INTRODUCTION
Mercaptopurine (MP) is a purine analog that prevents the creation of RNA and proteins and is an antagonist to natural purines necessary for DNA replication [1]. MP treats leukemia, inflammation, and cancer and inhibits the immune system. Its usage is limited, though, because of severe adverse effects such as gastrointestinal distress, bone marrow suppression, and liver damage [2]. Lymphocytes absorb the prodrug after being metabolized in the gastrointestinal tract system and liver. The purine salvage enzyme breaks down MP to produce thioguanine nucleotides and thioinosine monophosphate [3]. The amount of purines available for DNA synthesis is further decreased by methylthioinosine monophosphate, a potent purine de novo synthesis inhibitor. When synthesized thiopurine nucleotides replace native purines, cells experience purine deprivation, which stops the production of DNA, RNA, and proteins and reduces cell proliferation and cytotoxicity [4,5].
The metabolites thioguanine can harm myeloid, liver, and renal tissue without benefitting life [4,5]. Both acute and long-term MP consumption can cause hepatotoxicity. During the first several months of treatment, jaundice, tiredness, and increased enzymes are common symptoms of acute liver damage [4]. Persistent therapy may lead to the development of portal hypertension and nodular regenerative hyperplasia, which would ultimately cause liver failure. When thiopurines, such as MP, suppress the immune system in males with inflammatory bowel disease, an uncommon consequence is hepatosplenic T-cell lymphoma [3].
The adverse effects of synthetic medications are drawing attention to the therapeutic evaluation of medicinal plants using a systematic research approach. Since the conventional treatment of liver issues is associated with a number of detrimental side effects, botanical medications are commonly used [6].
Ephedra fragilis (E. fragilis) is a member of the Ephedraceae botanical family. This family’s plants are well known for their many ephedrine alkaloids, the most prominent of which are ephedrine and pseudoephedrine [6,7]. The primary phytochemical components of Ephedra plants that are thought to be responsible for these pharmacological characteristics are flavonoids, alkaloids, phenolic acids, and other chemicals. The Ephedra plant contains more than 80% alkaloids, including ephedrine and pseudoephedrine. A study on one Ephedra species discovered hydroalcoholic extract had a more significant impact than aqueous extract and had higher antioxidant, anti-inflammatory, and antiviral properties [7,8]. Ephedra fragilis and Ephedra Alte, used interchangeably for the same common name Alenda in Jordan, are very stimulating plant that has been used for a long time in Jordan under the name Alenda or pine joints [9]. It is also well known and respected in the herbalist traditions of many other nations. It is widely utilized and well-studied in the Tefillah area in southern Jordan, where its anticancer properties have been established in multiple studies [8-10].
The liver is an essential organ that manages chemical metabolism, secretion, storage, and detoxification [11]. Liver cirrhosis is the leading cause of death in hepatic disorders, which represents a severe threat to public health. Even with the advances in contemporary medicine, no entirely effective drugs can protect the entire organ or improve liver function. Plant-based phytochemicals are crucial to the search for safer and more productive pharmaceutical substitutes. Viral infections, malignancies, alcoholism, drug overdoses, and nonalcoholic fatty liver disease are examples of hepatic illnesses. Treatment-induced liver impairment can result in treatment withdrawal, and liver transplantation is necessary for the majority of end-stage liver disease situations. Hepatoprotective herbs, such as milk thistle, turmeric, and N-acetylcysteine, can potentially prevent or treat liver damage [12]. The hepatoprotective ability of E. fragilis, Alenda, against MP consumption has not been the subject of any scientific investigation, previously according to published research. Few investigations have established the detrimental effects of E. fragilis and the efficacy of several species of Ephedra as hepatoprotective activity.
This research aims to assess the hepatoprotective and antioxidant properties of the aerial parts of the E. fragilis plant. There is a link between oxidative stress and inflammation; free radicals can promote inflammation [8]. Thus, this work assessed the hepatoprotective qualities of E. fragilis aerial portion utilizing MP-intoxicated rats as an experimental paradigm.
EXPERIMENT
Chemicals
The Sigma-Aldrich Company provided the analytical reagent grade chemicals and used reagents.
Plant material
Mutah University authenticated the E. fragilis plant, which was harvested on March 1st, 2022, and 2023, from Tafillah, Jordan, authenticated by Dr. Saleh Al-Quran (Department of Biology, Mutah University, Karak, Jordan). A drug development lab received the voucher specimen and assigned specimen number ER008 for deposit. The aerial portion was dried, ground for a short while, and then stored in the dark at room temperature. The mixture was filtered using filter paper 48 hours after 33% ethanol was added. The resultant extracts were then concentrated at 40°C ± 5°C under reduced pressure and then lyophilized for 48 hours [13].
Preliminary phytochemical screening
Using conventional techniques, phytochemical screening assays were carried out to determine if phytoconstituents were present in E. fragilis extracts.
