INTRODUCTION
Diabetic foot ulcer infection (DFUI) is a complication of diabetes mellitus resulting from uncontrolled blood glucose levels. Elevated blood glucose levels lead to nerve damage, rendering the patient incapable of feeling pain in the wound. Additionally, high glucose levels hinder the flow of nutrient- and oxygen-rich blood to the wound, slowing down the healing process. Deterioration of the ulcer may eventually lead to lower extremity amputation (LEA) in the patient, with approximately 20% of DFUI cases requiring this intervention. Apart from LEA, DFUI increases the cost of hospital care. The Indonesian government spends around $1,040 annually per patient on treating DM patients with DFUI in hospitals [1]. In line with several studies, Driver et al. [2] in the United States noted that diabetic foot ulcer (DFU) patients incur treatment costs five times higher than patients without DFU. Alavi et al. [3] reported an average cost of $44,790 per patient for lower limb amputation and subsequent rehabilitation treatment. Earlier studies by Borsse’n (1990) and Moss (1992) reported that DFU patients incurred significant treatment costs compared to non-DFU patients, primarily due to prolonged hospitalization resulting in the development of complications such as infection, acute ischemia, and gangrene [4]. DFUI can affect 19%–34% of DM patients, with its incidence increasing in line with the progression of the disease’s complexity. A study by Ndosi et al. [5] reported inadequate outcomes in DFU patients, particularly those with diabetes-related DFU, based on a large prospective study. By the year’s end, only 46% of patients had healed from ulcers (with recurrence in 10% of cases), 15% had died, and 17% had undergone LEA.
The deterioration of DFU patients’ conditions is associated with various risk factors, including comorbidities and simultaneous infection developments, during prolonged hospitalizations, thus impacting the clinical outcomes of DFUI patients [6]. Previous studies have also reported that complications in DFU patients, particularly long-term vascular complications, are risk factors for DFU onset [4], occurring in up to 90% of DFU patients [7]. For instance, the incidence of peripheral neuropathy is relatively high at 3% among DFU patients, while renal dysfunction is confirmed at 25.7% [8]. Additionally, peripheral artery disease (PAD) contributes to inducing hypoxia in the circulation of the lower extremities, leading to ulcer onset. In this regard, PAD and neuropathy are highly effective predictors of amputation in DFUI patients [9]. This is further supported by the EURODIALE study’s findings, which identified PAD as an independent predictor of non-healing wounds in 23% of DFU patients during a 1-year follow-up [10]. A retrospective study covering the period from 2,000 to 2,009 in China reported risk factors for amputation in DFU patients, including PAD, elevated white blood cell count (WBC), high-sensitivity C-reactive protein, and lower lipid levels [11]. Meanwhile, the most common comorbidities in DFU patients are hypertension, ischaemic heart disease, and cerebrovascular disease [12].
Amputation is a significant risk factor assessed in DFUI patients. A national study in Scotland from 2018 to 2021 reported that DFUI patients faced a heightened risk of amputation compared to patients without ulcers, with an amputation-free survival rate of 84.5% over 2 years. DFUI patients with healed ulcers have a lower amputation-free survival rate over 2 years compared to those without previous ulcers [13]. Additionally, infection leads as a risk factor for short-term amputation in hospital-admitted DFUI patients [14]. A longitudinal study involving 1,666 diabetic patients showed a heightened risk of amputation associated with increasing severity of infection [15].
DFUI is also frequently associated with mortality in DM patients. Complications and mortality in DFUI patients are closely related to disease severity. Wound progression increases the risk of further wound progression, leading to amputation, and if left untreated, may result in death [16]. The mortality rate attributable to DFUI within 5 years ranges from 50% to 70% [17]. A prospective nonrandomized cohort study in a Mediterranean country (Central Greece) reported a mortality rate of 17.5% over a 12-month period [18]. Similarly, a study in Indonesia showed that the mortality rate for DFUI patients reached 10.7% over a 3-year observation period [19]. One approach to reducing the mortality rate in DFUI patients is to identify the factors affecting mortality in DFUI based on the characteristics of the DM population in Indonesia. There have been limited recent studies concerning DFUI outcomes. Based on these problems, the aim of this study is to analyze the factors affecting mortality in DFUI at the national referral hospital in Jakarta, Indonesia.
MATERIALS AND METHODS
Study design and ethical approval
This is an observational study using a retrospective cross-sectional design. It was conducted at Dr. Cipto Mangunkusumo Hospital (RSCM), a national referral hospital located in Jakarta, Indonesia. Data for DFUI patients were collected from January 2019 to December 2022. Infections severity grading based on the IWGDF/IDSA guideline 2023 [20]. A total of 259 eligible DFUI patients, with ulcers ranging from no infection to severe infection grade, were included in this study. Data were extracted from patient medical records obtained from the admission medical record unit, electronic health records (EHRs), hospital integrated system, and the foot registry system in the metabolic endocrine division. Primary and secondary diagnoses of DFI were accessed from EHR. Approval for this study was granted by the Ethical Committee of RSCM-Faculty of Medicine, Universitas Indonesia, with the reference number KET-1192/UN.F1/ETIK/PPM.00.02/2022.
Population and sample
The population in this study comprises all DFUI patients. The inclusion criteria for this study are DFUI patients hospitalized from January 2019 to December 2022; aged >18 years; and including all grades of ulcers with at least one period of in-hospital admission. The exclusion criteria comprise incomplete medical records, patients not registered in the foot registry, those not classified as DFUI patients and incomplete clinical data. Secondary data were obtained from patient medical records and the foot registry, with DFUI diagnosis based on ICD-10 codes: E.10.5, E.11.5, and E11.9.
The sample size for this study was determined using a formula for a cross-sectional design, with the following parameters:
The prevalence of mortality in DFUI cases (P) is 10.7% [21]; the value of represents the statistical confidence level (1.96) at a 95% confidence level (95% CI); and the margin of error (d) is 5%. This gives the following calculation formula:
where
N = sample size
Z( = the statistical confidence level for a 95% CI
P = the proportion of DFUI patients with high-grade ulcers who experience
mortality
d = margin of error.
Based on the sample calculation, the number of samples meeting the criteria is 147 individuals. The number of samples meeting the inclusion criteria is 259 individuals. In line with the study by Chen [22], it stated that the model’s predictive performance data with 100+events do not need to be validated.
Demographic data
The demographic data included age, sex, occupation, family status, education, and type of insurance used. Anthropometric data included the duration of DM, determined from the time of diagnosis based on the 2019 ADA criteria, which include fasting blood sugar levels ≥126 mg/dl; a 2-hour oral glucose tolerance test ≥200 mg/dl; a hemoglobin A1c test ≥6.5% using standardized NGSP and DCCT methods; or examination of plasma glucose when ≥200 mg/dl with classic complaints or hyperglycaemic crisis (11.1 mmol/l) [23]. Body mass index values were calculated based on the patient’s body weight and height, adjusted for body surface area [24–26]. History of DFUI, amputation type including major and minor amputation, and smoking were obtained from the patient’s medical history (anamnesis).
