INTRODUCTION
Monocytes are pivotal components of the innate immune system. They are crucial in combating infections, controlling inflammation, and tissue repair and remodeling via inflammatory and anti-inflammatory responses. The dysregulation of monocytes is characterized by increased proinflammatory phenotypes. This could potentially initiate or exacerbate immunopathological conditions such as allergies, autoimmune diseases, chronic inflammation, and immunosenescence in elderly individuals [1,2]. Recently, the concept of trained immunity has been introduced which involves the epigenetic changes and metabolic reprogramming of the innate immune cells including monocytes. This adaptive-like response of the innate immune system enhances its reaction to subsequent infections or stressors after an initial encounter [3]. Studies on trained immunity have implications for developing vaccines and therapies, as enhancing innate immune memory might offer improved defenses against infectious diseases and immune modulation in chronic inflammatory conditions [4]. Therefore, the strategies to modulate the balance of monocyte immune response are challenging.
A healthy diet or functional food has gained attention for its impact on modulating the human immune system. Functional foods contain antioxidants, fiber, prebiotics, or phytochemicals that can prevent diseases or improve overall health. Growing evidence reveals that bioactive components in such foods influence the molecular regulatory mechanisms such as epigenetics involved in gene expression [5,6]. Mushrooms, one of the functional foods, are now recognized as a source of nutraceuticals and have been revered in traditional medicine for centuries [7]. Extensive research has unequivocally shown the remarkable health benefits of edible mushrooms for their anti-cancer, antioxidant, anti-microbial, and immunomodulatory effects. However, these advantages have been demonstrated in some mushroom species, whereas most mushrooms are still unknown [8,9]. Furthermore, the insight mechanisms that lead to the activity have not been thoroughly explored. Several pieces of evidence demonstrated that different mushroom strains, species, and extraction methods affect bioavailability and bioactivity [10–12]. Thus, an investigation into commonly available and affordable mushroom species, alongside an analysis of their nutritional components through extraction methods mirroring traditional culinary techniques, could yield accurate data that more authentically reflects the transformations occurring in real cooking processes.
Pleurotus pulmonarius (P. pulmonarius), the Indian oyster, is an easily cultivated and affordable mushroom that is widely consumed worldwide and has significant economic value. P. pulmonarius is high in dietary fiber and rich in bioactive compounds such as polysaccharide components, proteins, vitamins, and minerals. Literature studies have reported the anticancer, antioxidant, and immunomodulatory effects of P. pulmonarius extracts [12]. The studies on the immunomodulatory activities of P. pulmonarius are limited and mainly focused on the effect of bioactive compounds extracted from methanol or organic solvent [12–15]. The specific bioactive compounds extracted from hot water that are responsible for immunomodulatory activities on monocytes and the pharmacological network have not been thoroughly explored.
This study is the first to demonstrate the profile of water-soluble compounds in P. pulmonarius. Pleurotus pulmonarius was extracted using hot water to simulate traditional food and alternative medicine use. A comprehensive analysis using untargeted liquid chromatography with tandem mass spectrometry (LC-MS/MS) was performed to identify bioactive compounds in P. pulmonarius. Genes associated with monocyte immune responses, metabolic control, and epigenetic regulation were gathered and the tentative compound-target proteins were identified. Furthermore, the interactions between these bioactive compounds and monocyte-targeted proteins were investigated using an in silico molecular docking approach. Additionally, the pharmacokinetic properties and the biological activity of potential compounds were evaluated using ADMET and PASS analysis, respectively. Overall, this study provides valuable insights into the immunomodulatory benefits of P. pulmonarius, especially in monocyte function, and highlights specific compounds for further investigation into potential preventive or therapeutic applications in inflammation-related diseases.
MATERIALS AND METHODS
Pleurotus pulmonarius crude extract preparation
The mushroom was identified as a Voucher specimen: MZ395974. The extraction of P. pulmonarius was performed using hot water followed by ethanol precipitation [16,17]. Initially, the dried mushrooms were ground into a fine powder. Subsequently, 100 mg of this powder was extracted with 1,000 ml of distilled water at a ratio of 1:10 (w/v) at a temperature of 95°C ± 5°C for 1 hour. The mixture was then meticulously filtered through 11 µm of filter paper (Whatman, England), allowing the mushroom residue to undergo two additional extraction rounds, with the supernatant being collected each time. After this, the supernatant was further filtered through 0.2 µm of SFCA syringe filter (Corning, USA) and precipitated for 18 hours in 80% ethanol (v/v) at 4°C. The mixture was then centrifuged for 10 minutes at 4,500 rpm and 4°C to facilitate component separation. After removing the supernatant, the residue was washed with absolute ethanol and incubated at 70°C to ensure complete evaporation of the ethanol.
Component analysis by liquid chromatography with tandem mass spectrometry
To find bioactive molecules comprised in P. pulmonarius, a crude extract was submitted to the Institute of Systems Biology (Universiti Kebangsaan, Malaysia). The untargeted compound was analyzed using LC-MS/MS. In brief, 20 ul of P. pulmonarius solution was injected. Then the gradient elution was performed through a Thermo Scientific C18 column operating on an UltiMate 3000 UHPLC system (Dionex), with a 22-minute total run time. Using ESI positive ionization, high-resolution mass spectrometry was performed through MicroTOF QIII (Bruker Daltonic, USA). M/Z was analyzed using Compass Data Analysis software (Bruker Daltonic, USA). Subsequently, in silico MS/MS fragmentation was performed, and the compounds were identified by comparing M/Z with the theoretical masses accessible through the public database.
In silico studies of potential compound-targeted proteins on monocyte immune response
Compound-protein target interaction and GO and Reactome analyses
To identify potential human protein targets for the proposed bioactive molecules from P. pulmonarius. The proposed bioactive compounds were ranked in ascending order based on their Area (%) values. The top seven compounds have been selected as potential immunomodulators based on their area percentage and being identified in mushrooms. Then, the compounds’ simplified molecular input line entry system (SMILES) was obtained from PubChem (https://pubchem.ncbi.nlm.nih.gov/) and imported into the SwissTargetPrediction web tool (http://www.swisstargetprediction.ch/). The targets of proposed bioactive compounds acquired from SwissTargetPrediction with a probability score greater than 0.04 were chosen as potential targets in this study. Those compounds without target information were excluded. [18].
Next, the GeneCards database (https://www.genecards.org/) was used to select the targets that have been reported to be expressed in monocytes together with those involved in immune response, metabolic pathways related to immune response, and epigenetic regulation of immune response. In addition, CD36, a well-known receptor for long chain-fatty acid chains, was selected as the target protein for diacylglycerol [19]. A Venn diagram to identify intersecting genes was plotted. These genes were then used to construct a protein-protein interaction (PPI) network in the STRING database.
Furthermore, the MCODE analysis (default parameter) in Cytoscape software (Version 3.10.2) was applied to identify core target proteins based on the highest clustering scores in the network [20]. Subsequently, enrichment analysis on these core target proteins was studied using GO ontology and Reactome pathway analysis to determine their functions. Then, a bar plot was generated using R studio (Version 2024.04.2).
