INTRODUCTION
The development of targeted drug delivery systems has become a major focus in pharmaceutical and biotechnology research. One promising approach in targeted drug delivery is the use of glycoproteins. Glycoproteins are molecules consisting of proteins covalently bonded to oligosaccharides, playing crucial roles in various biological processes, including cell recognition, cell adhesion, and signal transduction [1]. In the context of drug delivery, glycoproteins have several advantages that make them ideal candidates as drug delivery agents. First, glycoproteins have the ability to recognize and bind to specific receptors on the surface of target cells, allowing for more precise drug delivery [2]. Second, the biocompatibility and biodegradability of glycoproteins make them safe for use in biological systems without causing significant side effects [3]. However, despite their great potential, the use of glycoproteins in targeted drug delivery faces several challenges. The main challenge is the stability of glycoproteins in the bloodstream. Glycoproteins are susceptible to degradation by protease and glycosidase enzymes present in the body, which can reduce their effectiveness as drug delivery agents [4]. Additionally, the production and modification processes of glycoproteins for medical applications require advanced technology and high costs [5]. The potential for glycoproteins in drug delivery also extends to their role as stabilizers for hydrophobic drugs. By forming complexes with glycoproteins, hydrophobic drugs can be solubilized and protected from premature degradation, allowing them to reach targeted cells more effectively. This strategy leverages the unique structure of glycoproteins to provide both stability and specificity in drug transport [6].
Pentagamavunon-1 (PGV-1) is a synthetic curcumin analog that has garnered interest for its potential anticancer properties. PGV-1 has shown cytotoxic effects against various cancer cell lines, including breast cancer, colon cancer, and lung cancer cells [7-9]. PGV-1 inhibits cancer cells by inducing cell cycle arrest, promoting apoptosis, reducing inflammation, interfering with specific cancer-related signaling pathways, inhibiting angiogenesis, and possibly interacting with cancer-specific receptors, depending on the cancer type [7,8]. PGV-1 also inhibits enzymes that metabolize reactive oxygen species (ROS), leading to increased ROS levels and resulting in cell death [10]. Similar to curcumin, PGV-1 may have limited solubility in aqueous solutions, which can affect its efficacy in cell culture systems [11]. Glycoprotein complexes could indeed be a potential solution to overcome this problem by increasing their solubility in an aqueous environment and preventing them from precipitating out of solution.
Glycation of bovine serum albumin (BSA) with carbohydrates modifies the protein’s surface, enhancing its solubility in aqueous environments. This increased solubility ensures that drugs bound to BSA remain dissolved in biological fluids, preventing precipitation and enhancing bioavailability [12]. Glycation of BSA with lactose enhances drug delivery by improving the solubility and stability of drug-BSA complexes. Glycated BSA, such as BSA+Lactose (BSA+Lac), incorporates sugar residues that are recognized by specific receptors on cell surfaces, such as those involved in cancer cells [13]. This targeting ability increases the efficiency of drug delivery to specific cells and tissues. The modified BSA can more effectively penetrate cancer cell membranes through receptor-mediated endocytosis. This targeted approach ensures that a higher concentration of the drug is delivered directly into the cancer cells, increasing the therapeutic impact [14].
Research and innovation continue to address these challenges, including the development of techniques to protect glycoproteins from enzymatic degradation and the improvement of more efficient production methods. With these advancements, it is expected that glycoproteins can be more widely used as targeted drug delivery agents, enhancing the effectiveness of drug therapies and reducing unwanted side effects. Therefore, further research on the use of glycoproteins as targeted drug delivery agents is essential. This study specifically aims to develop a drug delivery complex using PGV-1 and glycated BSA (BSA+Lac NPs) to enhance the cytotoxic effect on cancer cells, demonstrating the potential of glycoprotein-based nanoparticles in targeted cancer therapy. Therefore, further research on the use of glycoproteins as targeted drug delivery agents is essential. Understanding the mechanisms of glycoproteins, developing methods to increase their stability, and optimizing their production processes are key steps to realizing the full potential of glycoproteins in modern medical therapy.
METHODS
Materials
BSA and Lac were purchased from Sigma. Glycated BSA (BSA+Lac), which has been prepared previously [15]. PGV-1 was synthesized, purified, and characterized by the Cancer Chemoprevention Research Center at the Faculty of Pharmacy, Gadjah Mada University (CCRC-UGM). Phosphate buffered saline (PBS) (1×, pH 7.4) was obtained from Fujifilm. Cisplatin and the Dulbecco modified Eagle medium (DMEM) with high glucose and penicillin–streptomycin were procured from Fujifilm Wako Pure Chemical Corp., Osaka, Japan, and fetal bovine serum (FBS) from Hyclone, Logan, UT. All other reagents were of analytical grade.