Physical examination
PAD was defined by ankle-brachial index scores of less than or equal to 0.9 or above, vascular ultrasound results, and/or CT angiography results. Hypertension was defined by a blood pressure greater than 140/90 or a history of hypertension, while dyslipidemia was defined by an abnormal lipid profile with a triglyceride level of 150 mg/dl, an LDL level of 100 mg/dl, an HDL level of 50 mg/dl, or a history of dyslipidemia [27]. CAD was defined as a type of heart disease where the arteries are unable to deliver oxygen-rich blood to the heart based on ECG (Electrocardiogram) or coronary intervention confirmation.
Laboratory examination
HbA1c represents the percentage of glucose bound to hemoglobin in the blood, with a treatment target value of <7 [26,28]. An initial HbA1c value ≥6.5 (48 mmol/mol) indicates the onset of diabetes, prompting hospitalization for the patient [28]. An HbA1c value >7% indicates uncontrolled glycaemic status [29]. Random blood glucose (RBG) is the blood glucose level at any given time, which can vary depending on the nutritional intake entering the body.
Leukocytes, also known as WBCs, aid the body in fighting various infectious diseases as part of the immune system. When the leukocyte count is >12,000/mm3 or 4 G/l, or when >10% of immature forms are present [30,31], it indicates leukocytosis. Leukocytosis, characterized by a leukocyte count > 10 × 103/ μl, clinically indicates the presence of inflammation in the body.
Wound and infection assessment
The characteristics of foot ulcers consist of ulcer grade and infection grade following the IWGDF/IDSA consensus classification [29,31,32].
Outcome analysis
The clinical outcomes observed in this study are mortality, amputation, and length of stay (LOS). The primary outcome variable in this study is mortality, defined as the number of deaths occurring during hospitalization, identified based on death records recorded in the patient’s vital status report in the medical records.
The clinical outcomes of amputation and LOS serve as independent variables that act as predictive factors for mortality in DFUI patients. Amputation refers to the number of amputations during hospitalization. LOS is the duration of a patient’s hospital stay, from admission until discharge home.
Statistical analysis
All statistical analyses were conducted using IBM SPSS Statistics, version 28, and Microsoft Excel version 16.75.2, licensed 2019 for Mac. The initial stage of data analysis involved frequency analysis to determine the number and percentage of cases descriptively presented in various categories such as age, sex, education level, occupation, family status, insurance, PEDIS ulcer grade, severity of infection, type of diabetes, duration, BMI, smoking status, comorbidities, and amputation status. Continuous variables such as LOS were calculated and presented as total value, mean, and standard deviation. Data were presented as mean values (standard deviation, SD) and median (min–max). Independent variables in this study were transformed into nominal scale with binomial categories. Bivariate analysis was performed using the chi-Square test, with mortality as the dependent variable. Subsequently, all variables with a p-value < 0.25 were included in the full model multivariate analysis using the backward stepwise LR method. The logistic regression test using the backward stepwise LR method was conducted by including all variables in the model and removing independent variables one by one. In the final modeling stage for the cross-sectional study, variables with a p-value < 0.05 were included in the multivariate analysis. If the variable had the highest OR value, it was considered to influence the dependent variable. Therefore, the results of the multivariate analysis for variables with a p-value < 0.05 were considered predictive factors for mortality in DFUI patients.
The backward (step-down) selection approach includes all candidate variables. The model begins with all candidate variables. At each phase, the variable with the least significance is eliminated. If no nonsignificant variables are left, this process will stop. The user sets the significance threshold for eliminating variables from the model [33].
RESULTS
This study involved 259 DFUI patients who met the inclusion criteria and were evaluated retrospectively. Secondary data were obtained from patient medical records from January 2019 to December 2022, based on ICD-10 diagnoses. Patients who were readmitted due to recurrent ulcers were excluded from the study criteria. Sociodemographic data are presented in Table 1.
The majority of patients were over 50 years old, with females comprising 52.1% of the sample. Nearly 60% of patients were unemployed (primarily retired individuals and homemakers), while 74.9% were married. Educationally, 82.2% had attained some level of education, and healthcare expenses were covered by the government through the National Health Insurance (JKN) for 96.9% of patients. Based on Table 2, a majority of the patients were diagnosed with Type 2 Diabetes Mellitus (T2DM) (98.5%), with a diabetes duration exceeding 5 years (58.3%). The grade of infection is directly proportional to the ulcer grades, namely severe infection grade (64.1%) and high-grade ulcers (≥ Grade 3) (54.8%). Interestingly, however, non-smoking patients accounted for a higher percentage compared to those with a history of smoking, at 61.4%. The most common comorbid conditions included hypertension (51.4%), CAD (14.3%), PAD (51.4%), and dyslipidemia (34.7%). Meanwhile, laboratory test results for blood glucose levels indicated that the majority of patients had an RBG level of ≥200 mg/dl (53.3%). Patients admitted to the hospital with serious infection conditions, indicated by a high leukocyte count of >12,000 mm3, accounted for 74.9% of cases. More than 50% of patients exhibited an HbA1c value >7% (50.2%). Furthermore, BMI calculations revealed that almost 60% had a BMI of ≥23kg/m2 (59.5%). Nearly half of the patients underwent amputation during the treatment period, 44.8%, while 57.9% had a LOS of less than 20 days.
Variables with a p-value < 0.25 were included in the backward elimination analysis, and binary logistic regression in multivariate analysis (Table 3). The variables included were severity of infection, CAD, PAD, dyslipidemia, blood glucose level, leukocyte level, BMI, and amputation. Variables with a p-value > 0.25 were not included in the model.