Molecular docking and PASS analysis
Bulk molecular docking was conducted on the core target proteins and compounds to determine which compounds primarily bind to each target. The structure of core target proteins was obtained from the Protein databank (PDB) (https://www.rcsb.org/). The structure of predicted compounds was generated by the IUPAC name using Biovia Draw (Version 24.1.NET). Using the Discovery Studio visualizer, water molecules were removed, and energy was minimized. Molecular docking was performed by Autodock tools (Version 1.5.7). Hydrogen atom and Kallmandata charge were added, and the coordinate files were generated. Torsion root detection was performed for ligand preparation, and the coordinate files were generated. The size and coordinates of the grid boxes were set with a spacing of 0.375°A, the center of the grid box and docking parameters are shown in Table 1. Docking analysis was performed using the Lamarckian genetic algorithm with default parameters. The Discovery Studio visualizer was used to visualize the hydrogen bond between the targeted protein and ligand.
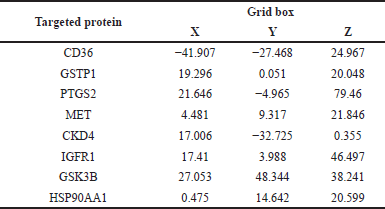 | Table 1. Grid box of the x, y, and z centers for each target compound. [Click here to view] |
For molecular docking validation, the redocking method was used to verify the molecular docking method and parameters. The co-crystal structure of the targeted proteins and native ligands was downloaded from the PDB (https://www.rcsb.org/). The structure was then separated, and molecular docking was performed, as mentioned above. Moreover, the potential of a substance to exhibit immunomodulatory activity was assessed using the PASS server (https://www.way2drug.com/passonline/predict.php) [21]. The schematic diagram of the methodology is presented in Figure 1.
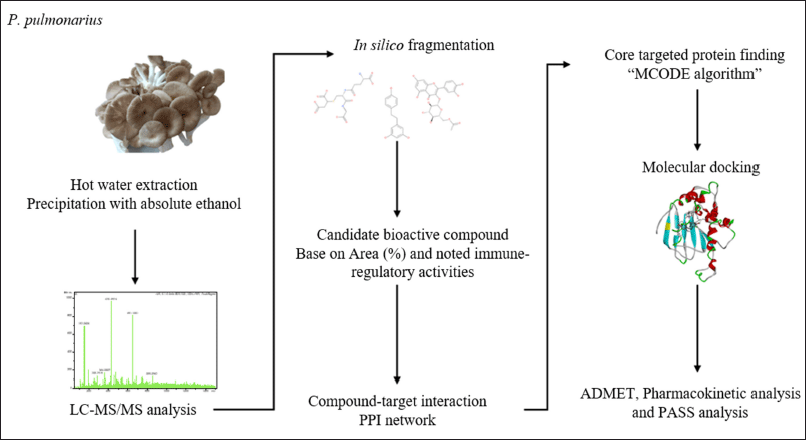 | Figure 1. Schematic diagram of methodology. [Click here to view] |
Absorption, distribution, metabolism, excretion, and toxicity (ADMET) analysis, and pharmacokinetic properties
The pharmacokinetic properties of the candidate compounds were assessed using ADMET analysis (the SwissADME and pkCSM online tools). The analysis included absorption, distribution, metabolism, excretion, toxicity, and drug-likeness evaluation based on Lipinski’s rule of five, allowing no more than one violation. Intestinal absorption and hepatotoxicity were also considered. The compound’s structure was analyzed using the SMILES notation.
RESULTS AND DISCUSSION
Identification of PP crude extract contains bioactive molecules using untargeted LC-MS/MS analysis
Pleurotus pulmonarius was extracted by hot water extraction to recapitulate its traditional culinary and folk medicine applications. LC-MS/MS analysis was performed to identify water-soluble bioactive compounds in P. pulmonarius crude extract. A total of 169 chromatographic peaks were detected in ESI-positive mode. In silico MS/MS fragmentation was carried out using molecular ion peak [M+H] + compared with the theoretical mass, 36 bioactive molecules were proposed (Table 2). We found that 12 out of 36 proposed bioactive compounds, namely diacylglycerol, phosphatidylethanolamine, phosphatidylinositol, glutathione, quercetin, dihydroresveratrol, aspartic acid, isorhamnetin, catechin, myricitrin, apigenin, and oleic acid had previously been reported in mushrooms [12,22–28]. The proposed twelve bioactive compounds reported in mushrooms are labeled (Fig. 2), and the immunomodulatory activities of each compound are presented in Table 3. These