The study focuses on detecting tannins, saponins, alkaloids, and flavonoids in plant extracts. Distilled water and ferric chloride are added to a plant extract to produce blue and green/black colors, which are used to evaluate the tannin content. A layer of foam detects saponins. Alkaloids are calculated by adding 2% hydrochloric acid to the residue in a water bath at 100°C. After filtering, the mixture is split into two equal parts. Sodium hydroxide is added to a solution to detect flavonoids and flavones, causing the solution to become yellow. Following treatment with 5N hydrochloric acid, the solution becomes orange for flavones and colorless for flavonoids [13].
Determination of phenolic
The total phenol content of the hydroalcoholic extract of E. fragilis was determined using the Folin–Ciocalteu technique. About 200 µl of the sample solution and 250 µl of 1.0-N Folin’s reagent were combined. After 5 minutes, approximately 1,300 µl of 20% sodium Na2CO3 was added, and the mixture was vigorously agitated. Using UV/visible (double beam Perkin Elmer spectrophotometer), the absorbance was measured at 760 ± 2 nm following 45 minutes of incubation at 25°C ± 2°C. A calibration curve was made using gallic acid standards. Gallic acid equivalents (GEs) expressed the phenolic content [13,14].
Determination of flavonoids
Aluminum chloride and a hydroalcoholic sample solution determined the total flavonoid content. After 35 minutes of incubation at 25°C ± 2 °C, the samples’ absorbance at 500 ± 5 nm was determined. Standard quantities of a quercetin methanolic solution were used to generate a calibration curve, and the total flavonoid content was expressed using quercetin equivalents or QE [13,14].
Free radical scavenging activity/2,2-diphenyl-1-picrylhydrazyl
The study used 2,2-diphenyl-1-picrylhydrazyl (DPPH) to measure free radical scavenging activity in extract and reference samples. The samples were treated with DPPH ethanolic solution and then subjected to absorbance measurements at 520 ± 2 nm. Ascorbic acid is the standard, and an ethanolic DPPH solution was used as a negative control [13,14].
Animals
In this study, laboratory-bred Wistar rats (female) weighing 150–180 g were procured from the Laboratory Animal Research Center of the Applied Science University located/in Amman, Jordan. For the duration of the experiment, they were kept in a facility with a constant temperature of 25°C ± 2°C and regular cycles of light and dark (12/12 h). The Applied Science University Faculty of Pharmacy’s ethics committee approved all experimental protocols and techniques intending to lessen the suffering of the animals (Ethics approval references number: 2023-PHA-57). These rules adhered to globally acknowledged norms that govern the care and use of animals in experiments [14,15].
In vivo study
Rats were divided into seven groups. Each group comprised six rats, and each group received MP, Silymarin, and E. fragilis orally for 21 days following Table 1 [14-18]. The positive control group was the silymarin group, while the negative control group was the hydroalcoholic water. Doses were selected according to reported safe values for E. fragilis extract, while therapeutic doses were previously used for MP and Silymarin [14-19].
 | Table 1. Experiment design. Seven groups of six rats each were set up in a random manner. [Click here to view] |
As illustrated in Table 1, animal groups were treated as follows—C: normal control (rats treated with 1 ml/day of hydroalcoholic water); MP: mp (40 mg/kg /day); S: silymarin (50 mg/kg); E: E. fraglisis; extract (30 mg/Kg); E/MP: E. fraglisis; extract (30 mg/Kg) and MP (40 mg/kg); S/MP: silymarin (50 mg/kg) and MP (40 mg/kg); E/S/MP: E. fraglisis; extract (15 mg/Kg); silymarin (25 mg/kg); and MP (20 mg/kg). The protocol adhered to several published protocols evaluating hepatoprotective activity for plant extracts using silymarin as standard material for its hepatoprotective activity at a dose of 50-mg/kg body weight [15]. Ephedra dose was selected according to previously reported hepatoprotective dose against deltamethrin hepatic injury with a dose of about 30-mg/kg body weight in conjunction with the control group administered the same dose for more pronounced safety evaluation [10,15].
Assessment of hepatic injury (biochemical markers)
Conventional biomarkers for liver injury fall into two categories: those that indicate a disturbance in the liver’s homeostasis or normal function and those that offer distinct indications of cellular and tissue damage [11]. The liver processes bile acids and other endogenous compounds, synthesizes proteins, and excretes metabolic waste products including urea and bilirubin. Conventional indicators of compromised liver function include changes in plasma bile acids, plasma total bilirubin (TB), and plasma total plasma protein brought on by medications or illnesses [1-4]. These indicators may be evaluated because injured or dying cells frequently release them into the bloodstream [6-10,12-19]. This group of enzymes includes gamma-glutamyl transferase, glutamate dehydrogenase, and aspartate aminotransferase (SGPT). Other readily available alternatives include triglycerides, albumin (ALB), and total protein (TP). Accordingly, these indicators are used in this study.
