INTRODUCTION
Cardiovascular diseases (CVDs)
CVDs are a major global health issue responsible for around 17.9 million deaths in 2019, with 85% of these instances involving heart attacks and/or strokes [1]. A significant risk factor for CVDs is dyslipidemia. This is a condition characterized by abnormal blood lipid levels, with the most common form involving high levels of total cholesterol (TC), triglycerides (TG), and low-density lipoprotein (LDL), together with low levels of high-density lipoprotein (HDL). Regulating lipid levels, particularly cholesterol, is thus a key strategy to reduce the risk of CVDs caused by dyslipidemia [2].
Dyslipidemia contributes to endothelial dysfunction and atherosclerosis, which are two major contributors of CVDs, in various ways. First, oxidized LDLs can lead to endothelial dysfunction and a sequence of biological processes resulting in atherosclerosis and arterial stiffness [3]. Second, dyslipidemia can cause an accumulation of iron in the aorta and elevate serum hepcidin levels, which can exacerbate endothelial dysfunction, oxidative stress, and inflammatory reactions [4]. Third, non-HDL cholesterol reduces the bioavailability of nitric oxide (NO) produced by endothelial nitric oxide synthase, which can then lead to the development of atherosclerosis [5].
Arterial stiffness is currently used as a prognostic indicator to identify the cardiovascular protective effects of test compounds in clinical practice [6], particularly in cases where dyslipidemia is associated with an increased incidence of arterial stiffness [7]. Arterial stiffness can be measured using the non-invasive pulse wave velocity (PWV) method in 1- and 3-month-old mice, with arterial stiffness increasing with age [8]. To induce the thickening of the aortic wall, Kurniati et al. [9] reported the use of a high-fat high-cholesterol (HFC) diet.
Use of herbal medicine in managing CVDs
Treating or managing diseases using herbal medicine has been a significant research interest in recent years, with various researchers focusing on their potential to manage CVDs. Garlic has been shown to lower blood pressure and cholesterol [10], while ginger has been shown to reduce blood pressure in hypertensive patients [11] and provide anti-inflammatory and antioxidant effects to help reduce vascular damage [12,13]. However, the evidence remains mixed, with the exact mechanism of action poorly understood. Further studies are needed to uncover the molecular pathways involved in their cardioprotective effects.
Dayak onion (Eleutherine americana (aubl.) Merr), the herb of interest in this research, has been studied for its protective effects on cardiovascular function, including its ability to prevent dyslipidemia. Eleutherine americana extracted using water and ethanol was shown to reduce TC and LDL levels [14]. It was further observed to exhibit anti-atherogenic activity through in silico methods, with compounds such as eleutherinoside A, ß-Sitosterol, eleuthoside B, and eleutherinoside B [15] playing a role in its anti-atherogenic activity by acting as agonists of Peroxisome Proliferated-Activated Receptor γ [16]. Eleutherine americana extracted using n-hexane was found to reduce atherosclerosis by hindering foam cell development through a decrease in the uptake of oxidized LDL while concurrently promoting cholesterol efflux [17]. Eleutherine americana was also found to have antihypertensive properties in a fructose-induced hypertension animal model, being able to effectively regulate systolic and diastolic blood pressure and heart rate (HR). A potential mechanism underlying its blood pressure-lowering effect is its diuretic action to stimulate increased urine output [18].
However, the effects of E. americana on arterial stiffness have not been reported, which is the interest of this research. This study thus aims to contribute to existing studies on the cardiovascular protective effects of E. americana by examining the effects of ethanol extract of E. americana as an antidyslipidemia agent and its implications on arterial stiffness in an experimental dyslipidemia model on rats induced by an HFC diet.
MATERIALS AND METHODS
Materials
Eleutherine americana was obtained from Lembang, West Java, Indonesia, with determination certificate No. 48/BH/02/2021 issued by Herbarium Jatinangor, Plant Taxonomy Laboratory, Faculty of Mathematics and Natural Sciences, Padjadjaran University. Cholesterol kit (Proline), Triglyceride kit (Proline), HDL kit (Sekisui), LDL kit (Sekisui), 96% Ethanol, Na-CMC, Cholesterol Powder (Bioworld), cholic acid, and other chemicals commonly used for phytochemical screening were provided by the laboratory department of Bhakti Kencana University. Propylthiouracil (PTU) and generic simvastatin were purchased from a pharmacy.
Animals
24 male Wistar rats used as the animal model in this study were acquired from D-Wistar, Indonesia. The rats were 2 months old and had body weights (BWs) ranging from 200 to 250 g. Once acquired, they were housed within enclosures maintained at 25°C and a 12-hour dark-light cycle. The rats had unrestricted access to water and their respective diets. The research protocol used in this study complies with ethical standards and has been approved by the Ethics and Research Committee of the Faculty of Medicine, Padjadjaran University (approval number: 268/UN6.KEP/EC/2021).
Preparation and extraction of E. americana
Eleutherine americana was thoroughly cleansed under running water to remove impurities such as soil, particles, and other plant components. Then, the clean E. americana was chopped to smaller sizes and dried in an oven at 45°C. Finally, the dried E. americana was powdered and stored in an airtight jar.