water-soluble bioactive compounds are mainly involved in antioxidant activity and anti-inflammatory activity. Moreover, diacylglycerol and glutathione have been revealed to enhance monocyte and macrophage responses via trained immunity [19,29,30]. Previous studies have shown that mushrooms are rich sources of β-glucans and natural polysaccharides [31]. However, due to the large molecular size of these compounds, the limitations of LC/MS-MS methods prevent their analysis. In this study, we detected disaccharides, but their specific types could not be identified (data not shown). Therefore, additional analytical methods should be employed to further investigate such compounds.
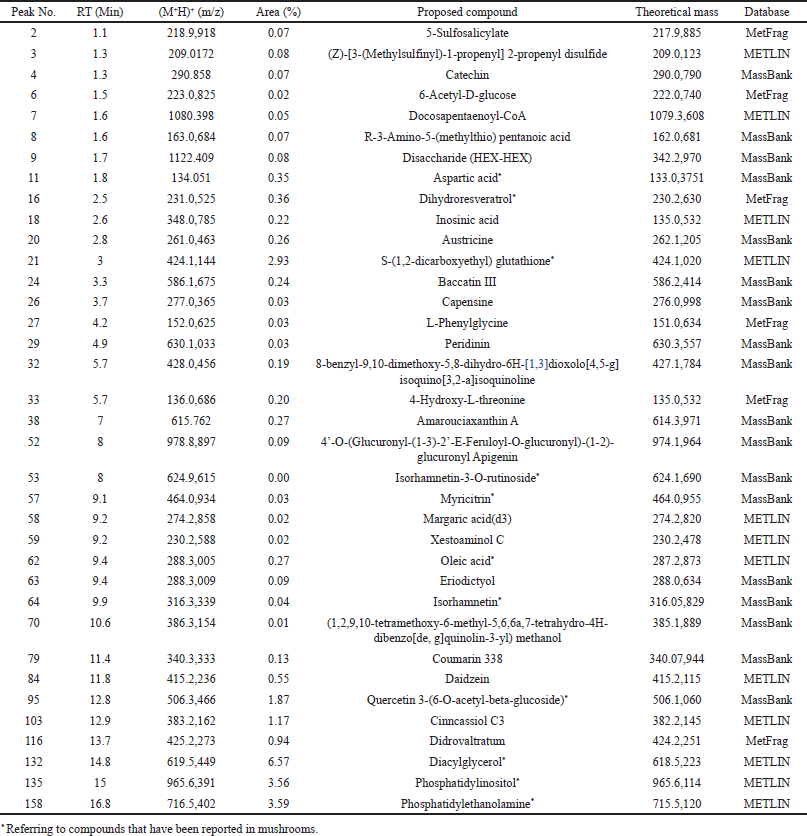 | Table 2. The compounds found in the extract of Pleurotus pulmonarius analyzed by LC-MS/MS and in silico fragmentation, ordered by retention time (RT). [Click here to view] |
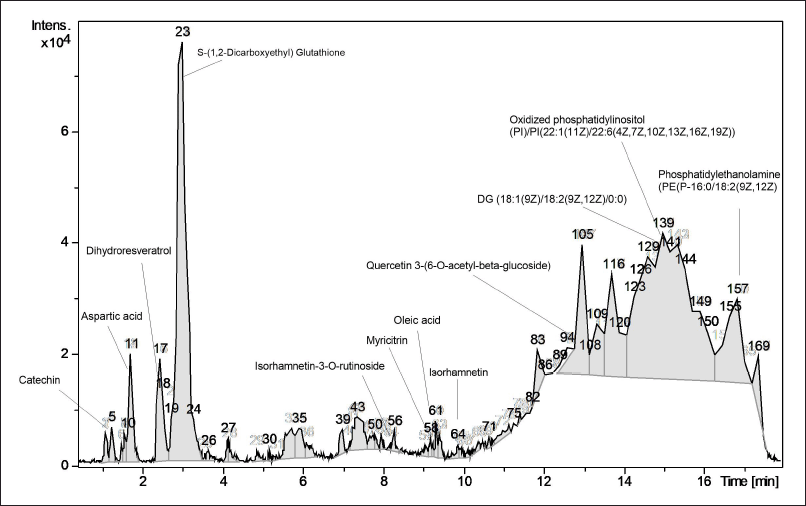 | Figure 2. In silico MS/MS fragmentation with M/Z was used to predict the compounds. Thirty-six bioactive molecules were proposed. Chromatograms are presented, and the 12 bioactive compounds that have been previously reported in mushrooms are labeled. [Click here to view] |
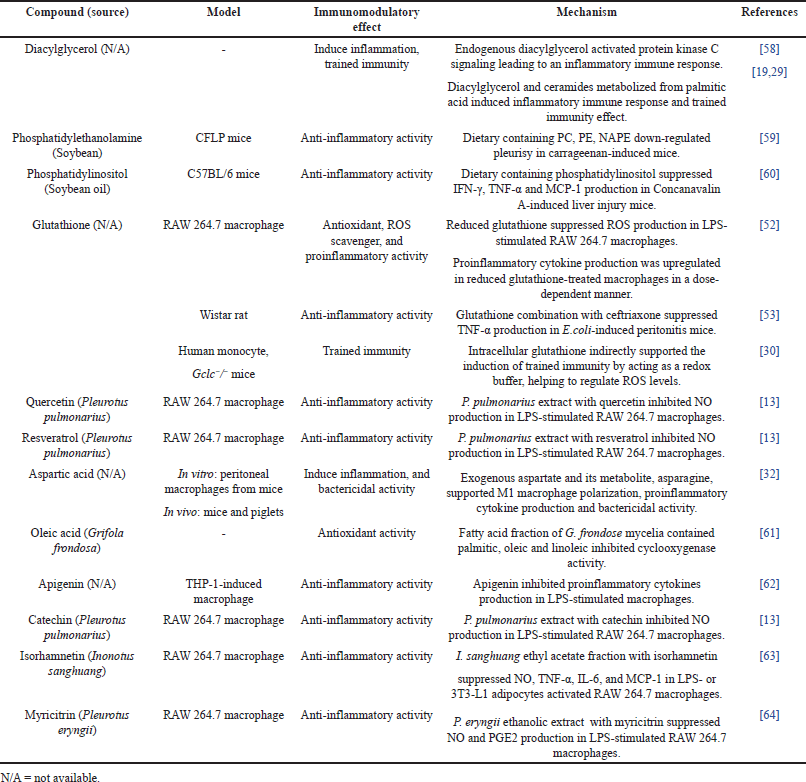 | Table 3. Immunomodulatory properties of the proposed bioactive compounds ordered by area (%). [Click here to view] |
Compound-protein target interaction and pharmacy network of P. pulmonarius crude extract
The proposed bioactive compounds were ranked in ascending order based on their area percentage values to predict the functions and interactions between bioactive compounds and monocytes. The top seven compounds based on their area percentage were selected and proposed as potential immunomodulators, including diacylglycerol, phosphatidylethanolamine, phosphatidylinositol, glutathione, quercetin 3-(6-O-acetyl-beta-glucoside), dihydroresveratrol, and aspartic acid. Potential target proteins for each compound were identified using the SwissTargetPrediction. Intersecting between these potential target proteins and monocyte-related target proteins was discovered and visualized in the Venn diagram (Fig. 3A). We could not find the intersecting targets between aspartic acid and monocyte. Although, the aspartate ion form of aspartic acid has been reported to induce inflammation in macrophages [32]. Since amino acid metabolism contributes to immune responses, we hypothesized that aspartic acid possibly promotes inflammation through its role as a precursor rather than directly interacting with proteins.
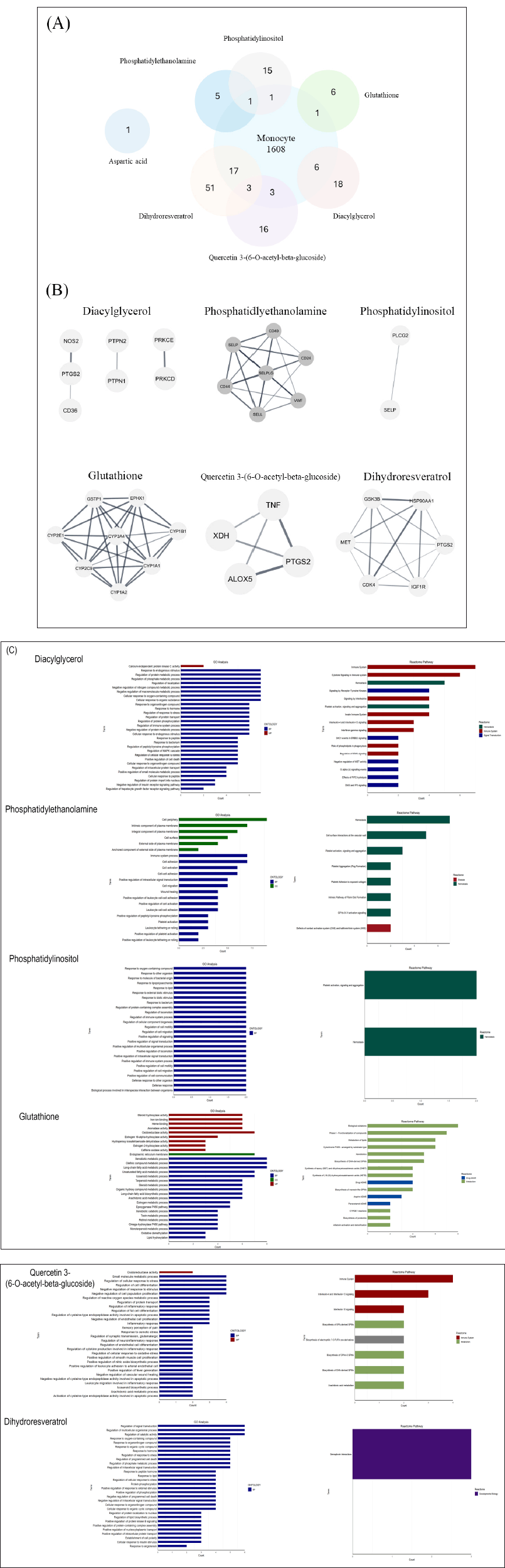 | Figure 3. The illustration of the Venn diagram demonstrated an intersecting target between monocyte proteins and potential targets of each bioactive compound (a), the core cluster of the PPI network (b), and functional analysis of core target proteins (c). GO Gene Ontology, BP biological process, CC cellular component, MF molecular function. [Click here to view] |
Moreover, the MCODE analysis revealed the core target proteins for glutathione, quercetin, and dihydroresveratrol, as presented in the cluster network (Fig. 3B). However, the core target proteins of diacylglycerol could not be identified. This may be due to the low abundance of the protein or a lack of connectivity of this protein (Fig. 3B). Furthermore, GO ontology and Reactome pathway analyses were performed to identify the function of each core targeted protein, which is involved in immune regulation and homeostasis in inflammatory responses (Fig. 3C).
