INTRODUCTION
Cancer is one of the main public health problems in the world causing around 10 million deaths in 2022 [1,2]. Cancer is defined as a heterogeneous group of malignant diseases characterized by unregulated proliferation and invasion of abnormal cells into different tissues and organs of the body. This uncontrolled division process originates from genetic alterations that modify the control mechanisms of the cell cycle, apoptosis, and genomic stability [3]. Among the main causal factors of cancer are genetic mutations that can be inherited or acquired throughout life. In addition, lifestyle factors, such as tobacco use, alcohol, poor diet, and lack of physical activity, also contribute significantly to the risk of developing certain types of cancer. The most common cancers are breast, lung, colon and rectum, and prostate cancer. About 10 million deaths, or one in six deaths, were caused by cancer in 2020, making it the second most common cause of death worldwide, according to the WHO [2].
Surgery, radiation therapy, chemotherapy, and other treatments are available for this illness. Despite serious side effects and a high rate of treatment failures brought on by drug resistance, chemotherapy is still the most often utilized form of treatment. Surgery, radiation therapy, chemotherapy, and other treatments are available for this illness. Chemotherapy is the most widely utilized treatment modality, despite its severe side effects and high rate of drug resistance-related treatment failures [4]. For these reasons, in order to combat resistance and increase the efficacy of chemotherapy, it is imperative to create new medications with lower toxicity and higher selectivity [5].
Heterocycles are continuously being exploited to find novel lead compounds. Heterocycles make up most natural products, such as carbohydrates, alkaloids (such as morphine, atropine, and reserpine), proteins, amino acids, nucleic acids, hemoglobin, hormones, enzyme cofactors, vitamins, and proteins. Furthermore, due to its intriguing pharmacological properties, and adaptability, and that these compounds may be effective against a variety of metabolic pathways and cellular processes involved in cancer pathogenesis, the heterocyclic nucleus is present in many therapeutically approved drugs, including anticancer medications such as methotrexate, vinblastine, vincristine, daunorubicin, 5-fluorouracil (5-FU), and doxorubicin [6-10].
Nowadays, molecular hybridization is used for rationally designing new therapeutic alternatives [11,12]. This method creates novel hybrid compounds that may be more effective than their original pharmacophores by covalently combining the pharmacophoric moieties of several bioactive substances [13-17]. Another approximation used in drug discovery is molecular conjugation. It consists of the structural modification of compounds that are biologically active.
One of the methodologies used to carry out the molecular hybridization and conjugation is the so-called click reaction, which is the name given to a class of reactions that are quick, easy to employ, adaptable, regiospecific, and yield large amounts of product. An illustration of this reaction is the CuI-catalyzed Huisgen 1,3-dipolar cycloaddition of azides with terminal alkynes to produce 1,2,3-triazoles (Fig. 1) [18,19].
 | Figure 1. General representation of the click reaction for obtaining hybrid molecules. [Click here to view] |
Morten Meldal and Barry Sharpless were granted the 2022 Nobel Prize in Chemistry for establishing the principles of click chemistry and advancing chemistry into the functionalism time. Carolyn Bertozzi, who started mapping cells with click chemistry and expanded its use, shares the award with them. Currently, her bioorthogonal protocols are helping to develop more focused cancer therapies [20].
Click reaction can be carried out with readily available reagents, starting ingredients, and environmentally friendly solvents such as water. Furthermore, this reaction can be carried out with straightforward workup and purification techniques for product separation under ambient reaction conditions. The production of 1,4-disubstituted-1,2,3-triazoles in a regiospecific manner with extremely high yields can be easily accessed with this synthetic technique. The resulting triazole has advantageous physicochemical characteristics (such as a propensity to form hydrogen bonds, stacking and dipole–dipole interactions, and familiarity with amide bonds in terms of planarity and distance), which enhances target binding and cell permeability and is highly resistant to biotransformation [11].
A method that helps in providing a macroscopic perspective of substantial volumes of scientific literature is bibliometric analysis. This information can be used to assess the research patterns during a defined timespan. In click reaction, there are several reviews available, including advances in triazole and their pharmacological application [2-24,19], constructing liquid separation membranes for water treatment, combination for analysis of food hazard factors, and preparation of biomedical hydrogels, among others [25].
In the case of cancer, 1,2,3-triazole-containing hybrids and conjugates are a useful scaffold for the development of novel drug candidates because they could exert dual or multiple anticancer mechanisms of action [18,26,27].
The current study summarizes the new 1,2,3-triazole-incorporating hybrids and conjugates reported in the last years. In addition, a bibliometric study of these compounds in cancer was carried out.
METHODS
Database selection
This study relied exclusively on the Scopus database to conduct a bibliometric analysis of triazole-based click chemistry for anticancer applications. Scopus was selected due to its extensive coverage across disciplines, including chemistry and biomedical sciences, as well as its rigorous indexing criteria, which support reliable bibliometric insights. Expanding the search to include additional databases, such as Web of Science or PubMed, could provide further insights but would likely involve considerable overlaps with the Scopus-indexed studies.
Search strategy
The bibliographic data retrieved include the most relevant works related to “click reaction” and “hybrid.” The search query was designed using the terms “Click reaction” and “hybrid,” connected with the Boolean operator “AND.” The final query was as follows:
First query: (TITLE-ABS-KEY (“click reaction”) AND TITLE-ABS-KEY (hybrid)) AND (LIMIT-TO (DOCTYPE, “ar”) OR LIMIT-TO (DOCTYPE, “re”))
Timespan: 2007 to August 25, 2024.
Second query: (TITLE-ABS-KEY (“click reaction”) AND TITLE-ABS-KEY (“1,2,3-triazoles”) AND TITLE-ABS-KEY (hybrid)) AND (LIMIT-TO (DOCTYPE, “ar”) OR LIMIT-TO (DOCTYPE, “re”))
Timespan: 2008 to November 21, 2024.
Keywords, abstract, and article title were used to conduct the search, which was then further refined to only include articles and reviews. Duplicate records were removed using the Mendeley Reference Manager to minimize limitations or potential biases in the bibliometric analysis. A schematic of the methods employed in this investigation is shown in Figure 2.