To make the ethanol extract, the powdered E. americana was macerated in 96% ethanol for 72 hours and then filtered. Subsequently, the filtrate was evaporated using a rotary evaporator at a controlled temperature of 50°C [18], resulting in a yield of 7.83% w/w, i.e., 78.3 g of extract from 1,000 g of dried E. americana. A 96% ethanol extract was used as prior studies show that an ethanol extract resulted in a greater extraction yield compared to an aqueous extract, with a 96% ethanol extract exhibiting the highest total phenolic content and the most potent antioxidant activity compared to lower ethanol concentrations [19,20].
Physiochemical and phytochemical analysis
Physiochemical analysis using methods described by the World Health Organization was conducted to determine water content; total ash; water-soluble extractive value and ethanol-soluble extractive value of the ethanol extract of E. americana [21,22].
Phytochemical analysis using a standard procedure was conducted on the ethanol extract of E. americana to determine the presence of flavonoids, alkaloids, saponins, tannins, and terpenoids [23,24].
Diet preparation
HFC diet and normal diet [for normal control (NC) group] were prepared by mixing all ingredients and pressing using a feed press, according to the composition shown in Table1. The suspension of cholesterol, cholic acid, and thiouracil in vegetable oil was made with a composition of 200 mg/kg BW cholesterol, 0.2% cholic acid, and 12.5 mg/kg BW PTU and administered orally to the rats.
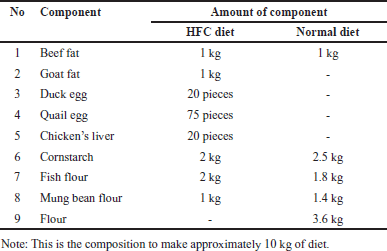 | Table 1. Composition of normal and HFC diet. [Click here to view] |
Experimental design
The rats were acclimatized in their enclosures for 7 days, and then randomly grouped into six groups (n = 4) based on Federer’s [25] method. Federer’s method calculates the minimum number of animals required using the formula (t-1) (n-1) >15, where (t) is the number of groups and (n) is the number of animals [25]. Rats were administered the test compounds orally and fed their diets once a day for 60 days, with a 2-hour interval between the feeding of the test compound and the diet. The test compounds and diets administered to each of the six groups are summarized in Table 2. At the end of the experiment, HR and PWV were measured and blood samples were collected for lipid profile analysis for each rat. Then, the rats were euthanized, and their aortas were collected for histopathological examination.
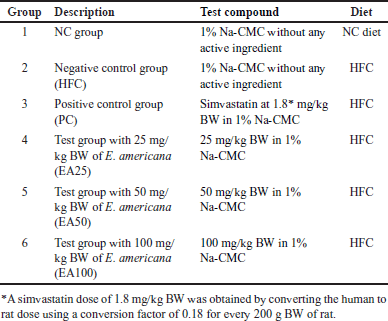 | Table 2. Test compounds and diets administered to the rats. [Click here to view] |
HR measurement
HR measurement was conducted using an electrocardiogram (ECG) device, which is a non-invasive method described by Hasimun et al. [26]. Analysis of the ECG patterns involved the determination of the distance (time elapsed) between two successive R-waves of the QRS signal (R-R intervals), with shorter R-R intervals indicative of an elevated HR [27].
Arterial stiffness measurement
Arterial stiffness was measured indirectly through PWV using ECG and photoplethysmogram sensors [8]. A higher PWV index indicates increased arterial stiffness.
Blood sampling and lipid profile analysis
Blood samples were collected via the Retro-Orbital Vein of the rats for lipid profile analysis. Before blood sampling, the rats fasted for 8 to 12 hours with access to only water. Then, they were anesthetized using CO2, and their blood was collected using a hematocrit capillary pipe. The blood samples were centrifuged at 3,000 rpm for 5 minutes and the resulting serum was stored at −20°C before lipid profile analysis.
The lipid profile analysis on TC, TG, HDL, and LDL levels was done using Proline and Sekisui kits. For TC and TG, 10 µl of serum was mixed with 1,000 µl of reagent and incubated at 37°C for 10 minutes. Then, TC and TG levels of the resulting mixture were determined through Microlab 300 (ELITech) at a wavelength of 500 nm. For HDL and LDL, 3 µl of serum was mixed with 300 µl of reagent 1 from the Sekisui kit and incubated at 37°C for 5 minutes. Subsequently, 100 µl of reagent 2 from the Sekisui kit was added and incubated at 37°C for 5 minutes. HDL and LDL levels of the resulting mixture were determined through Microlab 300 at a wavelength of 600 nm.
