INTRODUCTION
Diabetes mellitus, a non-communicable disease, adds a considerable burden to healthcare systems worldwide. The International Diabetes Federation reported that around 537 million adults had diabetes in 2021, with estimates suggesting an increase to 643 million by 2030 [1]. American Diabetes Association in its annual report 2023 states that “Research, quality of care, food, nutrition, and access health equity” will help in fighting against diabetes [2].
The maintenance, survival, and proliferation of beta cells are influenced by various transcription factors, including Neurogenin 3, Paired Box 6, Neurogenic Differentiation 1, Musculoaponeurotic Fibrosarcoma Oncogene Homolog A (MAFA), and Pancreatic and duodenal homeobox 1 (PDX1) [3]. Among these, PDX1 is a cardinal transcription factor that attaches to the promoter region of the Insulin gene, resulting in enhanced insulin production [4–6]. The expression of PDX1 is epigenetically confined to beta cells [7] and is particularly expressed in regenerating beta cells. Absence of PDX1 leads to diabetes mellitus [4]. Beta-cell regeneration in adults occurs from existing beta cells. However, there is ample evidence that beta cells can regenerate through dedifferentiation, redifferentiation, and trans-differentiation, among which alpha-to-beta cell trans-differentiation is considered a valuable therapeutic strategy for diabetes. Studies have demonstrated that in the treatment of autoimmune diabetes, endogenous alpha cells can be trans-differentiated into beta cells through gene therapy involving viruses and the increased expression of PDX1 and MAFA [3].
Numerous people worldwide use alternative and complementary medicines for diabetes treatment, with the belief that drugs from plant sources have fewer side effects than their synthetic counterparts [8,9]. Salacia species are among the numerous herbal plants used to treat diabetes [10]. Preliminary investigations conducted by our research group demonstrated the presence of phenol, flavonoids, tannins, and flavanols in the aqueous extract of Salacia oblonga roots. High-performance liquid chromatography (HPLC) analysis revealed the presence of 16 metabolites, including ponkoranol, salasol, mangiferin, and kotalanol (data not provided), consistent with previous studies [11]. Salacia oblonga has been reported to exhibit various biological properties, including antimicrobial, anti-inflammatory, antimutagenic, antilipidemic, and nephroprotective effects [11]. The antidiabetic properties of these phytochemicals manifest through the inhibition of α-glucosidase enzyme, stimulation of insulin secretion, enhancement of glucose uptake via stimulation of glucose transporter 4 (GLUT4), and inhibition of aldose reductase and peroxisome proliferator-activated receptor (PPAR) ligands, thereby mitigating diabetic complications [12,13]. Tea and meals containing Salacia oblonga have been evaluated in clinical trials for their efficacy against diabetes, yielding positive results [14,15]. However, the mechanism of action on pancreatic islets remains elusive, necessitating further investigation.
Building on existing evidence, this study tried to validate the antidiabetic properties of Salacia oblonga, focusing on its role in insulin and glucagon secretion, along with the modulation of PDX1 expression.
MATERIALS AND METHODS
Chemicals
Streptozotocin (#14653) 99.4% HPLC grade powder was purchased from SISCO Research Laboratory Private Limited (Mumbai, India). Analytical-grade glibenclamide (#G0382) was purchased from TCI Co. LTD (Tokyo, Japan). Unconjugated polyclonal anti-PDX1 (#PA5-78024) and anti-glucagon (#PA5-89937) antibodies were procured from Thermo Fisher Scientific (USA). The insulin polyclonal antibody (HB125) and poly excel Horseradish Peroxidase/Diaminobenzidine (HRP/DAB) (#PEH002) were purchased from Biogenex (USA). Ketamine hydrochloride was purchased from NEON Laboratories Limited (Mumbai, India). Analytical-grade products were used for all other chemical substances.
Preparation of extract
Salacia oblonga roots are obtained from the local market. The root was subjected to macroscopic and microscopic (quantitative and powder) analyses and authenticated by the Department of Pharmacognosy, Siddha Central Institute, Chennai. The roots were then shade-dried, cleaned, and powdered using a dry blender. 100 g of the dried powder were soaked in 3 l of distilled water and heated to 50°C for 15 minutes. The obtained solution was cooled and filtered through a Whatman paper 1. The filtrate was freeze-dried and lyophilized to obtain an aqueous extract. The obtained extract was stored in a dry container until further use. The extract yield was 3.28%.
Experimental animals
Institutional ethics committee approval was obtained for animal experiments (1/IAEC/SVMCH/01/2023). Male Wistar albino rats were acclimatized for 10 days and housed under standard conditions (temperature, 26°C ± 1°C; humidity, 57%, 12 hours day/night cycle). Autoclaved multigrain food pellets and reverse osmosis-treated water were provided ad libitum. Thirty male rats weighing between 200 and 250 g were randomly distributed into five groups, each comprising six animals.
Group I: Control
Group II: Normal rats administered with S. oblonga (200 mg/kg body weight)
Group III: Untreated diabetic group
Group IV: Diabetic rats treated with glibenclamide (2 mg/kg body weight)
Group V: Diabetic rats treated with S. oblonga (200 mg/kg body weight)
Induction of diabetes mellitus
Before inducing diabetes, initial fasting blood glucose levels of all rats were measured using an Accu check active device on blood samples from the tail vein. A single intraperitoneal dose of streptozotocin (40 mg/kg body weight) in 50 mM cold citrate buffer (pH 4.5) was administered [16]. Ten percent sucrose water was fed for 2 days to avoid hypoglycemia. Three days after diabetes induction, the experimental animals were tested for fasting blood glucose levels, and animals with more than 250 mg/dl of blood glucose level were considered diabetic and taken up for the study. The non-diabetic groups were intraperitoneally administered a citrate buffer solution.
Administration of experimental drugs
The dosage of the experimental drugs was determined based on previous studies that have demonstrated superior outcomes under comparable experimental conditions [17,18]. Salacia oblonga was given 200 mg/kg body weight orally for groups II, and V. Two milligrams per kilogram of body weight of glibenclamide was administered orally to Group IV. Distilled water and 0.5% carboxymethyl cellulose were used as the vehicles for S. oblonga and glibenclamide, respectively. Groups I and III received distilled water during the same period.
