INTRODUCTION
The immune system is essential in protecting the body against infectious diseases and pathogens, as well as supporting cell cycle homeostasis [1]. Two groups of cells, macrophages and lymphocytes, play crucial roles in innate and adaptive immunity, respectively [2]. Furthermore, Pattern Recognized Receptors found on the surface of innate immune cells identify foreign microbes for destruction through defense mechanisms, such as NK cells and macrophage-mediated phagocytosis [3]. In adaptive immunity, microorganisms are recognized by T and B lymphocytes through antigen-specific surface receptors, then eliminated by cytotoxic responses (neutrophils, CD45+ lymphocytes, CD8+ T cells, monocytes, and B lymphocytes) and antibody production [4].
Natural materials are increasingly used as immunostimulants due to their stronger tolerance ability and minimal side effects [5]. The World Health Organization estimates that approximately three-quarters of the global population currently use herbs and other forms of traditional medicine to treat various diseases [6]. Ciplukan (Physalis angulata L) is among such plants applied in traditional medicine for many years.
Physalis angulata L belongs to the Solanaceae family widely distributed in tropical and sub-tropical regions. This plant is traditionally used to treat malaria, asthma, hepatitis, dermatitis, and rheumatism [7]. The components include phenolic acids (21.51%), flavonoids (15.59%), chalcones (1.08%), coumarins (7.53%), other phenolic compounds (16.13%), amino acid-related compounds (10.75%), fatty acid-related compounds (6.45%), and terpenoids (20.97%) [8]. Daltro et al. [9] reported that ethanol extract from P. angulata L stems reduced the production of NO, interleukin 6, interleukin 12, and TNF-a in activated macrophage cells without affecting cell viability. Wang et al. [10] found that Physagulin A, C, and H isolated from the stems and leaves inhibited the production of NO, IL-6, IL-12, and TNF-a in RAW 264.7 cells without initiating toxicity. Additionally, P. angulata L fruit callus extract can prevent the induction of IL-1B, (TNF-α), IL-6, IL-12, cyclooxygenase-2 (COX-2), and inducible nitric oxide synthase (iNOS), as well as increase the levels of arginase 1, IL-10, and mannose receptor C [11].
The activity of P. angulata L extract which addressed it as an immunomodulator has been widely reviewed in various studies above, but existing studies on the use of P. angulata L fruit extracts specific as an immunostimulatory agent are still limited. Kusumaningtyas et al. [12] have been reported that P. angulata L fruit ethanol extract at a concentration of 1 mg/100 µl increased lymphocyte cell proliferation with a stimulation index of 204.09% [12], and Marin Sisley et al. [13] found that inducing a 17.2 mg/kg dose of P. angulata L herb extract for 6 hours extract elevated the average activation of immune system response by 58.8% [13]. However, in vitro activity of P. angulata L fruit extract against macrophage cells and in vivo effects on male Wistar rats as immunostimulants have not been widely explored. Therefore, this study aimed to characterize as well as investigate in vivo and in vitro immunostimulatory effect of P. angulata L fruit extract in male Wistar rats and RAW 264.7 cells.
MATERIALS AND METHODS
Material
The materials used in this study included P. angulata L fruit, ethanol 70%, methanol, water, gallic acid, folin ciocalteu reagent, male Wistar rats, RAW 264.7 cells (Elabscience, Texas, USA), Dulbecco’s Modified Eagle medium (DMEM), Fetal Bovine Serum (FBS), penicillin-streptomycin, WST-1 reagent, and a Nitric oxide (NO) kit (Catalog no.MAK367, Sigma).
Extraction of P. angulata L fruit
This research was carried out from August 2021 to December 2023. The ripe fruit of P. angulata L with yellow–purple color of epicarp, age 2–3 months was collected in the summer season from the Ciwidey plantation area, Bandung, West Java, Indonesia. The samples were then identified at the National Research and Innovation Agency, Bogor. Fruit samples were dried as well as macerated with a 1:1 mixture of 70% ethanol and water, then evaporated using a rotavapor. The resulting thick extract was granulated with mannitol and aerosil, dried, and powdered.