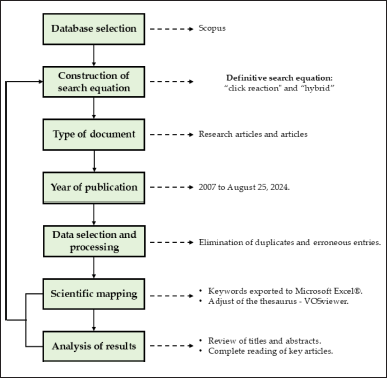 | Figure 2. Research methodology for bibliometric analysis. [Click here to view] |
Bibliometric data export
Citation and bibliographical information, abstracts, and keywords were among the bibliographic data that were obtained for research publications and reviews. CSV files of the data were retrieved from Scopus and exported to Excel®. Afterward, data analysis and visualization were conducted using VOSviewer 1.6.19 [28].
Bibliometric indicators
Furthermore, bibliometric indicators were assessed, including the number and growth of published articles and reviews, subject areas, co-occurrence keyword overlay and network visualizations, top journals and institutions, and international collaboration networks.
RESULTS
Evolution of published papers, journals, and institutions related to triazole-based click reactions
For the first query, a total of 594 articles were retrieved from Scopus. The increase in published research on click reactions and hybrids between 2007 and August 25, 2024, is depicted in Figure 3. During this period, a notable increase in the number of articles was observed, starting from 2012. The primary areas of focus in these studies were: (i) chemistry, (ii) materials science, and (iii) chemical engineering, representing, respectively, 35.7%, 20.4%, and 11.8% of the records. Furthermore, China, India, and the United States ranked first, second, and third, respectively, followed by Iran and Germany as the countries with the most published articles and reviews in this field.
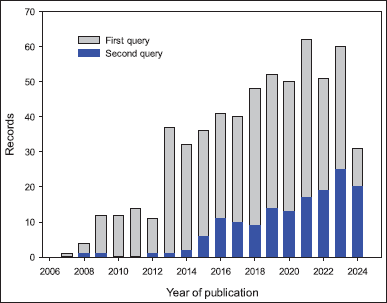 | Figure 3. Development of published research on hybrids produced by click reactions based on triazoles. [Click here to view] |
Likewise, Osmania University, the Chinese Academy of Sciences, and the People’s Republic of China’s Ministry of Education were found to be the top three universities in this discipline. These prestigious universities are located in the previously mentioned top-ranked nations (China and India), as was to be expected.
Similarly, 152 records were obtained for the second query, and the evolution of the number of published studies showed a similar pattern. Nevertheless, 44.7%, 17.6%, and 16.5% of the records were related to: (i) chemistry, (ii) toxicology pharmacology and pharmaceutics, and (iii) genetics, biochemistry, and molecular biology.
Bibliometric networks of hybrids obtained via triazole-based click chemistry
The co-occurrence network of the authors’ keywords for the first query has 33 nodes and 7 clusters (Fig. 4a). Notably, 1,2,3-triazoles, molecular docking, hybrids, and drug delivery (green light, and green and blue clusters) stand out as key topics. In addition, Figure 4b highlights the research topics that were the focus between 2016 and 2021. It is evident that in 2021, studies concentrated on molecular docking as well as anticancer activity (see yellow nodes in Fig. 4b). This suggests that these are emerging topics in the field. Furthermore, the second query also confirms the mentioned topics as keys and emerging areas (Fig. 4c and d). Furthermore, Figure 5 presents the countries’ collaboration networks. India, United States, China, and Germany display the largest nodes inside this network. Based on the described results, the discussion in this study is focused on the anticancer activity of hybrids via click reaction and molecular docking studies.
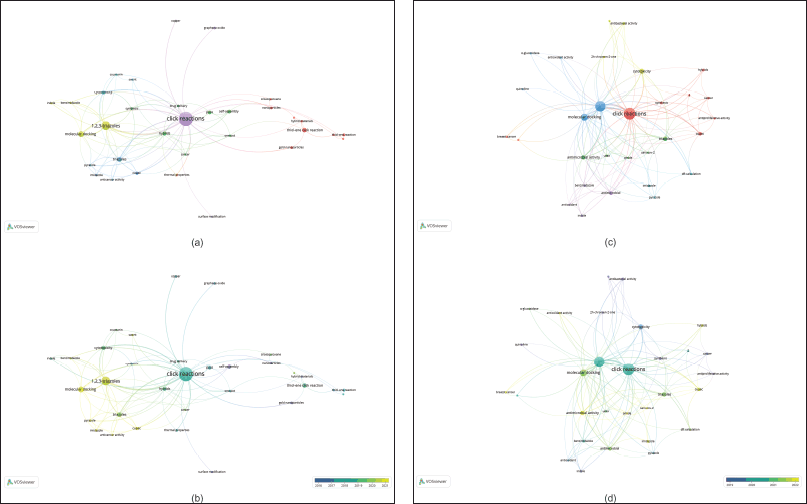 | Figure 4. Bibliometric network of research on hybrids produced by click reactions based on triazoles. First query: a) map of research topics. b) Time-overlapping research-topic map. Keep in mind that a keyword must appear at least five times. Second query: c) map of research topics. d) Time-overlapping research-topic map. Note: the minimum number of occurrences of a keyword is three. [Click here to view] |
DISCUSSION
Anticancer activity of hybrids obtained via click chemistry
Figure 6 displays several hybrids obtained via click chemistry that have demonstrated efficacy against various cancer lines. The structure–activity relationship (SAR) analysis was based on the publication of Dhiman et al. [29] and Kumari et al. [30]. These hybrids are discussed as follows.
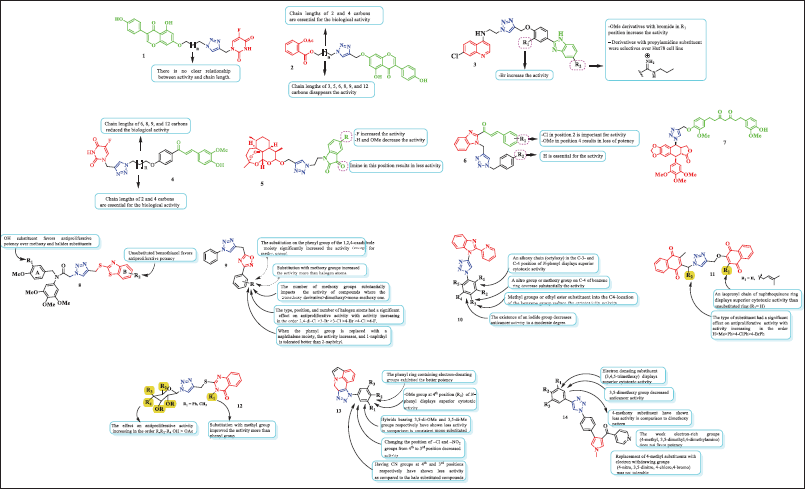 | Figure 6. Chemical structure of hybrids obtained via click chemistry. [Click here to view] |
Hybrid 5-FU-genistein 1 was cytotoxic against SW480 and SW620 adenocarcinoma de colon cell lines with inhibitory concentrations around 62.73 and 50.58 µM, respectively. Compared to genistein alone, the reference drug (5-FU), and its equimolar mixture, this molecule was more selective. In addition, the hybrid induced apoptosis in SW620 cells, likely due to the extrinsic pathway in response to p53 activation (Tp53), as demonstrated by the increase in caspases 3/8 and tumor suppressor protein levels. These effects, which included cell cycle arrest at the S-phase and G2/M, were dependent on both time and concentration. By using molecular docking research, it was found that hybrid 1 attached to the Tp53 and caspases-3/7 protein with an effective binding affinity that ranged from −7.9 to −9.7 kcal/mol when compared to those inhibitors that are currently on the market. MD simulations demonstrated that the hybrid 1/caspase-3 complex showed to have a stable and equilibrium RMSD along the 100-ns MD simulation in addition to validating the stability and logic of docking solutions [15].