Blood samples were drawn from the rats’ tail veins during the trial’s first, tenth, and twenty-first days. Chloral hydrate was used to conduct animal anesthesia. The TP (Arcomex, Jordan), ALB, TB, glutamic-oxaloacetic transaminase (SGOT), SGPT, and alkaline phosphatase (ALP) (Beacons Diagnostic Pvt Ltd, India) diagnostic kits were all evaluated [1-4, 6-10,12-19]. According to Eidi et al. [19], liver tissue samples were obtained for histological examination, and the same pathologist assessed and assigned a rating. The data analysis investigator is blinded to the fact that the specimens or animals served as controls and treatments.
Histopathology
About 5-µm-thick tissue slices and 10% formalin in saline solution were used for the histological analysis of the liver. After being dried in alcohol, the slices were wrapped in paraffin. A histopathologist will stain and evaluate the samples using hematoxylin and eosin (H&E). The liver slices were quantitatively graded to assess the acute hepatic injury [14].
A histopathologist used a light microscope to examine these slices after they had been stained with H&E, as previously mentioned in [15,19]. A quantitative scoring system was used to evaluate the histological characteristics of acute hepatic injury in the liver sections. Significant hepatocyte necrosis, lymphocyte and Kupffer cell infiltration, lipid alteration, and hyaline degeneration were all shown by the histological results. Hyaline degeneration, lipid change, lymphocyte, and Kupffer cell infiltration, and considerable hepatocyte necrosis were among the histological findings. The French Metavir Cooperative Study Group [20] methodology for evaluating liver pathology was followed. A score of 0 indicates no discernible cell damage. Localized hepatocyte injury is seen in less than 25% of the tissue when the score is 1 [15,19].
Statistical analysis
The SPSS22 program, Tukey’s test, and the analysis of variance test were used to assess the results of the biochemical parameter analysis. Statistical significance was reached when the p-value, which shows a difference, was less than 0.05. Results expressed as average ± standard error of the mean (Av ± SEM). To assess for data normality and homogeneity, the Shapiro–Wilk and Levene tests were applied.
RESULTS
Phytochemical, total phenols, flavonoids, and DPPH analysis
Both Wagners and Dragendorff yield positive scores for alkaloids. Moreover, saponins and tannins produce positive results for the qualitative phytochemical investigation.
The E. fragilis ethanol:water (33%) extract had a total phenolic content of 46. 7 ± 1.7 mg GE per gram of dry extract (mean ± SEM). The flavonoid amount in 1 g of dry extract was 76.6 ± 1.3 mg QE (mean ± SEM). Moreover, an IC50 of 0.126 mg.ml−1 was obtained from the DPPH Free Radical Scavenging Assay. The extract yielded 3% w/w per gram of plant dry powder utilized.
Serum biochemical parameters
As indicated in Table 2, the plasma biochemical parameters were assessed in each of the rats on days 0, 10, and 21 of the assessment. As demonstrated in Table 2 and Figure 1, rats treated with MP exhibited significantly higher levels of ALP on day ten and continued to increase substantially on day 21 (144.6 IU.l−1) (p = 1*10–7) of the study, as well as significantly higher levels of SGPT (73.8 IU.L−1) and SGOT (95.6 IU.l−1) on days 10 and 21 of evaluation (p = 5*10–6; 2*10–5). ALB was reduced to 2.5 mg.l−1, although the difference was insignificant (p = 0.86). On day 21 of the research, total bilirubin significantly increased to 12.1 mg.l−1 (p = 9*10−12).