Standardization
The dried extract was standardized by assessing moisture content, density, loss on drying, total ash content, water-soluble compounds, and ethanol-soluble compounds based on the evaluation procedure in practical pharmacognosy [14].
Metabolite identification using LC-ESI-MS
Physalis angulata L fruit extract was analyzed using an LC-ESI-MS instrument (Waters Acquity UPLC I-Class and XEVO G2-XS QTOF). During this process, 10 mg of extract samples were weighed, dissolved in 10 ml of methanol, and inserted into a 5 µl microsyringe. The samples were eluted with a gradient system using a mobile phase of water/formic acid (99.9/0.1 [v/v]) and acetonitrile/formic acid (99.9/0.1 [v/v]). The resulting separation chromatogram was processed with Masslynx 4.1 software to obtain data in the form of the peak areas and m/z spectra of each peak. Furthermore, predicted compounds were interpreted using ChemSpider and PubChem [15].
Determination of total phenolic content
The total phenolic content in the samples was determined using the Folin-Ciocalteu colorimetric method. Furthermore, gallic acid served as a standard, and the results were presented in the form of mg gallic acid equivalent/100 g P. angulata L fruit extract [9].
In vivo immunostimulatory effect
The immunostimulatory effect of P. angulata L fruit extract was determined according to Adnyana et al. [16] and Fitria et al. [17] with some modifications. Male Wistar rats of 2–3 months old, weighing 150–250 g, were obtained from SITH, Bandung Institute of Technology. Handling and treatment of these test animals adhered to the ethics approval no. 269/HRECC.FODM/VI/2021. The rats were divided into four groups of six members each, then adapted. Treatments including 300 mg P. angulata L fruit extract/70 kg BW, a gallic acid equivalent dose of 300 mg P. angulata L fruit extract/70 kg BW, negative control, and positive control, were orally administered to groups 1, 2, 3, and 4, respectively, for 14 days. Hematological measurements of leukocytes, lymphocytes, monocytes, and granulocytes were conducted on day 14 using a hematology analyzer.
Cell culture
The entire RAW 264.7 cells (Elabscience, Texas, USA) were cultured in DMEM, supplemented with 10% FBS and 1% penicillin-streptomycin, then incubated at 37°C with 5% CO2 [18].
Cell viability
RAW 264.7 cells at 7 × 103 were cultured in 96 well plates and incubated for 24 hours. Approximately 50 µl of P. angulata L fruit extract with concentrations of 1,000 ppm, 500 ppm, 250 ppm, 125 ppm, 62.5 ppm, 31.25, and 15, 63 ppm were added to each well, then incubated for another 24 hours. Subsequently, WST-1 reagent was added and incubated for 2 hours before measuring absorbance with a microplate reader at a wavelength of 450/620 nm [17].
Nitric oxide assay
RAW 264.7 cells (1 × 105) were cultured in 24 well plates and incubated for 24 hours at 37°C with 5% CO2. The previously used medium was discarded, and then 500 µl of P. angulata L fruit extract dissolved in FBS-free medium was added to the wells at concentrations of 500 ppm, 250 ppm, and 125 ppm. After incubation for 48 hours, 100 µl of supernatant collected from each sample was reacted with 100 µl of Griess reagent (Catalog no.MAK367, Sigma) according to the manufacturer’s instructions. NO concentration was calculated based on the NO2 standard curve, and absorbance was measured at 540 nm using a microplate reader [19].
Statistical analysis
Collected data were analyzed with one-way ANOVA using Minitab® statistical software version 21.3.1. Subsequently, the values obtained from this process were considered to be significantly different when p < 0.05.
RESULTS AND DISCUSSION
Standardization of P. angulata L fruit extract
Standardization of P. angulata L fruit extract was conducted by assessing moisture content, density, loss on drying, total ash content, water-soluble compounds, and ethanol-soluble compounds. The result is presented in Table 1. The results of the moisture content test showed that P. angulata L fruit extract met the moisture requirements (< 10%), which was the threshold for the water content of the extract to avoid microbial contamination and enzymatic hydrolysis [20]. The results of loss on drying test also indicated that P. angulata L fruit extract met the requirements, which was < 11%, where loss on drying indicated the amount of compounds lost after drying at a temperature of 105°C. The total ash content obtained also met the requirements, which was < 16.6%, where the ash content indicated the level of inorganic compounds, including light metals, heavy metals, and transition metals present in the extract. High levels of metals in the extract can affect its safety [13]. The results also indicated that ethanol-soluble compounds in P. angulata L fruit extract were higher than water-soluble compounds [20]. In general, the test results signified that P. angulata L fruit extract fulfilled the standardization parameters, leading to suitability for traditional medicine development.