Molecular docking of P. pulmonarius-derived candidate compounds and the potential target proteins
Next, molecular docking analysis of selected core target proteins was performed to investigate the candidate compounds’ interaction with the potential target proteins. The crystal of the possible target proteins was obtained from a PDB. We performed the redocking of the native ligand with the targeted protein to validate the molecular docking protocol. Subsequently, the validated protocol was applied to the in-silico screening of the bioactive compound and target protein interaction. The binding affinities of native ligands from redocking experiments are presented in Table 4. Molecular docking of well-known activators or inhibitors sourced from the Drug Bank database is shown in Table 5, detailing their binding affinities. Molecular docking results of the candidate compounds, including binding affinity and 2D diagram structure of binding, are shown in Table 6 and Figure 4, respectively.
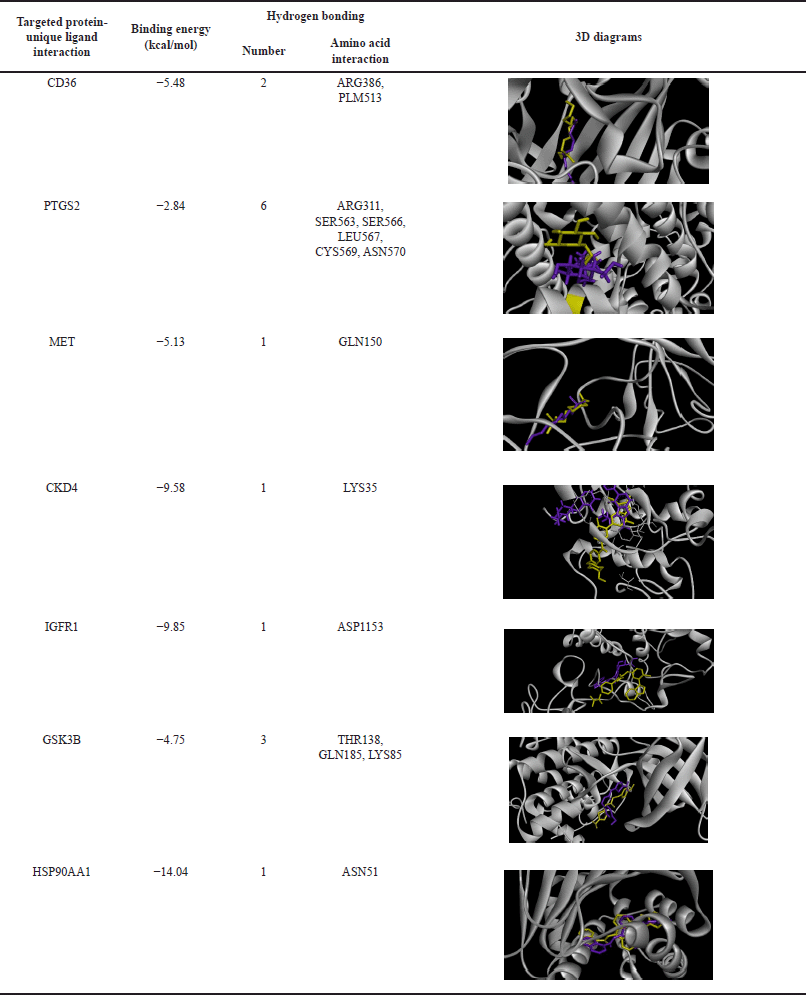 | Table 4. Hydrogen bond interaction between targeted protein and original unique ligand. [Click here to view] |
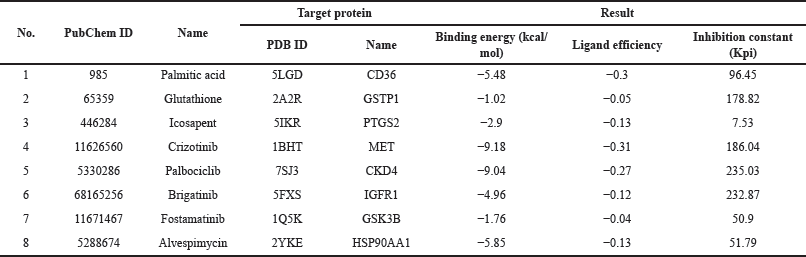 | Table 5. Molecular docking results of activators and inhibitors on the targeted protein. [Click here to view] |
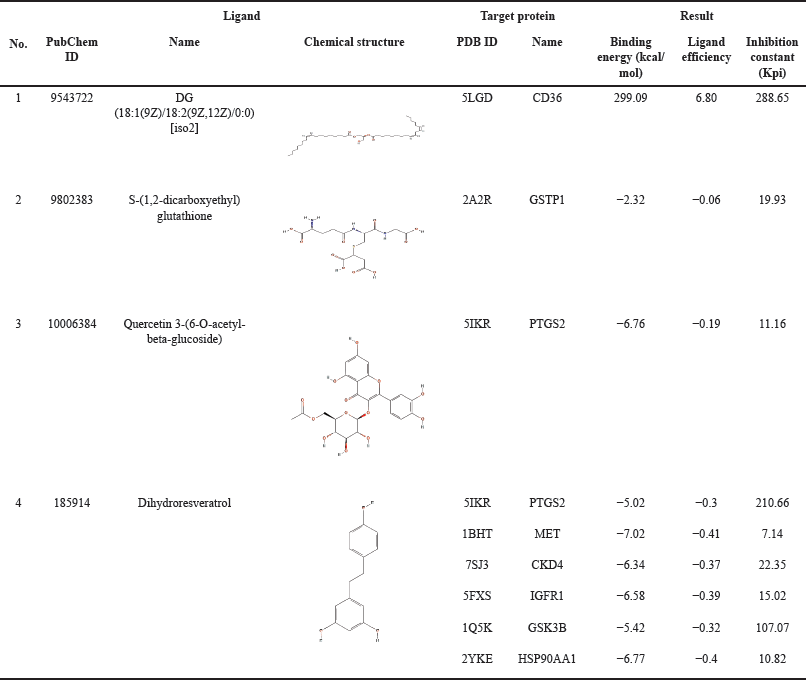 | Table 6. Chemical structure and molecular docking results. [Click here to view] |
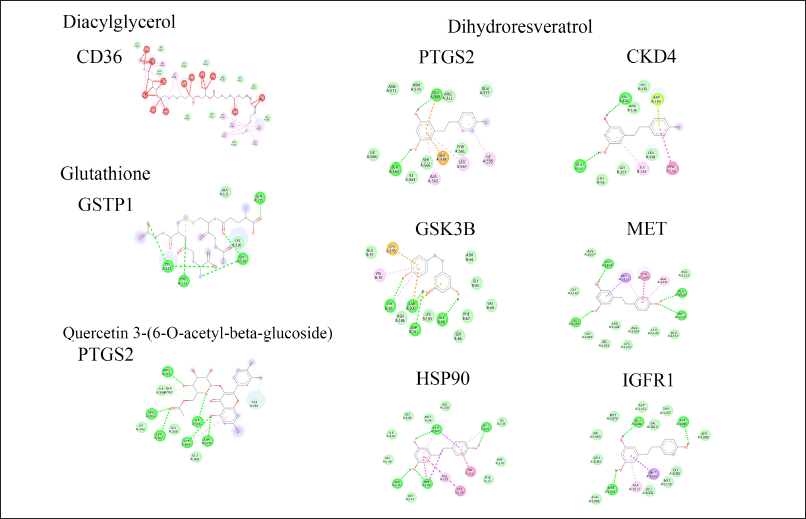 | Figure 4. A 2D diagram represents the molecular docking analysis of P. pulmonarius candidate compounds and potential targeted proteins. [Click here to view] |
The finding demonstrated the unfavorable interactions between diacylglycerol and the CD36 fatty acid transporter, which may be related to the high molecular weight of these compounds. Additionally, no interactions between phosphatidylethanolamine, phosphatidylinositol, and the targeted protein were observed (data not shown). Interestingly, glutathione, quercetin, and dihydroresveratrol demonstrated promising potential proinflammatory inhibitors, with their binding affinities surpassing those of known inhibitors (−2.32 kcal/mol for glutathione; −6.76 kcal/mol for quercetin and −5.02 to −7.02 kcal/mol for dihydroresveratrol).