Preparation of complex between BSA, BSA+Lac and PGV-1
BSA nanoparticles (BSA NPs) and BSA+Lac nanoparticles (BSA+Lac NPs) were prepared. BSA and BSA+Lac were dissolved in a 50 mM NaCl aqueous solution to achieve a concentration of 0.24 mM. The pH of the solution was then adjusted to 7.0 using 3M NaOH. A 2.0 ml aliquot of the solution was placed in a glass tube, and the solution was heated in a water bath at 60°C–70°C for 5 minutes. Immediately afterward, the tube was transferred to an ice bath for 10 minutes to rapidly cool the solution and stabilize the formed nanoparticles. Using the dispersion of BSA NPs and BSA+Lac NPs, 40 μM of each was prepared. To enhance stability and improve solubility, 0.1% (w/v) polyethylene glycol (PEG) was added to the dispersions. Subsequently, 1× PBS at pH 7.4 was added to maintain isotonic conditions. An ethanolic solution of PGV-1 was prepared at a concentration of 100 μM. This solution was then added to the nanoparticle dispersion, ensuring gentle mixing to promote an even distribution of PGV-1 within the nanoparticles. The resulting dispersions were placed in darkness for 3 hours for equilibration to form PGV-1+BSA NPs and PGV-1+BSA+Lac NPs. The complexes were then freeze-dried using an Alpha 3–4 LSCbasic (Martin Christ, Germany) for 24 hours. The obtained freeze-dried PGV-1+BSA NPs and PGV-1+BSA+Lac NPs were used for scanning electron microscopy (SEM) and Fourier transform infrared spectroscopy (FT-IR) analysis.
Measurement of size distribution
Dispersion containing each complex PGV-1+BSA NPs and PGV-1+BSA+Lac NPs was prepared. The size and polydispersity index were assessed using a Zetasizer Nano (Malvern Instruments, UK) at 25°C.
Transmittance electron microscopy (TEM)
The complex morphology was examined using a TEM (H-7000, Hitachi, Japan). A drop of sample was applied to a 200-mesh copper grid, negatively stained with 2% phosphotungstic acid for 30 seconds, dried under a heat lamp, and then observed with the TEM.
Scanning electron microscopy (SEM)
The morphology of the freeze-dried complexes was observed by using a scanning electron microscopy (SEM) JEOL JSM-6510LA. The samples were attached to an aluminum stub using adhesive carbon tape.
Fourier transform infrared spectroscopy (FT-IR)
The FT-IR spectra of BSA, Lactose, PGV-1, PGV-1+BSA physical mixture, PGV-1+BSA NPs, PGV-1+BSA+Lac physical mixture, and PGV-1+BSA+Lac NPs were recorded using a Bruker TENSOR 27 FT-IR spectrometer (USA) to confirm the successful synthesis of PGV-1-BSA-Lac NPs and PGV-1-BSA NPs. Each sample was thoroughly mixed with KBr and then compressed into pellets. These pellets were analyzed within the range of 4,000 to 400 cm−1.
Cell culture
HepG2, T47D, and Vero cell lines were obtained from the cell line collection of the CCRC, UGM, Indonesia. The cells were maintained using high-glucose DMEM (Gibco) with supplementary of 10% FBS (Gibco), 1.5% penicillin-streptomycin (Gibco), and incubated in a 5% CO2 incubator at 37°C.
Cytotoxic MTT assay
Cells were cultured in 96-well plates at a density of 1 × 104 cells per well and divided into two groups: untreated and treated. Cells were incubated with varying concentrations of PGV-1, ranging from 0.1 to 5 µM for HepG2 and T47D cells and 1 to 125 µM for Vero cells. After 24 hours (Vero cells) and 48 hours (HepG2 and T47D cells) of incubation, the culture medium was removed, and the cells were rinsed with PBS (Sigma). A solution of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (Sigma) at a concentration of 5 mg/mL in PBS was diluted in DMEM at a 1:9 ratio. Then, 100 μl of this diluted reagent was added to each well. Following a 3-hour incubation, the reaction was stopped by adding 10% sodium dodecyl sulfate in 0.01 N HCl. The plates were left to incubate overnight at room temperature in the dark. Afterward, the plates were shaken for 10 minutes to ensure the complete dissolution of the purple formazan crystals. The amount of purple formazan was measured at 550 nm using a microplate reader (BIO-RAD 680XR). Cell viability was calculated as follows:
Absorbance data was converted into the percentage of viable cells and analyzed statistically using a correlation method, followed by a significance test for the percentage of viable cells. The IC50 was then calculated using the logarithmic probit method to establish the linearity between the log concentration and the percentage of viable cells. The selectivity index (SI) was determined as the ratio of the IC50 for the normal cell line (Vero) to the IC50 for the respective cancerous cell lines (HepG2 and T47D).
Senescence-associated β-galactosidase (SA-βGal) assay
The evaluation of β-galactosidase expression related to senescence was conducted using the senescence-associated β-galactosidase assay . In this process, Vero cells (2 × 104 cells per well) were first seeded in a 24-well plate and incubated for 24 hours. After that, samples and cisplatin were exposed to cells and then incubated for 24 hours. Following incubation, the cells were washed twice with 1X PBS. A fixation buffer was then applied and left for a designated time, followed by another wash with 1X PBS. Next, 650 µl of X-Gal solution was added, and the cells were incubated at 37°C. After 72 hours, the cells were examined under a microscope (Olympus CKX-41) at 200× magnification. The appearance of blue cells indicated β-galactosidase-positive cells, which are markers of senescence.