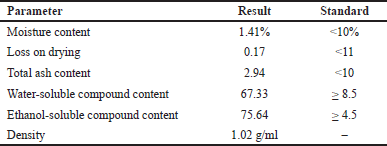 | Table 1. Standardization results of P. angulata L fruit extract. [Click here to view] |
Metabolite identification by LC-ESI-MS
A total of 16 metabolite compounds were identified from P. angulata L fruit extract using LC-ESI-MS (Fig. 1) according to mass accuracy and fragmentation patterns. These compounds belonged to gallic acid derivatives, coumarin, flavonoids, diterpenoids, sesquiterpene, and triterpene compounds. Table 2 shows the retention time, mass, and name of the isolated compounds. Gallic acid is found to improve immunomodulatory activity by enhancing the phagocytic capacity of macrophages, lysosomal volume, nitric release, and intracellular calcium levels while downregulating MAPK-related phagocytic signaling pathways in murine macrophages [21]. This compound also produces therapeutic effects against infection by disrupting intracellular signaling pathways, inducing cytokine production, and preventing bacterial proliferation in mouse spleens [22]. Flavonoids have potential preventive activity against oxidative cell destruction as well as the ability to block tumor induction, stimulation, and progression [23]. However, a group of terpenoids known as andrographolide and boswellic acid suppress NO and iNOS production, along with pro-inflammatory mediators [24]. Coumarins produce anti-inflammatory effects in lipopolysaccharide-stimulated mouse macrophages (RAW264.7) in an in vitro edema model, inhibiting the protein expression of nitric oxide synthase and COX-2 [25]. Additionally, cummarins have been reported to block several inflammatory signaling pathways [26]. Based on the observations in this study, the immunostimulatory activity of P. angulata L is attributed to the gallic acid and flavonoid content.
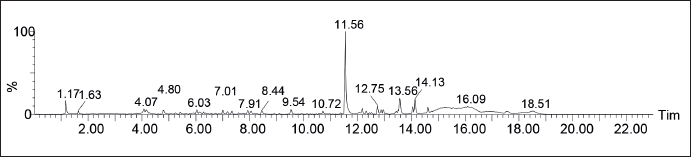 | Figure 1. Chromatogram of P. angulata L fruit extract (P. angulata L) sample using UPLC-QToFMS/MS method. [Click here to view] |
 | Table 2. Metabolite identification results of P. angulata L fruit extract (P. angulata L) using UPLC-QToFMS/MS method. [Click here to view] |
Total phenolic content of P. angulata L fruit extract
Total phenolic content was determined through the Folin-Ciocalteu colorimetric method and recorded as gallic acid equivalents. The principle of this method is the oxidation reaction of the hydroxyl group of phenolic compounds with Folin-Ciocalteu reagent to form a blue molybdenum-tungsten complex detectible by a spectrophotometer [27]. Based on Table 3, the total phenolic content of P. angulata L fruit extract was 2.895 mg/g gallic acid. A previous study stated that the total phenolic content equivalent to gallic acid from water and ethanol extract of P. angulata L fruit was 0.46 and 0.51 mg/G, respectively [11]. Meanwhile, de Oliveira et al. [28] reported the total phenolic content for water-soluble and water-insoluble fractions of ripe P. angulata L fruit to be 749.10 and 3847.72 mg/100 g of extract, respectively [28]. Iwansyah et al. [29] estimated the total phenolic content of P. angulata L fruit juice as 18.74 mg GAE/L [29]. The difference in total phenolic content is influenced by the polarity of the solvent used for extraction and the non-specific characteristics of the folin ciocalteu reagent, which can be easily reduced by non-flavonoid compounds [30]. A literature study showed that phenolic acid content of P. angulata L fruit decrease during the ripening process due to being used as an energy source in plant respiration and a carbon source in the synthesis of sugars alongside other compounds [31].