Animal sacrifice and tissue sample collection
Weekly fasting blood glucose analysis was performed using blood collected from the tail veins of the restrained rats. After 8 weeks, the rats were sacrificed using an intraperitoneal injection of ketamine (100 mg/kg body weight). Two milliliters of blood samples collected from retro-orbital sinus were processed by centrifugation at 4,000 rpm for 10 minutes. The serum obtained was stored at −20°C for later examination. Pancreatic tissue was dissected from euthanized animals and fixed in neutral buffered formalin for histopathological and immunohistochemical analyses. For gene and protein expression studies, pancreatic tissues were stored at −80°C.
Estimation of biochemical parameters
An Accu-Check Active glucometer (Roche, Basel, Switzerland) was utilized to determine the levels of glucose in fasting blood samples [19]. Urea levels were estimated using a reagent kit (# Z5030016, CUSA Bioscience, USA). Creatinine level was measured using the Jaffe method [20]. Total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), and triglycerides were measured using an enzymatic colorimetric method with commercially available kits from Life Span Biosciences, USA (#LS-K314). All tests were performed using an autoanalyzer (Mindray BS 240, India) [21]. Rat insulin (#IN374S, Calbiotech, USA) and glucagon (#E-EL-R0425, Elabscience, USA) ELISA kits were used to estimate the fasting serum insulin and glucagon levels, respectively. Experiments were conducted following the manufacturer’s protocols [22]. An ELISA reader was utilized to measure the optical densities of the solutions at 450 nm.
Histopathological analysis
Neutral buffered formalin-fixed pancreatic tissue was processed using an Epredia (STP 120) automatic tissue processor and embedded in paraffin. A 4-micron thickness serial section was cut using a LEICA (RM2235) microtome. Standard protocols were followed to conduct haematoxylin and eosin (H&E) staining. A BestScope microscope was utilized to examine and obtain images of the stained slides.
Immunohistochemistry
Tissue sections (3-micron) placed on positively charged slides (Pathnsitu) were incubated at 60°C–70°C for 20 minutes. Antigen retrieval was performed using TRIS EDTA buffer, followed by endogenous peroxidase masking with H2O2. Primary antibodies (1:100 for insulin and glucagon; 1:1000 for PDX1) were applied for 45 minutes, followed by poly excel target binder (12 minutes), poly excel HRP secondary antibody (12 minutes), and DAB chromogen mixture (5 minutes), with buffer solution washes between each step. Slides were counterstained with Harris Hematoxylin, observed under a microscope, and photographed.
Gene expression study
Pancreatic tissue stored at −80°C was transferred to TRIZOL and processed for real-time quantitative polymerase chain reaction using the SYBER green chemistry method (#RR820A, Takara, Japan) [23]. The extraction of RNA was carried out using an IGB MarbanX RNA extraction kit, India (#IGB-R- 27017-B), after which cDNA was synthesized using a RevertAid First Strand cDNA Synthesis Kit (#K1622, THERMO FISHER Scientific, USA). Table 1 contains a list of the primers utilized in this study. The comparative mRNA expression levels of Pdx1, insulin (Ins1), and glucagon (Gcg) were evaluated, with the Glyceraldehyde 3-Phosphate Dehydrogenase (Gapdh) gene serving as a control. Each test was repeated thrice, and the average values were calculated.
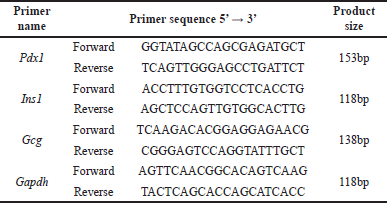 | Table 1. Primer sequence used in the experiment. [Click here to view] |
Western blot analysis
Pancreatic tissue (100 μg) was homogenized and lysed in a buffer containing 1% protease inhibitor (#ML 051, Himedia Laboratories) for 60 minutes at 4°C. Samples were centrifuged at 10,000 rpm for 15 minutes at 4°C. The supernatant was collected, and protein concentration was measured using a Bradford protein assay kit. Protein samples (40 μg) were separated via 10% SDS-PAGE and transferred to a PVDF membrane through electrotransfer. Membranes were blocked with TTBS containing 5% non-fat milk for 1 hour at room temperature, followed by overnight incubation at 4°C with GAPDH (1:1000) and unconjugated polyclonal PDX1 (1:1000). The membranes were washed three times with Tris-buffered saline and incubated with HRP-labeled goat anti-rabbit secondary antibody (1:500) for 1 hour at room temperature. After three additional washes with Tris-buffered saline containing 0.5% Tween, PDX1 proteins were visualized using enhanced chemiluminescence reagent and film exposure [24]. Specific protein band intensity was analyzed using Adobe Photoshop CS6 (version 12.0).
Morphometric analysis
Islets diameter and numerical density were measured in H& E-stained slides at 100X magnification. The fractional volume of insulin, glucagon, and PDX-1 immunopositive cells was measured from their corresponding immunohistochemically stained slides at 400X magnification. These measurements were made using an ocular micrometer and a reticule having 121 intersections. Ten random sections were selected from each animal in each group. Five random fields were examined from each section. In total, 50 fields were observed per animal [25]. The following formulae were used to calculate the above parameters.
(L - Maximum length of islet, B - Maximum breath of islet, perpendicular to L)
(NA- Number of islets observed with in the reticule, A- Area of the reticule in mm2, D- Mean diameter of islet in mm, T- Section thickness in mm)
(Pint – number of intersections falling on immune positive cells, Ptot – number of intersections on total section)
Statistical analysis
Results are presented as mean ± standard error of the mean (SEM). To analyze the differences between groups, a one-way analysis of variance (ANOVA) followed by Bonferroni’s post-hoc test was performed using IBM SPSS software version 29 (NY, USA). The p value was set at < 0.05.
RESULTS
Effects on body weight, food, and water consumption
The body weights were measured weekly; animals in Groups I and II exhibited normal weight gain (Table 2). All animals with diabetes showed a significant initial weight loss. Without treatment, Group III animals continued to lose weight throughout the experiment. In contrast, with an improvement in blood glucose levels, groups IV and V gradually gained weight, which was significantly higher than that of group III. The food and water consumption of the animals were calculated daily and compared between groups (Table 2). Food and water consumption showed a significant increase in group III (diabetic untreated) compared with the control. However, with treatment, the food and water intake of Groups IV and V animals was significantly reduced compared to that of Group III animals.