Atherogenic index plasma (AIP) is a marker to predict the risk of atherosclerosis using TG and HDL levels, and there is a clinical correlation between high AIP values and cardiovascular events [28]. AIP was calculated using TG and HDL levels using Equation 1:
Aortic histopathology
Aortic histopathology of the rat’s aorta was done using the hematoxylin and eosin staining method. After the rats were sacrificed, their aortic tissues were collected and rinsed with physiological saline and then fixed in 10% formalin. Following fixation, the tissues were dehydrated using increasing concentrations of ethanol (50%, 70%, 80%, 90%, and 96%) for 1 hour each, followed by three times 100% ethanol for 1 hour each. Thereafter, the tissues were cleared in xylol three times for 1 hour each. The tissues were then embedded in paraffin and sectioned at a thickness of 4 to 5 µm. The sections were mounted on glass slides and stained with HE and then examined under a microscope [29]. Parameters observed under the microscope included histopathological features, aortic wall thickness, and the number of foam cells. Progression of atherosclerosis, which eventually leads to arterial stiffness, is indicated by the aortic wall thickness and the amount of foam cells.
Data analysis
GraphPad Prism 10.2.2 statistical software (GraphPad Software, San Diego, CA) was used to analyze the data. Results were expressed as the mean ± SD, and one-way ANOVA followed by Tukey’s multiple comparison testing was used to compare the differences between treatment groups. Statistical significance was considered at p < 0.05, p < 0.001, and p < 0.0001.
RESULTS AND DISCUSSION
Results
Physicochemical and phytochemical analysis
Tables 3 and 4 present the results of physicochemical analysis and phytochemical screening of the ethanol extract of E. americana, respectively. The water content, total ash, water-soluble extractive value, and ethanol-soluble extractive value were 5.5, 5.6, 15.0, and 10.0% w/w respectively, which comply with the quality requirement specified by Herbal Pharmacopeia and Indonesian Materia Medica [30]. In addition, the ethanol extract of E. americana contained flavonoids, alkaloids, saponin, tannins, and terpenoids.
 | Table 3. Physicochemical parameters of E. americana. [Click here to view] |
 | Table 4. Qualitative phytochemical analysis of E. americana. [Click here to view] |
HR and arterial stiffness analysis
Figure 1 shows the results of HR and PWV analysis after the experiment. There were no significant differences in HR parameters of the positive control (SC), and treatment groups EA25, EA50, and EA100 compared to the NC group (p > 0.05). However, there were significant differences in PWV of EA25 group compared to the NC group (p < 0.0001). In addition, there were significant differences in HR and PWV parameters of the SC group, EA50 and EA100 compared to the negative control group (HFC) (p < 0.0001), but there were no significant differences in PWV of EA25 group compared to the HFC group (p > 0.05). The results show that E. americana at 50 and 100 mg/kg BW may prevent arterial stiffness as it decreases HR value, through a decrease in PWV value.
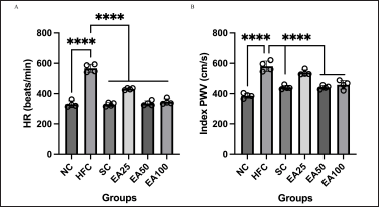 | Figure 1. Arterial stiffness is a pivotal biomarker for assessing the risk of cardiovascular events. The ethanol extracts of E. americana at 25, 50, and 100 mg/kg BW can significantly influence hemodynamics on the 60th day of treatment by reducing HR (A) and arterial stiffness as indicated by PWV values (B). Therefore, the ethanol extracts of E. americana were beneficial for controlling and preventing endothelial dysfunction or arterial stiffness. The data are expressed as the mean ± SD (n = 4). ****p < 0.0001 compared to the HFC group. [Click here to view] |
Figure 2 shows the ECG patterns for all six groups in this study. NC group (Fig. 2A), SC group (Fig. 2C), EA50 group (Fig. 2E), and EA100 group (Fig. 2F) had the longest distances between the R-R intervals at around 0.2 seconds. Meanwhile, HFC group (Fig. 2B) and EA25 (Fig. 2D) had shorter distances between the R-R intervals at around 0.15 seconds. A short R-R interval suggests an elevation in the HR.
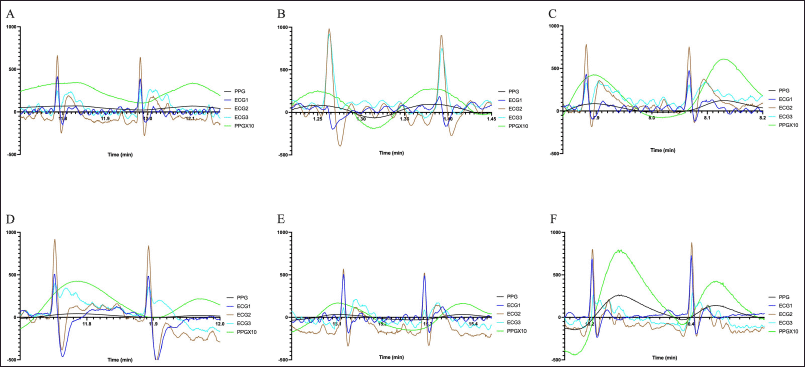 | Figure 2. ECG patterns for all groups, NC group (A), HFC group (B), SC group (C), EA25 group (D), EA50 Group (E), and EA100 Group (F). [Click here to view] |
Lipid profile analysis
Figure 3 shows the analysis of TC, TG, LDL, and HDL levels. Compared to the NC group, the HFC group had higher TC, TG, and LDL levels (p < 0.0001) and lower HDL levels (p < 0.05). TC, TG, and LDL levels showed significant differences for SC group and all treatment groups (EA25, EA50, and EA100) when compared to HFC group (p < 0.0001). However, no significant differences were observed for SC group and all treatment group (EA25, EA50, and EA100) when compared to NC group (p > 0.05). In addition, HDL levels showed significant differences for SC group (p < 0.001) and all treatment groups (EA25, EA50, and EA100) (p < 0.0001) when compared to HFC group. Put together, the results indicate that E. americana at 25, 50, and 100 mg/kg BW may confer antidyslipidemia effects through decreased TC, TG, LDL, and increased HDL levels, and the effect is similar to the SC group.