ASA-Genistein hybrids 2a (n = 0) and 2b (n = 2) exhibited concentration- and time-dependent action against SW480 cells, showing that when treated with 300 µM, the cell viability decreased by 40.49% and 40.39%, respectively, over a 48-hour period. Docking analysis displayed that these compounds suppress Polo-like kinase 1 activity with a binding affinity of about −10 kcal/mol. Furthermore, our study indicates that in SW480 colorectal cancer cells, these hybrids may elicit synergistic lethal effects via regulating the Bcl-2 (B-cell lymphoma 2) protein system and cyclooxygenase-2 with Vina scores between −10.1 and −9.3 kcal/mol [31]. The quinoline–benzimidazole hybrid 3 (R1 = H and R2 = propylamidine) exhibited potent and selective antiproliferative response over lymphoma cell line growth, HuT78 cells, with an IC50 = 10.86 µM and SI = 9.2. In comparison to untreated cells, this hybrid reduced the number of cells in the G1 and G2/M phases by 68%, p < 0.05, increasing the fraction of subG0/G1 phase cells. The alterations in other cell cycle phases were not statistically significant. The docking analysis on the TAO2 kinase domain (PDB: 2GCD) showed that compound 3 had high binding energy (−140.44 kcal/mol), which is due to the hydrophobic surface of the ATP-binding cleft of TAO2, along with a large number of hydrogen bonds and van der Waals interactions [32].
Hybrids 5-FU-curcumin 4a (n = 0) and 4b (n = 3) displayed good activity against 5-FU-resistant colon cancer cells (SW-480) with inhibitory concentrations at around 17.37 and 2.43 µM, respectively). Furthermore, compound 4b was active over the SW620 cell line (IC50 = 7.51 ± 1.47 µM). These hybrids were more cytotoxic and selective than curcumin alone, 5-FU, the reference drug, and the equimolar mixture of curcumin and 5-FU. Determining the importance of the length of the aliphatic chain that connects the triazole, which binds to 5-FU, the chalcone analog of curcumin was one of the objectives of the biological evaluation in connection to the SAR. The results of the IC50 value analysis showed that the hybrid compounds’ biological activity improved when the linker’s chain length increased to five carbons. The data therefore demonstrated that the hybrids with chain lengths of 6, 8, 9, and 12 carbons reduced the biological potency. In addition, compounds 4b in SW620 and hybrids 4a and 4b in SW480 produced a significant increase in the population in the sub-G0/G1 phase in both cell lines, and compound 4b in SW620 caused S-phase cell cycle arrest [13]. Dihydroartemisinin-isatin hybrids 5a (R = H) and 5b (R = F) were investigated for their antiproliferative effectiveness against A549, doxorubicin-resistant A549 (A549/DOX), and cisplatin-resistant A549 (A549/DDP). The result showed that these compounds exhibited good activity over all cell lines (5a: 8.32, 12.1, and 10.7 µM; 5b: 7.54, 9.89, and 8.77). From this study, the substituents at positions C-3 and C-5 of the isatin moiety significantly affected the activity, according to the structure–activity connections, while the activity was decreased by the imine at position C-3 and increased by the fluoro group at position C-5 [33].
Triazole–benzimidazole–chalcone hybrid 6 (R1 = 2-Cl and R2 = H) exhibited IC50 values of 6.23, 5.89, and 10.7 µM against T47-D, MDA-MB-231, and PC3 cancer cells, as well as the prostate PC3 cancer cell line [34]. This study demonstrated that the benzyl group attached to the 1,2,3-triazole moiety and the chloro substituents at the chalcone ring of the triazole–benzimidazole–chalcone skeleton enhanced the cytotoxic effects in both breast and prostate cancer cell lines. Podophyllotoxin–tetrahydrocurcumin hybrid 7 was evaluated over HCT 116 and HeLa human cancer cell lines. The result showed that this compound exhibited IC50 values of 18 and 83 µM [35].
Recently, Wu et al. [36] reported that triazole hybrids containing benzothiazole 8 possess potent anti-proliferative activities against esophageal cancer cells Kyse30 and EC-109. The SAR for this series revealed that alkoxy and halide groups do not favor activity on A-ring, while the 2-hydroxysustituition on A-ring and unsubstituted benzothiazole (B-ring) was essential for the major antiproliferative activity (IC50 of 0.042 and 0.038 μM, respectively) [36].
On the other hand, the apoptotic antiproliferative action of 1,2,3-triazole bearing a 1,2,4-oxadiazole framework 9 was reported in 2024 by Mahmoud. Comparing these hybrids to Erlotinib (GI50 = 33 nM), most of them demonstrated strong antiproliferative efficacy with GI50 values ranging from 28 to 104 nM. The SAR analysis showed that the activity was considerably enhanced by substitution on the 1,2,4-oxadiazole moiety’s phenyl group, indicating that methoxy group substitution increased the activity more than halogen atom substitution. Besides, the activity of hybrids is greatly impacted by the number of methoxy groups in the order trimethoxy derivative>dimethoxy>mono-methoxy one. The antiproliferative activity of compounds was significantly influenced by the type, position, and number of halogen atoms; activity increased in the order 2,4-di-Cl >3-Br >3-Cl >4-Br >4-Cl >4-F. The activity increases when a naphthalene moiety is added in place of the phenyl group, and 1-naphthyl is more tolerable than 2-naphthyl [37].