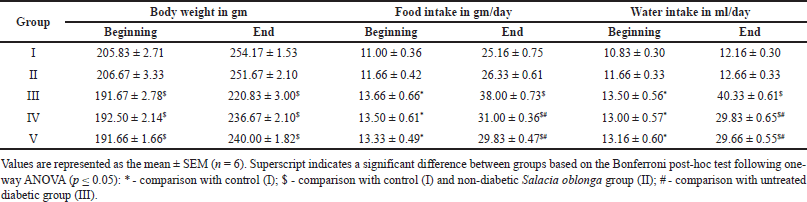 | Table 2. Body weight, Food, and water intake values at the beginning and end of the study. [Click here to view] |
Effects of fasting glucose, insulin, and glucagon
Fasting blood glucose levels at the beginning and end of treatment are shown in Table 3. The blood glucose level gradually increased in the diabetic untreated rats (Group III) and decreased significantly in the diabetic treated rats (Groups IV and V), but did not reach the level of the control group. Group II rats treated with S. oblonga showed a slight decrease in blood glucose levels, indicating the impact of S. oblonga on normoglycemic conditions. A similar trend was observed for the glucagon levels (Table 3).
 | Table 3. Fasting blood glucose, insulin, and glucagon levels of animals. [Click here to view] |
Compared to non-diabetic rats (Groups I and II), diabetic rats (Groups III, IV, and V) showed generally lower insulin levels due to beta cell destruction by streptozotocin. Salacia oblonga and glibenclamide-treated diabetic rats showed a significant increase in insulin levels (Table 3).
Effects on urea, creatinine, and lipid profiles
The levels of urea, creatinine, total cholesterol, LDL, and triglycerides were higher in Group III untreated animals, indicating a deleterious effect of hyperglycemia on fat metabolism and renal function (Table 4). Animals in groups IV and V displayed a significant reduction in the above-mentioned parameters, suggesting that S. oblonga and glibenclamide could rectify the disturbances in fat metabolism and renal damage caused by diabetes. In contrast, HDL levels were lower in Group III and moderately improved in Groups IV and V. There was no discernible difference between Groups I and II, except for the total cholesterol level, which was significantly lower in Group II.
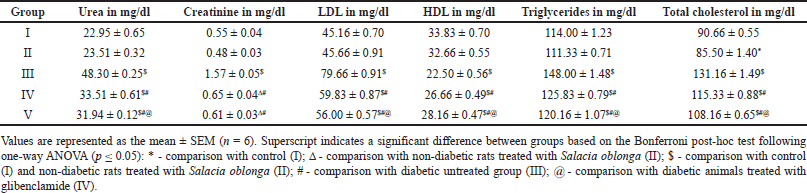 | Table 4. Urea, creatinine, and lipid profile of different groups at the end of 8 weeks. [Click here to view] |
Histopathological changes
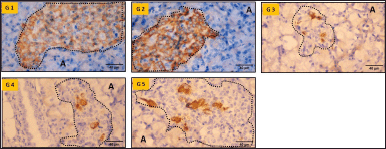
H&E-stained sections from group I (control) showed normal exocrine and endocrine portions of the pancreas. Group II showed increased eosinophilia with prominent zymogen granules in the cytoplasm of serous acini (Fig. 1). Untreated diabetic rats exhibited atrophic and inflammatory changes in islets, such as reduction in islet diameter, reduction in cell population, presence of empty vacuoles, lymphocytic infiltration, and indistinct demarcation between the exocrine and endocrine portions. These atrophic and inflammatory changes were diminished in diabetic rats treated with S. oblonga and glibenclamide (Groups IV and V).
 | Figure 1. H & E-stained photomicrographs of the pancreas (40X magnification). G1—Group I, G2—Group II, G3—Group III, G4—Group IV, G5—Group V, A—Acini, Dotted outline—Islet of Langerhans, Red arrow—Atrophic changes, Black arrow—Degranulated nucleus, Red circle—Lymphocytic infiltration. Scale bar = 40μm, n = 6. [Click here to view] |
Immunohistochemistry
Insulin immunohistochemistry revealed insulin-immunopositive cells in the central region of the islets in Groups I and II, consistent with their typical anatomical location (Fig. 2). Insulin-immunopositive cells were reduced in untreated diabetic rats (group III) because of the specific destruction of beta cells by streptozotocin. Immunopositive cell numbers improved in groups IV and V compared with those in group III. Group V animals demonstrated moderately more beta cells than did group IV animals (Fig. 2, G4 and G5).
 | Figure 2. Insulin immunohistochemistry of pancreatic tissue (40X magnification). G1—Group I, G2—Group II, G3—Group III, G4—Group IV, G5—Group V, A—Acini, Dotted outline—Islet of Langerhans. Scale bar = 40μm, n = 6. [Click here to view] |
The pancreatic tissues of groups I and II exhibited glucagon-positive cells in the periphery of the islets, indicating the normal location of the alpha cells (Fig. 3). In diabetic rats (Groups III, IV, and V), positively stained cells were observed in the center, in addition to the periphery, which was more pronounced in Group III. Treatment with S. oblonga and glibenclamide led to a moderate reduction in glucagon-positive cells. These observations were consistent with the serum glucagon levels and indicated an increase in the alpha cell mass in diabetic animals.