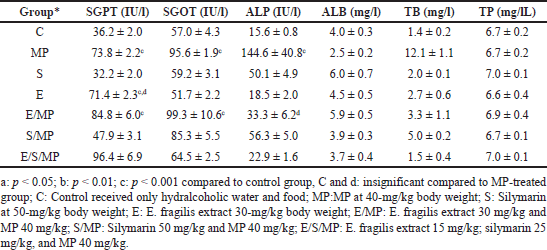 | Table 2. Effect of oral administration of E. fragilis Arial part hydroalcoholic extract at a dose of 30-mg/kg body weight on serum AST, ALT, ALP, TB, ALB, and TP serum levels in MP-induced hepatotoxicity in rats at day 21. Each column represents mean ± SEM for 6 rats. [Click here to view] |
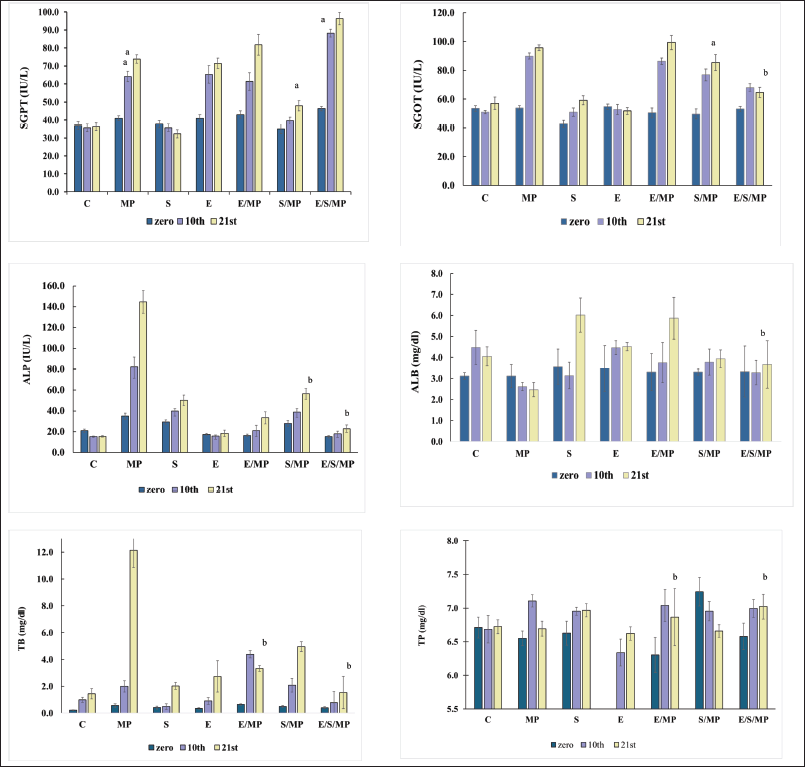 | Figure 1. Effect of oral administration of E. fragilis Arial part hydroalcoholic extract at a dose of 30-mg.kg−1 body weight on serum AST, ALT, ALP, TB, ALB, and TP serum levels in MP-induced hepatotoxicity in rats at days: 0, 10, and 21, for six rats. (A) and d: insignificant compared to MP-treated group (B); *: C: Control received only distilled water and food; MP:MP at 40-mg.kg−1 body weight; S: Silymarin at 50-mg.kg−1 body weight; E: E. fragilis extract 30-mg.kg−1 body weight; E/MP: E. fragilis extract 30 mg.kg−1 and MP 40 mg.kg−1; S/MP: Silymarin 50 mg.kg−1 and MP 40 mg.kg−1; E/S/MP: E. fragilis extract 15 mg.kg−1; silymarin 25 mg.kg−1, and MP 40 mg.kg−1. The error bars represent the SEM of measurements in six samples (n = 6). * a: significant difference compared to the C group at p values < 0.05, b: insignificant difference compared to the C group at p values > 0.05. [Click here to view] |
However, compared to group C, the administration of E. fragilis significantly increased SGPT levels to 71.4 IU.l−1 (p = 2*10−5) and insignificantly different from the MP group (p = 1.0) while maintaining other parameters as expected and no significant difference against the control group. Similarly, silymarin has no impact on all parameters, which shows its harmless effect on liver function. Combination E/MP increased SGPT 84.8 IU.L−1 (p = 9*10−13); SGOT 99.3 IU.l−1 (p = 1*10−8), low increment effect against ALP 33.3 IU.l−1 not significant (p = 0.999) compared to group C. TB did not increase significantly at 3.3 mg.l−1 (p = 0.905), TP 6,9 mg.l−1, and ALB 5.9 mg.l−1 (p = 0.942).
The silymarin combination shows a better effect on SGPT levels than E/MP, so it gets a value of 47.9 IU.l−1, while SGOT significantly increased to 85.3 IU.l−1 (p = 0.005), ALP 56.3 IU.l−1 not significantly higher than C (p = 0.576). Similarly, normal levels were obtained for ALB, TB, and TP. Furthermore, triple combination E/S/MP shows different behavior; SGPT significantly increased 96.4 IU.l−1 (p = 9*10−13), while there was no significant increase in all other parameters: SGOT 64.5 IU.l−1, TP 7 mg.l−1, ALP 22.9 IU.l−1, TB 1.5 mg.l−1, and ALB 3.7 mg.l−1 (p = 1.0).
Histopathological assessment
The liver sections showed considerable hepatocyte necrosis, hyaline degeneration, fatty modification, lymphocyte and Kupffer cell infiltration, and quantitative scoring to evaluate acute hepatic injury (Fig. 2). According to The French Metavir Cooperative Study Group [20] methodology, liver pathology is assessed using a scoring system where 0 denotes no cell damage and 1 represents less than 25% localized hepatocyte damage in the tissue [21].
