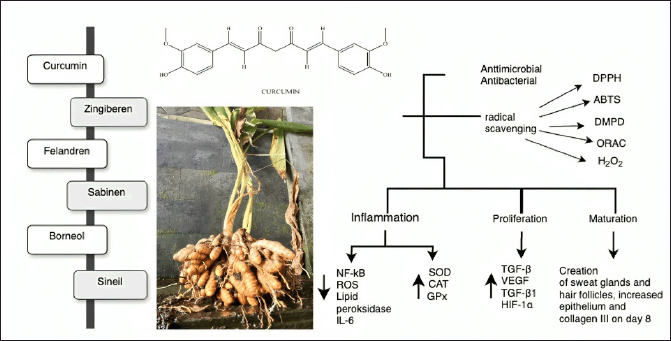 | Figure 10. Bioactive and pharmacological target of C. longa L on wound healing. [Click here to view] |
Curcumin exhibits radical scavenging activity, as assessed using 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), N,N-dimethyl-p-phenylenediamine (DMPD), oxygen radical absorbance capacity (ORAC), and hydrogen peroxide scavenging assays. Turmeric can also chelate ferrous ions, lowering their availability to bacteria. Compared with tocopherol, curcumin more significantly inhibits lipid peroxidation [104,105]. The antimicrobial properties of curcumin are crucial in promoting the rapid healing of wounds [106,107]. Curcumin stops bacteria from growing in several ways, such as preventing the formation of biofilms and attachment of bacteria to host receptors. Curcumin also decreases bacterial virulence factors and damages bacterial DNA, proteins, cell walls, cell membranes, and other parts of bacterial cells [108].
In an in vivo experiment, the topical application of curcumin to a burn site and incision wound stimulated the formation of new blood vessels (angiogenesis), deposition of collagen, formation of granulation tissue, and levels of hydroxyproline to accelerate wound healing in less than 14 days [101,107,109]. A previous study demonstrated that curcumin-coated silver nanoparticles (AgNPs) improved wound healing by enhancing the proliferation of L929 fibroblasts and expediting the restoration of the epithelial layer [106]. Applying a curcumin-loaded chitosan/alginate sponge increased wound contraction by 90% in the first 12 days after injury compared with a wound contraction rate of 74% in the control group [110].
Curcumin affects wound healing in multiple phases. During the inflammatory phase, curcumin may inhibit the NF-kB transcription factor, remove reactive oxygen species (ROS), inhibit lipid peroxidase, reduce the amount of IL-6 produced by macrophages, and increase the levels of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) [105,111]. During the proliferation phase, the curcumin-treated wounds showed increased TGF-β levels, which improved fibroblast proliferation and granulation tissue development with increased VEGF, TGF-β1, and hypoxia-inducible factor (HIF-1α) expression in neovasculogenesis. The remodeling phase was characterized by the development of sweat glands and hair follicles, a fully regenerated epithelial layer, and higher concentrations of type III collagen than type I collagen after 8 days of treatment [103,111].
Jatropha multifida L.
Asia and Africa are home to extensive cultivation of J. multifida L., a member of the Euphorbiaceae family. Jatropha multifida, commonly known as jarak tintir, is underused, especially in Indonesia. This plant can react to climatic conditions, adjust to agroecological environments, and has accumulated various genetic variants. Jatropha multifida has multiple therapeutic properties, including antimicrobial, antifungal, antibacterial, hypoallergenic, cytotoxic, and antihypertensive activities and can be used as a medication to combat infections and promote wound healing (Fig. 11) [112].
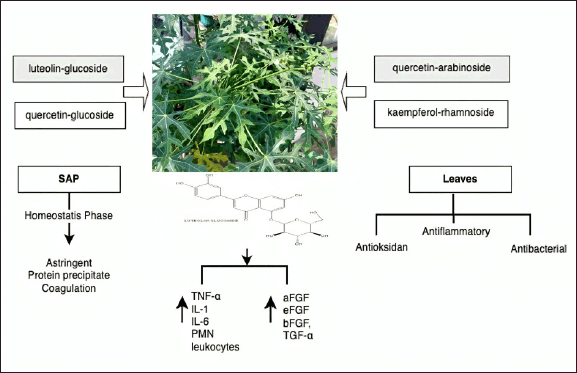 | Figure 11. Bioactive and pharmacological target of J. multifida L on wound healing. [Click here to view] |
In a previous study, J. multifida sap healed traumatic ulcers in rats when the sap was applied using an iota carrageenan poly (vinyl alcohol) hydrogel film, considerably enhancing wound healing, resulting in a 98% healing rate by day 10 of treatment [113]. Jatropha multifida sap also influenced the hemostasis phase by precipitating proteins through its astringent ability and affecting the cascade response of coagulation in the wound-healing process [114]. Jatropha multifida leaf extracts exhibit antioxidant, anti-inflammatory activities, and antibacterial activity. The mentioned activity is attributable to chemicals that were identified via liquid chromatography-mass spectrometry–electrospray ionization analysis and include luteolin glucoside, quercetin glucoside, quercetin arabinoside, and kaempferol rhamnoside. Interestingly, luteolin rhamnoside was the most abundant constituent in the J. multifida leaf extracts, with a concentration of 19.73 mg/gv [115].
Several authors have reported that luteolin in J. multifida extracts can regulate the production of proinflammatory cytokines, such as TNF-α, IL-1, and IL-6 [115,116]. The J. multifida–induced wound healing has also been reported to increase the levels of acidic fibroblast growth factor, epidermal fibroblast growth factor, bFGF, TGF-α, and TGF-β and reduce those of polymorphonuclear leukocytes [115,117,118].
Jatropha curcas L.
Jatropha curcas L., known as Jarak Pagar in Indonesia, is a highly adaptable plant with potential for medicinal uses. More than 170 officially identified species are included in the Jatropha genus [119]. Jatropha curcas is a small, dense shrub or diminutive tree that grows to 3–5 m but can reach 10 m under ideal conditions and flourishes at 15°C–40°C [120]. Sesquiterpenoids, triterpenes, alkaloids, flavonoids, phenolics, phytosterols, coumarins, neolignan, and lignans are among the many compounds found in J. curcas [121].
The phytochemical components of J. curcas exhibit numerous pharmacological activities, such as anti-inflammatory, antibacterial, antioxidant, anticancer, antiviral, antidiabetic, analgesic, hepatoprotective, anticoagulant, antifertility, and wound-healing properties (Fig. 12). All parts of J. curcas have been used for both traditional medicine and veterinary uses. The leaves, barks, and latexes are used to treat wounds, refractory ulcers, septic gums, and as a styptic in cuts and bruises [122,123].
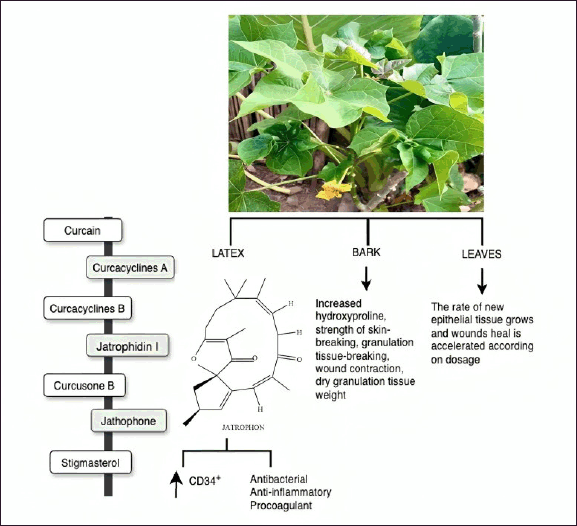 | Figure 12. Bioactive and pharmacological target of J. curcas on wound healing. [Click here to view] |
Applying an ointment containing a methanolic extract from the bark of J. curcas produced an increase in total protein and collagen content at the wound site, as shown by the hydroxyproline content of granulation tissues [122]. Furthermore, the extract enhanced wound contraction, granulation tissue formation, skin integrity, and the weight of dry granulation tissue, all of which contributed to improved healing processes [124]. Esimone et al. [125] assessed the effectiveness of a herbal ointment containing a methanolic extract derived from J. curcas leaves. Adding the extract to an ointment accelerated wound healing and reduced the time required to develop new epithelial tissue.