Molecular docking results uncovered the interaction between glutathione and glutathione S-transferase P (GSTP1). This interaction might support cellular redox status and modulation of host immune defense mechanisms to prevent cellular damage [33]. Moreover, the finding revealed that Quercetin 3-(6-O-acetyl-beta-glucoside) and dihydroresveratrol hold potential as anti-inflammatory agents through their ability to inhibit prostaglandin G/H synthase-2 (PTGS2). The PTGS2 enzyme plays a crucial role in converting arachidonic acid to prostaglandin H2, which promotes inflammatory immune responses [34]. Furthermore, dihydroresveratrol demonstrated the potential to bind to hepatocyte growth factor receptor (MET), cyclin-dependent kinase 4 (CKD4), glycogen synthase kinase-3 beta (GSK3B), and heat shock protein HSP 90-alpha (HSP90AA1). Such target proteins are involved in numerous signal transduction pathways linked to immune responses, including monocyte migration and proinflammatory cytokine production [35–38].
Previous studies have highlighted the role of PTGS2 in trained immunity [39], while the epigenetic regulation by GSK3B affects the expression of specific NF-κB-regulated genes [40]. Furthermore, the activity of GSK3B also influences the expression of MCP-1 and IL-6 [41]. Trained immunity or innate immune memory strengthens the responses of monocytes to subsequent stimuli; however, its dysregulation could result in hyperinflammation [42]. Therefore, modulating the activities of PTGS2 and GSK3B using Quercetin 3-(6-O-acetyl-beta-glucoside) and dihydroresveratrol may help control inflammation and slow disease progression in hyperinflammatory conditions. Notably, dihydroresveratrol can interact with multiple signaling pathways involved in immune regulation, including MET and CDK4. This offers new therapeutic opportunities for chronic inflammation-related diseases.
Research has established a significant connection between functional foods and immune responses, demonstrating their remarkable ability to promote inflammation and provide anti-inflammatory benefits [5,43]. Targeting specific proteins with bioactive compounds like quercetin 3-(6-O-acetyl-beta-glucoside) and dihydroresveratrol, found in P. pulmonarius, may play a crucial role in mitigating the inflammatory phenotype of monocytes. This strategic approach holds significant promise, particularly for conditions such as inflammatory bowel disease, which is characterized by excessive inflammation driven by monocytes [44–46]. On the other hand, insulin-like growth factor 1 (IGF1R) is pivotal in anti-inflammatory response [47]. Inhibiting IGF1R by dihydroresveratrol may contribute to supporting inflammatory phenotype.
Resveratrol has demonstrated both pro-inflammatory and anti-inflammatory effects, influencing immune responses in monocytes and macrophages in a cell type-specific and dose-dependent manner. These effects are associated with the differential upregulation of the Aryl hydrocarbon receptor and cytochrome P450 1B1 in these cell types [48].
Furthermore, the results from PASS analysis support the anti-inflammatory properties of quercetin 3-(6-O-acetyl-beta-glucoside) and dihydroresveratrol (Table 7). Quercetin 3-(6-O-acetyl-beta-glucoside) exhibited strong anti-inflammatory (Pa 0.773) and immunosuppressant (Pa 0.713) potential. This finding aligns with previous studies that have demonstrated quercetin’s ability to inhibit pro-inflammatory cytokines and downregulate immune cell activation [49]. Dihydroresveratrol displayed modest anti-inflammatory (Pa 0.366) and immunosuppressive effects (Pa 0.285). Notably, the parent compound resveratrol has shown both inflammatory and anti-inflammatory activities [48]. Therefore, further experimental validation and in vivo studies are crucial to fully verify its therapeutic potential.
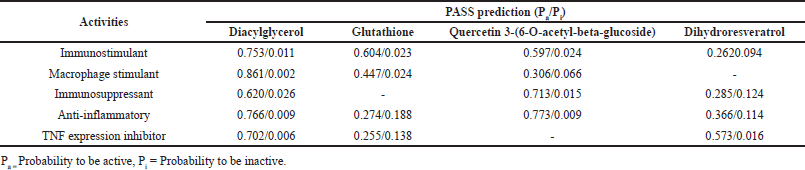 | Table 7. PASS analysis of potential target compounds for inflammatory and anti-inflammatory effects. [Click here to view] |
ADMET analysis and pharmacokinetic properties
To investigate the pharmacokinetic properties and drug-likeness of candidate bioactive compounds, we conducted an ADMET analysis, focusing on gastrointestinal (GI) absorption and hepatotoxicity. The results are summarized in Table 8. The predictions indicated that the GI system rarely absorbs diacylglycerol, glutathione, and quercetin 3-(6-O-acetyl-beta-glucoside). In contrast, resveratrol is well absorbed through passive human gastrointestinal absorption and can cross the blood-brain barrier (Fig. 5A). The physicochemical properties of each compound are illustrated in a bioavailability radar, which includes attributes such as lipophilicity, size, solubility, polarity, saturation, and flexibility (Fig. 5B). The colored area of the radar shows the most desirable zone for each bioavailability characteristic. According to Lipinski’s rule of five and the bioavailability properties assessed, dihydroresveratrol emerges as the only compound with a favorable absorption, distribution, and safety profile, indicating its potential as a drug-like compound.