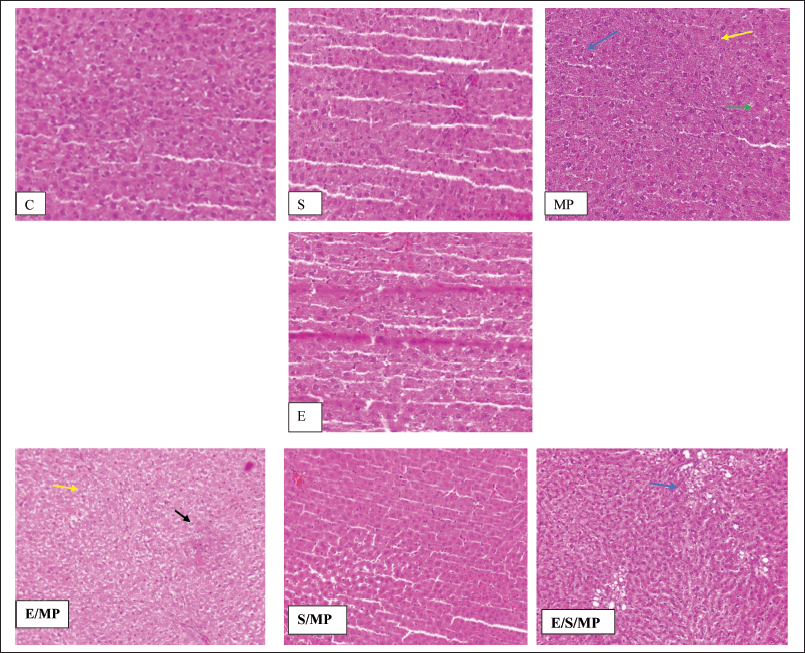 | Figure 2. Hepatic tissue sections: (A) and d: insignificant compared to MP-treated group (B); *: C: Control received only distilled water and food; MP:MP at 40-mg.kg−1 body weight; S: Silymarin at 50-mg.kg−1 body weight; E: E. fragilis extract 30-mg.kg−1 body weight; E/MP: E. fragilis extract 30 mg.kg−1 and MP 40 mg.kg−1; S/MP: Silymarin 50 mg.kg−1 and MP 40 mg.kg−1; E/S/MP: E. fragilis extract 15 mg.kg−1; silymarin 25 mg.kg−1, and MP 40 mg.kg−1. Blue arrow shows inflammation, yellow arrow shows edema, green arrow shows necrosis, and black arrow shows fatty degeneration. [Click here to view] |
Table 3 and Figure 2 display the histological findings of the current investigation on hepatic tissue evaluation. Hepatocyte necrosis, swelling and inflammation of cells, and fatty degeneration were among the morphological alterations analyzed and evaluated. The three most noticeable changes were fatty degeneration, inflammation, and cell swelling in that order. Changes in cell edema that were most visible were seen in group E/MP. Except for group E/S/MP, inflammation was most evident in these groups. MP-treated rats showed slight hepatic morphological changes, such as necrosis, cell edema, and inflammation, compared to the control group’s normal liver tissues. Compared to a standard control, the injection of Alenda caused minor cell edema and inflammation. It has been shown that co-administration of Alenda and MP was linked to more noticeable fatty degeneration and cell enlargement. On the other hand, the MP-induced necrosis improved in groups E/MP, S/MP, and E/S/MP. Interestingly, group E/MP showed signs of mitotic activity.
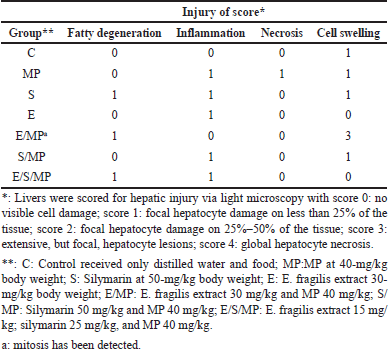 | Table 3. Histological injury score of liver under different treatments. [Click here to view] |
DISCUSSION
Liver cirrhosis is a leading cause of death in hepatic disorders, posing a significant public health threat. Despite advances in medicine, there are no effective drugs for protecting the liver or improving its function. Hepatic illnesses include viral infections, malignancies, alcoholism, drug overdoses, and nonalcoholic fatty liver disease. Treatment-induced liver impairment can lead to treatment withdrawal, and liver transplantation is necessary for end-stage liver disease. Plant-based phytochemicals are crucial for finding safer and more productive pharmaceutical substitutes.
Children with acute lymphoblastic leukemia are frequently treated with chemotherapeutic drugs such as methotrexate and MP, which can be harmful to the liver. Hepatoprotective substances, such as milk thistle extract silymarin and herbals, have been utilized to lessen adverse effects. Silymarin does not counteract the effects of chemotherapy and has been demonstrated to reduce liver damage significantly. The sole traditional medication approved for hepatoprotection in the United States, ursodeoxycholic acid, has been connected to mutagenesis, liver cell failure, and an elevated risk of hepatocellular carcinoma. Low survival rates, morbidity, and graft rejection are possible outcomes of hepatotoxic liver transplants. There is a growing market for safer, more potent hepatoprotective medications, with Ayurvedic botanicals leading the way. The development of hepatoprotective medications that are well-tolerated, affordable, and safe is now undertaken.