Curcain, curcacycline A and B, and jatrophidin I are the most common isolated compounds found in J. curcas latex [126,127], along with curcusone B, jatrophone (diterpene), and stigmasterol (triterpene) [128]. These compounds have therapeutic qualities that are useful for wound healing, such as anticancer, antioxidant, antibacterial, antiviral, antinematode, anti-inflammatory, procoagulant, and anticoagulant effects [126]. In vitro studies have shown that the J. curcas latex spray improved wound healing in human keratinocyte (HaCaT) and human fibroblast (BJ) cell lines as assessed using the scratch test. Indeed, wound healing was faster in formula-treated cells than with the positive control; moreover, the J. curcas spray and the biomarker compound, namely curacilyn, have similar antioxidant and wound-healing activities. Balqis et al. [123] observed a notable increase in the quantity of circulating CD34+ cells with a J. curcas treatment than that in the positive control at day 7 in their investigation of the angiogenesis activity of J. curcas latex in cream.
Lantana camara L.
Lantana camara (Verbenaceae family), often known as red/ wild sage, grows at 2,000 m and is a dominant and invasive plant in temperate, tropical, and subtropical climates. Lantana camara is now found in more than 60 countries in Central and South America, Asia, Africa, and Australia. Lantana camara, or tembelekan in Indonesia [129], is employed in herbal medicine to relieve skin itching, as an antiseptic for wounds, and externally to treat leprosy and scabies [130]. Place freshly crushed fruits and leaves directly into the affected area to treat wounds. Indigenous tribes have also traditionally used the fresh leaves of L. camara for measles [131].
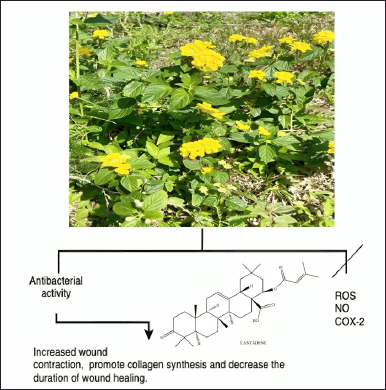
The finely crushed leaves of L. camara are used directly on the skin as a compress to heal cuts, wounds, and swelling in a specific area. An alternative method involves using powdered leaves and coconut oil to treat skin conditions, whereas the whole plant is usually used for herbal treatments (Fig. 13) [132]. Applying the L. camara extract significantly accelerated wound contraction (98%), encouraged collagen synthesis, and shortened the average healing time. Lantana camara was also shown to demonstrate potential as a medication in healing skin injuries, as shown by the effective repair of excision wounds in laboratory animals [130,133]. Lantana camara contains triterpenes lantadene A, reduced lantadene A, and lantadene B, which have antibacterial activity against S. aureus and Salmonella typhi. The antibacterial activity of the extract could explain its wound-healing activity in terms of decreasing the wound area in the excision wound model [130]. In vitro studies, molecular docking, and ADMET prediction demonstrated that the terpenes in L. camara decrease ROS, NO, and COX-2 levels, which are essential in reducing inflammation during the wound-healing phase [134].
 | Figure 13. Bioactive and pharmacological target of L camara on wound healing. [Click here to view] |
Melastoma malabathricum L.
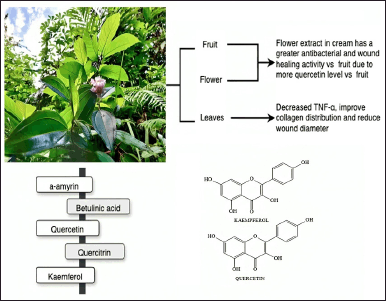
Melastoma malabathricum L. (Melastomataceae) is an indigenous plant species found in tropical and subtropical regions called Senduduk in Indonesia. The Melastoma genus contains approximately 4,000 species of this plant that are globally distributed. The Southeast Asian region has 22 species, two subspecies, and three variants under the Melastoma genus. Various uses of M. malabathricum have been recorded in traditional medicine, although clinical trials are lacking [56]. In Malay, Indian, and Indonesian traditional medicines, various plant components, such as leaves, roots, and barks, are used for treating illnesses and conditions such as diarrhea, dysentery, leucorrhea, hemorrhoids, cuts and wounds, postpartum infections, toothache, stomachache, flatulence, sore legs, and thrush [135,136].
Melastoma malabathricum, as shown in Figure 14, contains phytochemical compounds such as flavonoids, flavan-3-ols, triterpenes, tannins, anthocyanins, saponins, alkaloids, steroids, glycosides, phenolics, ursolic acid, malvidin-3,5-diglucoside, β-sitosterol, and β-sitosterol-3-oβ-dglucopyranoside [56]. The administration of M. malabathricum flower and fruit extract showed antibacterial and wound-healing activity due to the high quercetin content, which has a high antibacterial and wound-healing effect [137]. Anbu Jeba Sunilson et al. [138] investigated the wound-healing characteristics of M. malabathricum leaf ointment extract in excision and incision wound models in rats. Melastoma malabathricum improved wound contraction in excision wounds, promoting rapid closure, increasing wound strength, and facilitating tissue regeneration in 18 ± 2 days, whereas incision wounds showed significantly improved tensile strength after 10 days.
 | Figure 14. Bioactive and pharmacological target of M. malabathricum L. on wound healing. [Click here to view |
A combination of M. malabathricum and Psidium guajava leaf extract decreased blood glucose levels and wound area in rat feet [139]. Forming these extracts into a gel reduced TNF-α levels, wound width, and enhanced collagen distribution [140]. The inhibitory effects of M. malabathricum compounds on platelet-activating factor receptor binding with rabbit platelets revealed that a-amyrin and betulinic acid had considerable inhibitory effects of 67.3% and 64.3%, respectively. Furthermore, quercetin and quercitrin appeared to have moderate inhibitory action, with 57.% and 45.4% inhibition percentages, respectively [141].
Thin-layer chromatography was used to analyze extracts of M. malabathricum from various geographical regions in Indonesia, and it showed that the predominant ingredients were phenolic compounds, such as quercetin, quercitrin, and kaempferol. An acetone extract included several tannins, including strictinin and casuarinin. Quercetin and quercitrin were revealed as possible antioxidant agents, while kaempferol was found to have wound-healing effects for diabetes excisional and nondiabetic incisional wounds [142].
Piper betle L
Piper betle L., known as Sirih in Indonesia, is a widely recognized perennial trailing plant in the Piperaceae family. Indigenous to central and eastern Peninsular Malaysia, it is distributed across East Africa and many tropical Asian countries. Piper betle has been extensively studied for its diverse phytochemical constituents, exhibiting significant biological activity. The essential oil extracted from betel leaves primarily contains phenols and terpenes. Preliminary phytochemical analysis of aqueous and methanol extracts from betel leaves has identified the presence of alkaloids, flavonoids, tannins, sterols, phenols, glycosides, saponins, and terpenoids [143].
The phytochemical compounds present in P. betle (Fig. 15) have various pharmacological activities, such as anticancer, antitumor, and antiproliferative effects, as well as analgesic, anti-inflammatory, antinociceptive, antidepressant, antianxiety, anticholinesterase, anti-Alzheimer, hepatoprotective, antioxidant, immunomodulatory, cardioprotective, and wound-healing properties [143]. Limited documentation is available on the wound-healing effects of P. betle. Several studies have reported that extracts of P. betle stimulate the growth of fibroblasts, reduce the activity of 11β hydroxysteroid dehydrogenase-1, enhance collagen production, prevent the formation of ROS, and encourage the proliferation of NIH-3T3 cells [144,145]. An ethanolic extract of P. betle leaves was shown to contain hydroxychavicol, eugenol, safrole, caryophyllene, caryophyllene oxide, silicone oil, campestral, stigmasterol, vitamin E, and sitosterol, with the significant component being eugenol, which is responsible for the flavor and aroma of the leaves [146]. Essential oils obtained from plants are frequently applied to treat wounds. Eugenol is recognized for having antimicrobial properties due to its distinctive anti-inflammatory, antimicrobial, antifungal, antioxidant, antiseptic, and antiallergic characteristics [147]. Combining eugenol-loaded nanogel and PCL/C nanofiber-enhanced wound healing [148]. Eugenol-loaded cellulose–chitosan hydrogels promote fibroblast cell motility. In vitro scratch tests showed that this treatment lowered cPGES expression, limiting cell inflammatory responses and enhancing VEGF expression, which is responsible for a cascade of events involving angiogenesis and wound-healing processes [149].
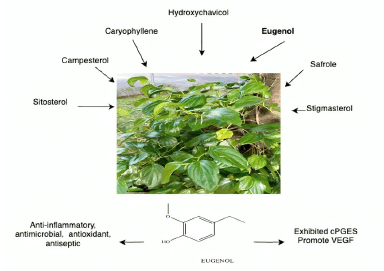 | Figure 15. Bioactive and pharmacological target of P. betle L. on wound healing. [Click here to view] |
Zingiber officinale L.