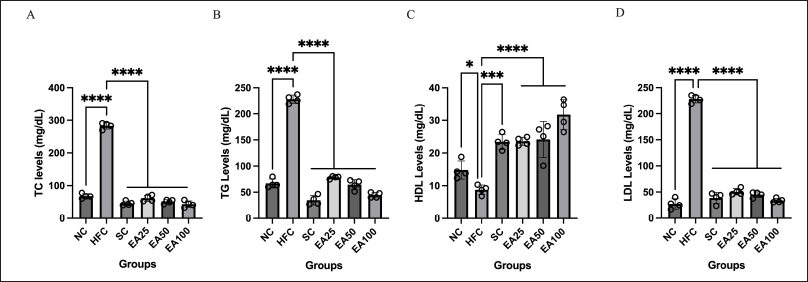 | Figure 3. Lipid profiles are crucial biomarkers for predicting cardiovascular events, particularly endothelial dysfunction. Ethanol extracts of E. americana at 25, 50, and 100 mg/kg BW influenced the lipid profiles on the 60th day of treatment by maintaining TC (A), TG (B), HDL (C), and LDL (D) levels within a normal state. Therefore, ethanol extracts of E. americana were beneficial for controlling and preventing dyslipidemia events. The data are expressed as the mean ± SD (n = 4). *p < 0.05, ***p < 0.001, ****p < 0.0001 compared to the HFC group. [Click here to view] |
Figure 4A shows the AIP values. There were no significant differences in the AIP value for SC group and all treatment groups (EA25, EA50, and EA100) when compared to NC group (p > 0.05). However, there were significant differences for NC group, SC group, and all treatment groups (EA25, EA50, and EA100) when compared to HFC group (p < 0.0001). The results show that E. americana at 25, 50, and 100 mg/kg BW may prevent atherosclerosis by decreasing AIP value, through decreasing TG and increasing HDL levels, and such an effect is similar to the SC group. AIP is a measure of the ratio of TG and HDL and reflects the balance between the protective and atherogenic lipoproteins. It thus correlates with the size of pro- and antiatherogenic lipoprotein particles and represents the atherogenic risk. Since AIP is an easily available cardiovascular risk marker, it has the potential for use as a useful measure of response to treatment for pharmacological intervention.
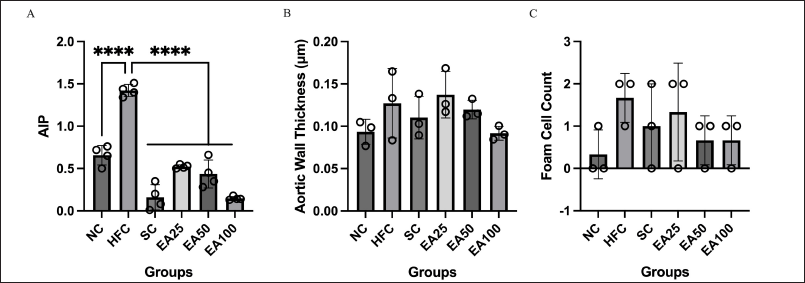 | Figure 4. AIP values (A), aortic wall thickness (B), and foam cell count (C) are crucial markers for predicting cardiovascular events, especially arterial stiffness and endothelial dysfunction. The EA25, EA50, and EA100 groups exhibit lower AIP values, foam cell count, and aortic wall thickness compared to the HFC group on the 60th day of treatment. Based on the AIP values, the ethanol extracts of E. americana were beneficial for preventing atherosclerosis. The data are expressed as the mean ± SD (n = 3–4), ****p < 0.0001 compared to the HFC group. [Click here to view] |
Among the three groups, EA100, with the highest dosage of E. americana, showed the highest effectiveness in reducing TC, TG, and LDL levels, increasing HDL levels, and lowering AIP values. Nevertheless, EA50 (50 mg/kg BW) itself has a similar performance to the SC group.