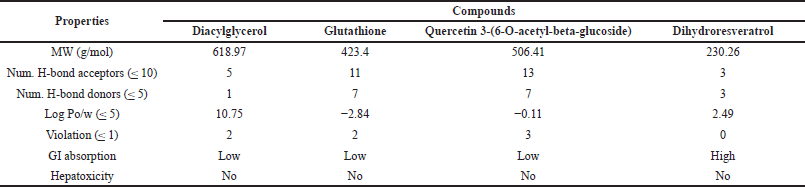 | Table 8. ADMET analysis and pharmacokinetic properties. [Click here to view] |
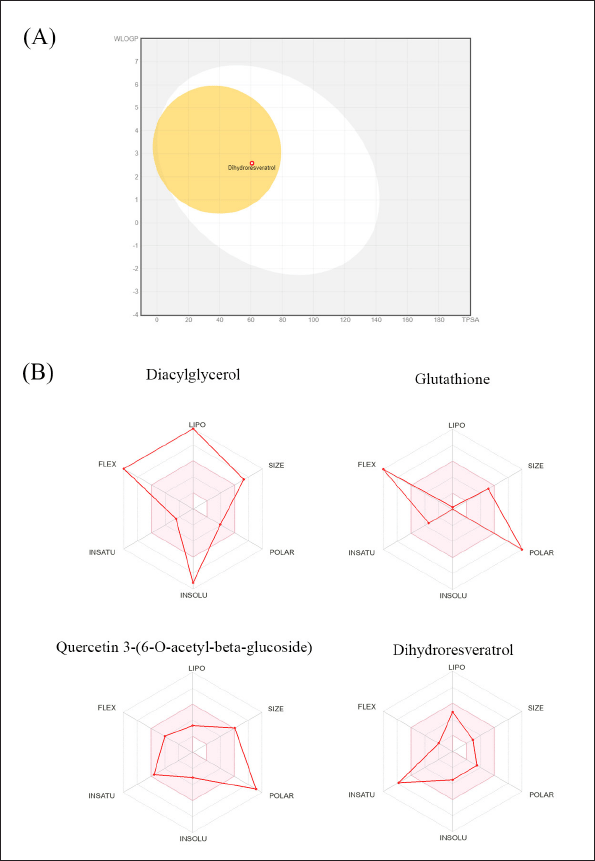 | Figure 5. The illustration of BOILED-Egg diagram (a) and bioavailability radar related to physiochemical properties of P. pulmonarius candidate compounds (b). [Click here to view] |
Limitations and future directions
This study identified water-soluble bioactive compounds from P. pulmonarius utilizing LC-MS/MS profiling and in silico molecular docking, emphasizing their potential as immunomodulators targeting monocyte immune responses. By employing advanced computational tools such as PASS analysis, ADMET profiling, and Reactome pathway analysis, we predicted interactions between bioactive compounds and immune-related proteins. This approach provides a comprehensive framework for understanding their immunomodulatory effects. However, it is essential to recognize the necessity for further experimental validation alongside a broader environmental context.
Biomedical implications
The bioinformatics analyses revealed compounds such as glutathione, quercetin, and dihydroresveratrol, which demonstrated significant binding affinities to monocyte-related target proteins involved in inflammatory processes. These findings are supported by existing experimental evidence on related compounds, underscoring their relevance [13,50–54]. Although the current study does not encompass wet laboratory experiments, the results provide a roadmap for future validation. It is recommended that cytokine modulation assays be conducted using monocyte-derived cell lines or primary monocytes to evaluate pro- and anti-inflammatory cytokine production through ELISA assays. In addition, in vitro binding studies should be performed to confirm the predicted interactions between compounds and proteins. Pathway-specific analyses targeting key immune pathways, such as NF-κB and JAK/STAT, may be investigated using qPCR, Western blotting, or reporter assays. Furthermore, in vivo models would serve as a suitable tool for assessing the bioavailability, pharmacokinetics, and therapeutic efficacy of the compounds. These experimental approaches are vital for translating computational findings into actionable biomedical applications.
Environmental implications
While the primary focus of this study is biomedical applications, the ecological behavior of P. pulmonarius-derived bioactive compounds warrants further research. Their interactions with environmental pollutants and behavior in aquatic ecosystems have not been thoroughly explored. Glutathione has been shown to reduce oxidative stress caused by heavy metals and pesticides in aquatic environments [55], and dihydroresveratrol possesses antioxidant properties that may help mitigate environmental oxidative damage [56,57]. Therefore, investigating the biodegradability and environmental fate of these compounds, along with their stability and interactions with pollutants, is essential. Moreover, exploring their potential in bioremediation to address pollutant accumulation in water bodies could be beneficial. Such studies could contribute to the broader understanding of natural immunomodulators and their potential applications in human health and ecosystem restoration.
CONCLUSION
Pleurotus pulmonarius is a highly accessible and cost-effective edible fungus worldwide. Our study involved the comprehensive profiling of water-soluble bioactive compounds in P. pulmonarius using untargeted LC-MS/MS analysis and revealed 36 bioactive compounds from the mushroom with hot-water extraction. The top seven candidate compounds found abundantly in the mushroom were selected, including diacylglycerol, phosphatidylethanolamine, phosphatidylinositol, glutathione, quercetin 3-(6-O-acetyl-beta-glucoside), dihydroresveratrol, and aspartic acid. The bioactive compounds responsible for modulating immune response in monocytes were further identified by in silico screening. We unveiled the potential anti-inflammatory activity, mainly attributed to glutathione, quercetin, and dihydroresveratrol in the extract. Furthermore, our ADMET analysis highlighted the promising potential of dihydroresveratrol as an immunomodulatory target due to its favorable absorption and bioavailability. This study provides valuable insights into the immunomodulatory potential of P. pulmonarius bioactive compounds, offering a strong foundation for future experimental validation which could contribute to significant implications for their application in functional food and alternative medicine, especially in monocyte-related inflammation in the future.
ACKNOWLEDGMENTS
The authors thank Miss Sunita Nilkhet for her technical support in the molecular docking analysis. The English editing of this manuscript was kindly performed in part by Dr. James Michael Brimson, Research, Innovation and International Affairs, Faculty of Allied Health Sciences, Chulalongkorn University. A.A. would like to thank the Graduate School, Chulalongkorn University for the research assistance scholarship.
LIST OF ABBREVIATIONS
ADMET, Absorption, distribution, metabolism, excretion, and toxicity; CKD4, Cyclin-dependent kinase 4; GSK3B, Glycogen synthase kinase-3 beta; GSTP1, Glutathione S-transferase pi 1; HSP90AA1, Heat Shock Protein 90 alpha family class A member 1; IGF1R, Insulin-like growth factor 1 receptor; LC-MS/MS, Liquid chromatography with tandem mass spectrometry; MET, Mesenchymal-epithelial transition, tyrosine kinase receptor; P. pulmonarius, Pleurotus pulmonarius; PPI, Protein-protein interaction; PTGS2, Prostaglandin G/H synthase-2; SMILES, Simplified molecular input line entry system.
AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took Fart in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This work was supported by Research Funding from the Faculty of Allied Health Sciences, Chulalongkorn University (AHS CU 62003).
