INTRODUCTION
Roselle (Hibiscus sabdariffa L. (Malvaceae)) is a shrub found mainly in tropical and subtropical areas of India, China, and Southeast Asia [1]. The calyx of roselle contains various phenolic compounds, including hydroxycoumarin, gallic acid, and catechin [2]. Water-soluble pigments, termed anthocyanins, make up 1.7%–2.5% of the dry weight of the roselle calyx [3]. Roselle extract, rich in anthocyanin has been proven to possess anti-oxidative [4] and anti-inflammatory properties, resulting in the reduction of tumor necrosis factor-α (TNF-α), interleukin 6 [5] and nitric oxide (NO) level [6]. Moreover, Roselle extract has been reported to induce apoptosis of human cancer cells including, leukemia, prostate, breast, and gastric cancer [7]. There are also reports of roselle extract suppressing human gastric carcinoma cell (AGS), by inducing cell cycle arrest and apoptosis [8,9].
Dietary fibers consist of indigestible forms of carbohydrate, plant-derived polysaccharides that are divided into soluble dietary fiber and insoluble dietary fiber [10]. They include the non-starch polysaccharides (cellulose, inulin, pectin [11], konjac glucomannan (KGM) [12]), analogous carbohydrates (methyl cellulose, hydroxypropyl methylcellulose (HPMC), and resistant starches) [13] and a seaweed-derived dietary fiber (alginate) [14]. Dietary fibers are attractive option for the new generation of environmentally sustainable drug delivery due to their biodegradability and low toxicity. They have been reported to be effective in site-specific drug delivery and controlled release applications in transdermal, inhalable, and gastrointestinal drug delivery [15]. The highly viscous nature of hydrated dietary fiber-based formulations leads to prolonged release of the drug and may protect the drug against adverse gastrointestinal conditions.
Gastroretentive floating systems are invented to prolong drug release in the stomach, thereby resulting in reduced dosing frequency and increased patient compliance [16]. One such design combines in situ gel-forming polymers (e.g., alginate and pectin) and CO2-generating agents (e.g., calcium carbonate or sodium bicarbonate). The gas bubbles are entrapped in the gel formed on contact with gastric fluid, resulting in the production of a floating raft structure [17]. Sodium alginate (SA), derived from brown algae and composed of L-guluronic and D-mannuronic acid, is the most commonly used polymer for in situ gelling formulations [17]. Alginate displays pH-sensitive gelation on contact with acid fluids and precipitates as alginic acid [18]. Crosslinking of alginate hydrogels by divalent metal cations to enhance gel strength and density provides a further advantage of this particular polysaccharide.
The purpose of this research was to formulate in situ gelling systems based on dietary fiber and incorporating roselle extract to prolong gastric delivery of anthocyanin. The stomach and small intestine are the principal sites of anthocyanin absorption [19,20]. The dietary fiber comprised SA, hydroxyethyl cellulose (HEC), HPMC, sodium carboxymethyl cellulose (SCMC), and KGM. SA was utilized as the gel-forming polymer, the others provided a release rate modifying function. SCMC, HEC, and HPMC are widely used as binders, disintegrants, mucoadhesive, and film-coating agents in oral dosage forms. KGM is employed as a gel-forming and release-retarding agent. Sodium bicarbonate was included as a flotation agent to generate CO2 on contact with gastric fluid. The anti-oxidative activity of the formulations was assessed using a 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging method. Anti-inflammatory activity was assessed by investigating their effect on NO production by lipopolysaccharide (LPS)-stimulated RAW264.7 murine macrophage cells. The anticancer potential was evaluated by measuring the cytotoxic effect of the formulations on AGS cell lines.
MATERIALS AND METHODS
Materials
Roselle extract was purchased from TCS Pacific Co., Ltd. (Bangkok, Thailand). SA, 2% w/v solution at 25?C = 2,000 cPs was from High Science Co., Ltd. (Songkhla, Thailand). SCMC, 1% w/v solution at 25?C = 1,960 cPs and HEC, 1% w/v solution at 25?C = 5,000 cPs were obtained from Krungthepchemi Co., Ltd. (Bangkok, Thailand). HPMC K4M, viscosity of 1% w/v solution at 25?C = 4,000 cPs was provided by Colorcon Asia Pacific Ltd. (Singapore). KGM was sourced from Asian bioplex Co., Ltd. (Chonburi, Thailand). Butylated hydroxytoluene (BHT) was purchased from Sigma Aldrich (Buchs, Switzerland) and ascorbic acid was provided by Chemsupply (Adelaide, Australia).