Ephedra fragilis, a member of the Ephedra genus, has been used in traditional Chinese medicine for over 5,000 years for its anti-inflammatory, anti-invasive, anti-angiogenic, antimicrobial, neuroprotective, hepatoprotective, and antioxidant properties, primarily due to flavonoids, alkaloids, and phenolic acids [22]. This being the case, research has been proposed to investigate the potential benefits of E. pachyclada extract, which has been shown to possess liver-protective properties against CCl4 liver inflammation [15]. The most common biochemical markers of liver damage are SGOT, SGPT, and ALP plasma levels.
The present investigation examines the course of liver damage resulting from repeated MP administrations and the potential hepatoprotective effect of concurrent consumption of E. fragais extract compared to silymarin hepatoprotectant.
Both Wagners and Dragendorff yield positive scores for alkaloids. Moreover, saponins and tannins produce positive results for the qualitative phytochemical investigation. These findings conform to the literature [11,23]. The E. fragilis ethanol:water (33%) extract had a total phenolic content of 46. 7± 1.7 mg GE per gram of dry extract (mean ± SEM). Compared to 13 mg, EAG/g previously reported in Algeria by Djahra et al. [10] 32.7 by Guenaou et al. [22], in Morrocco, and 30.5 by Kmail et al. [24]. The flavonoid content was 76.6 ± 1.3 mg QE per gram of dried extract, which is higher than previously reported by Guenaou et al. [22], 10.5 mgQE/g. A higher value can be attributed to several factors such as time of collection, part used, species, soil content, solvent, and exact extraction method. DPPH Free Radical Scavenging IC50 of 0.126 mg.ml−1 indicates intense antioxidant activity, compared to 0.9 mg.ml−1 reported by Djahra et al. [10] and reasonably comparable to ethyl acetate fraction extract accomplished by Guenaou et al. [22] that is 0.116 mg.ml−1.
The hydroalcoholic extract of E. fragilis successfully decreased the increase in plasma enzyme activity brought on by MP in the case of ALP, only besides a significant effect on TB levels. At the same time, there has been no considerable amelioration in SGPT or SGPOT. They are indicating weak effectiveness in decreasing MP-induced liver damage. Increases in the enzymes, as mentioned earlier, may be a sign of hepatobiliary network injury and inflammation. Finding specific patterns at aberrant levels aids in locating the damage’s origin. The hepatocellular disease is indicated by elevations in SGPT and SGOT that are not in line with ALP.
A sensitive indicator of hepatic injury is serum aminotransferase activity, which can change enzyme leakage due to hepatocyte transport activity and membrane permeability [25]. Hepatic tissue membranes sustain severe damage during MP treatment, exposing the bloodstream to SGOT and SGPT. Enzymatic defenses against reactive free radicals, such as catalase and superoxide dismutase, are present in hepatic cells [26]. Rats given MP showed signs of hepatotoxicity, as seen by elevated SGOT, SGPT, TB, and ALP plasma levels. This finding is consistent with previous studies that found MP-caused hepatotoxicity in drug-treated rats due to a considerable rise in aminotransferase levels [27]. These results are pretty different or disagree with the previous study that evaluated E. Alata against deltamethrin pesticide liver damage by Djahra et al. [10], where liver enzymes were reduced, and the extract shows an effect SGPT, SGOT, and ALP.
Hepatoprotective medications can prevent high ALP levels that are linked to drug-induced cholestasis. The amelioration effect against ALP levels when using E. fragilis in conjunction with MP indicates its probable selective effect against drug-induced cholestasis [28]. Drug-induced liver injury is a severe adverse reaction to drugs and xenobiotics, primarily affecting the liver. It can lead to cholestatic damage and is more common in older patients. There is a genetic predisposition to toxic cholestasis, and bile duct injury is a significant prognostic finding in this situation. E. fragilis could be a suitable protective element if additional study is done to investigate its effect in conjunction with some of these medicines.
An endogenous anion called bilirubin helps the liver produce and excrete bile. The liver gets hyperbilirubinemia if it cannot eliminate bilirubin because of hepatocyte damage. Total bilirubin levels indicate the degree of liver disease. MP treatment results in mixed hepatotoxicity and markedly lowered liver activity of catalase and superoxide dismutase.
The investigation’s findings show that E. fragilis extract may influence catalase and superoxide dismutase activities, indicating that the hepatoprotective action of Alenda extract may entail the modulation of hepatic antioxidant enzymes. This may suggest further targeted and focused study to determine how this impact, represented in biochemical output, is produced. Due to their ability to conjugate to decreased GSH, antioxidant enzymes are essential for detoxifying xenobiotics. Since free radicals affect the body’s different enzyme functions, they are a significant source of nonenzymatically caused lipid peroxidation in MP ingestion, and they may be connected to enzyme-induced lipid peroxidation [6]. Thus, when compared to normal rats, treated rats with MP may benefit from an E. fragilis hydroalcoholic extract to possibly counteract reduced superoxide dismutase and catalase activities. The possible hepatoprotective action of E. fragilis extract is not significantly different from that of silymarin, a well-known standard hepatoprotectant, concerning ALP levels.