Data analysis
The cytotoxic and senescence assay data were presented as mean ± SD and analyzed with SPSS 21.0 software. Statistical significance between the untreated group and different treatment groups was evaluated using one-way ANOVA. The corresponding p-values (**p <0 .01) are included in each figure within the experiments.
RESULT
Preparation and characterization of complex between BSA, BSA+Lac and PGV-1
The characteristics of the obtained complexes are summarized in Table 1. Native BSA and BSA+Lac (without heating) with an average size of 15–30 nm became BSA NPs and BSA+Lac NPs (heating at 60°C–70°C) with an average size of 100–300 nm. The PGV-1 complex was analyzed using TEM, with the results presented in Figure 1. Both BSA and the BSA+Lac complex exhibited a spherical morphology. In contrast, PGV-1 complexed with BSA and BSA+Lac formed an irregular surface structure, which is likely indicative of the incorporation of PGV-1 within the complex.
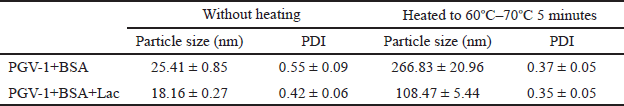 | Table 1. Characteristics of the complexes in 1 × PBS, pH 7.4 containing 0.1% PEG. [Click here to view] |
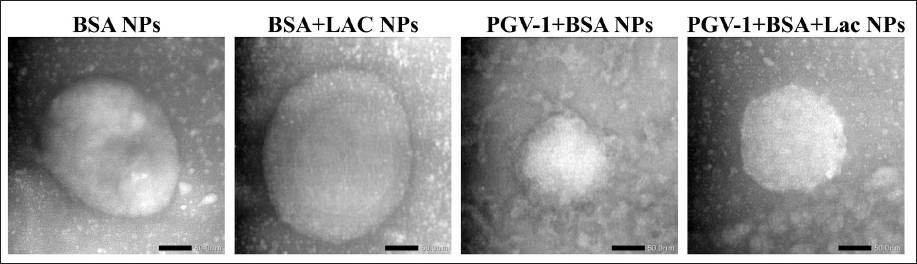 | Figure 1. TEM images at 80,000× magnification of BSA NPs, BSA+Lac NPs, PGV-1+BSA NPs, and PGV-1+BSA+Lac NPs. [Click here to view] |
The SEM images (Fig. 2) demonstrate clear morphological differences between BSA, BSA+Lac NPs, and PGV-1+BSA+Lac NPs. In the BSA image, the structure appears smooth and layered, characteristic of protein aggregates. With the addition of lactose (BSA+Lac NPs), the structure becomes rougher and more granular, indicating that heterogeneous particles are formed due to interactions between BSA and lactose. When PGV-1 is introduced (PGV-1+BSA+Lac NPs), the morphology undergoes a notable transformation, with dense nanoparticles forming and smaller aggregates evenly distributed, implying molecular interactions between PGV-1, BSA, and lactose, and creating a more complex nanoparticle formation.
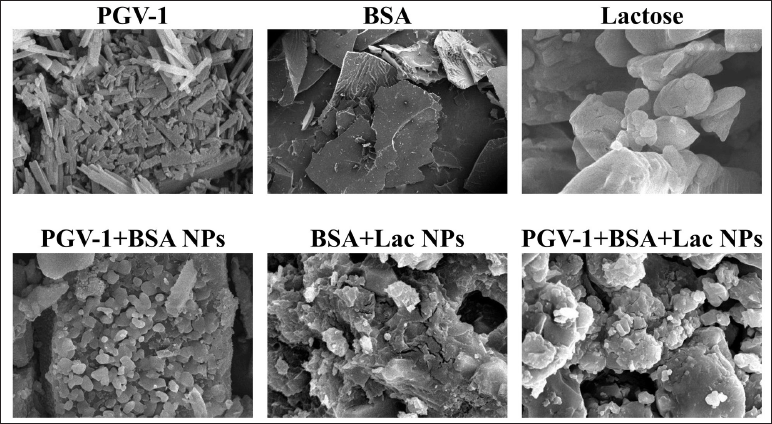 | Figure 2. SEM images at 5,000× magnification of PGV-1, BSA, Lactose, PGV-1+BSA NPs, BSA+Lac NPs, and PGV-1+BSA+Lac NPs. [Click here to view] |
According to the FT-IR spectra (Fig. 3) of BSA and BSA+Lac NPs, the amide I and amide II bands of BSA shifted from 1,658 to 1,656 cm−1 and 1,536 to 1,546 cm−1, respectively. The broad peak at 3,380 cm−1 of Lac (Fig. 3b) is related to the aromatic sugar group with O−H as the main functional group. As shown in Figure 3D, all the characteristic absorption peaks of Lac could be found in the FT-IR spectra of the BSA+Lac NPs. To determine whether PGV-1+BSA+Lac NPs were successfully synthesized, the PGV-1 spectrum (Fig. 3c) was compared to the spectrum of PGV-1+BSA+Lac NPs (Fig. 3e). A shift in the PGV-1-related peak from 3,372 to 3,405 cm-¹ was observed, and the broadening of the valley at 3,405 cm-¹ indicated enhanced hydrogen bonding [16]. Additionally, the PGV-1 peak at 1,259 cm-¹ slightly shifted to 1,261 cm-¹, while the peak at 1,154 cm-¹ remained unchanged. The amide I and amide II absorption peaks at 1,656 and 1,546 cm-¹, respectively, also showed no shift. However, the hydrogen bonding peak of BSA+Lac at 2,900 cm-¹ shifted to 2,919 cm-¹.