Zingiber officinale (ginger) is a spice, herb, and edible plant known as Jahe in Indonesia. Zingiber officinale is an herbaceous perennial represented by over 70 species endemic to Southeast Asia and develops tall (1 m) annual pseudostems (false stems composed of coiled leaves) with narrow leaf blades. Flowers with pale yellow petals and purple margins emerge directly from the rhizome on independent stalks in the inflorescences [150]. The rhizome of Z. officinale contains zingerone, gingerols, shogaol, flavonoids, diterpenoid, and sesquiterpenoids, of which zingerone, shogaol, and gingerols are the most potent substances with gingerols and shogaol considered to be responsible for the anti-inflammatory properties of ginger (Fig. 16). The main pharmacologically active compound found in ginger is 6-gingerol [151,152].
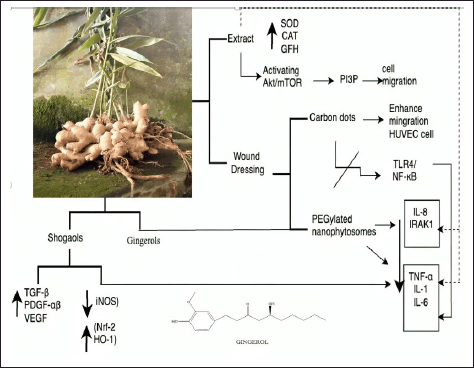 | Figure 16. Bioactive and pharmacological target of Z. officinale on wound healing. [Click here to view] |
Ginger extract has inhibitory effects on lipid peroxidation and enhances the intracellular functions of antioxidant enzymes, including SOD, CAT, and GPX. The ginger extract also improved the plasma antioxidant capacity and decreased lipid peroxidation in diabetic rats [153]. Ginger extract influences cell migration and heat tolerance in mouse fibroblast cells, phosphorylate protein kinase B (AKT) and mTOR, and activates the Akt/mTOR-signaling pathway. Ginger extract also increased phosphatidylinositol 3-phosphate levels in cells, which regulated cell morphology and stimulated cell migration [154]. Ginger extract–loaded hydrogel films significantly increased fibroblast proliferation, granulation tissue formation, collagen remodeling, and reepithelialization after 16 days of treatment [155].
To improve the practical use of ginger extract for clinical application, this has been incorporated into various DDSs. Zingiber officinale carbon dots can prevent the overexpression of proinflammatory mediators such as TNF-α, IL-1β, IL-6, and NO by blocking the phosphorylation pathway of TLR4/NF-κB in RAW264.7 cells, as well as increasing the migratory capacity of HUVEC cells [156]. The crude extract and 6-gingerol, as well as PEGylated nanophytosomes loaded with 6-gingerol, had a significant effect on downregulating TNF-α, IL-1β, IL-6, IL-8, and IRAK1 inflammation factors in MDA-MB-231 breast cancer cell lines cells. In addition, they improved the ability of human periodontal fibroblast cells to heal wounds [152].
Additional in vitro research has shown that an active compound in ginger, 10-shogaol, stimulated the proliferation of human dermal fibroblasts and normal epidermal keratinocytes and increased the production of TGF-β, PDGF-αβ, and VEGF growth factors in both cell lines; furthermore, in 12–24 hours, the fibroblasts and keratinocytes moved more quickly than in the vehicle control group in the wound-healing assay [139,157]. Poly(lactic-co-glycolic acid) PLGA/PLA-polyethylene glycol-folic acid-PEG-FA nanoparticles loaded with 6-shogaol (NPs-PEG-FA/6-shogaol) accelerated wound repair of colitis in DDS-treated mice, by regulating the expression levels of proinflammatory TNF-a, IL-6, IL-1b, and iNOS and anti-inflammatory Nrf-2 and HO-1 factors [158].
Herbal active compounds and molecular targets on wound healing
Research on wound healing now primarily addresses chronic or severe wounds, such as diabetic ulcers, burns, deep wounds, and infected wounds, which require extended time and substantial costs for healing. Several bioactive studies have explored the wound-healing potential of eleven medicinal plants traditionally used in Indonesia. These studies have investigated crude extracts, fractionations, and active compounds in various formulations, including simple ointments, nanoparticle formulations, AgNPs, electrospun nanofibers, and hydrogel films.
Although in vitro or in vivo tests have yielded experimental data for each listed plant, not every mechanism of action has been confirmed. Nevertheless, well-characterized compounds that have been demonstrated to have properties that promote wound healing include luteolin, quercetin, kaempferol [71,115], allicin [76], aloin, emodin, and b-sitosterol [82], ursolic acid [99], a-amyrin, betulinic, quercetin, quercitrin [141], curcumin [103], curcain, curcacycline A and B, jatrophidin, curcusone B, jatrophone, stigmasterol [126–128], lantadene [130] eugenol [147], and gingerols and shogaol [151,152].
Usually, each wound heals by a process that includes hemostasis, inflammation, proliferation, maturation, and remodeling. Plants used in traditional medicine share many of the same biological targets and pathways critical in the mammalian wound-healing cascade. The inflammation and proliferation phases are essential components of wound healing. Medicinal plants can target mitogenic pathways such as AKT, phosphoinositide 3-kinase, and smooth muscle actin (SMA) [102], as well as the proinflammatory NF-κB pathway [152], which involves caspases, ILs, TNF-α, TGF-β1, and cyclins [88,159]. The angiogenesis pathway includes VEGF, the ECM synthesis includes MMPs, and differentiation pathways include α-SMA [92,98].
Full-thickness, burn, diabetic, and infection wounds are wound models representing chronic wounds characterized by high levels of proinflammatory mediators and infection. Targets in inflammatory phases have been discovered, including CD8+ lymphocytes, ROS, IL-1, TNF-α, IL-6, IL-10, COX-1, and COX-2, and cells that function inside the wound tissue, such as macrophages-1, neutrophils, and platelets [160]. The content of herbal antioxidants can reduce the duration of the inflammatory phase in chronic wounds. Furthermore, antibacterial activities to the most common bacteria found in skin wound infections, such as S. aureus, help synergize the wound-healing activity in this phase [107,161].
Antioxidants from flavonoids and terpenes suppress the action of proteases and ROS formed from the accumulation of neutrophils in the wound area. They also protect SOD, CAT, and GPx from oxidative damage [162]. At the transition from the inflammatory phase to the proliferation phase, T lymphocytes, particularly CD4+ cells, play a crucial role in wound healing by secreting lymphokines that stimulate fibroblasts, which act as anti-inflammatory agents, and activate factors in major histocompatibility complex class II [84].
Angiogenesis occurs in the proliferation phase to compensate for vascular injury and accelerate the healing of wounds. CD31, also known as PECAM-1 and integrin 11, plays a role in endothelial cell migration and angiogenesis [84]. Together with growth factors such as VEGF, TGF-1, EFG, and HIF-1a, CD31 is involved in fibroblast proliferation and the formation of new blood vessels via angiogenesis [105,111,155]. Integrins have also been identified as essential in reepithelialization by enhancing keratinocyte proliferation and governing cell migration via the ECM.
Fibroblasts play a vital role in wound healing by preventing fibrin clot formation, producing the ECM, and building collagen structures. The migration and proliferation of fibroblasts in the wound area significantly impact the rate of wound healing. Collagen produced by fibroblasts binds the wound and influences the reepithelialization process, ultimately facilitating wound closure. During the final remodeling phase, collagen III is degraded and replaced by collagen I, whereas the epithelium is formed to complete the wound closure [8].
Strenght and limitations of the study
The ethnopharmacology and bioevidence of several medicinal plants in Indonesia for treating wound healing were clarified by this scoping review. This is the first and most recent scoping review regarding the active ingredient and molecular target of herbal plants used to treat wounds in Indonesia. In addition, it showed that most locals know the benefits of medicinal plants and commonly use traditional methods to treat wounds. It is important to note several limitations when presenting the review’s findings. The limitation is that while in vitro and in vivo studies have provided experimental data for each plant, the mechanisms of action for many of these plants remain unconfirmed. In addition, there are few clinical studies to support the findings from in vitro and in vivo preclinical investigations; third, there is a lack of toxicity and safety studies to confirm the efficacy of these treatments. Furthermore, most research omitted information about the formulation’s composition, standardization, and preparation methods.