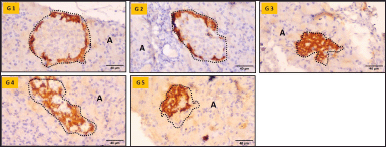 | Figure 3. Glucagon immunohistochemistry of the pancreas (40X magnification). G1—Group I, G2—Group II, G3—Group III, G4—Group IV, G5—Group V, A—Acini, Dotted outline—Islet of Langerhans. Scale bar = 40μm , n = 6. [Click here to view] |
Pancreatic sections stained with the PDX1 antibody showed positive nuclear staining in cells located at the center of the islets. PDX1 immunopositive cells were reduced in untreated diabetic rats, correlating with the reduced number of beta cells observed by insulin immunohistochemistry. The number of PDX1 immunopositive cells was significantly higher in the glibenclamide and S. oblonga-treated groups compared to the untreated group, suggesting an increase in PDX1 expression following treatment. The presence of PDX1-positive cells in the center of the islets indicated their expression in alpha cells as well as in beta cells of groups III, IV, and V (Fig. 4).
 | Figure 4. PDX1 immunohistochemistry of the pancreas (40X magnification). G1—Group I, G2—Group II, G3—Group III, G4—Group IV, G5—Group V, A—Acini, Dotted outline—Islet of Langerhans. Scale bar = 40μm, n = 6. [Click here to view] |
Effects on Pdx1, Ins1, and Gcg gene expressions
Relative quantification of gene expression using Gadph as a housekeeping gene is shown in Figure 5. Pdx1 expression was downregulated in Group III. The graph revealed that Pdx1 was upregulated in Groups IV and V. However, S. oblonga-treated animals showed higher Pdx1 gene expression than the untreated animals. Increased Pdx1 expression was also observed in Group II.
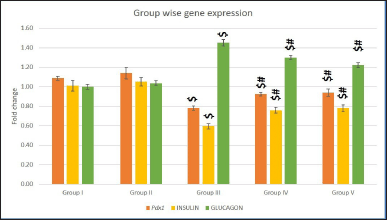 | Figure 5. Pdx1, Ins1, and Gcg gene expressions in different groups. Values are represented as mean ± SEM (Standard error of mean) (n = 6). Superscript indicates a significant difference between groups based on the Bonferroni post-hoc test following one-way ANOVA (p ≤ 0.05): $ - comparison with control (I) and non-diabetic Salacia oblonga group (IV); # - comparison with untreated diabetic group (III). [Click here to view] |
Streptozotocin-induced destruction of beta cells led to a lower expression of Ins1 in Group III. Groups IV and V showed 10% and 20% higher expression, respectively, than the untreated diabetic rats. A slight increase in Ins1 gene expression in non-diabetic rats treated with Salacia oblonga indicated that S. oblonga has a positive impact on insulin expression in normal beta cells.
In contrast to Ins1, Gcg expression was high in the diabetes-induced groups (Groups III, IV, and V). Group III animals exhibited a 40% increase in Gcg expression. The treatment groups exhibited reduced Gcg expression compared to the untreated groups. Nevertheless, the expression levels were notably higher than that of the control (20%–30%).
Effect on PDX1 protein expression
Analysis of PDX1 protein expression using western blotting showed poor bandwidth on the SDS page in Group III (Fig. 6A and B). In untreated diabetic animals, PDX1 protein expression was 1.5 folds less than that in control animals. Group IV diabetic rats treated with glibenclamide exhibited a mild increase in PDX1 expression. Compared to groups III and IV, PDX1 expression was 0.5-fold higher in group V, implying that S. oblonga had a greater impact on PDX1 expression than glibenclamide.
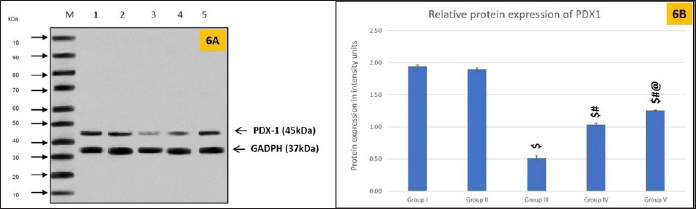 | Figure 6. PDX1 protein expression in pancreatic tissues. 6A is a representative gel image of PDX1 protein expression in the various groups. The y-axis indicates the molecular weight of the protein in kilo Dalton (kDa). In X-axis, M - Marker Lane, Lane 1: Group I, Lane 2: Group II, Lane 3: Group III, Lane 4: Group IV, and Lane 5: Group V. Fig. 6B shows the relative PDX1 protein expression levels in the different groups. Values are represented as mean ± SEM, n = 6. Superscript indicates a significant difference between groups based on the Bonferroni post-hoc test following one-way ANOVA (p ≤ 0.05): $ - comparison with control (I) and non-diabetic animals treated with Salacia oblonga (IV); # - comparison with diabetic untreated group (III), @ - comparison with diabetic animals treated with glibenclamide (IV). [Click here to view] |
Morphometric analysis
The reduction in the diameter and density of islets (Fig. 7A and B) indicated a disturbance in islet architecture and population due to STZ (Group III). These destructive changes were attenuated in the treated groups (IV, V), as evidenced by a significant increase in islet density and diameter. The fractional volume of insulin and PDX1 was significantly higher in group V animals compared to group III, demonstrating the protective effect of S. oblonga on diabetic islets. A significant increase in glucagon-positive alpha cells was observed in groups III–V (Fig. 7C). This increase in alpha cells may have contributed to the increase in islet diameter observed in groups IV and V (Fig. 7A).