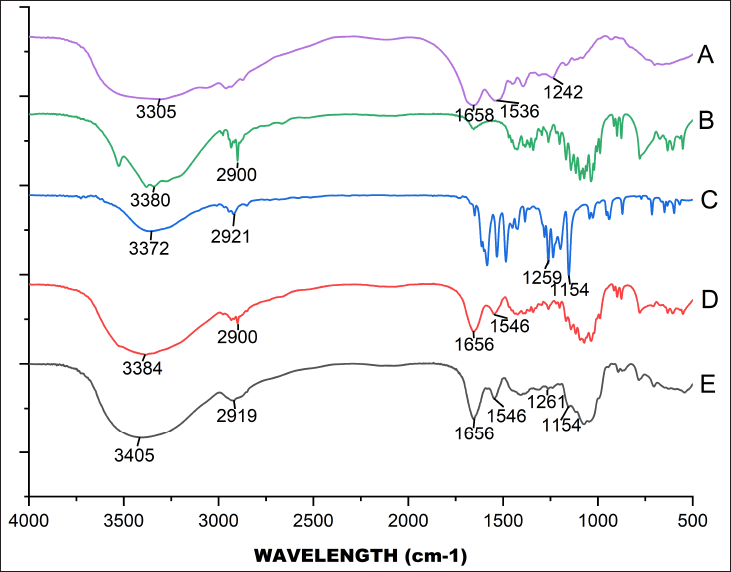 | Figure 3. FT-IR spectra for BSA (A), Lactose (B), PGV-1 (C), BSA+Lac NPS (D), and PGV-1+BSA+Lac NPs (E). [Click here to view] |
Cytotoxic effect in HepG2, T47D, and Vero cells
To evaluate the cytotoxic effects of PGV-1 and its complexes, individual cytotoxicity tests were initially performed using the MTT assay. PGV-1 (free form and complexes) was tested at concentrations ranging from 0.1 to 5 µM on HepG2 and T47D cells (Fig. 4a and b). The results indicated that the PGV-1+BSA+Lac complex was more cytotoxic than PGV-1+BSA and free PGV-1 in both HepG2 and T47D cells, with IC50 values of 0.049 and 0.398 µM, respectively. In HepG2 cells, the IC50 value of the PGV-1+BSA+Lac complex is 49× lower compared to its free form, while in T47D cells, the IC50 value of the PGV-1+BSA+Lac complex is 8× lower.
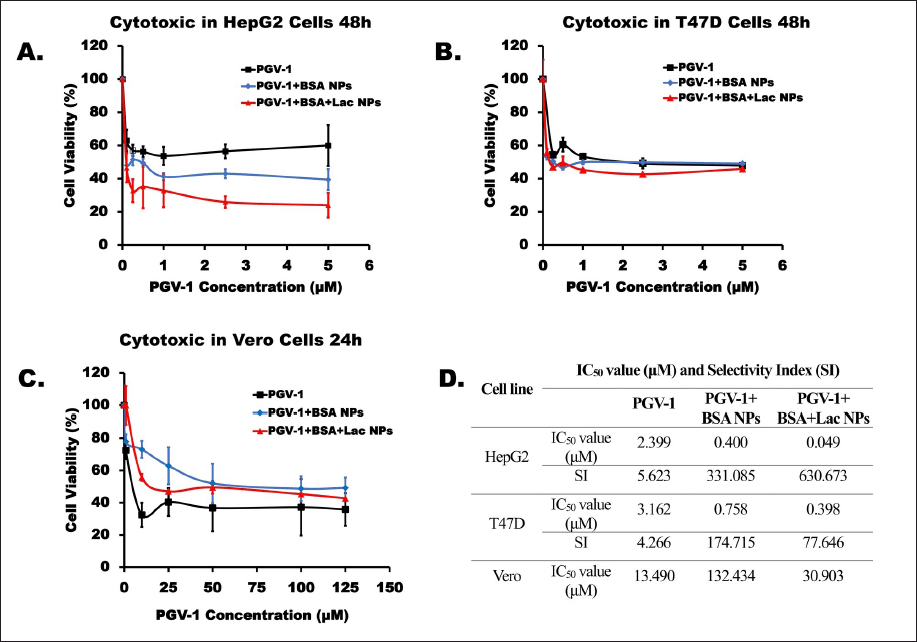 | Figure 4. Cytotoxic effects on HepG2 (A) and T47D (B) cells over 48 hours and Vero (C) cells over 24 hours. The cells were cultured for 24 hours and then exposed to samples of PGV-1, PGV-1+BSA NPs, and PGV-1+BSA+Lac NPs. Cell viability was assessed using the MTT assay in triplicate (n = 3). The IC50 and SI values for each sample are presented in the table. The SI is calculated by comparing the IC50 of the samples against Vero and HepG2 nor T47D cells. [Click here to view] |
The cytotoxicity of these compounds was also assessed in normal cells (Vero) using the MTT assay at concentrations up to 125 µM (Fig. 4c). The results demonstrated that the PGV-1+BSA complex exhibited the highest IC50 value (132.434 µM) compared to the PGV-1+BSA+Lac complex (30.903 µM) and free PGV-1 (13.490 µM). These findings suggest that the PGV-1 complex can reduce the cytotoxic effects of PGV-1 on normal cells, even though free PGV-1 is not toxic to normal cells.