CONCLUSION
The incidence of skin injuries continues to increase globally, leading to substantial annual financial costs. In response, we assessed Indonesia’s pharmacopeia and traditional medicine for plants recognized for their medicinal properties. Each botanical investigated contains chemical compounds linked to various pharmacological effects, including antimicrobial, anti-inflammatory, and antioxidant properties. In vitro and in vivo studies have demonstrated that medicinal plant extracts and their purified active components hold significant potential as wound-healing treatments due to their safety, diverse modes of action, and antibacterial activity. Novel wound-dressing formulations offer several advantages over standard dressings, addressing limitations of natural materials such as solubility and restricted activity at the wound site. Further studies should focus on elucidating the mechanisms of action of the most promising natural substances, particularly their effects on cell-surface receptors, to enhance their bioavailability and minimize adverse effects in wound healing.
ACKNOWLEDGMENTS
The authors are grateful to the Center for Education Financial Services (Puslapdik) and the Indonesian Endowment Funds for Education (LPDP) for their support through the Indonesian Educational Scholarship Program (Beasiswa Pendidikan Indonesia) as well as to the Faculty of Pharmacy, Gadjah Mada University, and Universitas Udayana.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This work was funded by the Directorate General of Higher Education, Research, and Technology, Ministry of Education, Culture, Research, and Technology, Republic of Indonesia for the PhD Grant (PDD) 2024, and the article processing charge was covered by the Center for Education Financial Services (Puslapdik) and Indonesian Endowment Funds for Education (LPDP), under number 01575/J5.2.3/BPI.06/9/2022.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Monika P, Chandraprabha MN, Rangarajan A, Waiker PV, Chidambara Murthy KN. Challenges in healing wound: role of complementary and alternative medicine. Front Nutr. 2022;8:791899. doi: CrossRef
2. Sharma A, Khanna S, Kaur G, Singh I. Medicinal plants and their components for wound healing applications. Futur J Pharm Sci. 2021;7:53. doi: CrossRef
3. Bahramsoltani R, Farzaei MH, Rahimi R. Medicinal plants and their natural components as future drugs for the treatment of burn wounds: an integrative review. Arch Dermatol Res. 2014;306:601–17. doi: CrossRef
4. Krzyszczyk P, Schloss R, Palmer A, Berthiaume F. The role of macrophages in acute and chronic wound healing and interventions to promote pro-wound healing phenotypes. Front Physiol. 2018;9:419. doi: CrossRef
5. Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound healing: a cellular perspective. Physiol Rev. 2019;99:665–706. doi: CrossRef
6. Velnar T, Bailey T, Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res. 2009;37:1528–42. doi: CrossRef
7. Kumar P, Kumar S, Udupa EP, Kumar U, Rao P, Honnegowda T. Role of angiogenesis and angiogenic factors in acute and chronic wound healing. Plast Aesthetic Res. 2015;2:243. doi: CrossRef
8. Desjardins-Park HE, Foster DS, Longaker MT. Fibroblasts and wound healing: an update. Regen Med. 2018;13:491–5. doi: CrossRef
9. Yadav S, Mishra AP, Kumar S, Negi A, Maurya VK. Herbal wound healing agents. In: Preparation of phytopharmaceuticals for the management of disorders. Academic Press; 2021 Jan 1. pp. 169–84. doi: CrossRef
10. Swastini DA, Martien R, Fachiroh J, Endro Nugroho A. Bibliometric analysis of Basella ssp. as an antioxidant. BIO Web Conf. 2023;75:1–8. doi: CrossRef
11. Nugroho AE, Anas Y, Arsito PN, Wibowo JT, Riyanto S, Sukari MA. Effects of marmin, a compound isolated from Aegle marmelos Correa, on contraction of the guinea pig-isolated trachea. Pak J Pharm Sci. 2011;24:427–33.
12. Bueno FG, Panizzon GP, Mello EV, Lechtenberg M, Petereit F, de Mello JC, et al. Hydrolyzable tannins from hydroalcoholic extract from Poincianella pluviosa stem bark and its wound-healing properties: phytochemical investigations and influence on in vitro cell physiology of human keratinocytes and dermal fibroblasts. Fitoterapia. 2014;99:252–60. doi: CrossRef
13. Xie Z, Paras CB, Weng H, Punnakitikashem P, Su LC, Vu K, et al. Dual growth factor releasing multi-functional nanofibers for wound healing. Acta Biomater. 2013;9:9351–9. doi: CrossRef
14. Putra KDA, Pradnyaswari GAD, Yustiantara PS, Wirasuta IMAG, Setyawan EI. The effect of sucrose and yeast extract on total phenolic, flavonoid, and anthocyanin of lactic-acid-fermented mangosteen fruit peel (Garcinia mangostana L.). Turkish J Pharm Sci. 2024;21:211–8. doi: CrossRef
15. Kartawinata K. Dua Abad Mengungkap Kekayaan Flora dan Ekosistem Indonesia. Jakarta, Indonesia: LIPI; 2010. Vol. 20. doi: CrossRef
16. Riset Kesehatan Dasar (Riskesdas). Badan Penelitian dan Pengembangan Kesehatan Kementerian RI tahun 2018. [cited 2023 Jul 18]. Available from: http://www.depkes.go.id/resources/download/infoterkini/materi_rakorpop_20%0A18/Hasil Riskesdas 2018.pdf
17. Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8:19–32. doi: CrossRef
18. Levac D, Colquhoun H, O’Brien KK. Scoping studies: advancing the methodology. Implement Sci. 2010;5:69. doi: CrossRef
19. Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169:467–73. doi: CrossRef
20. Google Maps. Indonesia. 2023 [cited 2023 Jun 04]. Available from: https://library.lsbu.ac.uk/harvardreferencing/googlemaps
21. Navia ZI, Suwardi AB, Baihaqi. Ethnobotanical study of medicinal plants used by local communities in Sekerak subdistrict, Aceh Tamiang, indonesia. Biodiversitas. 2021;22:4273–81. doi: CrossRef
22. Navia ZI, Adnan A, Harmawan T, Suwardi AB. Ethnobotanical study of wild medicinal plants in Serbajadi protected forest of East Aceh District, Indonesia. Biodiversitas J Biol Divers. 2022;23:4959–70. doi: CrossRef
23. Elfrida, Tarigan NS, Suwardi AB. Ethnobotanical study of medicinal plants used by community in Jambur Labu village, East Aceh, Indonesia. Biodiversitas. 2021;22:2893–900. doi: CrossRef
24. Aththorick TA, Berutu L. Ethnobotanical study and phytochemical screening of medicinal plants on Karonese people from North Sumatra, Indonesia. J Phys Conf Ser. 2018;1116:52008. doi: CrossRef
25. Rambey R, Onrizal O. Ethnobotany of medicinal plants in Asam Jawa Village, South Labuhanbatu, North Sumatra, Indonesia. IOP Conf Ser Earth Environ Sci. 2022;977:012112. doi: CrossRef
26. Susandarini R, Khasanah U, Rosalia N. Ethnobotanical study of plants used as food and for maternal health care by the malays communities in Kampar Kiri Hulu, Riau, Indonesia. Biodiversitas. 2021;22:3111–20. doi: CrossRef
27. Hakim N, Wakhidah AZ. Ethnobotany of medicinal plants from Lampung Tribe around Way Kambas National Park, Indonesia. Nusant Biosci. 2022;14:84–94. doi: CrossRef
28. Putri LSE, Dasumiati D, Kristiyanto K, Assuyuti YM, Malik C, Leuvinadrie LP, et al. Ethnobotanical study of herbal medicine in Ranggawulung Urban Forest, Subang District, West Java, Indonesia. Biodiversitas. 2016;17:172–6. doi: CrossRef
29. Nahdi MS, Martiwi INA, Arsyah DC. The ethnobotany of medicinal plants in supporting the family health in Turgo, Yogyakarta, Indonesia. Biodiversitas. 2016;17:900–6. doi: CrossRef
30. Nahdi MS, Kurniawan AP. The diversity and ethnobotanical study of medicinal plants in the southern slope of Mount Merapi, Yogyakarta, Indonesia. Biodiversitas. 2019;20:2279–87. doi: CrossRef
31. Asyam Ammar L, Kurniawati B, Anggorowati D, Putri Cahyaningsih A, Dwi Setyawan A. Ethnobotanical study of the medicinal plant used by local communities in Karst area of Pacitan District, East Java, Indonesia. Int J Trop Drylands. 2021;5:4–93. doi: CrossRef
32. Nugraha AS, Agustina RP, Mirza S, Rani DM, Winarto NB, Triatmoko B, et al. Phytochemistry and pharmacology of medicinal plants used by the Tenggerese Society in Java Island of Indonesia. Molecules. 2022;27:7532. doi: CrossRef
33. Bhagawan WS, Suproborini A, Prastya Putri DL, Nurfatma A, Putra RT. Ethnomedicinal study, phytochemical characterization, and pharmacological confirmation of selected medicinal plant on the northern slope of Mount Wilis, East Java, Indonesia. Biodiversitas. 2022;23:4303–13. doi: CrossRef
34. Supiandi MI, Mahanal S, Zubaidah S, Julung H, Ege B. Ethnobotany of traditional medicinal plants used by dayak desa community in sintang, West Kalimantan, Indonesia. Biodiversitas. 2019;20:1264–70. doi: CrossRef
35. Arbiastutie Y, Diba F, Masriani. Ethnobotanical and ecological studies of medicinal plants in a mangrove forest in Mempawah district, west Kalimantan, Indonesia. Biodiversitas. 2021;22:3164–70. doi: CrossRef
36. Milad M, Fouad F, Reri Y, Rini D, Yusintha T. Ethnobotanical study of medicinal plants in Natai Sedawak village, Sukamara Regency, Central Kalimantan, Indonesia. Plant Sci Today. 2022;10(1):1–4. doi: CrossRef
37. Husaini IPA, Maulany RI, Nasri N, Ngakan PO. Diversity and use of traditional medicinal plant species in Bantimurung-Bulusaraung National Park, Indonesia. Biodiversitas. 2022;23:5539–50. doi: CrossRef
38. Fathurrahman F, Nursanto J, Madjid A, Ramadanil R. Ethnobotanical study of “Kaili Inde” tribe in Central Sulawesi Indonesia. Emirates J Food Agric. 2016;28:337–47. doi: CrossRef
39. Mallaleng H, Avanti C. An ethnobotanical survey of indigenous knowledge on medicinal plants used by traditional healers of the Warmasen area, Raja Ampat, East Papua. Int Referee J Eng Sci. 2022;11:1–7.