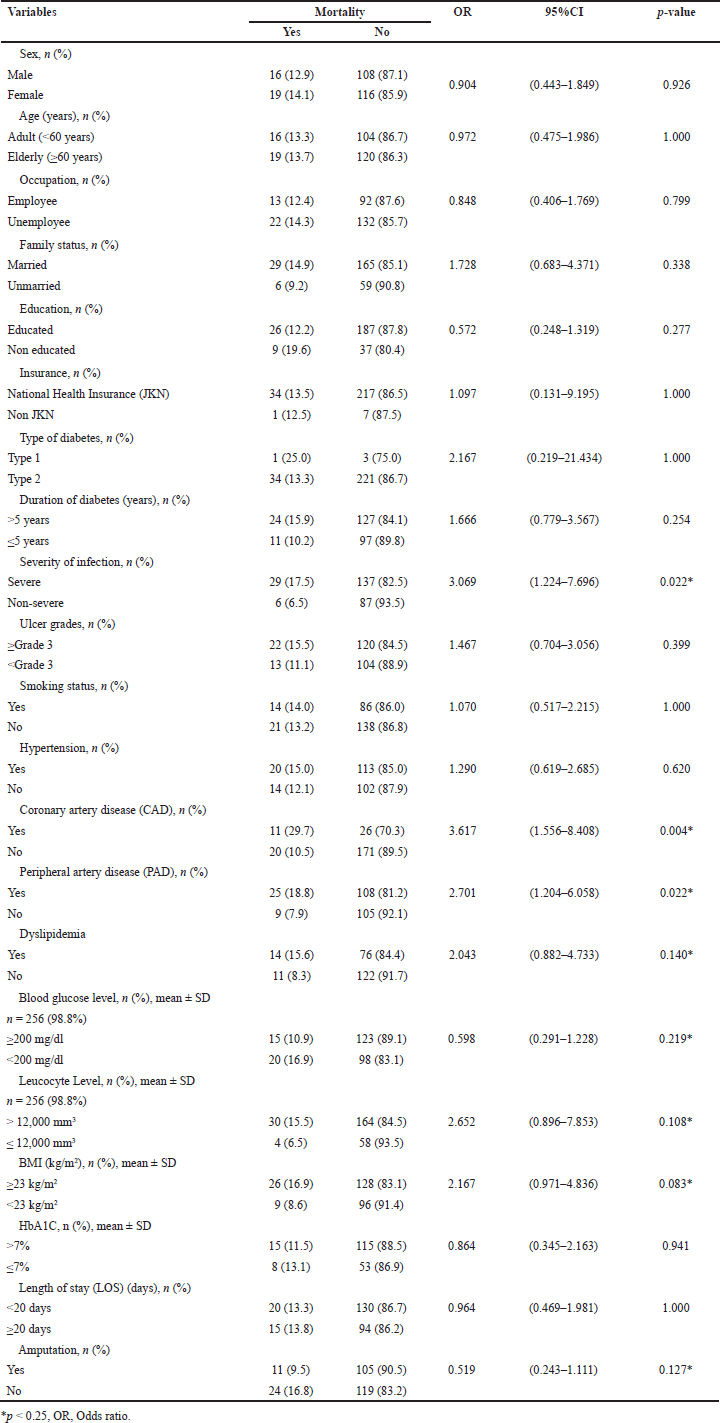 | Table 3. Bivariate analysis for factors related to mortality. [Click here to view] |
Predictors of mortality
The severity of infection and comorbid conditions affect the primary outcome, which is mortality. The odds ratio values for the risk factors for mortality are as follows: severity of infection 5.202 (95% CI; 1.397–19.375), p = 0.014), CAD 5.578 (95% CI; 2.037–15.272), p < 0.001), and dyslipidemia 2.309 (95% CI; 0.881–6.057), p = 0.089). In this study, DFUI patients with severe infection and comorbid CAD have a high risk of mortality with a p-value < 0.05, as shown in Table 4. This indicates that DFUI patients with CAD are at a 5.578 times higher risk of mortality compared to non-CAD DFUI patients. This finding is consistent with a study by Rastogi et al. [34] in India, reporting CAD as the most common risk factor contributing to mortality in DFU patients at 20% (p = <0.001). This result aligns with studies reporting a positive correlation between increased leukocyte levels (11.75 × 109 cells/l) in patients with amputation compared to non-amputation patients, which was 8.83×109 cells/l [35].
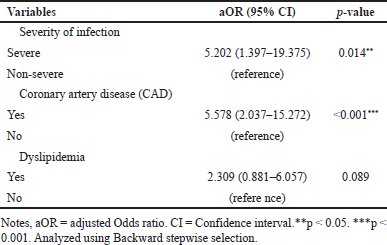 | Table 4. Multivariate analysis of factors affecting mortality in DFUI patients. [Click here to view] |
DISCUSSION
This study aims to determine the predictive factors and treatment outcomes of DFUI affecting the mortality of DFUI patients in Indonesia. We identified DFUI patients who were the subjects of the study, with an average age of 59.65 years (SD ± 10.83 years). This result is not significantly different from that reported by Yunir et al. [36], which was 61 years. Furthermore, the subjects of this study were predominantly elderly patients, accounting for 53.7%. This finding is consistent with that of Lo et al. [37], with an age range of 55–75 years at 74.7%, and Lu et al. [38], with an age range of 55–85 years at 86.1%. Other studies have reported an increased risk of mortality per year with a positive correlation between mortality and advanced age, with significant results (mean age 71 ± 10 years) compared to surviving patients (63 ± 13 years; p < 0.01) [36], and an increased risk of 1.08 times per year (95% CI; 1.06–1.11, p = 0.001) compared to adult DFUI patients [39].
As shown in Table 1, the research subjects were predominantly female, comprising 52.1%, with males at 47.9%, consistent with the findings of Yunir et al. [29], where the percentage of males was 48.8%. However, this contrasts with previous studies by Aviatin et al. [40], which had a majority of male subjects at 52.2%, and Lo et al. [37], where 64.4% of subjects were male. The gender variable difference remains a controversial topic in the development of DFUI treatment. According to Al-Rubeaan et al. [41], the higher prevalence among males is due to limitations in joint mobility and higher foot pressure, as well as lower awareness of foot care compared to females. However, a multi-centre study by Dinh and Veves [42] stated that with neuropathy, the risk of deterioration due to DFUI in females would be the same as that in males. The reported mortality rates for both genders show percentages that are not significantly different, at 30% and 25% [43]. Furthermore, 74.9% of the research subjects were married, with 82.2% having an educational background (ranging from elementary to higher education). This is consistent with the findings reported by Muhammad et al. [44], where over 60% of patients, namely 61.1%, were married and had received formal education ranging from elementary to higher education, at 65.6%. The majority of subjects in this study were unemployed, comprising homemakers and retirees at 59.5%. Other studies also indicate a relationship between education and occupation and DFU. Patients with low education and unemployment (especially housewives) are at higher risk of developing DFU. This is because the patient’s awareness of the risk of DFU is low, at only 39.5%, which can result in mortality [45]. Comprehensive therapeutic education related to DFU for DM patients needs to be improved because it can reduce the risk of mortality (OR 0.0096; 95% CI, 0.22–0.410; p = 0.0016) [46]. DFUI patients treated at RSCM overall are participants in the JKN programme, accounting for 96.9%. As a national referral hospital, RSCM facilitates the treatment of DFUI patients, where all treatment costs for these patients are covered by the Indonesian government. This is consistent with previous research by Yunir [47], where 85.3% of DFUI patients treated at RSCM used JKN as coverage insurance.
 | Table 1. Demographic characteristic of the subjects. [Click here to view] |
Almost all DFUI patients treated at RSCM, as indicated in Table 2, had more than one history of comorbidities upon admission (hospitalization). Sequentially, some of the comorbidities experienced by DFUI patients were hypertension (51.4%), PAD (51.4%), dyslipidemia (34.7%), and CAD (14.3%). These results are not significantly different from those of Yunir et al. [36], where hypertension and CAD were the most common comorbidities at 67.3% and 17.6%, respectively. Furthermore, a previous study by Aviatin et al. [40] also reported hypertension (75.2%) and dyslipidemia (46.9%), while Pratama’s study [48] reported dyslipidemia (86.7%), hypertension (63.0%), PAD (55.6%), and community-acquired pneumonia (21.5%). Pemayun et al. [49] reported a prevalence rate of PAD cases in DFUI patients at 40.4%, which is related to LEA caused by impaired wound healing due to inadequate circulation, and the occurrence of PAD (which does not present the possibility of revascularisation) significantly triggers the incidence of LEA (OR 6.80; 95% CI 2.67–17.32; p < 0.001).