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
CONSENT TO PARTICIPATE
All authors agree to publish the article.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Mildner A, Kim KW, Yona S. Unravelling monocyte functions: from the guardians of health to the regulators of disease. Discov Immunol. 2024;3(1):kyae014. CrossRef
2. Austermann J, Roth J, Barczyk-Kahlert K. The good and the bad: monocytes’ and macrophages’ diverse functions in inflammation. Cells. 2022;11(12):1979. CrossRef
3. Ochando J, Mulder WJM, Madsen JC, Netea MG, Duivenvoorden R. Trained immunity - basic concepts and contributions to immunopathology. Nat Rev Nephrol. 2023;19(1):23–37. CrossRef
4. Martín-Cruz L, Benito-Villalvilla C, Angelina A, Subiza JL, Palomares O. Trained immunity-based vaccines for infections and allergic diseases. J Allergy Clin Immunol. 2024;154(5):1085–94. CrossRef
5. Kim JH, Kim DH, Jo S, Cho MJ, Cho YR, Lee YJ, et al. Immunomodulatory functional foods and their molecular mechanisms. Exp Mol Med. 2022;54(1):1–11. CrossRef
6. Lorenzo PM, Izquierdo AG, Rodriguez-Carnero G, Fernández-Pombo A, Iglesias A, Carreira MC, et al. Epigenetic effects of healthy foods and lifestyle habits from the southern european atlantic diet pattern: a narrative review. Adv Nutr. 2022;13(5):1725–47. CrossRef
7. Bell V, Silva C, Guina J, Fernandes TH. Mushrooms as future generation healthy foods. Front Nutr. 2022;9:1050099. CrossRef
8. Motta F, Gershwin ME, Selmi C. Mushrooms and immunity. J Autoimmun. 2021;117:102576. CrossRef
9. Li H, Tian Y, Menolli N, Jr., Ye L, Karunarathna SC, Perez-Moreno J, et al. Reviewing the world’s edible mushroom species: a new evidence-based classification system. Compr Rev Food Sci Food Saf. 2021;20(2):1982–2014. CrossRef
10. Sharpe E, Farragher-Gnadt AP, Igbanugo M, Huber T, Michelotti JC, Milenkowic A, et al. Comparison of antioxidant activity and extraction techniques for commercially and laboratory prepared extracts from six mushroom species. J Agric Food Res. 2021;4:100130. CrossRef
11. Tepsongkroh B, Thaihuttakij C, Supawong S, Jangchud K. Impact of high pressure pre-treatment and hot water extraction on chemical properties of crude polysaccharide extract obtained from mushroom (Volvariella volvacea). Food Chem X. 2023;19:100864. CrossRef
12. Stastny J, Marsik P, Tauchen J, Bozik M, Mascellani A, Havlik J, et al. Antioxidant and anti-inflammatory activity of five medicinal mushrooms of the genus Pleurotus. Antioxidants. 2022;11(8):1569. CrossRef
13. Nguyen TK, Im KH, Choi J, Shin PG, Lee TS. Evaluation of antioxidant, anti-cholinesterase, and anti-inflammatory effects of culinary mushroom Pleurotus pulmonarius. Mycobiology. 2016;44(4):291–301. CrossRef
14. Olufemi AE, Terry AO, Kola OJ. Anti-leukemic and immunomodulatory effects of fungal metabolites of Pleurotus pulmonarius and Pleurotus ostreatus on benzene-induced leukemia in Wister rats. Korean J Hematol. 2012;47(1):67–73. CrossRef
15. Im KH, Baek SA, Choi J, Lee TS. In vitro and in vivo medicinal value of culinary-medicinal lung oyster mushroom Pleurotus pulmonarius var. stechangii (Agaricomycetes). Int J Med Mushrooms. 2020;22(8):763–74. CrossRef
16. Panthong S, Boonsathorn N, Chuchawankul S. Antioxidant activity, anti-proliferative activity, and amino acid profiles of ethanolic extracts of edible mushrooms. Genet Mol Res. 2016 Oct 17;15(4):1–14. CrossRef
17. Sangthong S, Pintathong P, Pongsua P, Jirarat A, Chaiwut P. Polysaccharides from Volvariella volvacea mushroom: extraction, biological activities and cosmetic efficacy. J Fungi (Basel). 2022;8(6):572. CrossRef
18. Que W, Chen M, Yang L, Zhang B, Zhao Z, Liu M, et al. A network pharmacology-based investigation on the bioactive ingredients and molecular mechanisms of Gelsemium elegans Benth against colorectal cancer. BMC Complement Med Ther. 2021;21(1):99. CrossRef
19. Seufert AL, Napier BA. A new frontier for fat: dietary palmitic acid induces innate immune memory. Immunometabolism. 2023;5(2):e00021.
20. Zhang K, Zhang C, Teng X, Wang K, Chen M. Bioinformatics and computational chemistry approaches to explore the mechanism of the anti-depressive effect of ligustilide. Sci Rep. 2023;13(1):5417. CrossRef
21. Filimonov DA, Lagunin AA, Gloriozova TA, Rudik AV, Druzhilovskii DS, Pogodin PV, et al. Prediction of the biological activity spectra of organic compounds using the Pass online web resource. Chem Heterocycl Compd (N Y). 2014;50(3):444–57. CrossRef
22. Sande D, Oliveira GPd, Moura MAFe, Martins BdA, Lima MTNS, Takahashi JA. Edible mushrooms as a ubiquitous source of essential fatty acids. Food Res Int. 2019;125:108524. CrossRef
23. Lin P, Yan ZF, Kook M, Li CT, Yi TH. Genetic and chemical diversity of edible mushroom Pleurotus species. Biomed Res Int. 2022;2022:6068185. CrossRef
24. Kalaras MD, Richie JP, Calcagnotto A, Beelman RB. Mushrooms: a rich source of the antioxidants ergothioneine and glutathione. Food Chem. 2017;233:429–33. CrossRef
25. Torres-Martínez BDM, Vargas-Sánchez RD, Torrescano-Urrutia GR, Esqueda M, Rodríguez-Carpena JG, Fernández-López J, et al. Pleurotus genus as a potential ingredient for meat products. Foods. 2022;11(6):779. CrossRef
26. Alam N, Yoon KN, Lee KR, Shin PG, Cheong JC, Yoo YB, et al. Antioxidant activities and tyrosinase inhibitory effects of different extracts from Pleurotus ostreatus fruiting bodies. Mycobiology. 2010;38(4):295–301. CrossRef
27. Liu K, Xiao X, Wang J, Chen CYO, Hu H. Polyphenolic composition and antioxidant, antiproliferative, and antimicrobial activities of mushroom Inonotus sanghuang. LWT - Food Sci Tech. 2017;82:154–61. CrossRef
28. Elhusseiny SM, El-Mahdy TS, Awad MF, Elleboudy NS, Farag MMS, Yassein MA, et al. Proteome analysis and in vitro antiviral, anticancer and antioxidant capacities of the aqueous extracts of Lentinula edodes and Pleurotus ostreatus edible mushrooms. Molecules. 2021;26(15):4623. CrossRef
29. Seufert AL, Hickman JW, Traxler SK, Peterson RM, Waugh TA, Lashley SJ, et al. Enriched dietary saturated fatty acids induce trained immunity via ceramide production that enhances severity of endotoxemia and clearance of infection. eLife. 2022;11:e76744. CrossRef
30. Su H, Huang J, Weng S, Zhang B, Zhang T, Xu Y. Glutathione synthesis primes monocytes metabolic and epigenetic pathway for β-glucan-trained immunity. Redox Biol. 2021;48:102206. CrossRef
31. Sari M, Prange A, Lelley JI, Hambitzer R. Screening of beta-glucan contents in commercially cultivated and wild growing mushrooms. Food Chem. 2017;216:45–51. CrossRef