40. Nababan V, Hakim L. Ethnobotanical study of early childhood medicinal plants used by the local people in South Bangka Regency. Indones Biosaintifika J Biol Biol Educ. 2020;12:414–21. doi: CrossRef
41. Sari DP, Hakim L. Medicinal plants for traditional treatment used by the Malays in South Bangka Regency. Indones Biosaintifika J Biol Biol Educ. 2022;14:125–34. doi: CrossRef
42. Sri Andila P, Warseno T, Syafitri W, Gede Tirta I. Ethnobotanical study of Hindu Society in Tabanan Bali and The Conservation Efforts. Dordrecht, The Netherlands: Atlantis Press International B.V.; 2022.
43. Ratnani DAS, Ketut Junitha I, Kriswiyanti E, Budiningsih DN. The ethnobotany of sense disease medical plant used by Ngis Manggis community Karangasem in Bali, Indonesia. Int J Appl Sci Sustain Dev. 2022;4:102–11.
44. Trimanto, Danarto SA, Ashrafuzzaman M. Ethnobotanical uses of plants by Brangkuah Community of Moyo Island, West Nusa Tenggara, Indonesia. J Bangladesh Agric Univ. 2019;17:325–37. doi: CrossRef
45. Jefrianto Ade Mela Y, Juliyanti Bria E, Morina Yostianti Tnunay I. Ethnobotany of semi-arid medicinal plants used by Bunaq Tribe in Lamaknen, Belu District, East Nusa Tenggara, Indonesia. Int J Trop Drylands. 2022;6:16–25. doi: https://doi.org/10.13057/tropdrylands/t060103.
46. Purwanti E, Mahmudati N, Faradila SF, Fauzi A. Utilization of plants as traditional medicine for various diseases: ethnobotany study in SumeNep, Indonesia. AIP Conf Proc. 2020;2231:040024. doi: CrossRef
47. Yakub A, Setyo Leksono A, Batoro J. Ethnobotany of Medicinal and Edible Plants of Tobelo Dalam Tribe in Aketajawe Lolobata National Park Area. J-PAL. 2019;10:2087–3522. doi: CrossRef
48. Tjitrosoepomo G. Taksonomi Tumbuhan Spermatophyta. Yogyakarta, Indonesia: Gajah Mada University Press; 2010.
49. Zougagh S, Belghiti A, Rochd T, Zerdani I, Mouslim J. Medicinal and aromatic plants used in traditional treatment of the oral pathology: the ethnobotanical survey in the economic capital Casablanca, Morocco (North Africa). Nat Products Bioprospect. 2019;9:35–48. doi: CrossRef
50. Sauini T, da Fonseca-Kruel VS, Yazbek PB, Matta P, Cassas F, da Cruz C, et al. Participatory methods on the recording of traditional knowledge about medicinal plants in Atlantic forest, Ubatuba, São Paulo, Brazil. PLoS One. 2020;15:1–18. doi: CrossRef
51. Zheng XL, Xing FW. Ethnobotanical study on medicinal plants around Mt.Yinggeling, Hainan Island, China. J Ethnopharmacol. 2009;124:197–210. doi: CrossRef
52. Ahmed HM. Ethnopharmacobotanical study on the medicinal plants used by herbalists in Sulaymaniyah Province, Kurdistan, Iraq. J Ethnobiol Ethnomed. 2016;12:8. doi: CrossRef
53. Jadid N, Kurniawan E, Himayani CES, Andriyani, Prasetyowati I, Purwani KI, et al. An ethnobotanical study of medicinal plants used by the Tengger tribe in Ngadisari village, Indonesia. PLoS One. 2020;15:e0235886. doi: CrossRef
54. Siew YY, Zareisedehizadeh S, Seetoh WG, Neo SY, Tan CH, Koh HL. Ethnobotanical survey of usage of fresh medicinal plants in Singapore. J Ethnopharmacol. 2014;155:1450–66. doi: CrossRef
55. Manju K, Jayaraj J, Shanthi M. Efficacy of botanicals against pulse beetle Callosobruchus maculatus (F.) in green Gram. Indian J Entomol. 2019;81:144–7. doi: CrossRef
56. Joffry SM, Yob NJ, Rofiee MS, Affandi MMRMM, Suhaili Z, Othman F, et al. Melastoma malabathricum (L.) Smith ethnomedicinal uses, chemical constituents, and pharmacological properties: a review. Evidence-Based Complement Altern Med. 2012;2012:258434. doi: CrossRef
57. Rahmah NL, Kamal SMM, Sulaiman A, Taip FS, Siajam SI. Optimization of phenolic compounds and antioxidant extraction from Piper betle Linn. leaves using pressurized hot water. J Appl Sci Eng. 2023;26:175–84. doi: CrossRef
58. Sharifi-Rad J, Mnayer D, Tabanelli G, Stojanovic-Radic ZZ, Sharifi-Rad M, Yousaf Z, et al. Plants of the genus Allium as antibacterial agents: from tradition to pharmacy. Cell Mol Biol. 2016;62:57–68. doi: CrossRef
59. Kumar A, Tewari SK. Origin, distribution, ethnobotany and pharmacology of Jatropha curcas. Res J Med Plant. 2015;9:48–59. doi: CrossRef
60. Martínez-Díaz Y, González-Rodríguez A, Rico-Ponce HR, Rocha-Ramírez V, Ovando-Medina I, Espinosa-García FJ. Fatty acid diversity is not associated with neutral genetic diversity in native populations of the biodiesel plant Jatropha curcas L. Chem Biodivers. 2017;14:e1600188. doi: CrossRef
61. Boyne RL, Osunkoya OO, Scharaschkin T. Variation in leaf structure of the invasive Madeira vine (Anredera cordifolia, Basellaceae) at different light levels. Aust J Bot. 2013;61:412–7. doi: CrossRef
62. Goncalves E, Herrera I, Duarte M, Bustamante RO, Lampo M, Velásquez G, et al. Global invasion of Lantana camara: has the climatic niche been conserved across continents? PLoS One. 2014;9:e111468. doi: CrossRef
63. Kaur A, Kaur S, Singh HP, Datta A, Chauhan BS, Ullah H, et al. Ecology, biology, environmental impacts, and management of an agro-environmental weed Ageratum conyzoides. Plants. 2023;12:2329. doi: CrossRef
64. Martín-Viaña NDLP, Lacarrere IGM, Amaro LT. Technological development and stability study of an antiulcerous drug of natural origin. Rev Cuba Plantas Med. 2007;12:1–7.