The clinical characteristics of DFUI patients are outlined in Table 2, where the majority are diagnosed with type 2 diabetes (98.5%), with an average diabetes duration of 9.03 years (SD ± 7.29 years). Research by Yazdanpanah et al. [50] reported that the type and duration of diabetes are associated with mortality in DFU. Patients with type 2 diabetes who receive insulin therapy are at higher risk of mortality, potentially due to these patients having longer-standing diabetes diagnoses and more severe complications. In a previous cross-sectional study of 123 DFU patients at RSCM, Jakarta, Indonesia, from 2010 to 2015, the duration of diabetes was reported with a median value and interquartile range (IQR) of 5 (2–12) years [29]. However, these figures may not be entirely accurate due to the underdiagnosis of diabetes mellitus cases in Indonesia, where many individuals are unaware of or undiagnosed with the condition. The prevalence of diabetes mellitus in Indonesia stands at 1.5% with a CI (1.5–1.5, 95% CI) [51].
Another characteristic obtained in this study is the BMI value of DFUI patients, which is 24.85 ± 4.95 (kg/m2). This result is consistent with research by Yunir [47], which reported a BMI value of 24.7 (kg/m2) and a median of 25.1 (4.84; 14.1–37.1, IQR; range) (kg/m2) [36]. Meanwhile, a prospective cohort study in Denmark involving 36 DFU patients reported a BMI value of 28.1 (kg/m2) [52]. The BMI value obtained in this study falls within the overweight category [24–26], which may contribute to an increased incidence of various diseases, with a positive correlation observed between mortality rates and comorbidity history [53].
Stratification and assessment of the DFUI ulcer grades in this study were based on the IWGDF 2019 guidelines [31,54]. The majority of DFUI patients’ wound conditions in this study were ≥Grade 3 (moderate to high) (54.8%) and
The measurement of HbA1c levels obtained from laboratory tests was 8.68 ± 2.41 (mean ± SD) (%) with more than half the patients (50.2%) having levels >7%. This aligns with the findings from a retrospective cohort study at RSCM on DFU patients during the early stage and throughout one year of the COVID-19 pandemic, where the value was 8.0 (6.8–10.2) (median, IQR) [27]. Next, based on the laboratory test results, the initial leukocyte count upon admission of DFUI patients was 19,970.71 ± 10,218.09 (mean ± SD) (/mm3) in 256 DFUI patients (98.8%). This indicates a positive correlation with the severity of infection criteria, predominantly observed in patients with severe infection. The initial blood glucose level (random blood sugar) of DFUI patients was 251.72 ± 154.42 (mean ± SD) (mg/dl) among 256 DFUI patients (98.8%). This suggests that the majority of patients experienced uncontrolled blood sugar conditions with a random blood sugar level ≥200 mg/dl. This finding is not significantly different from the results of the study by Aviatin et al. [40], which reported a random blood sugar level of 232; 60–173 mg/dl (median; min–max). Uncontrolled blood sugar levels are the primary cause of LEA [49]. Moreover, chronic hyperglycemia conditions trigger an increase in the virulence of several pathogens, reduced interleukin production in response to infection, decreased chemotaxis activity, phagocytosis, and polymorphonuclear leukocyte immobilization [55]. Uncontrolled HbA1c levels also contribute to increased mortality in DFU patients. Patients with uncontrolled blood sugar are 9.9 times more likely to experience mortality (95% CI 1.79–54.93) [56].
The clinical outcomes in this study, as shown in Table 2, include mortality as the primary outcome, followed by other clinical outcomes such as amputation and LOS. DFU and amputation serve as markers for the progression of diabetes stages [49]. Based on Table 2, the mortality rate is 13.5%, consistent with the findings of Yunir et al. (36), who reported no amputation or mortality events in 222 patients (83.1%) DFU patients at high risk of ulceration. This is in line with the ADVANCE obesity paradox theory (2008) and Sohn (2012), which suggest that obese patients with diabetes can reduce mortality risk through various hypotheses, including better mobilization of progenitor cells, reduced thromboxane production contributing to cardiovascular disease resistance, and better wound healing aspects [36]. Aviatin et al. [40] reported a 10.6% amputation rate with 7–14 days of hospitalization for DFU patients. The amputation rate in this study was 44.8%, with a mean HbA1c value of 8.68%, with 50.2% categorized as >7%. This is consistent with a study in Turkey from December 2019 to January 2021, which found a significant correlation (85%) between HbA1c values ≥10.1% and the development of PEDIS ulcer grade (Grade 3). Higher HbA1c values were statistically significantly associated with the progression of PEDIS ulcer grade (Grade 3) (p = 0.003) [57]. Amputation is recognized as a significant factor affecting mortality in DFU, as shown by various studies. A study on DFU in Spain in 2017 indicated that amputation affects mortality, with patients who underwent amputation being 2.24 times more likely to die compared to those who did not [58]. Both minor and major amputations increase the risk of mortality by 1.85 times and 2.96 times, respectively, in DFU patients [34]. Patients with a history of ulceration tend to have micro and macrovascular dysfunction, which subsequently impacts mortality [50].
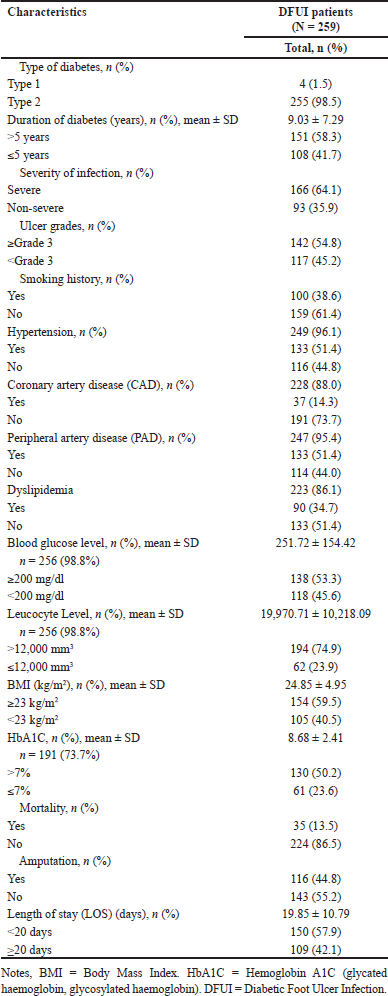 | Table 2. Clinical characteristic of the subjects. [Click here to view] |
The LOS in this study was 19.85 ± 10.79 (mean ± SD) days, with 57.9% of patients hospitalized for <20 days. This is consistent with studies conducted in several countries such as Singapore [37], Denmark [52], and China [38], where LOS ranged from 13.3 days to 18.10 ± 13.55 days (mean ± SD), depending on the severity of infection, severity of ulcer, and other existing complications. Lo et al. [37] reported LOS in DFU based on ulcer severity as ulcer-only, minor amputation, and major amputation, with mean values of 13.3, 20.5, and 59.6 days, respectively. The LOS for diabetic patients with DFU involving >50% of the lower extremity ulcer is longer than those without ulcers [59]. Despite a doubling of hospitalization rates over a 25-year period, the average LOS has decreased over the same period, from 35.3 days to 10.7 days [2].