On another side, the hybrid of 1,2,3-triazole containing the 2-(2-pyridinyl)-1H-benzo[d]imidazole ring 10 was recently prepared and their anticancer ability was investigated. An SAR analysis revealed that the in vitro anticancer activity was strongly correlated with the type of substituents attached to the N-phenyl group. Thereby, 1) N-phenyl exhibits greater cytotoxic action when an alkoxy chain (octyloxy) is present in its C-3 and C-4 positions; 2) a nitro group or methoxy group on C-4 of benzene ring decrease substantially the activity; 3) the cytotoxicity action is decreased when methyl or ethyl ester substituents are added to the benzene at C4 position; and 4) the presence of an iodide group moderately reduces anticancer activity [38]. In 2024, de Souza et al. [39] reported a new series of naphthoquinone-1H-1,2,3-triazole hybrids 11 exhibiting strong antiproliferative effects in a cell line of breast cancer (MCF-7) at lower concentrations, around 6 μM. An inspection of the structure–activity correlation revealed that an isoprenyl chain of naphthoquinone ring displays superior cytotoxic activity than an unsubstituted ring (R1 = H), as well as the type of substituent in R2 had a notable impact on antiproliferative activity, with potency increasing as follows: H>Me>Ph>4-ClPh>4-BrPh [39]. Abdel-Rahman et al. [40] examined a number of 1,2,3-triazole substituted/quinazolin hybrids 12 for their capacity to induce cytotoxicity to human cancer cell lines, which revealed excellent potency (IC50 range = 5.70–8.10 µM). A close view of the SAR determined that 1) replacement of acetylated glycosides with hydroxylated glycosides increases the effect on antiproliferative activity and 2) the substitution with methyl group on quinazoline ring improved the activity more than the phenyl group [40]. On another side, several quinoline-fused/1,2,3-triazole hybrid molecules 13 were recently reported and their anti-breast cancer activity against MCF-7, MDA-MB-468, and MDA-MB-231 cell lines was investigated. The IC50 values for most potent compounds were found in the range of 2.8–9.4 μM. The authors of the study used the SAR to determine that 1) the phenyl ring with electron-donating groups was the most potent of all the hybrids; 2) the methoxy group at position 4 (R2) of N-phenyl is the most cytotoxic; 3) hybrids with 3,5-di-OMe and 3,5-di-Me groups were less active than consistent mono substituted compounds; 4) moving the –Cl and –NO2 groups from position 4 to the third position reduced activity; and 5) having CN groups at the fourth and third positions, respectively, has demonstrated less activity than the halo substituted compounds [41]. Aravind and collaborators investigated recently the anticancer profile of 1,2,3-triazole incorporated pyrrole derivatives 14 against four types of human cancer cell lines such as human breast cancer (MCF-7), lung cancer (A549), colon cancer (Colo-205), and ovarian cancer (A2780). These compounds exhibited inhibitory concentration in the range of 0.02–11.8 μM. Further SAR studies indicated that electron-donating substituent (3,4,5-trimethoxy) displays the superior cytotoxic activity, 3,5-dimethoxy group decreased anticancer activity, and 4-methoxy substituent decreased activity in comparison to the dimethoxy pattern. The weak electron-rich groups (4-methyl, 3,5-dimethyl,4-dimethylamino) do not favor cytotoxic potency. In addition, the replacement of 4-methyl substituents with electron-withdrawing groups (4-nitro, 3,5-dinitro, 4-chloro, and 4-bromo) resulted in compounds with very poor activities [42].
Anticancer activity of conjugates obtained via click chemistry
Figure 7 shows several conjugates obtained via click chemistry that have been actives over various cancer lines. These conjugates are discussed as follows.
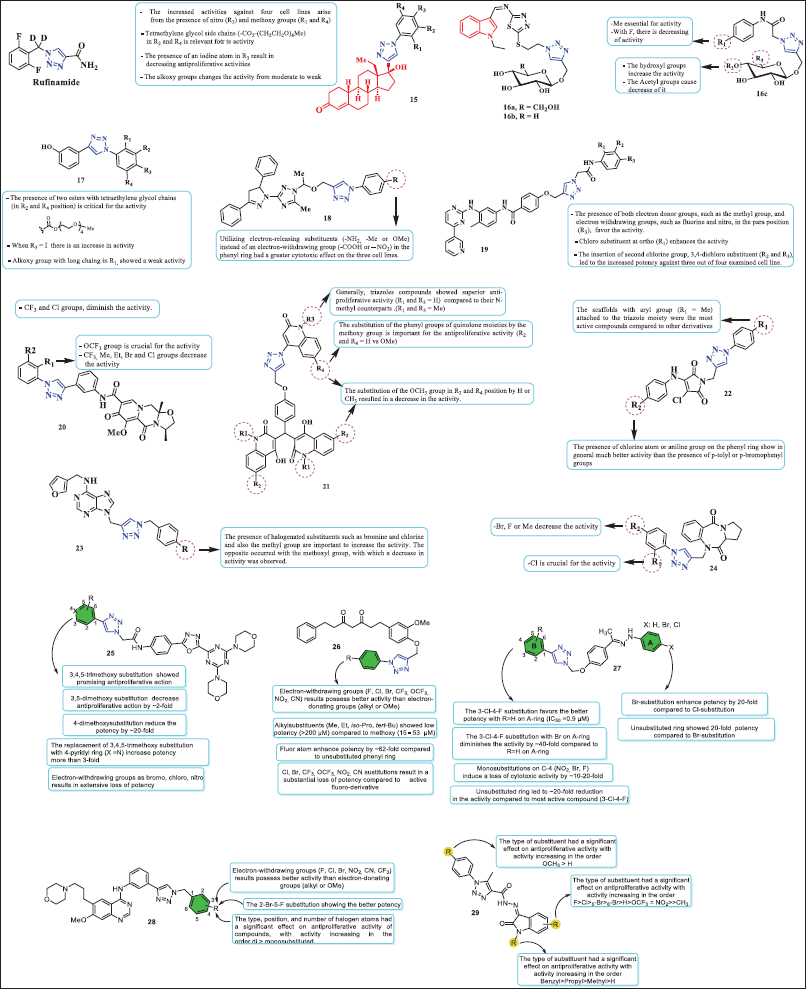 | Figure 7. Chemical structure of conjugates obtained via click chemistry. [Click here to view] |
Rufinamide is a sodium channel blocker that is being developed as an adjunctive treatment for patients with partial seizures. It differs structurally from antiepileptic medications currently on the market and works better for generalized seizures in kids with Lennox-Gastaut syndrome, a severe kind of infantile epilepsy. This drug is marketed as BANZEL® [43].