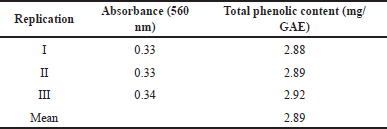 | Table 3. Total phenolic content of P. angulata L fruit extract. [Click here to view] |
Immunostimulatory effect in Male Wistar rats
The immunostimulatory effect in male Wistar rats was determined using leukocyte, lymphocyte, monocyte, and granulocyte counts. The measurement results showed that rats induced with 300 mg/70 kg BW P. angulata L fruit extract had increased leukocyte counts, compared to the negative control, gallic acid, and positive control (Fig. 2). Leukocytes are white blood cells essential to the immune system and capable of protecting the body against infection. Foreign materials that enter the body are surrounded, ingested, and eliminated by leucocytes [32].
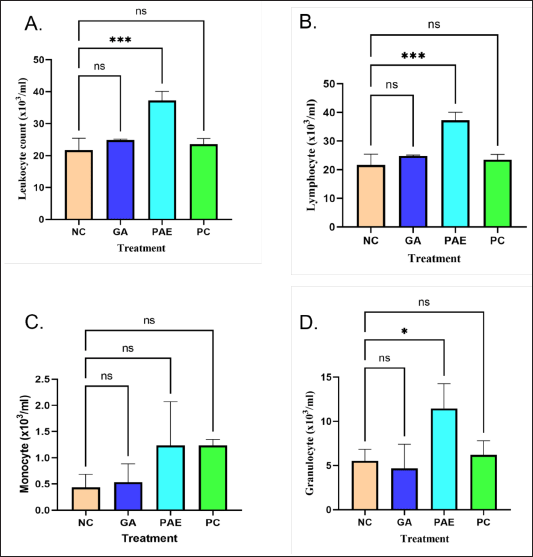 | Figure 2. Immunostimulatory activity of P. angulata L fruit extract with parameters of (A) leukocytes (B) lymphocytes (C) monocytes (D) granulocytes. NC = Negative control; GA = Gallic Acid; PAE = Physalis angulata Extract; PC = Positive Control. [Click here to view] |
Parameters of lymphocytes and granulocytes were found to present similar patterns in this study. Lymphocytes are a type of leukocytes that contribute to the immune response and antibody production. These comprise 20%–40% of all leukocytes in an adult individual and play an important role in the immune system due to being cells that define the features of the immune response to pathogenic bacteria alongside other foreign substances. Meanwhile, granulocytes, with types mainly including neutrophils, eosinophils, and basophils, are a sub-group of leukocytes containing granules that release enzymes when the immune system is attacked due to infection, allergy, or asthma [33].
The experimental animals treated with P. angulata L extract showed the same amounts of monocytes as the positive control group. Monocytes are circulating immune cells in the mononuclear phagocytic system that become macrophages when migrating in tissues [34]. In adult humans, monocytes are derived from precursor cells driven by the cytokine M-CSF (Monocyte/Macrophage-Colony Stimulating Factor) into the bone marrow. These precursors mature into monocytes then enter the circulation and migrate into tissues to form macrophages. Monocytes are phagocytic, recruited rapidly at sites of infection or tissue injury, and capable of producing inflammatory mediators [35]. Leukocyte, lymphocyte, monocyte, and granulocyte counts in male Wistar rats can all be elevated by P. angulata L fruit extract induction.
Viability test
The viability test results showed that 500 ppm of P. angulata L extract was not harmful to RAW 264.7 cells (Fig. 3). At 250 ppm, 125 ppm, and 62.5 ppm concentrations, the maximum survival rate was observed. All concentrations led to a total survival rate greater than 100%, suggesting P. angulata L fruit extract as non-cytotoxic.