The bivariate test results presented in Table 3 show that severity of infection, CAD, PAD, dyslipidemia, RBG level, leukocyte level, BMI, and amputation have p-values < 0.25, meaning they significantly impact mortality in DFUI patients. Data from the EURODIALE study, which involved 1,229 diabetic patients with new foot ulcers, reveal infection rates of up to 58% in patients with new foot ulcers and PAD conditions, with infections accounting for 31% or one-third of the total sample [60]. Meanwhile, ulcer severity emerges as a significant predictor of mortality [61–63]. CAD stands out as the leading cause of death, as reported in a cohort study involving 2,880 DFU and non-DFU patients, where more than 20% or one in five death cases attributed to infections occur more frequently in the DFU group [34]. The MEDFUN study in Nigeria also highlighted a close association between PAD and mortality, with statistically significant values (OR 2.252, 95%CI 1.278–3.969; p = 0.005), along with severe clinical outcomes such as lower probability of healing, prolonged healing times, increased incidence of ulcer recurrence, and amputation [64]. Pre-existing CAD and PAD conditions have been associated with mortality, as evidenced by a retrospective cohort study over 3 years, with univariate analysis results of 30.6% and 36.7% respectively [65]. A recent study conducted in Arad (Romania) from 2020 to 2022 reported an increase in triglyceride levels as a mortality biomarker in DFUI patients, with significant findings for triglycerides (TG) (126.9 ± 56.2 mg/dl vs. 165.8 ± 79.0 mg/dl, p = 0.004) and total cholesterol (133.6 ± 43 mg/dl vs. 164.6 ± 44.4 mg/dl, p = 0.002) [66]. Mader et al. (55) reported a correlation between poor glycaemic control and mortality; however, Rubio et al. [61] stated that DFUI patients without ischemia with HbA1c < 7% (53 mmol/mol) and HbA1c 8% (64 mmol/mol) have the same risk of death, with HR 1.43 (95% CI 1.02–2.0). In Indonesia, achieving controlled blood glucose and HbA1c levels in DFUI patients poses significant challenges due to various factors, including RSCM being the national referral hospital and the final referral for critical cases. Suboptimal hemoglobin levels and declining kidney function in patients post-blood transfusion further complicate emergency situations. In line with two previous studies, low medication adherence rates, low-income levels, and difficulties in accessing healthcare facilities due to demographic and other conditions contribute to the challenges of controlling blood glucose[19,67]. Consequently, RSCM often faces shortages of HbA1c reagents.
In this study, the majority of DFUI patients (74.9%) exhibited high leukocyte counts, exceeding 12 × 103 cells/μl upon admission. Based on the IDSA/IWGDF classification, this indicates leukocytosis with severe infection and serves as an infection marker related to wound size, as consistently observed in previous studies where severe infection triggers systemic inflammatory response syndrome [29,68,69]. Results from an RCT study (SIDESTEP) also highlighted a significant association between elevated WBC and clinical failure, with a 1.80 times increase with p <0.001 for every 1 standard deviation (2971 cells/mm3) in WBCs [70].
Our findings also indicate that BMI is another predictive factor affecting mortality. Over 50% of DFUI patients in our study exhibited high BMI and HbA1c values (≥ 23kg/m2 and >7%). In line with previous studies, high BMI values are associated with poor clinical outcomes, specifically delayed healing and amputation, which can impact mortality [19,71,72]. In contrast, a retrospective cohort study over 10 years in Italy from 2009 to 2019 reported that low BMI is associated with end-stage chronic degenerative diseases such as kidney and heart failure as predictors of mortality [73]. Amputation is considered a predictive factor for mortality, as underscored by recent research. In a study focusing on T2DM patients with high-risk ulcers, the adjusted hazard ratio for amputation or death was reported as 2.39 (95% CI 1.36–4.20; p = 0.003) [36]. In the group of patients with a history of ulcers, the occurrence of amputation or death followed a ratio of approximately 1:2, while in patients without prior ulcer history, this ratio was approximately 1:5. A study in Norway in 2017 found that in T2DM patients with foot ulcers, the likelihood of amputation or death in univariate analysis was 3.12 times higher (95% CI 3.03–3.21) [74]. Furthermore, a cohort study conducted in Lazio from 2012 to 2015 yielded similar results. Mortality rates in patients undergoing major and minor LEAs in the first year were 33% and 18%, respectively, and by the fourth year, these rates increased to 65% and 45%, respectively [75]. Over a 12-year follow-up period, approximately 7 out of 10 patients undergoing major lower limb amputation for the first time were reported to have died. This outcome is affected by various factors, including advanced age, gender (female), proximal amputation level, and preoperative low platelet and albumin levels, as well as parameters such as hemoglobin, erythrocyte sedimentation rate, and platelets and albumin at their lowest levels. Conversely, sodium, C-reactive protein, and neutrophil to lymphocyte ratio at the time of discharge were at their highest levels, impacting mortality at 1 month, 3 months, 6 months, and 12 months postoperatively [76]. Mortality in cases of DFU with amputation exceeds 70% at a 5-year follow-up, rising to 74% at a 2-year follow-up in patients with end-stage renal failure (ESRF) [43]. Lavery et al. [15] highlighted that ESRF patients face a heightened risk of limb loss following revascularisation procedures and have poorer survival outcomes [37].
Based on the results of multivariate analysis (binary logistic regression) using the backward stepwise LR method, binary logistic regression yielded [OR 5.202 (95% CI; 1.397–19.375), p = 0.014] and CAD [OR 5.578 (95% CI, 2.037–15.272); p < 0.001]. This multivariate analysis was conducted to determine the OR value of the predictive variables, where severity of infection showed a significant result with p = 0.014, while another factor was CAD with p < 0.001. This confirms that DFUI patients with CAD face a mortality risk 5.578 times higher than those without CAD. These findings align with a study in India by Rastogi et al. [34], which identified CAD as the predominant risk factor for mortality in DFU patients, accounting for 20% of cases (p = <0.001). Other studies have also reported a positive correlation between elevated leukocyte levels (11.75 × 109 cell/l) in amputated patients, contrasting with 8.83 × 109 cell/l in nonamputated individuals [35]. Moreover, comorbidities such as infection, CAD, and PAD also affect mortality in DFU patients. A meta-analysis highlights infection and CAD as the two primary drivers of DFU patient mortality, accounting for nearly 50% of deaths within 5 years. Infections cause 24.8% of deaths (95% CI 16.0–33.5%), while CAD accounts for 46.6% (95% CI 33.5–59.7%) [77]. Additionally, PAD emerges as a mortality predictor in DFU, with patients with PAD being 5.9 times more likely to die compared to those without (95% CI 1.37–25.33) [56]. Consistent with this, meta-analysis findings by Chen et al. [77] indicate that PAD influences mortality (HR 1.882, 95% CI 1.592–2.225) in DFU patients. The mortality rate attributable to minor amputations in diabetes and/or PAD patients is very high, reaching 3.5% in the first month, 20% in the first year, 28% in the third year, 44.1% in the fifth year, 51.3% in 6–7 years, and 58.5% in 8–9 years [78]. However, in our study, PAD was not identified as a risk factor for mortality, potentially due to limitations in data reporting, where PAD data was available for only 95.4% of cases.