Furthermore, by comparing the IC50 ratios of normal cells to cancer cells, the selectivity of these compounds was determined (Fig. 4d). The results revealed that PGV-1 was selective for HepG2 and T47D cancer cells, with SI values of 5.623 and 4.266 for Vero cells, respectively. PGV-1+BSA complex treatment showed SI values for HepG2 and T47D cells of 331.085 and 174.715, respectively, and the PGV-1+BSA+Lac complex showed SI values for HepG2 and T47D cells of 630.673 and 77.646, respectively. Since the SI values for both PGV-1 and the complexes were greater than 3, it can be concluded that these compounds exhibit high selectivity for the tested cancer cells.
Cellular senescence in Vero cells
The potential of PGV-1 as an anticancer agent often affects not only cancer cells but also normal cells. Our investigation revealed that treatment with PGV-1, both in its free form and in complex form, significantly (p < 0.01) reduced the number of β-galactosidase-positive cells compared to Cisplatin (Fig. 5b), which is a known senescence-inducing agent [17]. Furthermore, when comparing PGV-1 in its free form to its complex form, the PGV-1+BSA+Lac NPs exhibited a significant (p < 0.01) decrease in the number of β-galactosidase-positive cells. These findings suggest that the number of normal cells undergoing senescence is lower with the PGV-1+BSA+Lac complex compared to its free form, and even more so compared to Cisplatin.
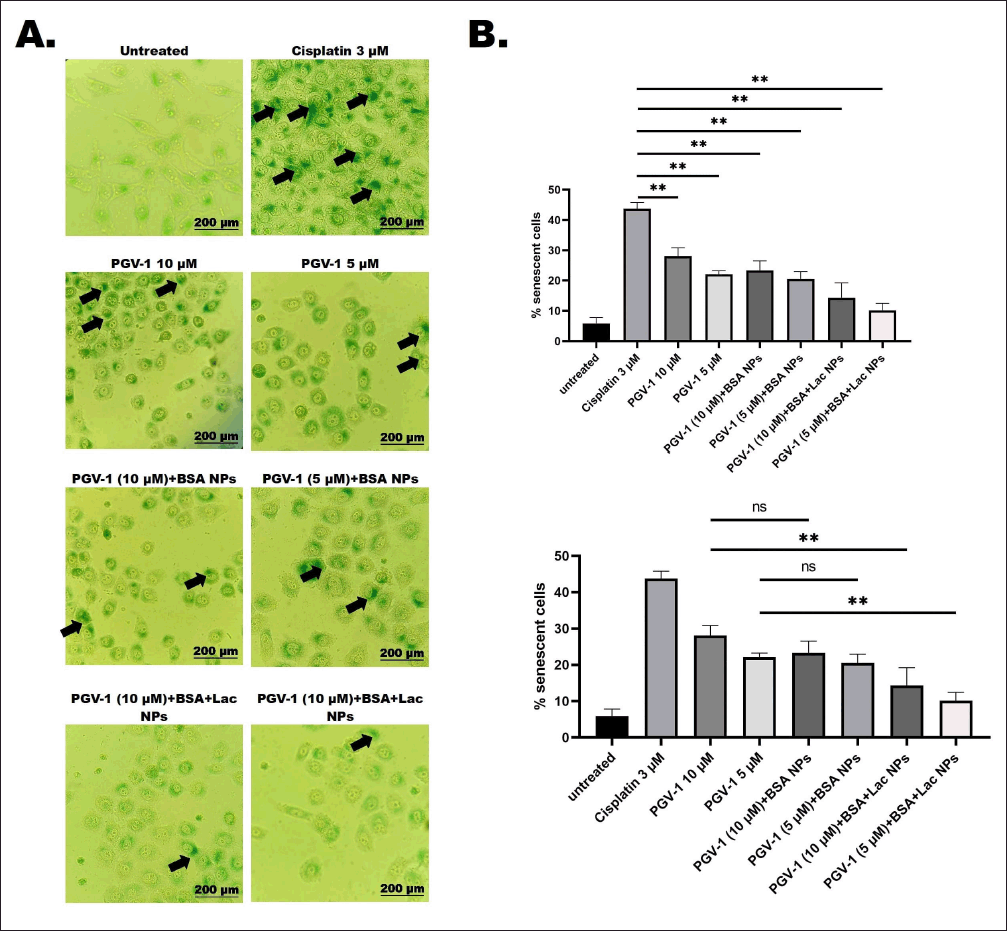 | Figure 5. Cellular senescence in vero cells following treatment with PGV-1 and the complexes. Senescent cells were identified using the SA-β-galactosidase staining assay. Vero cells were seeded at 2 × 10? cells/ml and treated for 24 hours with PGV-1 (5 and 10 μM) and its complexes at the same concentrations. Afterward, senescence was assessed through β-galactosidase staining. For the positive control, cells were exposed to 3 μM cisplatin for 24 hours. The proportion of β-galactosidase-positive (senescent) cells was calculated (n = 3). (A) Cellular morphology of Vero cells was observed after 72 hours of staining under a 200× inverted microscope. Black arrows show accumulation of SA-β-Galactosidase on the cells (B) Quantification of senescent Vero cells was performed (ns: not significant; **p < 0.01). [Click here to view] |
DISCUSSION
To produce nanoparticles from BSA and BSA+Lac, we utilized a heat-induced aggregation method [18,19]. Since PGV-1 is heat-sensitive, we adopted a two-step process to form the complex with BSA, BSA+Lac, and PGV-1. This involved first preparing the BSA and BSA+Lac nanoparticles, followed by combining them with PGV-1. In the heat-induced aggregation technique, the size of the resulting protein aggregates is influenced by the heating temperature and solution conditions, including protein concentration, pH, and ionic strength [19,20]. This size increase reflects the nature of the heat-induced aggregation process, where protein molecules aggregate due to thermal energy. Heating proteins like BSA denatures their structure, exposing hydrophobic regions and enabling aggregation into larger particles. The transformation from small (native BSA) to larger nanoparticles is likely driven by structural changes due to heating [21]. These larger nanoparticles are more suitable for complexing with bioactive molecules like PGV-1, which can benefit from enhanced stability and controlled release properties.