65. Okunade AL. Ageratum conyzoides L. (Asteraceae). Fitoterapia. 2002;73:1–16. doi: CrossRef
66. Kamboj A, Saluja A. Ageratum conyzoides L.: a review on its phytochemical and pharmacological profile. Int J Green Pharm. 2008;2:59. doi: CrossRef
67. Debbarma M, Pala NA, Kumar M, Bussmann RW. Traditional knowledge of medicinal plants in tribes of Tripura in Northeast, India. Afr J Tradit Complement Altern Med AJTCAM. 2017;14:156–68. doi: CrossRef
68. Dash G, Pn M. Wound healing effects of Ageratum conyzoides Linn. Int J Pharma Bio Sci. 2011;2:369–83.
69. Arulprakash K, Murugan R, Ponrasu T, Iyappan K, Gayathri VS, Suguna L. Efficacy of Ageratum conyzoides on tissue repair and collagen formation in rats. Clin Exp Dermatol. 2012;37:418–24. doi: CrossRef
70. Sukmawan YP, Alifiar I, Nurdianti L, Ningsih WR. Wound healing effectivity of the ethanolic extracts of Ageratum conyzoides L. leaf (White and Purple Flower Type) and Centella asiatica and astaxanthin combination gel preparation in animal model. Turk J Pharm Sci. 2021;18:609–15. doi: CrossRef
71. Bafila CBDK, Andissa NO, Moussoungou ASU, Moulari B, Abena AA. In vivo and in vitro healing potential of aqueous extract ointment Ageratum conyzoïdes Linn. applied to excision and incision wounds induced in Wistar rat and human cells. Asian J Biomed Pharm Sci. 2020;10:29–37.
72. Kim JS, Kim JH. Updated molecular phylogenetic analysis, dating and biogeographical history of the lily family (Liliaceae: Liliales). Bot J Linn Soc. 2018;187:579–93. doi: CrossRef
73. Ahmed S, Fahim J, Abdelmohsen U. Chemical and biological studies on Allium sativum L. (1952-2020): a comprehensive review. J Adv Biomed Pharm Sci. 2022;5:1–22. doi: CrossRef
74. Harris JC, Cottrell SL, Plummer S, Lloyd D. Antimicrobial properties of Allium sativum (garlic). Appl Microbiol Biotechnol. 2001;57:282–6. doi: CrossRef
75. Ait Abderrahim L, Taïbi K, Ait Abderrahim N, Boussaid M, Rios-Navarro C, Ruiz-Saurí A. Euphorbia honey and garlic: biological activity and burn wound recovery. Burns. 2019;45:1695–706. doi: CrossRef
76. Sasi M, Kumar S, Kumar M, Thapa S, Prajapati U, Tak Y, et al. Garlic (Allium sativum L.) bioactives and its role in alleviating oral pathologies. Antioxidants. 2021;10:1847. doi: CrossRef
77. Pazyar N, Feily A. Garlic in dermatology. Dermatol Rep. 2011;3:e4. doi: CrossRef
78. Santiago JL, Galan-Moya EM, Muñoz-Rodriguez JR, de la Cruz-Morcillo MA, Redondo-Calvo FJ, Gracia-Fernandez I, et al. Topical applications of thiosulfinate-enriched Allium sativum extract accelerates acute cutaneous wound healing in murine model. Chin J Integr Med. 2020;26:1–7. doi: CrossRef
79. Alhashim M, Lombardo J. Mechanism of action of topical garlic on wound healing. Dermatol Surg. 2018;44:630–4. doi: CrossRef
80. Duthie G, Campbell F, Bestwick C, Stephen S, Russell W. Antioxidant effectiveness of vegetable powders on the lipid and protein oxidative stability of cooked Turkey meat patties: implications for health. Nutrients. 2013;5:1241–52. doi: CrossRef
81. Sharifi E, Chehelgerdi M, Fatahian-Kelishadrokhi A, Yazdani-Nafchi F, Ashrafi-Dehkordi K. Comparison of therapeutic effects of encapsulated Mesenchymal stem cells in Aloe vera gel and Chitosan-based gel in healing of grade-II burn injuries. Regen Ther. 2021;18:30–7. doi: CrossRef
82. Sánchez M, González-Burgos E, Iglesias I, Gómez-Serranillos MP. Pharmacological update properties of Aloe vera and its major active constituents. Molecules. 2020;25:1324. doi: CrossRef
83. Takzaree N, Hadjiakhondi A, Hassanzadeh G, Rouini MR, Manayi A, Zolbin MM. Transforming growth factor-β (TGF-β) activation in cutaneous wounds after topical application of Aloe vera gel. Can J Physiol Pharmacol. 2016;94:1285–90. doi: CrossRef
84. Prakoso YA, Kurniasih. The effects of Aloe vera cream on the expression of CD4+ and CD8+ lymphocytes in skin wound healing. J Trop Med. 2018;2018:6218303. doi: CrossRef
85. Noori AS, Mageed NF, Abdalameer NK, Mohammed MKA, Mazhir SN, Ali AH, et al. The histological effect of activated Aloe vera extract by microwave plasma on wound healing. Chem Phys Lett. 2022;807:140112. doi: CrossRef
86. Movaffagh J, Khatib M, Fazli Bazzaz S, Taherzadeh Z, Hashemi M, Seyedian Moghaddam A, et al. Evaluation of wound-healing efficiency of a functional Chitosan/Aloe vera hydrogel on the improvement of re-epithelialization in full thickness wound model of rat. J Tissue Viability. 2022;31(4):649–56. doi: CrossRef
87. Kaewsrisung S, Sukpat S, Issarasena N, Patumraj S, Somboonwong J. The effects of oral Aloe vera on the efficacy of transplanted human endothelial cells and the expression of matrix metalloproteinases in diabetic wound healing. Heliyon. 2021;7:e08533. doi: CrossRef
88. Hormozi M, Assaei R, Boroujeni MB. The effect of Aloe vera on the expression of wound healing factors (TGFβ1 and bFGF) in mouse embryonic fibroblast cell: in vitro study. Biomed Pharmacother. 2017;88:610–6. doi: CrossRef
89. Shafaie S, Andalib S, Shafaei H, Montaseri A, Tavakolizadeh M. Differential biological behavior of fibroblasts and endothelial cells under Aloe vera gel culturing. Int J Mol Cell Med. 2020;9(3):234–46.
90. Betriksia D, Syahrial Hamid I, Hermanu LS. Uji Potensi Ekstrak Daun Binahong (Anredera cordifolia (Ten.) Steenis) Terhadap Peningkatan Ketebalan Jaringan Granulasi dan Waktu Penyembuhan Luka Bakar Tikus. J Pharm Sci Pract. 2018;5:11–7.
91. Situmorang GA, Yamamoto Z, Ichwan M, Prayugo B. Anredera cordifolia leaves extract accelerates the wound healing of normal and hyperglycemic rats. Pharmaciana. 2022;12:39. doi: CrossRef
92. Maharani ES, Puspitawati R, Gunawan HA. Antibacterial effect of binahong (Anredera cordifolia (Ten.) Steenis) leaf infusion against black pigmented bacteria. J Phys Conf Ser. 2018;1073:032013. doi: CrossRef
93. Sukrama DM, Wihandani DM, Manuaba AP. Topical Binahong (Anredera cordifolia) leaf extract increases interleukin-6 and VEGF (Vascular Endothelial Growth Factor) during burn wound healing in wistar rats infected with Pseudomonas aeruginosa. Biol Med. 2017;09:1. doi: CrossRef
94. Samirana PO, Swastini DA, Subratha IDGPY, Ariadi KA. Uji Aktivitas Penyembuhan Luka Ekstrak Etanol Daun Binahong (Anredera Scandens (L.) Moq.) pada Tikus Jantan Galur Wistar. J Farm Udayana. 2016;5:19-23.