In vitro tests of the anticancer properties of 1,2,3-triazole levonorgestrel conjugates 15a (R1 = R4 = OMe, R3 = NO2, and R2 = H) and 15b (R2 = R4 = -CO2-(CH2CH2O)4Me, R1 = R2 = H) were conducted on four human cancer cell lines: colon cancer (HCT116), hepatoblastoma (HePG2), prostate cancer (PC3), and breast adenocarcinoma (MCF-7). These compounds displayed activity comparable to the standard drug doxorubicin. Conjugate 15a showed IC50 values of 9.70, 16.18, 12.11, and 26.89 µM, while compound 15b exhibited IC50 values of 8.14, 10.69, 17.47a, and 7.48 µM. The conjugates showed moderate toxicity over normal cell line WI38 (15a: IC50 = 68.36 μM and 15b: IC50 49.74 μM). The molecular docking showed that the activity could be attributed to the presence of nitro and methoxy groups in 15a. In addition to their hydrophobic interactions with the cytochrome P450 17A1 residue, the two tetraglyme groups connected to the aromatic ring in 15b facilitate the creation of hydrogen bonds [44].
The anticancer properties of 1,2,3-triazole-based glycosides containing 1,3,4-Thiadiazolyl, Indolyl, and Arylacetamide scaffolds were reported by Elganzory et al. Conjugates 16a, 16b, and 16c (R1 = Me and R2 = R3 = H) exhibited the best activity against MCF-7 human breast cancer cells among these compounds (IC50 = 0.5, 4.2, and 0.6 µM, respectively). These conjugates were further tested in human colorectal cancer cells (HCT-116), yielding IC50 values of 15.5, 4.6, and 11.4 µM. Furthermore, in contrast to the untreated MCF-7 cells, conjugates 16a and 16c caused a notable cell cycle arrest at the sub-G1 and S phases. In addition, with IC50 ranges of 0.21–0.38, 1.50–1.95, and 2.18–2.77 µM, respectively, compounds 16a, 16b, and 16c demonstrated strong potential inhibitory effects against the EGFRWT, EGFRT790M, and HER-2 enzymes. Finally, docking experiments have shown that the natural ligands erlotinib, gefitinib, and TAK-285 are easily integrated into the binding pockets of EGFRWT, EGFRT790M, and HER-2 by similar H-bonding with the essential amino acids Met769, Met793, and Met801, respectively [45].
Conjugate 17 (R2 and R4 = esters with tetraethylene glycol chains) was examined against three cancer cell lines and one nontumoral cell line: breast cancer (MCF-7), colorectal carcinoma/colon cancer (HCT-116), hepatocellular carcinoma (HepG2), and human lung fibroblasts (WI38, control cell line). In each tumor cell line tested, this derivative showed high activity (IC50 = 13.67, 9.92, and 7.92). According to molecular docking, this compound established various amounts of hydrogen bonds that improve its complex stability within the TGF-bR1 pocket and, in turn, inhibit downstream pathways implicated in the growth and proliferation of cancer cells [46].
Conjugate 18 (R = -NH2) exhibited cytotoxic activity over HepG-2, HCT-116, and MCF-7 cells, with IC50 = 12.22, 14.16, and 14.64µM, respectively. According to the SAR analysis, utilizing electron-releasing substituents (-NH2 group) instead of an electron-withdrawing group (−COOH or –NO2) in the phenyl ring had a greater cytotoxic effect on the three cell lines. According to molecular docking studies, this molecule had a score of −17.01 kcal/mol and bound to the EGFR active site in a similar manner as lapatinib, forming three H bonds with Thr854 (via H2O), Met793, and Cys797 by binding OCH2 to the triazole (at N-2) and amino group [47].
On the one hand, compound 19 (R3 = NO2 and R4 = Me) exhibited IC50 = 19.13, 28.87, and 16.57 µM against PC3, Panc1, and MDA-MB-231, respectively [48]. On the other hand, derivative 20 (R1 = OCF3 and R2 = H) was active with an IC50 against lung cancer = 6.06 µΜ and also induced apoptosis and triggered ROS generation [49]. With IC50 values of 1.2 ± 0.2 µM and 1.4 ± 0.2 µM, conjugate 21 (R1 = R3 = H and R2 = R4 = OMe) demonstrated activity against the MCF-7 and Panc-1 cell lines. In addition, tests of Caspase-3/8/9, Cytochrome C, BAX, and Bcl-2 validated the compound’s cell cycle arrest at the G2/M phase [50]. Maleimide-1,2,3-triazole conjugate 22 (R1 = Me and R2 = Br) showed an IC50 = 1.32, 0.80, and >10.6 mM over HCT-116, T47D, and VERO, respectively [51]. Purine-triazole conjugates 23a (R = Cl) and 23b (R = Br) exhibited an IC50 = 22.3 µM and 22.9 µM for MCF-7, respectively. In addition, these compounds showed IC50 = 9.3 µM and 16.7 µM for MDA-MB-231 [52]. Pyrrolobenzodiazepine embrace 1,2,3-triazole 24 (R1 = Cl and R2 = H) showed cytotoxic activity on SNB-75 with a percent inhibition of 43.45% [53].