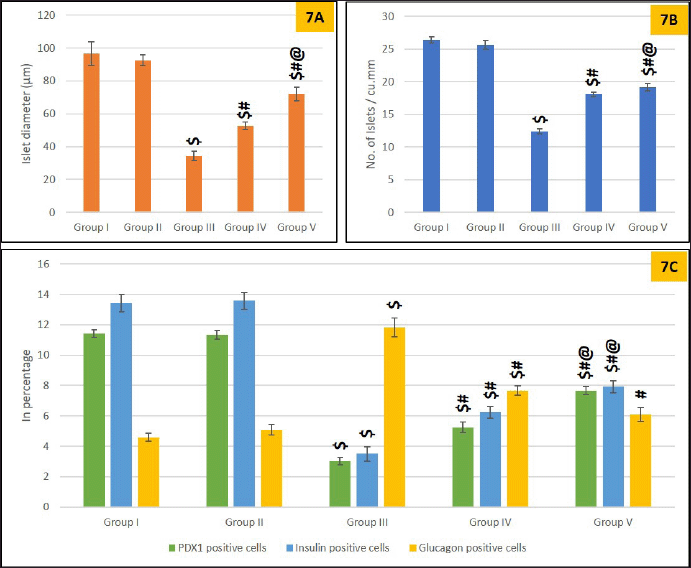 | Figure 7. Morphometric analysis of the islets. 7A is showing islet diameter, 7B is showing islet density, and 7C is showing fractional volume of PDX1, Insulin glucagon in various groups. Values are represented as mean ± SEM, n = 6. Superscript indicates a significant difference between groups based on the Tukey post-hoc test following one-way ANOVA (p ≤ 0.05): $ - comparison with control (I) and non-diabetic animals treated with Salacia oblonga (IV); # - comparison with diabetic untreated group (III), @ - comparison with diabetic animals treated with glibenclamide (IV). [Click here to view] |
DISCUSSION
Previous studies have demonstrated that S. oblonga inhibits alpha-glucosidase activity and that stimulation of insulin secretion corrects hyperglycemia in diabetes [26]. Previous studies conducted by Deepak et al. [17], Krishnakumar et al. [27], and Bhat et al. [28] utilizing petroleum ether extract (250 mg/kg), hydroalcoholic extract (50 and 100 mg/kg), and methanolic extract (50–400 mg/kg) of S. oblonga root, respectively, demonstrated increased insulin levels and reduced fasting glucose levels in STZ-induced diabetic animals. In the present study, although the extract used and the dosage differed, the efficacy of S. oblonga in restoring glycemic control (Table 3) was consistent with these findings [17,27,28]. Although blood sugar levels were lower than those in untreated animals, they were not within the normal range. This indicated the need for prolonged treatment with S. oblonga. Amelioration of hyperglycemia brought about the associated symptoms of diabetes under control, such as polyphagia and polydipsia, and diabetic animals gained weight and displayed signs of recovery (Table 2).
In addition to glucose metabolism, S. oblonga administration corrected diabetes-induced disturbances in fat and protein metabolism. This was evident from an increase in HDL levels and a reduction in total cholesterol, LDL, triglycerides, urea, and creatinine levels (Table 4). These observations are consistent with those of a previous study conducted by Bhat et al. [28] demonstrating the multifaceted role of S. oblonga in managing diabetes and its complications. Mangiferin, an important component of S. oblonga is shown to activate PPAR-α expression, which improves hyperlipidemia and hepatic steatosis in diabetic animals [29].
Streptozotocin selectively destroys beta cells and generates reactive oxygen species, which accelerate cellular destruction. This damage was confirmed through histopathological observations of H&E-stained sections and morphometric analysis from Group III animals, which showed a reduction in islet cell number and diameter [30], along with lymphocytic infiltration and dilated blood vessels (Fig. 1). These changes are characteristic of oxidative stress and corroborate the findings of other studies [25]. Furthermore, chronic hyperglycemia increases oxidative stress, which, in turn, causes cellular dysfunction by increasing lipid peroxidation. Beta cells have a low intrinsic antioxidant defense mechanism, making them susceptible to oxidative stress, resulting in decreased insulin secretion from unaffected beta cells. This was evident in group III insulin immunohistochemistry and hormonal assays (Fig. 2 and Table 3) [31].
The well-established antioxidant properties of S. oblonga likely played a protective role in STZ-induced diabetes, as evidenced by the improved histopathological features observed in Group V animals (Fig. 1). Although the islet size was comparatively smaller than that of the control, the beta cells displayed better proliferation than the untreated group, with positive insulin immunohistochemistry [32], suggesting that the treatment not only mitigated cell destruction but also promoted the recovery of insulin-producing cells (Figs. 3and 7). The results from insulin gene expression studies and hormone assays reinforced these findings, revealing improved functional beta cell mass and increased insulin levels (Table 3). Similar improvements in insulin levels have been previously reported [27,33].
It is well established that the transcription factor PDX1 is essential for insulin gene activation and maintenance of adult beta cell mass [34]. Glucotoxicity results in cytoplasmic translocation of PDX1 from the nucleus, leading to insulin gene downregulation and reduced insulin biosynthesis [35]. In diabetic animals administered S. oblonga, Pdx1 gene, and PDX1 protein expression were upregulated and localized in the nuclei of beta cells, confirming its availability to activate its downstream target insulin gene (Figs. 4–6). This action was evident from the upregulation of the insulin gene, increased insulin-immunopositive cells, and increased serum insulin levels (Figs. 2, 5, 7 and Table 3).
Few other plant extracts and compounds isolated from them have been shown to modulate Pdx1 expression. The ethanolic extract of Scrophularia striata, rice bran phenolic extract, Periploca laevigata extract, cinnamon extract, and avocado extract have demonstrated the ability to upregulate Pdx1 and Ins1 expression, both in vitro and in vivo. In many cases, this effect on Pdx1 expression has been attributed to inhibition of the JNK pathway, along with upregulation of insulin receptor β and Glut4 expression [36–39].
Tectorigenin, an isoflavone isolated from Pueraria thomsonii and hypericin, a chromophore produced by Hypericum perforatum, have also been shown to upregulate pdx1 expression and enhance insulin production by reducing endoplasmic reticulum stress and activating extracellular signal-regulated kinase (ERK). The antioxidant properties of tectorigenin are considered to be key to this pharmacological action. Similar mechanisms involving the JNK pathway, insulin receptor β, Glut4, and ERK should be explored to establish the molecular interactions involved in the antidiabetic activity of S. oblonga [40,41].
Another notable observation was an increase in alpha cell number and glucagon secretion after the induction of diabetes. Research has shown that both type 1 and type 2 diabetes are associated with an expansion of the alpha cell population and excessive glucagon production in response to the loss of beta cells [42]. In rodent models, self-replication of existing beta cells is the main source of beta cell regeneration in adults. Dedifferentiation, redifferentiation, and trans-differentiation are other plausible methods for beta cell regeneration [3]. However, the depletion of more than 90% of beta cells triggers alpha-to-beta cell trans-differentiation, leading to a sufficient beta cell mass to restore normoglycemia [43]. In the present study, the selective destruction of beta cells by streptozotocin could have been more than 90%, resulting in alpha-to-beta cell trans-differentiation to replenish the lost beta cell mass. This was evident from the increased number of glucagon-positive cells observed in Groups III, IV, and V (Figs. 3and 7).