95. Kintoko K, Karimatulhajj H, Elfasyari TY, Ihsan EA, Putra TA, Hariadi P, et al. Pengaruh Kondisi Diabetes pada Pemberian Topikal Fraksi Daun Binahong dalam Proses Penyembuhan Luka. Maj Obat Tradis. 2017;22:103
96. Laksmitawati DR, Widyastuti A, Karami N, Afifah E, Rihibiha DD, Nufus H, et al. Anti-inflammatory effects of Anredera cordifolia and piper crocatum extracts on lipopolysaccharide-stimulated macrophage cell line. Bangladesh J Pharmacol. 2017;12:35–40. doi: CrossRef
97. Swastini DA, Udayana INK, Arisanti CIS. Cold cream combination of Garcinia mangostana L. Anredera cordifolia (ten.) and Centella asiatica extracts on burn healing activity test. Res J Pharm Technol. 2021;4(5):2483–6.doi: CrossRef
98. Hanafiah OA, Hanafiah DS, Bayu ES, Abidin T, Ilyas S, Nainggolan M, et al. Quantity differences of secondary metabolites (Saponins, tannins, and flavonoids) from binahong plant extract (Anredera cordifolia (Ten.) Steenis) treated and untreated with colchicines that play a role in wound healing. World J Dent. 2017;8:296–9. doi: CrossRef
99. Istyastono EP, Yuliani SH. Scarless wound healing gel with Binahong (Anredera cordifolia (Ten) Steenis) leaves extract and celecoxib as the active ingredients. AIP Conf Proc. 2016;1755:160001. doi: CrossRef
100. Aarthi S, Suresh J, Prasath D. Variability and association analysis of curcumin content with yield components in turmeric (Curcuma longa L.). Electron J Plant Breed. 2018;9:295–303. doi: CrossRef
101. Adeliana, Usman AN, Ahmad M, Arifuddin S, Yulianty R, Prihantono. Effectiveness of turmeric (Curcuma Longa Linn) gel extract (GE) on wound healing: pre-clinical test. Gac Sanit. 2021;35:S196–8. doi: CrossRef
102. Kulyal P, Acharya S, Ankari AB, Kokkiripati PK, Tetali SD, Raghavendra AS. Variable secondary metabolite profiles across cultivars of Curcuma longa L. and C. aromatica Salisb. Front Pharmacol. 2021;12:659546. doi: CrossRef
103. Kabir MT, Rahman MH, Akter R, Behl T, Kaushik D, Mittal V, et al. Potential role of curcumin and its nanoformulations to treat various types of cancers. Biomolecules. 2021;11:1–39. doi: CrossRef
104. Fereydouni N, Darroudi M, Movaffagh J, Shahroodi A, Butler AE, Ganjali S, et al. Curcumin nanofibers for the purpose of wound healing. J Cell Physiol. 2019;234:5537–54. doi: CrossRef
105. Merrell JG, Mclaughlin SW, Tie L, Laurencin CT, Chen AF, Nair LS, et al. Curcumin loaded poly(ε-Caprolactone) nanofibers: diabetic wound dressing with antioxidant and anti-inflammatory properties NIH public access author manuscript. Clin Exp Pharmacol Physiol. 2009;36:1149–56. doi: CrossRef
106. Maghimaa M, Alharbi SA. Green synthesis of silver nanoparticles from Curcuma longa L. and coating on the cotton fabrics for antimicrobial applications and wound healing activity. J Photochem Photobiol B Biol. 2020;204:111806. doi: CrossRef
107. Mohammadi MR, Rabbani S, Bahrami SH, Joghataei MT, Moayer F. Antibacterial performance and in vivo diabetic wound healing of curcumin loaded gum tragacanth/poly(ε-caprolactone) electrospun nanofibers. Mater Sci Eng C. 2016;69:1183–91. doi: CrossRef
108. Ak T, Gülçin I. Antioxidant and radical scavenging properties of curcumin. Chem Biol Interact. 2008;174:27–37. doi: CrossRef
109. Kulac M, Aktas C, Tulubas F, Uygur R, Kanter M, Erboga M, et al. The effects of topical treatment with curcumin on burn wound healing in rats. J Mol Histol. 2013;44:83–90. doi: CrossRef
110. Dai M, Zheng X, Xu X, Kong X, Li X, Guo G, et al. Chitosan-alginate sponge: preparation and application in curcumin delivery for dermal wound healing in rat. J Biomed Biotechnol. 2009;2009:595126. doi: CrossRef
111. Panchatcharam M, Miriyala S, Gayathri VS, Suguna L. Curcumin improves wound healing by modulating collagen and decreasing reactive oxygen species. Mol Cell Biochem. 2006;290:87–96. doi: CrossRef
112. Hanafi, Sirait SM, Setyawati SR. Phytochemical, antimicrobial and total phenol test of coral plants “Betadin” leaf methanol extract (Jatropha muitifida linn). Orient J Chem. 2020;36:567–71. doi: CrossRef
113. Thomas N, Rusdin A, Tulsyahra M, Wathoni N, Kuswandi A. Accelerated wound healing ability of Jatropha sap by iota carrageenan-poly (vinyl alcohol) hydrogel film. J Adv Pharm Technol Res. 2020;11:226–32. doi: CrossRef
114. Victorien DT, Jean Robert K, Jacques DT, Julien S, Jean-Marc A, Aléodjrodo EP, et al. Hemostatic activity screening and skin toxicity of sap of Jatropha multifida L. (Euphorbiaceae) used in traditional medicine (Benin). Asian Pacific J Trop Dis. 2012;2:S927–32. doi: CrossRef
115. Dah-Nouvlessounon D, Chokki M, Agossou EA, Houédanou JB, Nounagnon M, Sina H, et al. Polyphenol analysis via LC-MS-ESI and potent antioxidant, anti-inflammatory, and antimicrobial activities of Jatropha multifida L. extracts used in Benin pharmacopoeia. Life. 2023;13:1898. doi: CrossRef
116. Cavalcante NB, Diego da Conceição Santos A, Guedes da Silva Almeida JR. The genus Jatropha (Euphorbiaceae): a review on secondary chemical metabolites and biological aspects. Chem Biol Interact. 2020;318:108976. doi: CrossRef
117. Juniarti, Aryenti, Yuhernita, Poerwaningsih EH, Jusuf AA, Freisleben HJ, et al. Effects of methanolic Jatropha multifida L. extract in wound healing assessed by the total number of PMN leukocytes and fibroblasts. MAKARA Sci Ser. 2013;16:178–82. doi: CrossRef
118. de Carvalho C, Vieira Mariano L, Negrão VS, Passarelli Gonçalves C, Cristina Ribeiro Marcucci M. Phenols, flavonoids and antioxidant activity of Jatropha multifida L. collected in Pindamonhangaba, Sao Paulo State, Brazil. J Anal Pharm Res. 2018;7:581–4. doi: CrossRef
119Carels N. Chapter 2 Jatropha curcas. A review. Adv Bot Res. 2009;50:39–86. doi: CrossRef
120. Kumar A, Sharma S. An evaluation of multipurpose oil seed crop for industrial uses (Jatropha curcas L.): a review. Ind Crops Prod. 2008;28:1–10. doi: CrossRef
121. Abdelgadir HA, Van Staden J. Ethnobotany, ethnopharmacology and toxicity of Jatropha curcas L. (Euphorbiaceae): a review. South Afr J Bot. 2013;88:204–18. doi: CrossRef
122. Sachdeva K, Garg P, Singhal M, Srivastava B, Jatropha IÒ, Llòparametersòsuchòasòwoundòcontraction WÒ, et al. Wound healing potential of extract of Jatropha curcas L . (Stem bark ) in rats. Pharmacogn J. 2011;3:67–72. doi: CrossRef
123. Balqis U, Darmawi, Iskandar CD, Salim MN. Angiogenesis activity of Jatropha curcas L. latex in cream formulation on wound healing in mice. Vet World. 2018;11:939–43. doi: CrossRef
124. Shetty S, Udupa SL, Udupa AL, Vollala VR. Wound healing activities of bark extract of Jatropha curcas Linn in albino rats. Saudi Med J. 2006;27:1473–6.
125. Esimone CO, Nworu CS, Jackson C. Cutaneous wound healing activity of a herbal ointment containing the leaf extract of Jatropha curcas L. (Euphorbiaceae). Int J Appl Res Nat Prod. 2008;1:1–4.
126. Almeida L, Santos Matos F, Bailão EF, Gonçalves P. Jatropha curcas L. Latex production, characterization, and biotechnological applications. In: Mulpuri S, Carels N, Bahadur B, editors. Jatropha, challenges for a New Energy Crop. Singapore: Springer. doi: CrossRef
127. Tinpun K, Nakpheng T, Padmavathi AR, Srichana T. In vitro studies of Jatropha curcas l. Latex spray formulation for wound healing applications. Turkish J Pharm Sci. 2020;17:271–9. doi: CrossRef
128. Sahidin S, Ardiansyah A, Muhammad T, Marianti M. Terpenoids from the stem bark of Jatropha plants. MAKARA Sci Ser. 2011;15:2–7.