Histopathology of aorta
Figure 5 shows the histopathological images of the aorta, and Figure 4B and 4C show the aortic wall thickness and foam cell count. Histopathological images of the aorta further confirmed the occurrence of early-stage atherosclerosis in the HFC group. In addition, the HFC group was found to have significantly higher AIP values when compared to the NC group (p < 0.05). There was also a slight thickening of the aortic wall and the formation of foam cells in the HFC group, although the results were not statistically significantly higher than the NC group (p > 0.05). This is consistent with the measurements of aortic wall thickness and foam cell count in the HFC group, which were higher compared to the NC group.
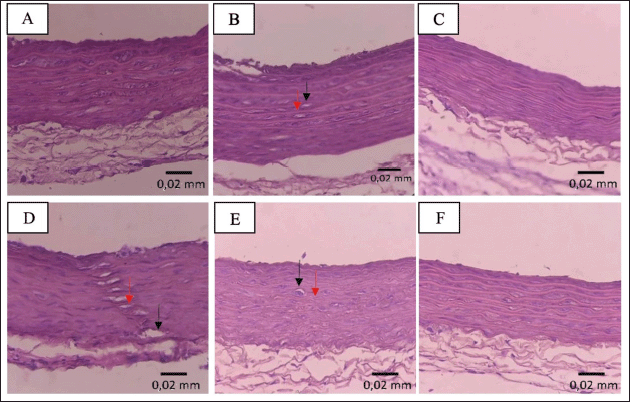 | Figure 5. Histopathological images of the aorta wall show the formation of foam cells and cell separation in the HFC (B), EA25 (D) and EA50 (E) groups. No foam cells and cell separation were observed in the NC (A), SC (C), and EA100 (F) groups. →Foam cells,→ = cell separation. [Click here to view] |
Overall results
The effects of E. americana in lowering TC, TG, LDL, and AIP levels, increasing HDL levels, and reducing HR and PWV (except for the EA25 group) were largely similar to the SC group, which received simvastatin at 1.8 mg/kg BW. The highest effective dosage of E. americana was 100 mg/kg BW, with 50 mg/kg BW yielding similar performance to 1.8 mg/kg BW of simvastatin.
DISCUSSION
This study confirmed previous research findings that an HFC diet can induce elevated TC, TG, and LDL levels, and decreased HDL levels (i.e., dyslipidemia) in male Wistar rats. Specifically, a significant increase in lipid profiles, namely TC, TG, and LDL, and a decrease in HDL were observed after the administration of an HFC diet for 60 days (Fig. 3). This study contributes to Kurniati et al.’s [9] study, which only examined lipid profiles and aortic wall thickness via histopathology, by including analyses of HR, PWV, and foam cell formation. The additional analyses used in this study showed that HFC-induced dyslipidemia results in gradual foam cell formation (leading to atherosclerosis), increased arterial stiffness or endothelial dysfunction, and elevated HR, eventually compromising cardiovascular function in the rats. The findings also align with prior research which suggests that increased oxidative stress plays a pivotal role in the development of arterial stiffness, thereby contributing not only to endothelial dysfunction but also to coronary artery disease [31]. Therefore, arterial stiffness can serve as an important biomarker for assessing the efficacy of cardiovascular therapy [32].
In this study, the HFC diet is able to induce dyslipidemia and arterial stiffness as it contains exogenous cholesterol, cholic acid, and PTU. Cholic acid increases the absorption of exogenous cholesterol by increasing bile acid production. This raises bile acid concentrations in the intestine and causes increased cholesterol absorption through the formation of cholesterol micelles by bile acids [33]. On the other hand, PTU is an antithyroid drug that inhibits thyroid cells and causes hypothyroidism [34]. Hypothyroidism downregulates lipoprotein metabolism by causing a decrease in LDL receptors in the liver, and results in an increase in lipid levels, particularly LDL [35]. The combined effects of exogenous cholesterol, cholic acid, and PTU thus raise LDL and TG and decrease HDL, thereby contributing to the increased incidence of atherosclerosis [36], the number of foam cells in the aortic wall, and arterial stiffness [37], which are consistent with this study’s research findings.
This study further showed that an ethanol extract of E. americana can improve lipid profiles and reduce cardiovascular events by preventing atherosclerosis and lowering arterial stiffness and HR, with effects similar to simvastatin. The observed effects of E. americana on lipid profiles corroborate with existing research and this study contributes by demonstrating its effects on arterial stiffness using PWV, HR, and aortic histopathology.
While various studies have investigated the source of antidyslipidemic activity of E. americana, there is still no clarity in the mechanism of action. Some studies attribute the antidyslipidemic activity to three compound groups (i.e., naphthalene, naphthoquinone, and anthraquinone), which can prevent the occurrence of atherosclerosis through a coronary vasodilator mechanism and facilitate blood flow in coronary arteries [17,38]. Other studies found that the antidyslipidemic activity comes from phytochemicals such as Eleutherol [39], Eleurherol A, Eleurherol B [40], Eleutherinoside A, Eleutherinoside B [41], Eleuthoside B, Elecanacin [42], isoeleutherin [43], and β-Sitosterol [15]. From a pathological perspective, understanding antidyslipidemic activity of E. americana alone is insufficient since atherosclerosis is a chronic disease with a prolonged and multi-stage development with early signs including the thickening of the aortic wall and foam cell formation. Current literature shows that dyslipidemia disrupts endothelial function by increasing the production of reactive oxygen species. These radicals deactivate NO, a key endothelial relaxation factor, and free radical exposure to endothelial cells then leads to LDL oxidation. Oxidized LDL can be captured by macrophages through scavenger receptors, and when exposed to oxidized LDL, macrophages transform into foam cells, causing cell-to-cell widening [44,45]. Further research is thus required to fully understand the antidyslipidemic activity of specific compounds in E. americana, including their protective effects on atherosclerosis.