However, compared to E. fragilis alone, the combination of E. fragilis and silymarin significantly improves and is less than that of the silymarin-treated group. This effect is reflected by the combination dose used. More investigation is needed to figure out the best combination from Alenda alone or combined with silymarin to get better benefits from the possible synergistic effect that might result. The study found that Silymarin reduces cell edema and inflammation, while E. fragilis extract reduces inflammation, necrosis, and liver lesions caused by MP. The extract may inhibit MP-triggered oxidative liver damage by preventing cells from being immediately affected by MP and decreasing damage caused by slow inflammation. This result aligns with previous studies showing E. fragilis extract’s ability to shield rat liver from oxidative damage caused by deltamethrin [11]. The results showed significant decreases in histopathological analysis, ALB, SGOT, SGPT, and ALP levels. According to Saidia et al. [29], E. alata Aqueous is effective in preventing and treating a variety of organ toxicities.
In summary, the improvement in ALP levels suggests that it has a likely selective impact on drug-induced cholestasis. Ephedra fragilis extract may influence catalase and superoxide dismutase activities, suggesting that the hepatoprotective action of Alenda extract may involve modulating hepatic antioxidant enzymes. That is supported by DPPH Free Radical Scavenging IC50 and high flavonoid content. Ephedra fragilis extract reduces inflammation, necrosis, and liver lesions caused by MP. The extract may inhibit MP-triggered oxidative liver damage by preventing cells from being immediately affected by MP and decreasing damage caused by slow inflammation.
CONCLUSION
The hydroalcoholic extract of E. fragilis was tested for hepatoprotective properties. The extract’s phytochemical composition ends with indications that alkaloids, tannins, and saponins will also get positive results. Ephedra fragilis extract has appreciated Free Radical Scavenging potential, substantial polyphenols, and remarkable flavonoid content. Furthermore, the data obtained indicate that the aerial portion is an aqueous extract of E. fragilis subsp. Alenda has a significant potential to be an anti-drug-induced cholestasis action via lowering ALP levels. These results provide biochemical support for the potential therapeutic use of E. fragilis in treating a few hepatic disorders, including drug-induced cholestasis.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
Ethical approval details is given in the ‘Experiment section’.
DATA AVAILABILITY
All data generated or analyzed during this study are included in this published article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Adam de Beaumais T, Fakhoury M, Medard Y, Azougagh S, Zhang D, Yakouben K, et al. Determinants of mercaptopurine toxicity in pediatric acute lymphoblastic leukemia maintenance therapy. Br J Clin Pharmacol. 2011;71:575–84. CrossRef
2. Suzuki A, Andrade RJ, Bjornsson E, Lucena MI, Lee WM, Yuen NA, et al. Drugs associated with hepatotoxicity and their reporting frequency of liver adverse events in VigiBase. Drug Saf. 2010;33(6):503–22.
3. Benkov K, Lu Y, Patel A, Rahhal R, Russell G, Teitelbaum J. Naspghan Committee on inflammatory bowel disease role of thiopurine metabolite testing and thiopurine methyltransferase determination in pediatric IBD. J Pediatr Gastroenterol Nutr. 2013;56:333–40. CrossRef
4. Pearson DC, May GR, Fick GH, Sutherland LR. Azathioprine and 6-mercaptopurine in Crohn’s disease. A meta-analysis. Ann Intern Med. 1995;123:132–42.
5. Present DH, Korelitz BI, Wisch N, Glass JL, Sachar DB, Pasternack BS. Treatment of Crohn’s disease with 6-mercaptopurine: a long-term, randomized, double-blind study. N Engl J Med. 1980;302:981–7.
6. Coelho T, Andreoletti G, Ashton JJ, Batra A, Afzal NA, Gao Y, et al. Genes implicated in thiopurine-induced toxicity: comparing TPMT enzyme activity with clinical phenotype and exome data in a pediatric IBD cohort. Sci Rep. 2016;6:34658. CrossRef
7. Flores ED, Trujillo J, Bautista E. A review of the Ephedra genus: distribution, ecology, ethnobotany, phytochemistry and pharmacological properties. Molecules. 2016;25(14):3283. CrossRef
8. Hikino H, Konno C, Takata H. Anti-inflammatory principle of Ephedra herbs. Chem Pharm Bull. 1980;28(10):2900–4.
9. Qasem JR. Ephedra alte (Joint-Pine) is an invasive, problematic, weedy species in Jordan’s forestry and fruit tree orchards. ScientificWorldJournal. 2012;2012(2012):971903. CrossRef
10. Djahra AB, Zoubiri F, Benkaddour M, Gouasmi S. Antioxidant and hepatoprotective activity of ephedra alata extracts against intoxication with deltamethrin pesticide in male rats. Pharmacophore. 2023;14(1):19–24.