129. Kato-Noguchi H, Kurniadie D. Allelopathy of Lantana camara as an invasive plant. Plants. 2021;10:1–10. doi: CrossRef
130. Nayak BS, Raju SS, Eversley M, Ramsubhag A. Evaluation of wound healing activity of Lantana camara L.—a preclinical study. Phyther Res. 2009;23:241–5. doi: CrossRef
131. De Wet H, Nciki S, van Vuuren SF. Medicinal plants used for the treatment of various skin disorders by a rural community in northern Maputaland, South Africa. J Ethnobiol Ethnomed. 2013;9:51. doi: CrossRef
132. Verma RK, Verma SK. Phytochemical and termiticidal study of Lantana camara var. aculeata leaves. Fitoterapia. 2006;77:466–8. doi: CrossRef
133. Ali Esmail Al-Snafi. Chemical constituents and pharmacological activities of Lantana camara—a review. Asian J Pharm Clin Res. 2019;12:10–20. doi: CrossRef
134. Vishwakarma RK, Negi A, Negi DS. Development of potential COX inhibitor as anti-inflammatory agents from leaves of Lantana camara by in-vitro analysis, molecular docking and ADMET prediction. J Indian Chem Soc. 2022;99:100694. doi: CrossRef
135. Begum D, Nath SC. Ethnobotanical review of medicinal plants used for skin diseases and related problems in Northeastern India. J Herbs Spices Med Plants. 2000;7:55–93. doi: CrossRef
136. Bhardwaj S, Gakhar SK. Ethnomedicinal plants used by the tribals of Mizoram to cure cuts & wounds. Indian J Tradit Knowl. 2005;4:75–80.
137. Isnaini, Oktaviyanti I, Budiarti LY. Antibacterial and wound healing activity of ethanolic extract Melastoma malabathricum L. Res J Pharm Technol. 2023;16:2210–4.
138. Anbu Jeba Sunilson J, James J, Thomas J, Jayaraj P, Varatharajan R, Muthappan M. Antibacterial and wound healing activities of Melastoma malabathricum. Afr J Infect Dis. 2008;2(2):68.
139. Maigoda TC, Siregar A, Podojoyo, Ridhowati S, Krisnasary A. Wound healing and blood sugar effect of Psidium guajava L. leaves and Melastoma malabathricum L. leaves on rats with diabetic foot ulcer. J Appl Sci. 2019;19:287–94. doi: CrossRef
140. Maigoda TC, Refdanita, Idramsyah. The effectiveness of Guava (Psidium guajava) and Senduduk leaves (Melastoma malabathricum L.) extract gel towards the inflammation markers and collagens on induced male rats (Sprague Dawley) with diabetes. Malaysian Appl Biol. 2022;51:157–62. doi: CrossRef
141. Mazura MP, Susanti D, Rasadah MA. Anti-inflammatory action of components from Melastoma malabathricum. Pharm Biol. 2007;45:372–5. doi: CrossRef
142. Mayasari D, Murti YB, Pratiwi SUT, Sudarsono S, Hanna G, Hamann MT. TLC-based fingerprinting analysis of the geographical variation of Melastoma malabathricum in Inland and Archipelago Regions: a rapid and easy-to-use tool for field metabolomics studies. J Nat Prod. 2022;85:292–300. doi: CrossRef
143. Biswal S. Phytochemical analysis and a study on the antiestrogenic antifertility effect of leaves of Piper betel in female albino rat. Anc Sci Life. 2014;34:16. doi: CrossRef
144. Lien LT, Tho NT, Ha DM, Hang PL, Nghia PT, Thang ND. Influence of phytochemicals in iper betle linn leaf extract on wound healing. Burn Trauma. 2015;3:1–8. doi: CrossRef
145. Ghazali NA, Elmy A, Yuen LC, Sani NZ, Das S, Suhaimi F, et al. Piper betel leaves induces wound healing activity via proliferation of fibroblasts and reducing 11β hydroxysteriod dehydrogenase-1 expression in diabetic rat. J Ayurveda Integr Med. 2016;7:198–208. doi: CrossRef
146. Begam KMF, Ravichandran P, Manimekalai V. Phytochemical analysis of some selected varieties of Piper betle L. Int J Curr Pharm Res. 2018;10:89. doi: CrossRef
147. Dashti A, Karamibonari AR, Farahpour MR, Tabatabaei ZG. Topical effectiveness of eugenol phytosome / chitosome hydrogels on the healing process of infected excision wounds. Colloids Surfaces A Physicochem Eng Asp. 2024;687:133482. doi: CrossRef
148. Noori F, Osanloo M, Moradi H, Jafarbeigloo H, Jirehnezhadyan M, Kouhpayeh S, et al. Fabrication, characterization, and in vivo implantation of eugenol-loaded nanogels and PCL/Cs electrospun nanofibers for wound healing applications. J Bioact Compat Polym. 2023;38:480–92. doi: CrossRef
149. Khadim H, Zeeshan R, Riaz S, Tabassum S, Ansari AA, Zulfiqar S, et al. Development of eugenol loaded cellulose-chitosan based hydrogels; in-vitro and in-vivo evaluation for wound healing. Colloids Surfaces A Physicochem Eng Asp. 2024;693:134033. doi: CrossRef
150. Cheshfar F, Bani S, Mirghafourvand M, Hasanpour S, Javadzadeh Y. The effects of ginger (Zingiber officinale) extract ointment on pain and episiotomy wound healing in nulliparous women: a randomized clinical trial. J Caring Sci. 2023;12:181–7. doi: CrossRef
151. Mošovská S, Nováková D, Kali?ák M. Antioxidant activity of ginger extract and identification of its active components. Acta Chim Slovaca. 2015;8:115–9. doi: CrossRef
152. Al-Samydai A, Al Qaraleh M, Alshaer W, Al-Halaseh LK, Issa R, Alshaikh F, et al. Preparation, characterization, wound healing, and cytotoxicity assay of PEGylated nanophytosomes loaded with 6-Gingerol. Nutrients. 2022;14:5170. doi: CrossRef
153. Afshari AT, Shirpoor A, Farshid A, Saadatian R, Rasmi Y, Saboory E, et al. The effect of ginger on diabetic nephropathy, plasma antioxidant capacity and lipid peroxidation in rats. Food Chem. 2007;101:148–53. doi: CrossRef
154. Sugimoto N, Katakura M, Matsuzaki K, Miyamoto M, Sumiyoshi E, Wada T, et al. Ginger facilitates cell migration and heat tolerance in mouse fibroblast cells. Mol Med Rep. 2021;23:250. doi: CrossRef
155. Khan BA, Ullah S, Khan MK, Uzair B, Menaa F, Braga VA. Fabrication, physical characterizations, and in vitro, in vivo evaluation of ginger extract-loaded gelatin/Poly(Vinyl Alcohol) hydrogel films against burn wound healing in animal model. AAPS PharmSciTech. 2020;21:323. doi: CrossRef
156. Li J, Fu W, Zhang X, Zhang Q, Ma D, Wang Y, et al. Green preparation of ginger-derived carbon dots accelerates wound healing. Carbon. 2023;208:208–15. doi: CrossRef
157. Chen CY, Cheng KC, Chang AY, Lin YT, Hseu YC, Wang HM. 10-Shogaol, an antioxidant from Zingiber officinale for skin cell proliferation and migration enhancer. Int J Mol Sci. 2012;13:1762–77. doi: CrossRef
158. Zhang M, Xu C, Liu D, Han MK, Wang L, Merlin D. Oral delivery of nanoparticles loaded with ginger active compound, 6-Shogaol, attenuates ulcerative colitis and promotes wound healing in a murine model of ulcerative colitis. J Crohns Colitis. 2018;12:217–29. doi: CrossRef
159. Duansak D, Somboonwong J, Patumraj S. Effects of Aloe vera on leukocyte adhesion and TNF-α and IL-6 levels in burn wounded rats. Clin Hemorheol Microcirc. 2003;29(3-4):239–46
160. Raziyeva K, Kim Y, Zharkinbekov Z, Kassymbek K, Jimi S, Saparov A. Immunology of acute and chronic wound healing. Biomolecules. 2021;11:1–25. doi: CrossRef
161. Shaheda Sultana S, Shaheda Sultana S, Rao KS, Vani T. Formulation and evaluation of herbal emulgel of Lantana camara leaves extract for wound healing activity in diabetic rats. WORLD J Pharm Pharm Sci. 2018;7:1617–35.
162. Comino-Sanz IM, López-Franco MD, Castro B, Pancorbo-Hidalgo PL. The role of antioxidants on wound healing: a review of the current evidence. J Clin Med. 2021;10:3558. doi: CrossRef
SUPPLEMENTARY MATERIAL
The supplementary material can be accessed at the link here: [https://japsonline.com/admin/php/uploadss/4530_pdf.pdf]