Compared to more traditional herbs like garlic, ginger, or ginseng, E. americana is lesser-known. However, current research, including ours, suggests potential benefits for cardiovascular health, with benefits as a vasodilator, anti-inflammatory effects, and antioxidant properties [46,47]. Eleutherine americana also has very low toxic potential. One study found that a 70% ethanol extract of E. americana exhibited an LD50 value exceeding 2,000 mg/kg BW in rats, with no observable toxic signs during administration [48], while another reported an LD50 value greater than 5,000 mg/kg BW in rats and classified it as practically non-toxic [49]. A separate study investigated the use of single and repeated doses of E. americana ethanol extract and found that it did not induce any alterations in clinical, hematological, or biochemical parameters in the rats [50]. Nevertheless, garlic, ginger, and ginseng do still have a more substantial body of clinical evidence supporting their use for cardiovascular health (although the strength and consistency of the evidence vary), while current studies for E. americana are still primarily in animal models and in vitro experiments. More human trials are thus required to establish the efficacy and safety of E. americana.
CONCLUSION
In conclusion, the administration of E. americana extract demonstrated overall improved health in rats induced with an HFC diet, maintaining lipid profiles within normal limits and preventing the occurrence of atherosclerosis and arterial stiffness. These findings suggest that E. americana has a positive impact on cardiovascular conditions, particularly dyslipidemia and arterial stiffness. Nevertheless, while there is great potential for E. americana to be used as a therapeutic agent for CVDs, further research is needed to fully understand its mechanisms of action, safety profile, and optimal dosage.
AUTHOR CONTRIBUTIONS
All authors made significant contributions to the conception and design, acquisition, analysis, and interpretation of data. They participated in drafting or critically revising the article for important intellectual content, collectively agreed to submit it to the current journal, provided final approval for the published version, and committed to being accountable for all aspects of the work. Furthermore, all authors fulfill the eligibility criteria for authorship according to the guidelines set forth by the International Committee of Medical Journal Editors (ICMJE).
FINANCIAL SUPPORT
Internal Grant from the Institute of Research and Community Service, Bhakti Kencana University.
CONFLICT OF INTEREST
The authors declare the absence of financial or any other conflicts of interest pertaining to this work.
ETHICAL APPROVALS
The research protocol complies with ethical standards and has obtained approval from the Ethics and Research Committee of the Faculty of Medicine, Padjadjaran University (letter number 268/UN6.KEP/EC/2021).
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. World Health Organization (WHO). Cardiovascular diseases (CVDs). Who.int [Internet]. Available from: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds)
2. World Health organization (WHO). Prevention of cardiovascular disease guidelines for assessment and management of cardiovascular risk. Geneva, Switzerland: WHO Press; 2007. 45–50 pp.
3. Dipiro JT, Yee GC, Posey LM, Haines ST, Nolin TD, Ellingrod V. Pharmacotherapy A pathophysiologic approach. 11th ed. New York, NY: Mc Graw Hill; 2020.
4. Yue Sun M, Zhang M, Ling Chen S, Ping Zhang S, Yu Guo C, Shang Wang J, et al. The influence of hyperlipidemia on endothelial function of FPN1 Tek-Cre mice and the intervention effect of tetramethylpyrazine. Cell Physiol Biochem. 2018;47:119–28. CrossRef
5. Darwin E, Elfi EF, Decroli E, Elvira D. The relationship between endothelial nitric oxide synthase with dyslipidemia in coronary heart disease. Maced J Med Sci. 2020;8(A):537–42. CrossRef
6. Bonarjee VVS. Arterial stiffness: a prognostic marker in coronary heart disease. Available methods and clinical application. Front Cardiovasc Med. 2018;5:64. CrossRef
7. Si XB, Liu W. Relationship between blood lipid and arterial stiffness in hypertension. Clin Invest Med. 2019;42(3):E47–55. CrossRef
8. Zakaria H, Hasimun P. Non-invasive pulse wave velocity measurement in mice. International Seminar on Sensor, Instrumentation, Measurement and Metrology (ISSIMM); Surabaya, Indonesia; 2017. pp 95–8. CrossRef
9. Kurniati NF, Garmana AN, Sakinah LN. The effect of ethanol extract of Punica granatum Linn. leaves on lipid profiles of dyslipidemic rat. Pharm Sci Res (PSR). 2021;8(2):86–92. CrossRef
10. Ried K. Garlic lowers blood pressure in hypertensive individuals, regulates serum cholesterol, and stimulates immunity: an updated meta-analysis and review. J Nutr. 2016;146(2):389S–96S. CrossRef
11. Hasani H, Arab A, Hadi A, Pourmasoumi M, Ghavami A, Miraghajani M. Does ginger supplementation lower blood pressure? A systematic review and meta-analysis of clinical trials. Phytother Res. 2019 Jun;33(6):1639–47. CrossRef
12. Abdi T, Mahmoudabady M, Marzouni HZ, Niazmand S, Khazaei M. Ginger (Zingiber officinale roscoe) extract protects the heart against inflammation and fibrosis in diabetic rats. Can J Diabetes. 2021;45(3):220–7. CrossRef
13. Ilkhanizadeh B, Shirpoor A, Khadem Ansari MH, Nemati S, Rasmi Y. Protective effects of ginger (Zingiber officinale) extract against diabetes-induced heart abnormality in rats. Diabetes Metab J. 2016;40(1):46–53. CrossRef
14. Febrinda AE, Yuliana ND, Ridwan E, Wresdiyati T, Astawan M. Hyperglycemic control and diabetes complication preventive activities of Bawang Dayak (Eleutherine palmifolia L. Merr.) bulbs extracts in alloxan-diabetic rats. Int Food Res J. 2014;21(4):1405–11.