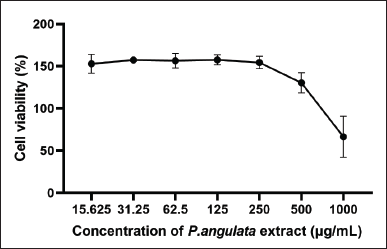 | Figure 3. Effect of P. angulata L fruit extract on RAW 264.7 cell viability. [Click here to view] |
Nitric oxide test
The test conducted showed that P. angulata L fruit extract triggered RAW 264.7 cells to produce higher NO compared to the control in a concentration-dependent manner (Fig. 4). NO is a harmful chemical produced by cells to facilitate the destruction of pathogenic bacteria and tumors as well as mediate biological reactions [19]. The effectiveness of P. angulata L fruit extract in eradicating harmful germs was signified by the elevation in NO levels. These results showed that P. angulata L fruit extract had immunostimulatory effects against macrophages. These also correlate with the characteristics of activated macrophage cells that can secrete several types of mediators including ROS, NO, and cytokines to protect the body from infection.
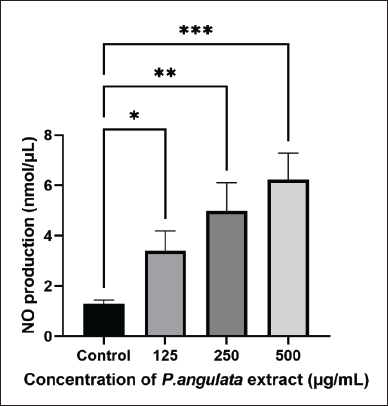 | Figure 4. Effect of P. angulata L fruit extract on NO levels of RAW 264.7 cells. [Click here to view] |
CONCLUSION
In conclusion, this study identified that P. angulata L fruit extract contained sesquiterpenes, triterpenes, coumarins, flavonoids, and derivatives of gallic acid. Physalis angulata L fruit extract had a total phenolic content of 2.895 mg/GAE. The increase of leukocyte, lymphocyte, monocyte, and granulocyte counts in the Wistar rats signified in vivo immunostimulant activity. Meanwhile, in vitro immunostimulatory effect was shown by lower toxicity to macrophages and enhanced NO generation from control cells.
ACKNOWLEDGMENT
This study was funded by the Center for Higher Education Fund, Ministry of Education, Culture, Research, and Technology of the Republic of Indonesia, and Indonesia Endowment Fund for Education (LPDP) from the Ministry of Finance of the Republic of Indonesia with registration number: 202101121279. The authors are also grateful to the Forensic Laboratory Center, Sentul West Java for providing UPLC-QToFMS/MS instrument, the Translational Pharmaceutical Research Laboratory of Padjajaran University for assisting with cell culture, and Bandung Institute of Technology for offering access to valuable facilities.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took Fart in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors declare that there is no conflict of interest.
ETHICAL APPROVALS
The study protocol was approved by the Health Research Ethical Clearance Commission of School of Pharmacy, Bandung Institute of Technology, Indonesia (Approval No. 269/HRECC.FODM/VI/2021).