A recent publication described a new library of 1,2,3-triazole-incorporated 1,3,4-oxadiazole-triazine derivatives 25. Compounds in this study show exceptional in vitro anticancer activity against the cancer cell lines PC3 and DU-145 (prostate cancer), A549 (lung cancer), and MCF-7 (breast cancer). The SAR studies of these derivatives determined that when electron-donating groups as methoxy were attached to the phenyl moiety (in green) favored, exceptionally the potency in comparison to electron-withdrawing group (Cl, Br, and NO2). The 3,4,5-trimethoxy-substitution showed favorable activity than those 3,5-dimethoxy, 4-methoxysubstituted derivatives. Interestingly, the most effective molecule in this series, the 4-pyridyl moiety (X = N), demonstrated more potency than the 3,4,5-trimethoxy-substitution against PC3, A549, MCF-7, and DU-145 cell lines, with IC50 values of 0.17 ± 0.063 μM, 0.19 ± 0.075 μM, 0.51 ± 0.083 μM, and 0.16 ± 0.083 μM, respectively [54]. The anticancer activity of a set of 1,2,3-triazole linked tetrahydrocurcumin derivatives 26 was examined by Duan et al. [35] against four cancer cell lines, including human cervical carcinoma (HeLa), human lung adenocarcinoma (A549), human hepatoma carcinoma (HepG2), and human colon carcinoma (HCT117). The SAR examination of these compounds determined that electron-withdrawing group (F, Cl, Br, CF3, OCF3, NO2, and CN) favors activity than electron-donating groups (alkyl or OMe). Alkylsubstituents (Me, Et, iso-Pro, and tert-Bu) showed no significant potency (>200 uM) compared to methoxysustituent (15–53 µM). Furthermore, attached Cl, Br, CF3, OCF3, NO2, and CN groups to the phenyl ring result in a substantial loss of potency compared to fluoro-substitution (IC50 = 1.09 ± 0.17 μM), which displayed superior anticancer activity [55]. More recently, Das and colleagues reported the cytotoxicity activity against MCF-7 (breast cancer cell line) of a series of 1,2,3-triazole-containing hydrazones 27 with inhibitory concentrations between 0.9 to 42 μM. A general SAR analysis revealed that when Br is attached to C-4 on A-ring the activity is higher than Cl substitution by 3–10-fold. In particular, the 2,4,5-Cl-substitution (on B-ring) and unsubstituted A-ring (R =H) were essential for the potent cytotoxic activity (IC50 ~0.9 μM), whereas a 2,4,5-Cl-substitution (on B-ring) with bromo on A-ring diminished notably the cytotoxic potency by ~40-fold. When the B-ring was monosubstituted on C-4 (NO2, Br, and F), a loss of cytotoxic activity by ~10–20-fold was observed. Finally, unsubstituted B-ring led to ~20-fold reduction in the activity compared to most potent compound (3-Cl-4-F) [56]. In addition, a set of 20 new gefitinib derivatives that contain the 1,2,3-triazole moiety 28 were recently reported, and potential anticancer responses to human lung adenocarcinoma cells (NCI-H1437) and EGFR wild-type human nonsmall cell lung cancer cells (NCI-H1299, A549) were examined. A comparative structure–activity analysis of the anticancer response determined that in general, electron-withdrawing groups (F, Cl, Br, NO2, and CN) results possess better activity than electron-donating groups (alkyl or OMe). The antiproliferative activity of compounds was significantly influenced by the type, location, and quantity of halogen atoms, with activity increasing in the order di > monosubstituted, being the 2-Br-5-F, the most active substitution pattern showing the higher cytotoxic concentrations (IC50 ~1.83–3.94 μM) [57]. The antiproliferative effects of isatin-grafted phenyl-1,2,3-triazole derivatives 29 against PC3 (prostate cancer) and PANC1 (pancreatic cancer) cells were recently studied by Elsebaie et al. [58] The IC50 values ranged from 0.13 to 2.34 μM and from 0.10 to 1.56 μM. The SAR studies of these derivatives determined that 1) the antiproliferative activity of the N-aryltriazole ring was significantly influenced by the type of substituent, with potency enhancing in the sequence OCH3 > H; 2) for the isatin-fused phenyl ring, the antiproliferative activity increasing in the order F>Cl>5-Br>6-Br>H>OCF3 = NO2>>CH3; and 3) for the N-substitution on isatin, the antiproliferative response increased in the order Benzyl>Propyl>Methyl>H [58].
CONCLUSION
An increasing amount of attention has been given to the development of innovative chemopreventive pharmaceutical medications because of the high incidence and mortality rates of cancer as well as the adverse side effects of conventional chemotherapy therapies. Triazole-based chemistry has attracted great interest in drug discovery through the synthesis of anticancer agents, including hybrid molecules and conjugates. This is mainly due to its versatility and the use of environmentally friendly solvents such as water for its synthesis. In this review, we showed the hybrids and conjugates, which exhibited anticancer activity based on different in vitro and in vivo studies. Here, we also showed that molecular docking revealed the possible mechanism of action of some compounds described in this study. Finally, it is important to continue exploring the great potential of triazole-based chemistry to develop new therapeutic alternatives against cancer. Furthermore, a substantial amount of reviews and articles have been published throughout the past 20 years, with increasing since 2012. Furthermore, bibliometric network analysis revealed molecular docking studies as an emerging topic.
ACKNOWLEDGMENTS
The authors acknowledge the University of Antioquia and the Ministry of Science, Technology and Innovation (Minciencias), through the Program 92332 “Progress in the development of preventive and therapeutic alternatives based on nanobioconjugates for the treatment of colorectal cancer in Colombia”. NanoBioCancer 2.0 GAT 2.0. Code: 121092092332, Contract: 621-2022. Projects: Nanovehicles 92355 and Preclinical 92391.
AUTHOR CONTRIBUTIONS
Each author accepted responsibility for all parts of the work, consented to have the work submitted to the journal in publication, and made substantial contributions to the conception and design, data collection, analysis, and interpretation. They were also involved in the authoring or critical review of the article for significant intellectual substance.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
The authors confirm that the data supporting the findings of this study are available within the article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021 Jan 12;71(1):7–33.
2. WHO. Cancer today. Geneva, Switzerland: WHO; 2022. Available from: https://Gco.Iarc.Fr/Today/En/Dataviz/Bars?Mode=cancer&group_populations=1&sexes=0&populations=90 0&types=0_1&sort_by=value1
3. NIH. What is cancer? 2021. Available from: https://Www.Cancer.Gov/about-Cancer/Understanding/What-Is-Cancer
4. Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019 Sep 11;69(5):363–85.
5. Mansoori B, Mohammadi A, Davudian S, Shirjang S, Baradaran B. The different mechanisms of cancer drug resistance: a brief review. Adv Pharm Bull. 2017 Sep 25;7(3):339–48.
6. Debela DT, Muzazu SG, Heraro KD, Ndalama MT, Mesele BW, Haile DC, et al. New approaches and procedures for cancer treatment: current perspectives. SAGE Open Med. 2021 Jan 12;9:20503121211034366.
7. Lee EM, Jiménez-Fonseca P, Galán-Moral R, Coca-Membribes S, Fernández-Montes A, Sorribes E, et al. Toxicities and quality of life during cancer treatment in advanced solid tumors. Current Oncol. 2023 Oct 19;30(10):9205–16.
8. Pearce S. Therapeutics the role of heterocycles in anti-cancer drug design [Internet]. Available from: https://www.cancer.gov/publica
9. Hossain M, Habib I, Singha K, Kumar A. FDA-approved heterocyclic molecules for cancer treatment: synthesis, dosage, mechanism of action and their adverse effect. Heliyon. 2024 Jan;10(1):e23172.
10. Kumar Singh R. Key heterocyclic cores for smart anticancer drug–design. Bentham Book imprint. Singapure: Bentham Science Publishers Pte. Ltd.; 2022.