TEM analysis, as depicted in Figure 1, shows that the BSA and BSA-Lac complexes maintain a spherical shape, a characteristic shape of many protein-based nanoparticles. However, when PGV-1 is introduced, the surface morphology becomes irregular. This irregular surface suggests the integration of PGV-1 within the protein complex. The incorporation of PGV-1 likely disrupts the uniform aggregation of BSA and BSA-Lac, altering the nanoparticle’s surface structure. The structural analysis using SEM and TEM confirmed that the PGV-1 complexes exhibited distinct morphological changes compared to unmodified BSA, indicating the successful incorporation of PGV-1 into the nanoparticle complexes. The irregular surface structures observed for the PGV-1 complexes may reflect the physical interactions between BSA NPs, BSA+Lac NPs, and PGV-1, which could contribute to their enhanced functional properties [22].
In addition, the enhanced cytotoxicity and selectivity of PGV-1 when complexed with BSA and BSA+Lac may be attributed to several key mechanisms. First, glycation of BSA with lactose potentially improves the bioavailability and cellular uptake of PGV-1 by increasing solubility in aqueous environments. This glycation could also promote selective interactions with cancer cells, where certain sugar-binding receptors facilitate the binding or uptake of these glycated molecules [23].
Furthermore, PGV-1’s improved cytotoxicity within the BSA+Lac complex aligns with findings from other studies on curcumin analogs and protein carriers. Similar research demonstrated that curcumin derivatives showed higher cellular uptake and selectivity in cancer cells when bound to glycosylated proteins, as these structures can engage with sugar-recognizing receptors overexpressed in cancer cells [11]. This comparison supports the notion that glycoprotein-based drug carriers can facilitate drug entry and enhance efficacy specifically in cancerous cells while reducing toxicity in non-cancerous cells.
Moreover, PGV-1+BSA+Lac NPs showed higher selectivity indices, suggesting that glycation plays a crucial role in preventing cytotoxic effects on normal cells while maintaining anticancer activity. Future studies should investigate the role of specific transporters, such as glucose transporters and lactate transporters, in cancer cells that may contribute to the uptake of glycosylated compounds like PGV-1+BSA+Lac [24]. Examining these transport pathways could provide insight into how glycation modifications enhance selectivity and cytotoxicity in cancer cells.
The present study demonstrates the enhanced effectiveness of PGV-1 when complexed with BSA and BSA+Lac nanoparticles, particularly in the context of targeted drug delivery and cytotoxicity against cancer cells. PGV-1, as a curcumin analog, exhibits significant anticancer potential, but its limited solubility in aqueous environments is a known challenge. By forming complexes with BSA NPs and BSA+Lac NPs, the solubility and bioavailability of PGV-1 were significantly improved. This improvement in solubility is attributed to the glycation of BSA with lactose, which alters the protein’s surface and enhances its interaction with aqueous environments. This modification potentially prevents PGV-1 from precipitating, allowing better dispersion in cancer cells [25].