DATA AVAILABILITY
All data generated and analyzed are included in this study.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Rajanna M, Bharathi B, Shivakumar BR, Deepak M, Prashanth DS, Prabakaran D, et al. Immunomodulatory effects of andrographis paniculata extract in healthy adults—an open-label study. J Ayurveda Integr Med. 2021 Jul 1;12(3):529–34. CrossRef
2. Duque GA, Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol. 2014;5(OCT):1–12. CrossRef
3. Marshall JS, Warrington R, Watson W, Kim HL. An introduction to immunology and immunopathology. Allergy, Asthma Clin Immunol [Internet]. 2018;14(s2):1–10. CrossRef
4. Alhazmi HA, Najmi A, Javed SA, Sultana S, Al Bratty M, Makeen HA, et al. Medicinal plants and isolated molecules demonstrating immunomodulation activity as potential alternative therapies for viral diseases including COVID-19. Front Immunol [Internet]. 2021 May 13;12:1–24. CrossRef
5. Rasheed HMF, Rasheed F, Qureshi AW, Jabeen Q. Immunostimulant activities of the aqueous methanolic extract of Leptadenia pyrotechnica, a plant from Cholistan desert. J Ethnopharmacol [Internet]. 2016 Jun;186:244–50. CrossRef
6. Musthaba M, Baboota S, Athar TM, Thajudeen KY, Ahmed S, Ali J. Patented herbal formulations and their therapeutic applications. Recent Pat Drug Deliv Formul [Internet]. 2010 Nov 1;4(3):231–44. Available from: http://www.eurekaselect.com/openurl/content.php?genre=article&issn=1872-2113&volume=4&issue=3&spage=231.https://doi.org/10.2174/187221110793237538
7. Rivera DE, Ocampo YC, Castro JP, Barrios L, Diaz F, Franco LA. A screening of plants used in Colombian traditional medicine revealed the anti-inflammatory potential of Physalis angulata calyces. Saudi J Biol Sci [Internet]. 2019 Nov;26(7):1758–66. CrossRef
8. Barbosa Lima LG, Montenegro J, De Abreu JP, Barros Santos MC, Pimenta do Nascimento T, Da Silva Santos M, et al. Metabolite profiling by UPLC-MSE, NMR, and antioxidant properties of amazonian fruits: mamey apple (Mammea Americana), Camapu (Physalis Angulata), and Uxi (Endopleura Uchi) Larissa. Mol Nutr Food Res. 2020;25:342. CrossRef
9. Daltro SRT, Santos IP, Barros PL, Moreira DRM, Tomassini TCB, Ribeiro IM, et al. In vitro and in vivo immunomodulatory activity of Physalis angulata concentrated ethanolic extract. Planta Med. 2021;87(1–2):160–8. CrossRef
10. Wang L, Lu S, Wang L, Xin M, Xu Y, Wang G, et al. Anti-inflammatory effects of three withanolides isolated from Physalis angulata L. in LPS-activated RAW 264.7 cells through blocking NF-κB signaling pathway. J Ethnopharmacol [Internet]. 2021;276(March):114186. CrossRef
11. Rivera D, Ocampo Y, Franco LA. Physalis angulata calyces modulate macrophage polarization and alleviate chemically induced intestinal inflammation in mice. Biomedicines. 2020 Feb 1;8(2):24. CrossRef
12. Kusumaningtyas R, Laily N, Limandha P. Potential of ciplukan (Physalis Angulata L.) as source of functional ingredient. Procedia Chem. 2015;14:367–72. CrossRef
13. Marin Sisley GM, Max Horna FY, Rios Isern F, Aranda-Ventura J, Villacres Vallejo J. Actividad inmunoestimulante del extracto acuoso liofilizado de la planta entera de Physalis angulata L. en ratas albinas cepa Holtzman. Rev Peru Med Integr. 2017;2(1):38–46. CrossRef
14. Khandelwal KR. Practical pharmacognosy. 19th ed. Pune, India: Nirali Prakashan; 2008.
15. Mutiah R, Rachmawati E, Fitrianingsih AA, Zahiro SR. Metabolite profiling of anticancer compounds in Saussure lappa based on UPLC-QToFMS/MS. Pharm Educ. 2023;23(4):37–42. CrossRef
16. Adnyana IK, Yulinah E, Maeistuti N, Setiawan F. Evaluation of ethanolic extracts of mullaca (Physalis Angulata L.) herbs for treatment of lupus disease in mice induced pristane. Procedia Chem. 2014;13:186–93. CrossRef
17. Fitria A, Hanifah S, Chabib L, Uno AM, Munawwarah H, Atsil N, et al. Design and characterization of propolis extract loaded self-nano emulsifying drug delivery system as immunostimulant. Saudi Pharm J [Internet]. 2021;29(6):625–34. CrossRef