Lam et al. [44] confirmed that only one-third of the proliferated alpha cells in adult mice with type 1 diabetes expressed glucagon-positive markers, whereas, the rest expressed alpha cell marker [Aristaless-related homeobox (ARX)] and developmental transcription marker [sex-determining region Y-box 9 (SOX-9)]. He justified alpha cell plasticity as the reason for this alpha cell mass increase, where it may transdifferentiate into undifferentiated cells or dedifferentiate into other endocrine cells of the islets [44]. Trans-differentiated beta cells of alpha cell origin have been shown to express insulin and PDX1(characteristic of beta cells), and have reduced expression of glucagon [45]. The signaling pathways involving PI3K/AKT/FOXO1 and MAPK/STAT3 may be associated with this process of trans-differentiation [46]. The results of the current study aligned with this observation. The islets of the treated groups (IV and V) showed reduced glucagon gene and protein expression and increased expression of PDX1 and Insulin gene and protein expression compared to the untreated diabetic group, signifying the trans-differentiation of alpha-to-beta cells (Table 3 and Figs. 2–7). The present study does not rule out the possibility of dedifferentiation and redifferentiation of beta cells, and these possibilities were not explored, as they were beyond the scope of this study.
The observed hyperglucagonemia can be explained by paracrine, autocrine, and juxtracrine mechanisms occurring between the endocrine cells of the islets. In the context of diabetes, when insulin secretion is impaired, alpha cells experience a loss of glucose-sensing capabilities due to diminished paracrine serotonin stimulation, consequently leading to hyperglucagonemia [47]. Furthermore, hyperglucagonemia exerts a trophic effect on the alpha cells via autocrine regulation. The loss of ephrin ligands after beta cell loss affects juxtacrine regulation between alpha and beta cells via the Eph/ephrin system, leading to uninhibited glucagon secretion [42].
Van der Meulen and Huising [43] showed that alpha to beta trans-differentiation can be experimentally induced by the overexpression of the PAX4 factor and by deleting the ARX factor from alpha cells. However, overexpression of PAX4 renders cells unresponsive to hyperglycemia, whereas ARX depletion improves glucose tolerance [43]. Furthermore, the literature suggests that appropriate stimuli, such as depletion of ARX from alpha cells, may induce the expression of beta cell markers such as PDX1, enabling their conversion into insulin-producing cells [45,48,49]. In the present study, animals in groups IV and V showed improvement in serum insulin and fasting blood glucose levels, indicating that the trans-differentiated cell mass is glucose-responsive and plays a plausible role in ARX depletion. Further research is required to determine the role of S. oblonga in the modulation of serotonin and ARX expression, activation of the Eph/ephrin system, and involvement of PI3K/AKT/FOXO1 and MAPK/STAT3 signaling pathways, which play a pivotal role in alpha-to-beta cell trans-differentiation.
This study provides scientific validation of the traditional use of S. oblonga in the management of diabetes. These findings suggest that S. oblonga has the potential to ameliorate experimental diabetes by enhancing insulin secretion and inducing alpha-to-beta cell trans-differentiation, possibly mediated by the upregulation of PDX1 expression. This provides a foundation for the development of new antidiabetic therapies using natural compounds to stimulate endogenous insulin production and potentially regenerate beta-cell mass. However, before such treatments can be considered for clinical use, additional studies are required to confirm these results in human subjects and to fully understand the safety profile of the S. oblonga extract. This study identified S. oblonga as a promising candidate for further research into diabetes treatment.
Limitations
While this study suggests that S. oblonga treatment leads to these cellular and molecular changes, it does not provide mechanistic insights into the molecular pathways and specific signaling mechanisms involved in the trans-differentiation of alpha-to-beta cells and the upregulation of PDX1 expression. Attempts were not made to display the co-expression of insulin and glucagon immunohistochemically which could have confirmed the presence of both these proteins in the same cell establishing the trans-differentiation mechanism. Further research is needed to identify the active compounds within S. oblonga that are responsible for its antidiabetic action.
CONCLUSION
The present study conclusively demonstrated that the aqueous root extract of S. oblonga possesses significant antidiabetic properties, primarily through two mechanisms: upregulation of PDX1 expression and trans-differentiation of alpha-to-beta cells. Experimental findings revealed that treatment with S. oblonga resulted in a marked increase in insulin secretion and a notable decrease in fasting glucose levels, highlighting its potential to restore glycemic control under diabetic conditions. Treatment with S. oblonga also corrected the disturbances observed in fat and protein metabolism, demonstrating its utility in preventing diabetic complications. Moreover, the present study highlights the possibility of alpha-to-beta cell trans-differentiation as a mechanism for beta cell regeneration, as indicated by the decrease in glucagon gene and protein expression, and the increase in insulin gene expression in the treated groups. This finding is particularly significant, suggesting that beyond merely enhancing insulin secretion, S. oblonga may contribute to the replenishment of beta cell mass through the conversion of alpha cells, thus offering a novel avenue for diabetes therapy. Future research should address the molecular intricacies underlying the observed antidiabetic effects, with a focus on identifying the active compounds within S. oblonga responsible for these actions. Further studies are necessary to evaluate the clinical applicability of S. oblonga in human diabetes management. This includes assessing the safety profile, determining the optimal dosing, and evaluating the long-term effects of S. oblonga extract in human subjects.
ACKNOWLEDGMENT
The authors thank Dr. S. Kalaivani, Associate Professor of Pathology, and Dr. Senniappan, Associate Professor of Pharmacognosy from Sri Venkateswara Medical College Hospital and Research Centre, for assisting with the histopathological interpretation and preparation of the plant extracts, respectively.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This work received no specific grant from any funding agency in the public, commercial, or non-profit organization.
CONFLICT OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The experimental protocol was approved by the Scientific Review Committee and Institutional Animal Ethics Committee of the Sri Venkateswara Medical College Hospital and Research Centre (SVMC/SRC/2022/24/ctr 744 and 1/IAEC/SVMCH/01/2023, respectively).
DATA AVAILABILITY
Data supporting the findings of this study are available from the corresponding author upon reasonable request.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. International Diabetes Federation. IDF Diabetes Atlas, 10th edition. Brussels, Belgium: International Diabetes Federation; 2021. Available from: https://www.diabetesatlas.org
2. Annual Report. ADA [Internet]. [cited 2024 Jul 3]. Available from: https://diabetes.org/about-us/annual-reports
3. Kerper N, Ashe S, Hebrok M. Pancreatic β-cell development and regeneration. Cold Spring Harb Perspect Biol. 2022 May 27;14(5):a040741.