Limitation of the study
The study has several limitations that need to be acknowledged in the review. First, it is a retrospective cross-sectional study that utilized secondary data extracted from the medical records of DFUI patients and foot registries. This approach leads to difficulties in controlling for information bias, such as incomplete sociodemographic and medical data of patients, potentially affecting the comprehensiveness of the obtained information because some of the transition data that we tracked manually does not match. Second, the study was conducted at a single hospital (RSCM), limiting its generalizability to Indonesia’s broader DFUI population. The short follow-up period may also affect the accuracy of mortality data analysis. Third, the majority of patients admitted to RSCM present with multiple comorbidities and highly complex conditions, warranting further investigation into related comorbidity factors. Nonetheless, the study exhibits notable strengths compared to others. First, its location at a hospital serving as a national referral center in Indonesia enhances the validity and diversity of the DFUI patient data collected. Patients admitted to this facility come from various regions across Indonesia, rendering the study results highly applicable as reference data.
CONCLUSION
The factors affecting mortality as a long-term outcome in DFUI patients treated at RSCM correlate with the findings from various studies conducted worldwide. Based on the multivariate analysis results, the severity of infection and CAD emerge as significant factors impacting mortality in DFUI patients. However, further studies are needed to explore other aspects influencing DFUI mortality. Gathering additional information through such studies would enable comparisons with the findings obtained in this study.
ACKNOWLEDGMENTS
This paper is based on a PhD thesis, and financial support was provided by the Directorate of Research and Development, Universitas Indonesia, through PUTI Pascasarjana (Q3) Grant No. NKB-035/UN2.RST/HKP.05.00/2023.
AUTHOR CONTRIBUTION
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study was approved by the Ethical Committee of RSCM-Faculty of Medicine, Universitas Indonesia, with the reference number KET-1192/UN.F1/ETIK/PPM.00.02/2022.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Hidayat B, Ramadani RV, Rudijanto A, Soewondo P, Suastika K, Siu Ng JY. Direct medical cost of type 2 diabetes mellitus and its associated complications in Indonesia. Value Heal Reg Issues [Internet]. 2022;28:82–9. CrossRef
2. Driver VR, Fabbi M, Lavery LA, Gibbons G. The costs of diabetic foot: the economic case for the limb salvage team. J Vasc Surg [Internet]. 2010;52(3 SUPPL.):17S–22S. CrossRef
3. Alavi A, Sibbald RG, Mayer D, Goodman L, Botros M, Armstrong DG, et al. Diabetic foot ulcers: Part I. Pathophysiology and prevention. J Am Acad Dermatol [Internet]. 2014;70(1):1.e1–1.e18. CrossRef
4. Caruso P, Longo M, Gicchino M, Scappaticcio L, Caputo M, Maiorino MI, et al. Long-term diabetic complications as predictors of foot ulcers healing failure: a retrospective study in a tertiary-care center. Diabetes Res Clin Pract [Internet]. 2020;163:108147. CrossRef
5. Ndosi M, Wright Hughes A, Brown S, Backhouse M, Lipsky BA, Bhogal M, et al. Prognosis of the infected diabetic foot ulcer: a 12-month prospective observational study. Diabet Med. 2018;35(1):78–88.
6. Kim TG, Moon SY, Park MS, Kwon SS, Jung KJ, Lee T, et al. Factors affecting length of hospital stay and mortality in infected diabetic foot ulcers undergoing surgical drainage without major amputation. J Korean Med Sci. 2016;31(1):120–4.
7. Neto RM, Ansaldi MA, da Costa MESM, da Silva SO, Luz VHF. A case report of a multi-drug resistant bacterial infection in a diabetic patient treated in northeast Brazil. Diabet Foot Ankle. 2012;3:1–6.
8. Ajayi EA, Ajayi AO. Pattern and outcome of diabetic admissions at a federal medical center: a 5-year review. Ann Afr Med. 2009;8(4):271–5.
9. Mansoor Z, Modaweb A. Predicting amputation in patients with diabetic foot ulcers: a systematic review. Cureus. 2022;14(7):1–11.
10. Prompers L, Schaper N, Apelqvist J, Edmonds M, Jude E, Mauricio D, et al. Prediction of outcome in individuals with diabetic foot ulcers: focus on the differences between individuals with and without peripheral arterial disease. The EURODIALE Study. Diabetologia. 2008;51(5):747–55.
11. Li X, Xiao T, Wang Y, Gu H, Liu Z, Jiang Y, et al. Incidence, risk factors for amputation among patients with diabetic foot ulcer in a Chinese tertiary hospital. Diabetes Res Clin Pract [Internet]. 2011;93(1):26–30. CrossRef
12. Jiang Y, Ran X, Jia L, Yang C, Wang P, Ma J, et al. Epidemiology of type 2 diabetic foot problems and predictive factors for amputation in china. Int J Low Extrem Wounds. 2015;14(1):19–27.
13. Vadiveloo T, Jeffcoate W, Donnan PT, Colhoun HC, McGurnaghan S, Wild S, et al. Amputation-free survival in 17,353 people at high risk for foot ulceration in diabetes: a national observational study. Diabetologia. 2018;61(12):2590–7.
14. Barbern J, Granizo JJ, Aguilar L, Alguacil R, Sainz F, Menndez MA, et al. Predictive model of short-term amputation during hospitalization of patients due to acute diabetic foot infections. Enferm Infecc Microbiol Clin. 2010;28(10):680–4.
15. Lavery LA, Armstrong DG, Murdoch DP, Peters EJG, Lipsky BA. Validation of the infectious diseases society of America’s diabetic foot infection classification system. Clin Infect Dis. 2007;44(4):562–5.
16. Saleem S, Hayat N, Ahmed I, Ahmed T, Rehan AG. Risk factors associated with poor outcome in diabetic foot ulcer patients. Turkish J Med Sci. 2017;47(3):826–31.
17. McDermott K, Fang M, Boulton AJM, Selvin E, Hicks CW. Etiology, epidemiology, and disparities in the burden of diabetic foot ulcers. Diabetes Care. 2023;46(1):209–11.
18. Spanos K, Saleptsis V, Athanasoulas A, Karathanos C, Bargiota A, Chan P, et al. Factors associated with ulcer healing and quality of life in patients with diabetic foot ulcer. Angiology. 2017;68(3):242–50.
19. Pemayun TGD, Naibaho RM. Clinical profile and outcome of diabetic foot ulcer, a view from tertiary care hospital in Semarang, Indonesia. Diabet Foot Ankle [Internet]. 2017;8(1):1–8. CrossRef
20. Senneville É, Albalawi Z, Asten SA Van, Abbas ZG, Allison G, Aragón-sánchez J, et al. IWGDF/IDSA guidelines on the diagnosis and treatment of diabetes-related foot infections (IWGDF / IDSA 2023). Clin Infect Dis. 2024;79(1):286. CrossRef
21. Pemayun TGD, Naibaho RM. Diabetic foot ulcer Registry at a Tertiary Care Hospital in Semarang, Indonesia: an overview of its clinical profile and management outcome. Diabetes Manag. 2016;6(4):82–9.
22. Chen L. Overview of clinical prediction models. Ann Transl Med. 2020;8(4):71–71.
23. Elsayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 2. classification and diagnosis of diabetes: standards of care in diabetes—2023. Diabetes Care. 2023;46(January):S19–40.
24. World Health Organization Expert Consultation. Appropriate body mass index for Asian populations and its implications. Lancet. 2004;363:157–63.
25. Yoon JL, Cho JJ, Park KM, Noh HM, Park YS. Diagnostic performance of body mass index using the western pacific regional office of world health organization reference standards for body fat percentage. J Korean Med Sci. 2015;30(2):162–6.