11. Sampath Kumar HM, Herrmann L, Tsogoeva SB. Structural hybridization as a facile approach to new drug candidates. Bioorg Med Chem Lett. 2020 Dec;30(23):127514.
12. de Oliveira Pedrosa M, Duarte da Cruz RM, de Oliveira Viana J, de Moura RO, Ishiki HM, Barbosa Filho JM, et al. Hybrid compounds as direct multitarget ligands: a review. Curr Top Med Chem. 2017;9(17):1044–79.
13. Moreno-Quintero G, Betancur-Zapata E, Herrera-Ramírez A, Cardona-Galeano W. New hybrid scaffolds based on 5-FU/curcumin: synthesis, cytotoxic, antiproliferative and pro-apoptotic effect. Pharmaceutics. 2023 Apr 12;15(4):1221.
14. Castrillón-López W, Herrera-Ramírez A, Moreno-Quintero G, Coa JC, Naranjo TW, Cardona-Galeano W. Resveratrol/hydrazone hybrids: synthesis and chemopreventive activity against colorectal cancer cells. Pharmaceutics. 2022 Oct 24;14(11):2278.
15. Moreno-Quintero G, Castrillón-Lopez W, Herrera-Ramirez A, Yepes-Pérez AF, Quintero-Saumeth J, Cardona-Galeano W. Synthesis and chemopreventive potential of 5-FU/genistein hybrids on colorectal cancer cells. Pharmaceuticals. 2022 Oct 21;15(10):1299.
16. Cardona G W, Herrera R A, Castrillón L W, Ramírez Malule H. Chemistry and anticancer activity of hybrid molecules and derivatives based on 5-fluorouracil. Curr Med Chem. 2021 Sep 8;28(27):5551–601.
17. Singh RK, Kumar S, Prasad DN, Bhardwaj TR. Therapeutic journery of nitrogen mustard as alkylating anticancer agents: historic to future perspectives. Eur J Med Chem. 2018 May;151:401–33.
18. Kumar A, Yadav AK, Mishra V, Kumar D. Recent advancements in triazole-based click chemistry in cancer drug discovery and development. SynOpen. 2023 May 17;07(02):186–208.
19. Kaur J, Saxena M, Rishi N. An overview of recent advances in biomedical applications of click chemistry. Bioconjug Chem. 2021 Aug 18;32(8):1455–71.
20. The Nobel Prize in Chemistry 2022. 2022. Available from: https://www.nobelprize.org/prizes/chemistry/2022/popular-information/
21. Khandelwal R, Vasava M, Abhirami RB, Karsharma M. Recent advances in triazole synthesis via click chemistry and their pharmacological applications: a review. Bioorg Med Chem Lett. 2024 Nov;112:129927.
22. Yu W , Lu X, Xiong L, Teng J, Chen C, Li B, et al. Thiol-ene click reaction in constructing liquid separation membranes for water treatment. Small. 2024 Jun 11;20(25).
23. Zhou H, Zhang X, Qu B, Zhao F, Man C, Jiang Y, et al. Biosensing meets click chemistry: a promising combination for analysis of food hazard factors. Coord Chem Rev. 2024 Dec;520:216137.
24. Thirumurugan P, Matosiuk D, Jozwiak K. Click chemistry for drug development and diverse chemical–biology applications. Chem Rev. 2013 Jul 10;113(7):4905–79.
25. Li X, Xiong Y. Application of “Click” chemistry in biomedical hydrogels. ACS Omega. 2022 Oct 25;7(42):36918–28.
26. Zhao S, Liu J, Lv Z, Zhang G, Xu Z. Recent updates on 1,2,3-triazole-containing hybrids with in vivo therapeutic potential against cancers:aA mini-review. Eur J Med Chem. 2023 May;251:115254.
27. Mashayekh K, Shiri P. An overview of recent advances in the applications of click chemistry in the synthesis of bioconjugates with anticancer activities. ChemistrySelect. 2019 Dec 13;4(46):13459–78.
28. van Eck NJ WL. Software survey: vOSviewer, a computer program for bibliometric mapping. 2010.
29. Dhiman A, Sharma R, Singh RK. Target-based anticancer indole derivatives and insight into structure?activity relationship: a mechanistic review update (2018–2021). Acta Pharm Sin B. 2022 Jul;12(7):3006–27.
30. Kumari A, Singh RK. Morpholine as ubiquitous pharmacophore in medicinal chemistry: deep insight into the structure-activity relationship (SAR). Bioorg Chem. 2020 Mar;96:103578.
31. Lizeth GR, Gustavo MQ, Angie HR, Wilson CL, Andrés FY, Wilson CG. New hybrid scaffolds based on ASA/genistein: synthesis, cytotoxic effect, molecular docking, drug-likeness, and in silico ADME/Tox modeling. J Appl Pharm Sci. 2022;12.
32. Krstulovi? L, Miškovi? Špoljari? K, Rastija V, Filipovi? N, Baji? M, Glavaš-Obrovac L. Novel 1,2,3-triazole-containing quinoline–benzimidazole hybrids: synthesis, antiproliferative activity, in silico ADME predictions, and docking. Molecules. 2023 Oct 6;28(19):6950.
33. Hou H, Qu B, Su C, Hou G, Gao F. Design, synthesis and anti-lung cancer evaluation of 1, 2, 3-triazole tethered dihydroartemisinin-isatin hybrids. Front Pharmacol. 2021 Dec 16;12.
34. Djemoui A, Naouri A, Ouahrani MR, Djemoui D, Lahcene S, Lahrech MB, et al. A step-by-step synthesis of triazole-benzimidazole-chalcone hybrids: anticancer activity in human cells+. J Mol Struct. 2020 Mar;1204:127487.
35. Duan M, Mahal A, Mohammed B, Zhu Y, Tao H, Mai S, et al. Synthesis and antitumor activity of new tetrahydrocurcumin derivatives via click reaction. Nat Prod Res. 2022 Oct 18;36(20):5268–76.
36. Wu BW, Huang WJ, Liu YH, Liu QG, Song J, Hu T, et al. Design, synthesis and biological evaluation of 1,2,3-triazole benzothiazole derivatives as tubulin polymerization inhibitors with potent anti-esophageal cancer activities. Eur J Med Chem. 2024 Feb;265:116118.
37. Mahmoud MA, Mohammed AF, Salem OIA, Almutairi TM, Bräse S, Youssif BGM. Design, synthesis, and apoptotic antiproliferative action of new 1,2,3-triazole/1,2,4-oxadiazole hybrids as dual EGFR/VEGFR-2 inhibitors. J Enzyme Inhib Med Chem. 2024 Dec 31;39(1):2305856.