18. Lallo S, Hardianti B, Djabir YY, Ismail I, Indrisari M, Aswad M, et al. Piper retrofractum ameliorates imiquimod-induced skin inflammation via modulation of TLR4 axis and suppression of NF-κB activity. Heliyon [Internet]. 2023;9(9):e20151. CrossRef
19. Jung JY, Shin JS, Rhee YK, Cho CW, Lee MK, Hong HD, et al. In vitro and in vivo immunostimulatory activity of an exopolysaccharide-enriched fraction from Bacillus subtilis. J Appl Microbiol. 2015;118(3):739–52. CrossRef
20. Kunle. Standardization of herbal medicines—a review. Int J Biodivers Conserv. 2012;4(3):101–12. CrossRef
21. Yang K, Zhang L, Liao P, Xiao Z, Zhang F, Sindaye D, et al. Impact of gallic acid on gut health: focus on the gut microbiome, immune response, and mechanisms of action. Front Immunol. 2020;11(September):1–13. CrossRef
22. Reyes AWB, Arayan LT, Hop HT, Ngoc Huy TX, Vu SH, Min W, et al. Effects of gallic acid on signaling kinases in murine macrophages and immune modulation against Brucella abortus 544 infection in mice. Microb Pathog [Internet]. 2018 Jun;119:255–9. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0882401018305564
23. Farhan M, Rizvi A, Aatif M, Ahmad A. Current understanding of flavonoids in cancer therapy and prevention. Metabolites [Internet]. 2023 Mar 27;13(4):481. Available from: https://www.mdpi.com/2218-1989/13/4/481
24. Behl T, Kumar K, Brisc C, Rus M, Nistor-Cseppento DC, Bustea C, et al. Exploring the multifocal role of phytochemicals as immunomodulators. Biomed Pharmacother [Internet]. 2021;133(October 2020):110959. CrossRef
25. Flores-Morales V, Villasana-Ruíz AP, Garza-Veloz I, González-Delgado S, Martinez-Fierro ML. Therapeutic effects of coumarins with different substitution patterns. Molecules [Internet]. 2023 Mar 6;28(5):2413. Available from: https://www.mdpi.com/1420-3049/28/5/2413
26. Rostom B, Karaky R, Kassab I, Sylla-Iyarreta Veitía M. Coumarins derivatives and inflammation: review of their effects on the inflammatory signaling pathways. Eur J Pharmacol. 2022;922(February). CrossRef
27. Dewantara LAR, Ananto AD, Andayani Y. Penetapan Kadar Fenolik Total Ekstrak Kacang Panjang (Vigna unguiculata) dengan Metode Spektrofotometri UV-visible. Lumbung Farm J Ilmu Kefarmasian. 2021;2(1):102. CrossRef
28. de Oliveira AM, Malunga LN, Perussello CA, Beta T, Ribani RH. Phenolic acids from fruits of Physalis angulata L. in two stages of maturation. South Afr J Bot. 2020 Jul;131:448–53. CrossRef
29. Iwansyah AC, Luthfiyanti R, Ardiansyah RCE, Rahman N, Andriana Y, Hamid HA. Antidiabetic activity of Physalis angulata L. fruit juice on streptozotocin-induced diabetic rats. South African J Bot. 2022;145:313–9. CrossRef
30. Shi L, Zhao W, Yang Z, Subbiah V, Suleria HAR. Extraction and characterization of phenolic compounds and their potential antioxidant activities. Environ Sci Pollut Res [Internet]. 2022 Nov 6;29(54):81112–29. Available from: https://link.springer.com/10.1007/s11356-022-23337-6
31. Naczk M, Shahidi F. Extraction and analysis of phenolics in food. J Chromatogr A [Internet]. 2004 Oct;1054(1–2):95–111. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0021967304014098
32. Sinaga E. Analisis imunostimulan Ekstrak Etanol Daun Pirdot (Saurauia vulcani Korth.) Pada Tikus (Rattus norvegicus L.). Medan, Indonesia: Universitas Sumatera Utara; 2020.
33. Makepeace BL, Martin C, Turner JD, Specht S. Granulocytes in helminth infection—who is calling the shots? Curr Med Chem. 2012;19(10):1567–86. CrossRef
34. Italiani P, Boraschi D. From monocytes to M1/M2 macrophages: phenotypical versus functional differentiation. Front Immunol. 2014;5(OCT):1–22. CrossRef
35. Yang J, Zhang L, Yu C, Yang XF, Wang H. Monocyte and macrophage differentiation: circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomark Res [Internet]. 2014 Dec 7;2(1):1. Available from: https://biomarkerres.biomedcentral.com/articles/10.1186/2050-7771-2-1