15. Kamarudin AA, Sayuti NH, Saad N, Razak NAA, Esa NM. Eleutherine bulbosa (Mill.) Urb. Bulb: review of the pharmacological activities and its prospects for application. Int J Mol Sci. 2021;22(6747):1–20. CrossRef
16. Khotimah S, Kalim H, Rohman MS, Soeharto S. Anti-atherosclerotic activity of Eleutherine americana Merr. as the peroxisome proliferated-activated receptor γ agonist: in silico study. Res J Pharm Technol. 2020;13(3):1423–8. CrossRef
17. Khotimah S, Kalim H, Rohman MS, Soeharto S. Anti-atherosclerotic activity of n-Hexane extract of Eleutherine americana Merr. on human macrophage primary cell culture. J Appl Pharm Sci. 2020;10(3):44–51. CrossRef
18. Hasimun P, Zakaria H, Susilawati E, Jeany DW. Antihypertensive activity ethanolic extract of bulb Eleutherine americana merr on fructose-induced hypertension rats. Int J Pharm Pharm Sci. 2017;9(8):25–8. CrossRef
19. Pakki E, Tayeb R, Usmar U, Ridwan IA, Muslimin L. Effect of orally administered combination of Caulerpa racemosa and Eleutherine americana (Aubl) Merr extracts on phagocytic activity of macrophage. Res Pharm Sci. 2020;15(4):401–9. CrossRef
20. Mutiah R, Muzazanah Z, Annisa R, Rachmawati E, Fitrianingsih AA. Comparative metabolite profiling of ethanol and water extracts by UPLC-MS/MS and their cytotoxic effects on T47D cells. J Appl Pharm Sci. 2024;14(04):052–62. CrossRef
21. Balasubramaniam G, Sekar M, Badami S. Pharmacognostical, physicochemical and phytochemical evaluation of Strobilanthes kunthianus (Acanthaceae). Pharmacogn J. 2020 Jun 1;12(4):731–41. CrossRef
22. Balekundri A, Mannur V. Quality control of the traditional herbs and herbal products: a review. Futur J Pharm Sci. 2020 Dec;6(1):1–9. CrossRef
23. Shaikh JR, Patil M. Qualitative tests for preliminary phytochemical screening: an overview. Int J Chem Stud. 2020 Mar 1;8(2):603–8. CrossRef
24. Adusei S, Otchere JK, Oteng P, Mensah RQ, Tei-Mensah E. Phytochemical analysis, antioxidant and metal chelating capacity of Tetrapleura tetraptera. Heliyon. 2019;5(11):1–5. CrossRef
25. Federer WT. Experimental design: theory and application. New York, NY: Macmillan; 1963.
26. Hasimun P, Yani M, Agus S, Dewa AES. Prevention of hypertension and arterial stiffness by combination of Centella asiatica and Curcuma longa in rats. Asian J Biol Sci. 2019;12(2):173–79. CrossRef
27. Hasimun P, Sulaeman A, Hidayatullah A, Mulyani Y. Effect of Curcuma longa L. extract on noninvasive cardiovascular biomarkers in hypertension animal models. J Appl Pharm Sci. 2021;11(8):85–9. CrossRef
28. Niroumand S, Khajedaluee M, Khadem-Rezaiyan M, Abrishami M, Juya M, Khodaee G, et al. Atherogenic index of plasma (AIP): a marker of cardiovascular disease. Med J Islam Repub Iran. 2015 Jul 25;29:240.