RAW264.7 cells (TIB-71TM, murine macrophage cell line) and AGS cells (gastric adenocarcinoma cell line) were obtained from the American Type Culture Collection, ATCC (VA, USA). LPS, from Escherichia coli, indomethacin, Griess reagent, and DPPH were from Sigma Aldrich (Missouri, USA). Fetal bovine serum (FBS), Kaighn’s Modification of Ham’s F-12 Medium (F12K medium), Roswell Park Memorial Institute (RPMI)- 1640 medium, 3-(4,5-dime-thyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide (MTT), phosphate buffer saline (PBS), trypsin EDTA 0.25%, trypan blue solution and penicillin-streptomycin were provided by Gibco (Invitrogen, USA). Dimethyl sulfoxide (DMSO) was sourced from Amresco (OH, USA).
Formulation of in situ gelling systems of roselle extract
In situ gel formulations were prepared using the procedure described by Fungfoung et al. [21]. The composition of the formulations is shown in Table 1. SA was used as the gel-forming polymer, whereas SCMC, HEC, HPMC, and KGM were used to modify anthocyanin release rate. The formulations were prepared by dissolving 0.5, 0.75, and 1 g, respectively, of SA in 75 ml deionized (DI) water with (F8-F19) or without (F1, F2, F3) 0.25 g of SCMC, HEC, HPMC or KGM, respectively. The mixture was stirred by magnetic stirring to produce clear solution. Sodium bicarbonate (CO2 generating agent) was added (0.2 g), followed by roselle extract (5 g) and mixing was continued to obtain a homogenous liquid. The volume was adjusted to 100 ml with DI water and the formulations were stored in air-tight glass containers and protected from light at room temperature before use.
 | Table 1. Composition of in situ gelling liquid formulations containing roselle extract. [Click here to view] |
Physicochemical characterization of in situ gelling systems of roselle extract
Appearance
The homogeneity and color of the liquid formulations were evaluated by visual observation.
pH measurements
The pH of each formulation was determined using a digital pH tester (Mettler Toledo, Switzerland) at room temperature (25?C ± 1?C). Before testing, 1 g of the liquid formulation was weighed, diluted to 100 ml of DI water, and stirred to obtain a homogenous solution. Each measurement was performed in triplicate and the data were expressed as mean ± S.D.
Viscosity measurements
The viscosity of formulations was determined at 25?C ± 1?C by a Brookfield digital rheometer (DVIII, Ametek Brookfield, USA) with spindle No. 64 at rotation speeds between 10 and 50 rpm. The viscosity measurements were performed for triplicate samples.
Gel forming behavior
The gel-forming behavior of liquid formulations was investigated on contact with simulated gastric fluid (SGF) (0.1 N HCl, pH 1.2) and phosphate (PBS, pH 7.4) [21]. Briefly, 35 g of liquid formulation was added to 150 ml SGF or PBS with mild agitation and gelation was characterized in terms of gelling time and appearance of the formed gel.
Density of the formed gel
The gel density was determined using the method described by Fungfoung et al. [21]. Briefly, SGF (75 ml) was transferred to a 100 ml measuring cylinder and weighed (W1). The liquid formulation (10 g) was then added into the cylinder and allowed to form a floating gel for 30 minutes. After that, the final weight of the cylinder and final content volume were determined as W2 and V, respectively. The density of the formed gel (D) was calculated using the following equation:
D = W2 - W1 / V-75 g/cm3 (1)
Gel floating behavior
In situ gel formulation (35 ml) was introduced into SGF (900 ml) contained in a glass vessel (PTWS 120D, Pharma Test, Germany) at 37?C [21]. The length of time that the formed gel took to reach to the surface of the acidic SGF (floating lag time) and the duration of floating on the medium surface (floating time) was measured. Each experiment was performed in triplicate.
Gel strength
The strength of the formed gel was investigated using TA. XT plus texture analyzer (Stable Microsystems, United Kingdom) following the method of Issarachot et al. [22] with minor modification. Briefly, in situ gel formulation (20 ml) was gently added to 150 ml of SGF in a 250 ml beaker at 37?C. After 30 minutes, the resulting floating gel was placed in a Petri dish and compressed with a stainless-steel cylindrical probe (25 mm diameter; 2.00 mm/second pre-test, 1.00 mm/second test, 10.00 mm/second post-test speeds). The load (gm) required to rupture the gel was recorded. Each sample was tested in triplicate and results were reported as mean ± S.D.