32. Wang H, Zheng X, Liu B, Xia Y, Xin Z, Deng B, et al. Aspartate metabolism facilitates IL-1β production in inflammatory macrophages. Front Immunol. 2021;12:753092. CrossRef
33. Mazari AMA, Zhang L, Ye ZW, Zhang J, Tew KD, Townsend DM. The multifaceted role of Glutathione S-transferases in health and disease. Biomolecules. 2023;13(4):688. CrossRef
34. Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011;31(5):986–1000. CrossRef
35. Galimi F, Cottone E, Vigna E, Arena N, Boccaccio C, Giordano S, et al. Hepatocyte growth factor is a regulator of monocyte-macrophage function1. J Immunol. 2001;166(2):1241–7. CrossRef
36. Pandey P, Khan F, Upadhyay TK, Sharangi AB. Deciphering the immunomodulatory role of Cyclin-Dependent Kinase 4/6 inhibitors in the tumor microenvironment. Int J Mol Sci. 2023;24(3):2236. CrossRef
37. Cortés-Vieyra R, Silva-García O, Gómez-García A, Gutiérrez-Castellanos S, Álvarez-Aguilar C, Baizabal-Aguirre VM. Glycogen synthase kinase 3β modulates the inflammatory response activated by bacteria, viruses, and parasites. Front Immunol. 2021;12:675751. CrossRef
38. Tukaj S, W?grzyn G. Anti-Hsp90 therapy in autoimmune and inflammatory diseases: a review of preclinical studies. Cell Stress Chaperones. 2016;21(2):213–8. CrossRef
39. Hartung F, Esser-von Bieren J. Trained immunity in type 2 immune responses. Mucosal Immunol. 2022;15(6):1158–69. CrossRef
40. Beurel E, Grieco SF, Jope RS. Glycogen synthase kinase-3 (GSK3): regulation, actions, and diseases. Pharmacol Ther. 2015;148:114–31. CrossRef
41. Steinbrecher KA, Wilson W, 3rd, Cogswell PC, Baldwin AS. Glycogen synthase kinase 3beta functions to specify gene-specific, NF-kappaB-dependent transcription. Mol Cell Biol. 2005;25(19):8444–55. CrossRef
42. Mishra S, Arsh AM, Rathore JS. Trained innate immunity and diseases: bane with the boon. Clin Immunol Commun. 2022;2:118–29. CrossRef
43. Serafini M, Peluso I. Functional foods for health: the interrelated antioxidant and anti-inflammatory role of fruits, vegetables, herbs, spices and cocoa in humans. Curr Pharm Des. 2016;22(44):6701–15. CrossRef
44. Liao X, Liu J, Guo X, Meng R, Zhang W, Zhou J, et al. Origin and function of monocytes in inflammatory bowel disease. J Inflamm Res. 2024;17:2897–914. CrossRef
45. Karaky M, Boucher G, Mola S, Foisy S, Beauchamp C, Rivard M-E, et al. Prostaglandins and calprotectin are genetically and functionally linked to the inflammatory bowel diseases. PLoS Genet. 2022;18(9):e1010189. CrossRef
46. Mijan MA, Lim BO. Diets, functional foods, and nutraceuticals as alternative therapies for inflammatory bowel disease: present status and future trends. World J Gastroenterol. 2018;24(25):2673–85. CrossRef
47. Salminen A, Kaarniranta K, Kauppinen A. Insulin/IGF-1 signaling promotes immunosuppression via the STAT3 pathway: impact on the aging process and age-related diseases. Inflamm Res. 2021;70(10):1043–61. CrossRef
48. Feng L, Yasmeen R, Schoene NW, Lei KY, Wang TTY. Resveratrol differentially modulates immune responses in human THP-1 monocytes and macrophages. Nutr Res. 2019;72:57–69. CrossRef
49. Saeedi-Boroujeni A, Mahmoudian-Sani M-R. Anti-inflammatory potential of quercetin in COVID-19 treatment. J Inflamm. 2021;18(1):3. CrossRef
50. Tsai CF, Chen GW, Chen YC, Shen CK, Lu DY, Yang LY, et al. Regulatory effects of quercetin on M1/M2 macrophage polarization and oxidative/antioxidative balance. Nutrients. 2021;14(1):67. CrossRef
51. Xu J, Li Y, Yang X, Li H, Xiao X, You J, et al. Quercetin inhibited LPS-induced cytokine storm by interacting with the AKT1-FoxO1 and Keap1-Nrf2 signaling pathway in macrophages. Sci Rep. 2024;14(1):20913. CrossRef
52. Kwon DH, Lee H, Park C, Hong SH, Hong SH, Kim GY, et al. Glutathione induced immune-stimulatory activity by promoting M1-Like Macrophages polarization via potential ROS scavenging capacity. Antioxidants. 2019;8(9):413. CrossRef
53. Junita D, Prasetyo AA, Muniroh M, Kristina TN, Mahati E. The effect of glutathione as adjuvant therapy on levels of TNF-α and IL-10 in wistar rat peritonitis model. Ann Med Surg (Lond). 2021;66:102406. CrossRef
54. Li F, Han Y, Wu X, Cao X, Gao Z, Sun Y, et al. Gut microbiota-derived resveratrol metabolites, dihydroresveratrol and lunularin, significantly contribute to the biological activities of resveratrol. Front Nutr. 2022;9:912591. CrossRef
55. Jozefczak M, Remans T, Vangronsveld J, Cuypers A. Glutathione is a key player in metal-induced oxidative stress defenses. Int J Mol Sci. 2012;13(3):3145–75. CrossRef
56. Constantinescu T, Mihis AG. Resveratrol as a privileged molecule with antioxidant activity. Food Chem Adv. 2023;3:100539. CrossRef
57. Zhang Y, Wang X, Liu S, Wang J, Zheng P, Xu D, et al. Hydrogen nanobubbles enhancing antioxidant activity of glutathione peroxidase: superiority at the nanoscale over molecular scale. Nano Today. 2024;59:102510. CrossRef
58. Brose N, Neher E. Specificity emerges in the dissection of diacylglycerol- and protein kinase C-mediated signalling pathways. Proc Natl Acad Sci. 2002;99(26):16522–3. CrossRef
59. Eros G, Varga G, Váradi R, Czóbel M, Kaszaki J, Ghyczy M, et al. Anti-inflammatory action of a phosphatidylcholine, phosphatidylethanolamine and N-acylphosphatidylethanolamine-enriched diet in carrageenan-induced pleurisy. Eur Surg Res. 2009;42(1):40–8. CrossRef
60. Inafuku M, Nagao K, Inafuku A, Yanagita T, Taira N, Toda T, et al. Dietary phosphatidylinositol protects C57BL/6 mice from concanavalin A-induced liver injury by modulating immune cell functions. Mol Nutr Food Res. 2013;57(9):1671–9. CrossRef
61. Zhang Y, Mills GL, Nair MG. Cyclooxygenase inhibitory and antioxidant compounds from the Mycelia of the edible mushroom Grifola frondosa. J Agric Food Chem. 2002;50(26):7581–5. CrossRef
62. Zhang X, Wang G, Gurley EC, Zhou H. Flavonoid Apigenin inhibits lipopolysaccharide-induced inflammatory response through multiple mechanisms in Macrophages. PLoS One. 2014;9(9):e107072. CrossRef
63. Zhang M, Xie Y, Su X, Liu K, Zhang Y, Pang W, et al. Inonotus sanghuang polyphenols attenuate inflammatory response via modulating the crosstalk between macrophages and adipocytes. Front immunol. 2019;10:286. CrossRef
64. Lin JT, Liu CW, Chen YC, Hu CC, Juang LD, Shiesh CC, et al. Chemical composition, antioxidant and anti-inflammatory properties for ethanolic extracts from Pleurotus eryngii fruiting bodies harvested at different time. LWT—Food Sci Tech. 2014;55(1):374–82. CrossRef