29. Arbi S, Bester MJ, Pretorius L, Oberholzer HM. Adverse cardiovascular effects of exposure to cadmium and mercury alone and in combination on the cardiac tissue and aorta of Sprague-Dawley rats. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2021;56(6):609–24. CrossRef
30. Ministry of Health of Indonesia. Indonesian herbal pharmacopeia II: directorate general of pharmaceuticals and medical devices. Jakarta, Indonesia: Ministry of Health of Indonesia; 2017. 1–561 pp.
31. Heitzer T, Schlinzig T, Krohn K, Meinertz T, Münzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104(22):2673–8. CrossRef
32. Wang X, James CKJ, Allan DS, Giora ZF. Assessment of arterial stiffness, a translational medicine biomarker system for evaluation of vascular risk. Cardiovasc Ther. 2008;26(3):214–23. CrossRef
33. Woollett LA, Buckley DD, Yao L, Jones PJH, Granholm NA, Tolley EA, et al. Cholic acid supplementation enhances cholesterol absorptio in humans. Gastroenterology. 2004;126(3):724—31. CrossRef
34. Marie ACB, Terry LS, Patrick MM, Jill MK, Kelly CL, Brandn PB. Pharmacotherapy principles and practice. 5th ed. New York, NY: McGraw-Hill; 2019.
35. Andrew MS, Rikurou H, Madeha A, Nicholas FB, Jenny B, Ole S, et al. Effects of hypothyroidism and high-fat feeding on mRNA concentrations for the low-density-lipoprotein receptor and on acyl-CoA: cholesterol acyltransferase activities in rat liver. Biochem J. 1991;3(3):825–32. CrossRef
36. Linton MRF, Yancey PG, Davies SS, Jerome G, Linton EF, Song WL, et al. The role of lipids and lipoproteins in atherosclerosis. Endotext [Internet]. 2019 January 3 [cited 2021 Dec 28]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK343489/
37. Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109(23):27–32. CrossRef
38. Insanu M, Kusmardiyani S, Hartati R. Recent studies on phytochemicals and pharmacological effects of Eleutherine Americana Merr. Procedia Chem. 2014;13:221–8. CrossRef
39. Bianchi C, Ceriotti G. Chemical and pharmacological investigations of constituents of Eleutherine bulbosa (Miller) Urb. (Iridaceae). J Pharm Sci. 1975;64(8):1305–8. CrossRef
40. Chen DL, Hu MG, Liu YY, Li RT, Yu M, Xu XD, et al. New naphthalene derivatives from the bulbs of Eleutherine americana with their protective effect on the injury of HUVECs. Molecules. 2018;23(9):1–9. CrossRef
41. Paramapojna S, Ganzera M, Gritsanapana W, Stuppner H. Analysis of naphthoquinone derivatives in the Asian medicinal plant Eleutherine americana by RP-HPLC and LC–MS. J Pharm Biomed Anal. 2008;47:990–3. CrossRef
42. Hara H, Maruyama N, Yamashita S, Hayashi Y, Lee KH, Bastow KF, et al. Elecanacin, a novel new naphthoquinone from the bulb of Eleutherine americana. Chem Pharm Bull. 1997;45(10):1714–6. CrossRef
43. Alves TMA, Kloos H, Zani CL. Eleutherinone, a novel fungitoxic naphthoquinone from Eleutherine bulbosa (Iridaceae). Mem Inst Oswaldo Cruz. 2003;98(5):709–12. CrossRef
44. Kopaei MR, Setorki M, Doudi M, Baradaran A, Nasri H. Atherosclerosis: process, indicators, risk factors and new hopes. Int J Prev Med. 2014;5(8):927–46. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/pmc4258672/
45. Eisuke A. Interaction of hyperlipidemia and reactive oxygen species: insights from the lipid-raft platform. World J Cardiol. 2016;8(12):689–94. CrossRef
46. Fikriah I, Ismail S, Kosala K. In vitro evaluation of the vasodilatory activity of ethanol extracts of Eleutherine bulbosa bulbs and leaves. J Appl Pharm Sci. 2021;11(05):135–40. CrossRef
47. Han AR, Min HY, Nam JW, Lee NY, Wiryawan A, Suprapto W, et al. Identification of a new naphthalene and its derivatives from the bulb of Eleutherine americana with inhibitory activity on lipopolysaccharide-induced nitric oxide production. Chem Pharm Bull (Tokyo). 2008;56(9):1314–6. CrossRef
48. Wati H, Muthia R, Kartini, Setiawan F. Acute toxicity study of the ethanolic extract of Eleutherine bulbosa Urb in Wistar rats. Pharm Educ. 2021;21(2):143–7. CrossRef
49. Wahdaningsih S, Untari EK, Robiyanto. Acute toxicity test of ethanolic extract of dayak onion leaves (Eleutherine americana Merr.) toward wistar female rats using OECD 425 method. Dhaka Univ J Pharm Sci. 2019;18(2):171–7. CrossRef
50. Quadros Gomes AR, da Rocha Galucio NC, de Albuquerque KCO, Brígido HPC, Varela ELP, Castro ALG, et al. Toxicity evaluation of Eleutherine plicata Herb. extracts and possible cell death mechanism. Toxicol Rep. 2021;8:1480–7. CrossRef