38. Al-Qahtani SD, Al-Senani GM. Novel hybrid motifs of 2-(2-pyridinyl)-1H-benzo[d]imidazole-1,2,3-triazole: Synthesis, anticancer assessment, and in silico study. J Mol Struct. 2024 Dec;1318:139376.
39. de Souza AS, Dias DS, Ribeiro RCB, Costa DCS, de Moraes MG, Pinho DR, et al. Novel naphthoquinone-1H-1,2,3-triazole hybrids: design, synthesis and evaluation as inductors of ROS-mediated apoptosis in the MCF-7 cells. Bioorg Med Chem. 2024 Mar;102:117671.
40. Abdel-Rahman AAH, El-Bayaa MN, Sobhy A, El-Ganzoury EM, Nossier ES, Awad HM, et al. Novel quinazolin-4-one based derivatives bearing 1,2,3-triazole and glycoside moieties as potential cytotoxic agents through dual EGFR and VEGFR-2 inhibitory activity. Sci Rep. 2024 Oct 23;14(1):24980.
41. Lavunuri S, Venkata Nadh R, Banothu D, Kumar Rapeti S. Synthesis of quinoline fused 1,2,3-triazole derivatives via continuous CuAAC and C H arylation; anti-breast cancer, anti-EGFR and HER2 activities, computational studies. Tetrahedron Lett. 2024 Oct;150:155282.
42. Nalla S, Aravind S, Annam SC, Padmavathi KV, Syed T, Subbarao M. Synthesis and biological evaluation of 1, 2, 3-triazole incorporated pyrrole derivatives as anticancer agents. Synth Commun. 2024 Nov 15;54(21):1828–41.
43. Narayanrao R, Ramachandra D, Ramdas D. Process for the preparation of rufinamide. WO2010043849A1. 2010.
44. Alshamari AK. Design and synthesis of novel 1,2,3-triazole levonorgestrel derivatives via click chemistry. Anticancer activity and molecular docking. Russian J Organ Chem. 2022 Dec 8;58(12):1878–88.
45. Elganzory HH, Alminderej FM, El-Bayaa MN, Awad HM, Nossier ES, El-Sayed WA. Design, synthesis, anticancer activity and molecular docking of new 1,2,3-triazole-based glycosides bearing 1,3,4-thiadiazolyl, indolyl and arylacetamide scaffolds. Molecules. 2022 Oct 17;27(20):6960.
46. Alshamari AK. Anticancer activity and molecular docking of 1,2,3-triazole hybrids of phenol and 4-methoxy-2-methylphenyl: synthesis via click chemistry. Russian J Organ Chem. 2023 Mar 10;59(3):455–64.
47. El Azab IH, El-Sheshtawy HS, Bakr RB, Elkanzi NAA. New 1,2,3-triazole-containing hybrids as antitumor candidates: design, click reaction synthesis, DFT calculations, and molecular docking study. Molecules. 2021 Jan 29;26(3):708.
48. Firoozpour L, Moghimi S, Fallah Barzegar MH, Toolabi M, Salarinejad S, Bijanzadeh HR, et al. Synthesis, molecular docking, and biological evaluation of pyridin-3-yl-pyrimidin-2-yl-triazole derivatives as anti-cancer agents. Polycycl Aromat Compd. 2024 Mar 15;44(3):2062–76.
49. Guo Y, Sang D, Guo B, Wang D, Xu X, Wang H, et al. Synthesis and biological evaluation of novel 1,2,3-triazole hybrids of cabotegravir: identification of potent antitumor activity against lung cancer. Front Pharmacol. 2023 Sep 20;14:1265245.
50. El-Sheref EM, Elbastawesy MAI, Brown AB, Shawky AM, Gomaa HAM, Bräse S, et al. Design and synthesis of (2-oxo-1,2-Dihydroquinolin-4-yl)-1,2,3-triazole derivatives via click reaction: potential apoptotic antiproliferative agents. Molecules. 2021 Nov 10;26(22):6798.
51. Salameh BA, Abu-Safieh KA, Al-Hushki EH, Talib WH, Al-ataby IA, Al-Qawasmeh RA. New maleimide 1,2,3-triazole hybrids: design, synthesis, anticancer, and antimicrobial activities. Monatshefte für Chemie Chem Monthly. 2020 Oct 8;151(10):1609–19.
52. de O. Torres NMP, Cardoso G de A, Silva H, de Freitas RP, Alves RB. New purine-triazole hybrids as potential anti-breast cancer agents: synthesis, antiproliferative activity, and ADMET in silico study. Med Chem Res. 2023 Aug 6;32(8):1816–31.
53. Hadiyal SD, Lalpara JN, Parmar ND, Joshi HS. Microwave irradiated targeted synthesis of pyrrolobenzodiazepine embrace 1,2,3-triazole by click chemistry synthetic aspect and evaluation of anticancer and antimicrobial activity. Polycycl Aromat Compd. 2022 Aug 9;42(7):4752–68.
54. Oggu S, Akshinthala P, Katari NK, Nagarapu LK, Malempati S, Gundla R, et al. Design, synthesis, anticancer evaluation and molecular docking studies of 1,2,3-triazole incorporated 1,3,4-oxadiazole-Triazine derivatives. Heliyon. 2023 May;9(5):e15935.
55. Duan M, Mahal A, Alkouri A, Wang C, Zhang Z, Ren J, et al. Synthesis, anticancer activity, and molecular docking of new 1,2,3-triazole linked tetrahydrocurcumin derivatives. Molecules. 2024 Jun 25;29(13):3010.
56. Das VB, Poojary B, Kamat V, Hamzad S, Suman P. Synthesis, anticancer evaluation, and molecular docking study of 1,2,3-triazole-containing hydrazones as potential HER2 Kinase inhibitors. Russian J Organ Chem. 2024 Mar 19;60(3):502–12.
57. Gao E, Wang Y, Fan G lu, Xu G, Wu ZY, Liu ZJ, et al. Discovery of gefitinib-1,2,3-triazole derivatives against lung cancer via inducing apoptosis and inhibiting the colony formation. Sci Rep. 2024 Apr 22;14(1):9223.
58. Elsebaie HA, Abdulla MH, Elsayed ZM, Shaldam MA, Tawfik HO, Morsy SN, et al. Unveiling the potential of isatin-grafted phenyl-1,2,3-triazole derivatives as dual VEGFR-2/STAT-3 inhibitors: design, synthesis and biological assessments. Bioorg Chem. 2024 Oct;151:107626.