26. PERKENI. Pedoman Pengelolaan dan Pencegahan Diabetes Melitus Tipe 2 di Indonesia 2021. Juli 2021. PERKENI, editor. Jakarta, Indonesia: PB. PERKENI; 2021. pp. 1–119.
27. Yunir E, Tarigan TJE, Iswati E, Sarumpaet A, Christabel EV, Widiyanti D, et al. Characteristics of diabetic foot ulcer patients pre- and during COVID-19 pandemic: lessons learnt from a National Referral Hospital in Indonesia. J Prim Care Community Heal. 2022;13(71):1–8.
28. Elsayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 6. Glycemic targets: standards of care in diabetes—2023. Diabetes Care [Internet]. 2023;46(January):S97–110. Available from: http://diabetesjournals.org/care/article-pdf/46/Supplement_1/S97/693609/dc23s006.pdf by guest on 10 October 2023
29. Yunir E, Tahapary DL, Tarigan TJE, Harbuwono DS, Oktavianda YD, Kristanti M, et al. Non-vascular contributing factors of diabetic foot ulcer severity in national referral hospital of Indonesia. J Diabetes Metab Disord [Internet]. 2021;20(1):805–13. CrossRef
30. Lipsky BA, Senneville É, Abbas ZG, Aragón-Sánchez J, Diggle M, Embil JM, et al. Guidelines on the diagnosis and treatment of foot infection in persons with diabetes (IWGDF 2019 update). Diabetes Metab Res Rev. 2020;36(S1):1–24.
31. Senneville É, Albalawi Z, van Asten SA, Abbas ZG, Allison G, Aragón-Sánchez J, et al. IWGDF/IDSA guidelines on the diagnosis and treatment of diabetes-related foot infections (IWGDF/IDSA 2023). Diabetes Metab Res Rev. 2024 Mar;40(3):e3687.
32. Monteiro-Soares M, Russell D, Boyko EJ, Jeffcoate W, Mills JL, Morbach S, et al. Guidelines on the classification of diabetic foot ulcers (IWGDF 2019). Diabetes Metab Res Rev. 2020;36(S1):1–8.
33. NCSS Statistical Software LLC. Stepwise regression. Kaysville, UT: NCSS Statistical Software LLC; 2015. 1–9 pp.
34. Rastogi A, Goyal G, Kesavan R, Bal A, Kumar H, Mangalanadanam, et al. Long term outcomes after incident diabetic foot ulcer: multicenter large cohort prospective study (EDI-FOCUS investigators) epidemiology of diabetic foot complications study: epidemiology of diabetic foot complications study. Diabetes Res Clin Pract [Internet]. 2020;162(108113):1–8. CrossRef
35. Lin C, Liu J, Sun H. Risk factors for lower extremity amputation in patients with diabetic foot ulcers: a meta-analysis. PLoS One [Internet]. 2020;15(9 September):1–15. CrossRef
36. Yunir E, Hidayah CD, Harimurti K, Kshanti IAM. Three years survival and factor predicting amputation or mortality in patients with high risk for diabetic foot ulcer in fatmawati general Hospital, Jakarta. J Prim Care Community Heal. 2022;13(71):1–7.
37. Lo ZJ, Surendra NK, Saxena A, Car J. Clinical and economic burden of diabetic foot ulcers: a 5-year longitudinal multi-ethnic cohort study from the tropics. Int Wound J. 2021;18(3):375–86.
38. Lu Q, Wang J, Wei X, Wang G, Xu Y, Lu Z, et al. Cost of diabetic foot ulcer management in China: A 7-year single-center retrospective review. Diabetes, Metab Syndr Obes Targets Ther [Internet]. 2020;13:4249–60. CrossRef
39. Lynar SA, Robinson CH, Boutlis CS, Commons RJ. Risk factors for mortality in patients with diabetic foot infections: a prospective cohort study. Intern Med J. 2019;49(7):867–73.
40. Aviatin M, Sauriasari R, Yunir E, Risni HW. Evaluation of the use of antimicrobial therapy for treating diabetic foot infections in an Indonesia referral hospital: a retrospective cohort study. Infect Chemother. 2023;55(1):80–9.
41. Al-Rubeaan K, Al Derwish M, Ouizi S, Youssef AM, Subhani SN, Ibrahim HM, et al. Diabetic foot complications and their risk factors from a large retrospective cohort study. PLoS One. 2015;10(5):1–17.
42. Dinh T, Veves A. The influence of gender as a risk factor in diabetic foot ulceration. Wounds. 2008 May;20(5):127–31.
43. Moulik PK, Mtonga R, Gill G V. Amputation and mortality in new-onset diabetic foot ulcers stratified by etiology. Diabetes Care. 2003;26(2):491–4.
44. Muhammad FY, Pedro LM, Suleiman HH, Uloko AE, Gezawa ID, Adenike E, et al. Cost of illness of diabetic foot ulcer in a resource limited setting: a study from Northwestern Nigeria. J Diabetes Metab Disord. 2018;17(2):93–9.
45. Shamim M, Alhakbani M, Alqahtani M, Alharthi O, Alhaqbani Y. Knowledge, attitude, and practice regarding diabetic foot care among Saudi and non-Saudi diabetic patients in Alkharj. J Fam Med Prim Care [Internet]. 2017;6(2):169–70. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8138424/pdf/JFMPC-10-859.pdf
46. Coppola A, Montalcini T, Gallotti P, Ferrulli A, Pujia A, Luzi L, et al. A comprehensive therapeutic patient education may improve wound healing and reduce ulcer recurrence and mortality in persons with type 2 diabetes. Can J Diabetes [Internet]. 2023;47(1):73–7. Available from: https://www.canadianjournalofdiabetes.com/article/S1499-2671(22)00244-1/abstract#
47. Yunir E. Peran Faktor Metabolik, Neuropati Perifer, Inflamasi, Infeksi dan Hemostasis terhadap oksigenasi jaringan serta pengaruhnya terhadap proses penyembuhan luka kaki diabetik. Jakarta, Indonesia: Universitas Indonesia; 2016.
48. Pratama V. Analisis Efektivitas Biaya Antara Ampisilin/Sulbaktam dan Non-Ampisilin/Sulbaktam pada Pasien Infeksi Kaki Diabetik Rawat Inap di RSUPN DR. Cipto Mangunkusumo. Jakarta, Indonesia: Universitas Indonesia; 2022.
49. Pemayun TGD, Naibaho RM, Novitasari D, Amin N, Minuljo TT. Risk factors for lower extremity amputation in patients with diabetic foot ulcers: a hospital-based case-control study. Diabet Foot Ankle. 2015;6:29629.
50. Yazdanpanah L, Shahbazian H, Nazari I, Hesam S, Ahmadi F, Cheraghian B, et al. Risk factors associated with diabetic foot ulcer-free survival in patients with diabetes. Diabetes Metab Syndr Clin Res Rev [Internet]. 2018;12(6):1039–43. CrossRef
51. Indonesian Ministry of Health. National Health Research Report 2018. Balitbangkes. Jakarta, Indonesia: Indonesian Ministry of Health; 2019. pp. 1–628.