4. Zhang Y, Fang X, Wei J, Miao R, Wu H, Ma K, et al. PDX-1: a promising therapeutic target to reverse diabetes. Biomolecules. 2022 Dec;12(12):1785.
5. Gao T, McKenna B, Li C, Reichert M, Nguyen J, Singh T, et al. Pdx1 maintains β cell identity and function by repressing an α cell program. Cell Metab. 2014 Feb 4;19(2):259–71.
6. Fujimoto K, Polonsky KS. Pdx1 and other factors that regulate pancreatic β-cell survival. Diabetes Obes Metab. 2009 Nov;11(4):30–7.
7. Ebrahim N, Shakirova K, Dashinimaev E. PDX1 is the cornerstone of pancreatic β-cell functions and identity. Front Mol Biosci. 2022 Dec 15;9:1–18.
8. Aljulifi MZ. Paradigm of complementary and alternative medicine in type 2 diabetes. Type 2 diabetes in 2024 – from early suspicion to effective management. London, UK: IntechOpen; 2023. pp 1–34. doi: 10.5772/intechopen.1002422
9. Garrow D, Egede LE. Association between complementary and alternative medicine use, preventive care practices, and use of conventional medical services among adults with diabetes. Diabetes Care. 2006 Jan;29(1):15–9.
10. Sukhikh S, Babich O, Prosekov A, Kalashnikova O, Noskova S, Bakhtiyarova A, et al. Antidiabetic properties of plant secondary metabolites. Metabolites. 2023 Apr;13(4):513.
11. Kushwaha P, Singh A, Keshari A, Maity S, Saha S. An updated review on the phytochemistry, pharmacology, and clinical trials of Salacia oblonga. Pharmacogn Rev. 2016;10(20):109.
12. Li Y, Huang THW, Yamahara J. Salacia root, a unique Ayurvedic medicine, meets multiple targets in diabetes and obesity. Life Sci. 2008 May;82(21–22):1045–9.
13. Girón MD, Sevillano N, Salto R, Haidour A, Manzano M, Jiménez ML, et al. Salacia oblonga extract increases glucose transporter 4-mediated glucose uptake in L6 rat myotubes: role of mangiferin. Clin Nutr. 2009 Oct;28(5):565–74.
14. Nakata K, Taniguchi Y, Yoshioka N, Yoshida A, Inagawa H, Nakamoto T, et al. A mixture of Salacia oblonga extract and IP-PA1 reduces fasting plasma glucose (FPG) and low-density lipoprotein (LDL) cholesterol levels. Nutr Res Pract. 2011;5(5):435.
15. Williams JA, Choe YS, Noss MJ, Baumgartner CJ, Mustad VA. Extract of Salacia oblonga lowers acute glycemia in patients with type 2 diabetes. AJCN. 2007;86(1):124–30.
16. Banerjee A, Singh S, Prasad SK, Kumar S, Banerjee O, Seal T, et al. Protective efficacy of Tinospora sinensis against streptozotocin induced pancreatic islet cell injuries of diabetic rats and its correlation to its phytochemical profiles. J Ethnopharmacol. 2020 Feb;248:112356.
17. Deepak KGK, Challa S, Suhasin G, Nagesewara Rao Reddy N, Elansary HO, El-Ansary DO. Antidiabetic and antilipidemic activity of root extracts of Salacia oblonga against streptozotocin-induced diabetes in Wistar Rats. Processes. 2020 Mar 5;8(3):301.
18. Sokolovska J, Isajevs S, Sugoka O, Sharipova J, Paramonova N, Isajeva D, et al. Comparison of the effects of glibenclamide on metabolic parameters, GLUT1 expression, and liver injury in rats with severe and mild streptozotocin-induced diabetes mellitus. Med Kaunas Lith. 2012;48(10):532–43.
19. Kitphati W, Sato VH, Peungvicha P, Saengklub N, Chewchinda S, Kongkiatpaiboon S, et al. Antihyperglycemic activity of a novel polyherbal formula (HF344), a mixture of fifteen herb extracts, for the management of type 2 diabetes: evidence from in vitro, ex vivo, and in vivo studies. Heliyon. 2024 Oct;10(19):e38703.
20. Besseling PJ, Pieters TT, Nguyen ITN, de Bree PM, Willekes N, Dijk AH, et al. A plasma creatinine- and urea-based equation to estimate glomerular filtration rate in rats. Am J Physiol Ren Physiol. 2021 Mar;320(3):F518–24.
21. Ghezzi AC, Cambri LT, Botezelli JD, Ribeiro C, Dalia RA, de Mello MAR. Metabolic syndrome markers in wistar rats of different ages. Diabetol Metab Syndr. 2012 Apr;4(1):16.
22. Lei YC, Hwang JS, Chan CC, Lee CT, Cheng TJ. Enhanced oxidative stress and endothelial dysfunction in streptozotocin-diabetic rats exposed to fine particles. Environ Res. 2005 Nov;99(3):335–43.
23. Cheng Y, Yao XM, Zhou SM, Sun Y, Meng XJ, Wang Y, et al. The m6A methyltransferase METTL3 ameliorates methylglyoxal-induced impairment of insulin secretion in pancreatic β cells by regulating MafA expression. Front Endocrinol. 2022 Jul;13:910868.
24. Subramanian M, Thotakura B, Chandra Sekaran SP, Jyothi AK, Sundaramurthi I. Naringin ameliorates streptozotocin-induced diabetes through forkhead box M1-mediated beta cell proliferation. Cells Tissues Organs. 2018;206(4-5):242–53.
25. Subramanian M, Thotakura B, Chandra Sekaran SP, Jyothi AK, Sundaramurthi I. Naringin (4’,5,7-Trihydroxyflavanone 7-Rhamnoglucoside) attenuates β-cell dysfunction in diabetic rats through upregulation of PDX-1. Cells Tissues Organs. 2018;206(3):133–43.