In the cytotoxicity assays, the PGV-1+BSA+Lac NPs showed superior effectiveness in killing HepG2 and T47D cancer cells compared to free PGV-1 and PGV-1+BSA NPs. The IC50 values were markedly lower, suggesting that lactose modification plays a crucial role in enhancing drug delivery efficiency [26]. The sugar residues present in the BSA+Lac complex may facilitate receptor-mediated endocytosis in cancer cells, improving selective uptake of the drug [27]. Interestingly, the PGV-1+BSA+Lac NPs also demonstrated reduced toxicity toward normal cells (Vero), as indicated by their higher IC50 in normal cells. This reduced cytotoxicity toward non-cancerous cells is important, as it suggests that the complex can preferentially target cancer cells while sparing healthy tissues. The high SI of the PGV-1+BSA+Lac complex confirms its potential for selective cancer cell targeting, and the samples displaying SI values higher than three were considered to be highly selective [28].
This study has several limitations, including the fact that the experiments were conducted in vitro using cancer cells as well as normal cells, which does not allow confirmation of efficacy and safety in vivo. Additionally, the stability of the complexes in biological fluids, such as blood plasma, has not been tested, and their long-term toxicity and biocompatibility have not been thoroughly evaluated. Future studies could address these limitations by conducting in vivo tests to validate the selective targeting and therapeutic effects of the PGV-1+BSA+Lac complexes in animal models. In particular, studying the biodistribution, pharmacokinetics, and overall safety profile of the complexes will be essential for translating this promising formulation into clinical applications.
In conclusion, the complexation of PGV-1 with BSA+Lac not only enhances its cytotoxicity against cancer cells but also reduces toxicity in normal cells. These findings suggest that the PGV-1+BSA+Lac complex holds significant promise as a novel approach for targeted cancer therapy, offering a more effective and safer drug delivery system.
CONCLUSION
We developed a simple formulation method for preparing complexes of BSA NPs, BSA+Lac NPs, and PGV-1. The study demonstrated that the complexation of PGV-1 with BSA NPs and BSA+Lac NPs enhances cytotoxicity against HepG2 and T47D cancer cells. The PGV-1+BSA+Lac NPs exhibited the highest selectivity and reduced toxicity in normal cells, making them a promising candidate for targeted cancer therapy. Future research should focus on in vivo applications of this formulation to evaluate its biodistribution, stability, and pharmacokinetics in a living organism. These in vivo assessments will be crucial in advancing this nanoparticle complex toward clinical testing and potential therapeutic use in cancer patients. Although the current results show promise, additional study is necessary to assess the effectiveness and safety of this novel formulation.
ACKNOWLEDGMENTS
The authors thank eAsia-JRP 2023-2024, which provides the cell lines.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This work is funded by the “Doctoral Dissertation Research (Penelitian Disertasi Doktor/PDD)” program, the Ministry of Education, Culture, Research, and Technology of Indonesia (Grant No. 2725/UN1/DITLIT/Dit-Lit/PT.01.03/2024).
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Chen F, Huang G. Application of glycosylation in targeted drug delivery. Eur J Med Chem. 2019;182:111612. CrossRef
2. Chen D, Liu X, Lu X, Tian J. Nanoparticle drug delivery systems for synergistic delivery of tumor therapy. Front Pharmacol. 2023;14:1111991. CrossRef
3. Yusuf A, Almotairy ARZ, Henidi H, Alshehri OY, Aldughaim MS. Nanoparticles as drug delivery systems: a review of the implication of nanoparticles’ physicochemical properties on responses in biological systems. Polymers (Basel). 2023;15(7):1596. CrossRef
4. Lee MF, Poh CL. Strategies to improve the physicochemical properties of peptide-based drugs. Pharm Res. 2023;40(3):617–32. CrossRef
5. Birrer MJ, Moore KN, Betella I, Bates RC. Antibody-drug conjugate-based therapeutics: state of the science. JNCI J Natl Cancer Inst. 2019;111(6):538–49. CrossRef
6. Jain K, Kesharwani P, Gupta U, Jain NK. A review of glycosylated carriers for drug delivery. Biomaterials. 2012;33(16):4166–86. CrossRef
7. Lestari B, Nakamae I, Yoneda-Kato N, Morimoto T, Kanaya S, Yokoyama T, et al. Pentagamavunon-1 (PGV-1) inhibits ROS metabolic enzymes and suppresses tumor cell growth by inducing M phase (prometaphase) arrest and cell senescence. Sci Rep. 2019;9(1):1–12. CrossRef