52. Søndergaard LN ørregaar, Christensen AB undgaar, Vinding AL un, Kjær IL undin, Larsen P. Elevated costs and high one-year mortality in patients with diabetic foot ulcers after surgery. Dan Med J. 2015;62(4):A5050.
53. Nuttall FQ. Body mass index: obesity, BMI, and health: a critical review. Nutr Today. 2015;50(3):117–28.
54. Netten JJ van, Bus SA, Apelqvist J, Lipsky BA, Hinchliffe RJ, Game F, et al. IWGDF definitions and criteria for diabetic foot disease. Lancet Diabetes Endocrinol [Internet]. 2019;2019(1):1–15. CrossRef
55. Casqueiro J, Casqueiro J, Alves C. Infections in patients with diabetes mellitus: a review of pathogenesis. Indian J Endocrinol Metab. 2012;16(7):27.
56. Mader JK, Haas W, Aberer F, Boulgaropoulos B, Baumann P, Pandis M, et al. Patients with healed diabetic foot ulcer represent a cohort at highest risk for future fatal events. Sci Rep. 2019;9(1):1–6.
57. Akyüz S, Mutlu ABB, Güven HE, Ba?ak AM, Y?lmaz KB. Elevated HbA1c level associated with disease severity and surgical extension in diabetic foot patients. Ulus Travma ve Acil Cerrahi Derg. 2023;29(9):1013–8.
58. Rubio JA, Jiménez S, Álvarez J. Clinical characteristics and mortality in patients treated in a Multidisciplinary Diabetic Foot Unit. Endocrinol Diabetes y Nutr. 2017;64(5):241–9.
59. Stockl K, Vanderplas A, Tafesse E, Chang E. Costs of lower-extremity ulcers among patients with diabetes. Diabetes Care [Internet]. 2004;27(9):2129–34. Available from: https://watermark.silverchair.com/zdc00904002129.pdf? token=AQECAHi208BE49Ooan9kkhW_Ercy7Dm3 ZL_9Cf3qfKAc485ysgAAA0kwggNFBgkqhkiG9w0BBwagggM2MIIDMgIBADCCAysGCSqGSIb3DQEHATAeBglghkgBZQMEAS4wEQQMwf80O2 KGrqNfFnY3AgEQgIIC_ADqd-naZ0gDqKp7arggl1y6Nzz0CjVWfKMJJS8f
60. Prompers L, Huijberts M, Apelqvist J, Jude E, Piaggesi A, Bakker K, et al. High prevalence of ischaemia, infection and serious comorbidity in patients with diabetic foot disease in Europe. Baseline results from the Eurodiale study. Diabetologia. 2007;50(1):18–25.
61. Rubio JA, Jiménez S, Lázaro-Martínez JL. Mortality in patients with diabetic foot ulcers: causes, risk factors, and their association with evolution and severity of ulcer. J Clin Med. 2020;9(9):1–14.
62. Brennan MB, Hess TM, Bartle B, Cooper JM, Kang J, Huang ES, et al. Diabetic foot ulcer severity predicts mortality among veterans with type 2 diabetes. J Diabetes Complications [Internet]. 2017;31(3):556–61. CrossRef
63. Jalilian M, Jalali R. Prevalence of celiac disease in children with type 1 diabetes: a review. Diabetes Metab Syndr Clin Res Rev [Internet]. 2021;15(3):969–74. CrossRef
64. Adeleye OO, Ugwu ET, Gezawa ID, Okpe I, Ezeani I, Enamino M. Predictors of intra-hospital mortality in patients with diabetic foot ulcers in Nigeria: data from the MEDFUN study. BMC Endocr Disord. 2020;20(1):1–10.
65. Panuda JPP, Macalalad Josue AA, Buenaluz-Sedurante M. Factors associated with in-hospital mortality among patients with diabetes admitted for lower extremity infections. J ASEAN Fed Endocr Soc. 2019;34(1):36–43.
66. Ardelean A, Neamtu AA, Balta DF, Neamtu C, Goldis D, Rosu M, et al. Lipid profile paradox: investigating improved lipid levels in diabetic mellitus patients with foot ulcer infections—a prospective descriptive study. Diagnostics. 2023;13(23):1–15.
67. Sutanegara D, Darmono, Budhiarta AAG. The epidemiology and management of diabetes mellitus in Indonesia. Diabetes Res Clin Pract. 2000;50:S9–16.
68. Lipsky BA, Berendt AR, Cornia PB, Pile JC, Peters EJG, Armstrong DG, et al. 2012 infectious diseases society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54(12):132–73.
69. Lee KM, Kim WH, Lee JH, Choi MSS. Risk factors of treatment failure in diabetic foot ulcer patients. Arch Plast Surg. 2013;40(2):123–8.
70. Lipsky BA, Sheehan P, Armstrong DG, Tice AD, Polis AB, Abramson MA. Clinical predictors of treatment failure for diabetic foot infections: data from a prospective trial. Int Wound J. 2007;4(1):30–8.
71. Dutra LMA, Melo MC, Moura MC, Leme LAP, De Carvalho MR, Mascarenhas AN, et al. Prognosis of the outcome of severe diabetic foot ulcers with multidisciplinary care. J Multidiscip Healthc. 2019;12:349–59.
72. Sarfo-Kantanka O, Sarfo FS, Kyei I, Agyemang C, Mbanya JC. Incidence and determinants of diabetes-related lower limb amputations in Ghana, 2010-2015 - a retrospective cohort study. BMC Endocr Disord. 2019;19(1):1–8.
72. Gazzaruso C, Gallotti P, Pujia A, Montalcini T, Giustina A, Coppola A. Predictors of healing, ulcer recurrence and persistence, amputation and mortality in type 2 diabetic patients with diabetic foot: a 10-year retrospective cohort study. Endocrine [Internet]. 2021;71(1):59–68. CrossRef
74. Chamberlain RC, Fleetwood K, Wild SH, Colhoun HM, Lindsay RS, Petrie JR, et al. Foot ulcer and risk of lowerlimb amputation or death in people with diabetes: a national population-based retrospective cohort study. Diabetes Care. 2022;45(1):83–91.
75. Cascini S, Agabiti N, Davoli M, Uccioli L, Meloni M, Giurato L, et al. Survival and factors predicting mortality after major and minor lower-extremity amputations among patients with diabetes: a population-based study using health information systems. BMJ Open Diabetes Res Care. 2020;8(1):1–8.
76. Reiso?lu A, Turgut A, Filibeli M, ?ncesu M, Yalç?n E, Parlar O. Analysis of the factors affecting mortality after non-traumatic major lower extremity amputations. Acta Orthop Traumatol Turc. 2022;56(6):377–83.
77. Chen L, Sun S, Gao Y, Ran X. Global mortality of diabetic foot ulcer: a systematic review and meta-analysis of observational studies. Diabetes, Obes Metab [Internet]. 2023;25(1):36–45. Available from: https://dom-pubs.onlinelibrary.wiley.com/action/showCitFormats?doi=10.1111%2Fdom.14840
78. Yammine K, Hayek F, Assi C. A meta-analysis of mortality after minor amputation among patients with diabetes and/or peripheral vascular disease. J Vasc Surg [Internet]. 2020;72(6):2197–207. CrossRef