26. Chelladurai GRM, Chinnachamy C. Alpha amylase and Alpha glucosidase inhibitory effects of aqueous stem extract of Salacia oblonga and its GC-MS analysis. Braz J Pharm Sci. 2018 May 14;54(1):10.
27. Krishnakumar K, Augusti KT, Vijayammal PL. Anti-peroxidative and hypoglycaemic activity of Salacia Oblonga extract in diabetic rats. Pharm Biol. 2000;38(2):101–5.
28. Bhat BM, C V R, D’Souza V, Manjrekar PA. Antidiabetic and hypolipidemic effect of Salacia oblonga in streptozotocin induced diabetic rats. J Clin Diagn Res. 2012 Dec;6(10):1685–7.
29. Huang THW, Peng G, Li GQ, Yamahara J, Roufogalis BD, Li Y. Salacia oblonga root improves postprandial hyperlipidemia and hepatic steatosis in Zucker diabetic fatty rats: activation of PPAR-alpha. Toxicol Appl Pharmacol. 2006 Feb 1;210(3):225–35.
30. Martha A, Jacks T W, Garba SH, Dibai NI. Pancreatic morphology and morphometric analysis of streptozotocin-induced diabetes in albino rats treated with n-hexane extract of leptadenia hastata leaves. J Med Histol. 2019 Dec 1;2(2):173–80.
31. Kajimoto Y, Kaneto H. Role of oxidative stress in pancreatic β-cell dysfunction. In: Lee HK, Di Mauro S, Tanaka M, Wei YH, editors. Mitochondrial pathogenesis from gene and apoptosis to aging and disease. Berlin, Germany: Springer; 2004. pp. 168–76.
32. Adeyemi D, Komolafe O, Adewole S, Obuotor EM, Abiodun A, Adenowo T. Histomorphological and morphometric studies of the pancreatic islet cells of diabetic rats treated with extracts of Annona muricata. Folia Morphol. 2010 May 1;69:92–100.
33. Gomathi D, Ravikumar G, Kalaiselvi M, Devaki K, Uma C. Efficacy of evolvulus alsinoides (L.) L. on insulin and antioxidants activity in pancreas of streptozotocin induced diabetic rats. J Diabetes Metab Disord. 2013 Jul 8;12(1):39.
34. Vaulont S, Vasseur-Cognet M, Kahn A. Glucose regulation of gene transcription. J Biol Chem. 2000 Oct 13;275(41):31555–8.
35. Kitamura T, Nakae J, Kitamura Y, Kido Y, Biggs WH, Wright CVE, et al. The forkhead transcription factor Foxo1 links insulin signaling to Pdx1 regulation of pancreatic β cell growth. J Clin Invest. 2002 Dec 15;110(12):1839–47.
36. Babaiedarzi A, Ghanbari S, Mehrad seresht M, Nasiri M. Antidiabetic effects of Scrophularia striata ethanolic extract via suppression of Pdx1 and Ins1 expression in pancreatic tissues of diabetic rats. Sci Rep. 2022 Jun 13;12(1):9813.
37. Saji N, Francis N, Schwarz LJ, Blanchard CL, Santhakumar AB. Rice bran phenolic extracts modulate insulin secretion and gene expression associated with β-cell function. Nutrients. 2020 Jun 24;12(6):1889.
38. Trabelsi G, Mellado S, Kalboussi Z, Chekir-Ghedira L, Pascual M. Novel role of Periploca laevigata extracts as anti-diabetic and anti-inflammatory function in pancreatic b cells exposed to hyperglycaemia. BioRxiv [Preprint], 28 Nov. 2023. doi: https://doi.org/10.1101/2023.11.07.565784
39. Shehata NI, Abo Zeid SM, Abd El Aziz SA, Abdelgawad HM. Mitigation of streptozotocin-induced alterations by natural agents via upregulation of PDX1 and Ins1 genes in male rats. J Food Biochem. 2022 May;46(5):e14086.
40. Yao X, Li K, Liang C, Zhou Z, Wang J, Wang S, et al. Tectorigenin enhances PDX1 expression and protects pancreatic β-cells by activating ERK and reducing ER stress. J Biol Chem. 2020 Sep 11;295(37):12975–92.
41. Liang C, Hao F, Yao X, Qiu Y, Liu L, Wang S, et al. Hypericin maintains PDX1 expression via the Erk pathway and protects islet β-cells against glucotoxicity and lipotoxicity. Int J Biol Sci. 2019 Jun 2;15(7):1472–87.
42. Martínez MS, Manzano A, Olivar LC, Nava M, Salazar J, D’Marco L, et al. The role of the α cell in the pathogenesis of diabetes: a world beyond the Mirror. Int J Mol Sci. 2021 Sep 1;22(17):9504.
43. van der Meulen T, Huising MO. Role of transcription factors in the transdifferentiation of pancreatic islet cells. J Mol Endocrinol. 2015 Apr;54(2):R103–17.
44. Lam CJ, Cox AR, Jacobson DR, Rankin MM, Kushner JA. Highly proliferative α-cell-related islet endocrine cells in human pancreata. Diabetes. 2018 Apr;67(4):674–86.
45. Thorel F, Népote V, Avril I, Kohno K, Desgraz R, Chera S, et al. Conversion of adult pancreatic α-cells to β-cells after extreme β-cell loss. Nature. 2010 Apr 22;464(7292):1149–54.
46. Wang W, Zhang C. Targeting β-cell dedifferentiation and transdifferentiation: opportunities and challenges. Endocr Connect. 2021 Jul 21;10(8):R213–28.
47. Wendt A, Eliasson L. Pancreatic alpha cells and glucagon secretion: novel functions and targets in glucose homeostasis. Curr Opin Pharmacol. 2022 Apr;63:102199.
48. Yang YP, Thorel F, Boyer DF, Herrera PL, Wright CVE. Context-specific α- to-β-cell reprogramming by forced Pdx1 expression. Genes Dev. 2011 Aug 15;25(16):1680–5.
49. Collombat P, Xu X, Ravassard P, Sosa-Pineda B, Dussaud S, Billestrup N, et al. The ectopic expression of Pax4 in the mouse pancreas converts progenitor cells into alpha and subsequently beta cells. Cell. 2009 Aug 7;138(3):449–62.