8. Meiyanto E, Septisetyani EP, Larasati YA, Kawaichi M. Curcumin analog pentagamavunon-1 (PGV-1) sensitizes Widr cells to 5-fluorouracil through inhibition of NF-κB activation. Asian Pacific J Cancer Prev APJCP. 2018;19(1):49. CrossRef
9. Meiyanto E, Putri H, Larasati YA, Utomo RY, Jenie RI, Ikawati M, et al. Anti-proliferative and anti-metastatic potential of curcumin analogue, pentagamavunon-1 (PGV-1), toward highly metastatic breast cancer cells in correlation with ROS generation. Adv Pharm Bull. 2019;9(3):445. CrossRef
10. Meiyanto E, Novitasari D, Utomo RY, Susidarti RA, Putri DDP, Kato J. Bioinformatic and molecular interaction studies uncover that CCA-1.1 and PGV-1 differentially target mitotic regulatory protein and have a synergistic effect against leukemia cells. Indones J Pharm. 2022;225–33. CrossRef
11. Endah E, Wulandari F, Putri Y, Jenie RI, Meiyanto E. Piperine increases Pentagamavunon-1 anti-cancer activity on 4T1 breast cancer through mitotic catastrophe mechanism and senescence with sharing targeting on mitotic regulatory proteins. Iran J Pharm Res IJPR. 2022;21(1). CrossRef
12. Huang Y, Hu L, Huang S, Xu W, Wan J, Wang D, et al. Curcumin-loaded galactosylated BSA nanoparticles as targeted drug delivery carriers inhibit hepatocellular carcinoma cell proliferation and migration. Int J Nanomedicine. 2018;13:8309. CrossRef
13. Torres-Pérez SA, Torres-Pérez CE, Pedraza-Escalona M, Pérez-Tapia SM, Ramón-Gallegos E. Glycosylated nanoparticles for cancer-targeted drug delivery. Front Oncol. 2020;10:605037. CrossRef
14. Teran-Saavedra NG, Sarabia-Sainz JA, Silva-Campa E, Burgara-Estrella AJ, Guzmán-Partida AM, Ramos-Clamont Montfort G, et al. Lactosylated albumin nanoparticles: potential drug nanovehicles with selective targeting toward an in vitro model of hepatocellular carcinoma. Molecules. 2019;24(7):1382. CrossRef
15. Ledesma-Osuna A, Ramos-Clamont G, Vázquez-Moreno L. Characterization of bovine serum albumin glycated with glucose, galactose and lactose. Acta Biochim Pol. 2008;55(3):491–7. CrossRef
16. Bourassa P, Kanakis CD, Tarantilis P, Pollissiou MG, Tajmir-Riahi HA. Resveratrol, genistein, and curcumin bind bovine serum albumin. J Phys Chem B. 2010;114(9):3348–54. CrossRef
17. Li C, Xie N, Li Y, Liu C, Hou FF, Wang J. N-acetylcysteine ameliorates cisplatin-induced renal senescence and renal interstitial fibrosis through sirtuin1 activation and p53 deacetylation. Free Radic Biol Med. 2019;130:512–27. CrossRef
18. Zai K, Yuzuriha K, Kishimura A, Mori T, Katayama Y. Preparation of complexes between ovalbumin nanoparticles and retinoic acid for efficient induction of tolerogenic dendritic cells. Anal Sci. 2018;34(11):1243–8. CrossRef
19. Sponton OE, Perez AA, Carrara CR, Santiago LG. Complexes between ovalbumin nanoparticles and linoleic acid: stoichiometric, kinetic and thermodynamic aspects. Food Chem. 2016;211:819–26. CrossRef
20. Weijers M, Visschers RW, Nicolai T. Light scattering study of heat-induced aggregation and gelation of ovalbumin. Macromolecules. 2002;35(12):4753–62. CrossRef
21. Borzova VA, Markossian KA, Chebotareva NA, Kleymenov SY, Poliansky NB, Muranov KO, et al. Kinetics of thermal denaturation and aggregation of bovine serum albumin. PLoS One. 2016;11(4):e0153495. CrossRef
22. Yadav P, Yadav AB. Preparation and characterization of BSA as a model protein loaded chitosan nanoparticles for the development of protein-/peptide-based drug delivery system. Futur J Pharm Sci. 2021;7:1–9. CrossRef
23. Dall’Olio F, Malagolini N, Trinchera M, Chiricolo M. Mechanisms of cancer-associated glycosylation changes. Front Biosci. 2012;17(1):670. CrossRef
24. Adekola K, Rosen ST, Shanmugam M. Glucose transporters in cancer metabolism. Curr Opin Oncol. 2012;24(6):650–4. CrossRef
25. Balzarini J. Targeting the glycans of glycoproteins: a novel paradigm for antiviral therapy. Nat Rev Microbiol. 2007;5(8):583–97. CrossRef
26. Calvaresi EC, Hergenrother PJ. Glucose conjugation for the specific targeting and treatment of cancer. Chem Sci. 2013;4(6):2319–33. CrossRef
27. Pastuch-Gawo?ek G, Szreder J, Domi?ska M, Pielok M, Cichy P, Grymel M. A small sugar molecule with huge potential in targeted cancer therapy. Pharmaceutics. 2023;15(3):913. CrossRef
28. Wu X-L, Liu L, Wang Q-C, Wang H-F, Zhao X-R, Lin X-B, et al. Antitumor activity and mechanism study of riluzole and its derivatives. Iran J Pharm Res IJPR. 2020;19(3):217. CrossRef