11. Kwo PY, Cohen SM, Lim JK. ACG clinical guideline: evaluation of abnormal liver chemistries. Am J Gastroenterol. 2017;112:18–35. CrossRef
12. Fanoudi S, Alavi MS, Karimi G, Hosseinzadeh H. Milk thistle (Silybum Marianum) as an antidote or a protective agent against natural or chemical toxicities: a review. Drug Chem Toxicol. 2020;43:240–54.
13. Al-Nadaf AH, Bastoni HM, Hamdan DF. Microwave-assisted efficient extraction of phenolics from Juglans regia L.: pellicle; kernel unripe fruits; and leaves in different solvents. Int J Green Pharm. 2018;12(3):182.
14. Al-Nadaf A, Awadallah A, Hussein R. Juglans regia L. fruit pellicle extract-based bioreduction of silver nanoparticles: structural features and in vivo therapeutic effects against ethanol-induced peptic ulcers. J Pharm Pharmacogn Res. 2024;12(2):193–203. CrossRef
15. Al-Nadaf AH, Thiab S, Obidat R, AL-Arman S, Shahin NA. Pharmacological evaluation of the protective effect of hydroalcoholic extract of walnut (Juglans regia L.) leaf on 6-mercaptopurine-induced hepatotoxicity in rats. Phytomedicine Plus. 2024;4(4);100617. CrossRef
16. Al-Abbassi LM, Sharba IR, Al-Hatemy KT. Study the protective effect of Aloe vera gel extract against hepatotoxicity after being treated with azathioprine in adult rats. AIP Conf Proc. 2023;2475:020010.
17. Awadallah A, Al-Nadaf HA. Topical analgesic and anti-inflammatory properties of bioengineered Juglans Regia L. silver nanoparticles. Pak J Biol Sci. 2023;26(9):493–503. CrossRef
18. Tülümen T, Ayata A, Özen M, Sütçü R, Canatan D. The protective effect of capparis ovata on 6-mercaptopurine-induced hepatotoxicity and oxidative stress in rats. J Pediatr Hematol Oncol. 2015;37:290–4.
19. Eidi A, Moghadam JZ, Mortazavi P, Rezazadeh S, Olamafar S. Hepatoprotective effects of Juglans regia extract against CCl4-induced oxidative damage in rats. Pharm Biol. 2013;51(5):558–65.
20. The French Metavir Cooperative Study Group. Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis c. Hepatology. 1994;20(1):15–20.
21. Ghasemi M, Azarnia M, Jamali M, Mirabolghasemi G, Nazarian S, Naghizadeh MM, et al. Protective effects of Ephedra pachyclada extract on mouse models of carbon tetrachloride- induced chronic and acute liver failure. Tissue Cell. 2014;46:78–85. CrossRef
22. Guenaou I, Nait Irahal I, Errami A, Lahlou FA, Hmimid F, Bourhim N.. Bioactive compounds from Ephedra fragilis: extraction optimization, chemical characterization, antioxidant and antiglycation activities. Molecules. 2021;2;26(19):5998. CrossRef
23. Hussein SA, Barakat HH, Nawar MA, Willuhn G. Flavonoids from Ephedra aphylla. Phytochemistry. 1997;45(7):1529–32.
24. Kmail A, Lyoussi B, Zaid H, Saad B. In vitro assessments of cytotoxic and cytostatic effects of Asparagus aphyllus, Crataegus aronia, and Ephedra alata in monocultures and co-cultures of Hepg2 and THP-1-derived macrophages. Pharmacogn Commun. 2015;5(3):165–72.
25. Molander DW, Wroblewsk F, La Due JS. Transaminase compared with cholinesterase and alkaline phosphatase, an index of hepatocellular integrity. Clin Res Proc. 1955;3:20–4.
26. Pandit A, Sachdeva T, Bafna P. Drug-induced hepatotoxicity: a review. J Appl Pharm Sci. 2012;2:233–43.
27. Nipanikar SU, Chitlange SS, Nagore D. Pharmacological evaluation of hepatoprotective activity of AHPL/AYTAB/0613 tablet in carbon tetrachloride-, ethanol-, and paracetamol-induced hepatotoxicity models in Wistar albino rats. Pharmacognosy Res. 2017;9(Suppl 1):S41–7. CrossRef
28. Pinazo-Bandera JM, Toro-Ortiz JP, Andrade RJ, García-Cortés M. Drug-induced cholestasis: causative agents and challenges in diagnosis and management. Explore Dig Dis. 2023;2:202–22. CrossRef
29. Saidia S, Turki M, Al-Shaikh OA, Alghamdi KH. Ephedra alata subsp. Alenda (Ephedraceae) leaf extracts: phytochemical screening, anti-diabetic, anti-obesity and anti-toxic activities on diabetic-induced liver-kidney-testes toxicities and inhibition of α-amylase and lipase enzymes. Heliyon. 2022;8:e11954.