Analysis of total anthocyanin content (TAC)
The total amount of anthocyanin contained in the liquid formulations was measured according to the pH differential method [23], which was based on the reversible structural changes of anthocyanins at pH 1.0 and 4.5. Each liquid formulation was mixed with potassium chloride buffer (0.025 M, pH 1) and sodium acetate buffer (0.4 M, pH 4.5), respectively, and allowed to stand for 15 minutes before measuring the absorbance at 520 and 700 nm using a UV–vis spectrophotometry (UV-1900i, SHIMADZU Corporation, Japan). The absorbance of the sample (A) was calculated by the following equation:
A = (A520–A700) pH 1.0 – (A520–A700) pH 4.5 (2)
The TAC of the formulations was calculated by the following equation:
TAC (mg/l) = (A × MW × DF × 103) / (ε × 1) (3)
where MW is the molecular weight of cyanidin-3-glucoside (449.2 g/mol), DF is the dilution factor (pathlength = 1 cm), and ε is the molar absorptivity calculated as cyanidin-3-glucoside (26,900 l/mol).
In vitro release behavior of anthocyanin from formulations in SGF
The release of anthocyanin from formulations was tested using a USP-type-II paddle dissolution apparatus (PTWS 120D, Pharma Test, Germany) at 37?C and a rotation speed of 50 rpm. Individual samples of liquid formulation (35 ml) containing roselle extract were added to 900 ml of SGF and 5 ml were collected at 30, 60, 120, 180, 240, 300, 360, 420, and 480 minutes and replaced with fresh medium. The anthocyanin content was determined by UV-vis spectrophotometry (as mentioned in section 2.4). The drug release profiles were expressed as a plot of cumulative anthocyanin release versus time. Each sample formulation was determined in triplicate.
The percentage gel weight remaining at corresponding release times of 30, 60, 120, 180, 240, 300, 360, 420, and 480 minutes was investigated separately using the following equation:
% Gel weight remaining = (Wt) / Wi) × 100 (4)
where Wi is the initial weight of gel and Wt is the gel weight at predetermined time.
Antioxidant activity
The antioxidant activity of formulations was determined according to DPPH radical scavenging assay [5]. Roselle extract, in situ gel liquid formulation and liquid formulation without roselle extract (blank) were diluted to various concentrations (3.125 to 100 µg/ml). Test samples (20 µl) were mixed with 180 µl of 0.1 mM DPPH radical solution (in absolute methanol) in a 96-well plate. The mixture was retained in the dark at room temperature for 30 minutes, before measuring the absorbance at 515 nm on a SPECTROstar Nano microplate reader (BMG LABTECH, Germany). BHT and ascorbic acid were used as a positive standard. The percentage inhibition of scavenging activity was calculated by the following equation:
% Inhibition = [(Ac - As) / Ac] x 100 (5)
where Ac is the absorbance of control and As is the absorbance of sample.
Cytotoxic activity
The cytotoxicity activity of liquid formulations containing roselle extract, blank formulations, and non-formulated roselle extract was investigated using RAW264.7 macrophages and AGS cells, respectively, in combination with the MTT assay [21,24]. RAW264.7 cells were grown in the RPMI medium while AGS cells were grown in F12K medium. Both cell preparations were supplemented with 10% FBS and 1% Pen/Strep. RAW264.7 cells were seeded in a 96-well plate at a density of 5 × 104 cells/well, while AGS were seeded at 1 × 105 cells/well. Test samples were diluted with sterile DI water to achieve anthocyanin concentrations in the range 2.5–10 µg/ml, before adding to the cells, which were subsequently incubated at 37?C and 5% CO2 atmosphere for 24 hours. Indomethacin was used as the positive control. MTT solution (50 µl, 0.5 mg/ml) was added to each well and further incubated for 4 hours. The supernatant was removed and the formazan crystals were dissolved in DMSO. The absorbance of the resulting solution was measured after 4 hours at wavelength 570 nm using a SPECTROstar Nano microplate reader. The percentage cell viability was determined as follows:
% Cell viability = (absorbance of sample / absorbance of control) × 100 (6)
Investigation of anti-inflammatory activity
The anti-inflammatory activity of formulations incorporating roselle extract, blank formulations, and roselle extract was investigated by measuring the effect on NO production by LPS-stimulated RAW264.7 cells [25–27]. Cells were seeded at a density of 7 × 105 cells/well in 96-well plates and exposed to test samples containing anthocyanin concentrations in the range 2.5 to 10 µg/ml, with or without 0.1 µg/ml LPS at 37?C, under humidified 5% CO2 atmosphere. After 24 hours, the supernatant was transferred to separate 96-well plates for indirect measurement of NO production. Griess reagent was added to each well for reaction with nitrite to form an azo compound measurable at 570 nm using a SPECTROstar Nano microplate reader. NO production was quantified using the following equation:
% Inhibition = [(A–B) / (A–C)] × 100 (7)
where (A–C): NO2- concentration (µM) (A: LPS (+) and sample (-), B: LPS (+) and sample (+) and C: LPS (-) and sample (-)).
Statistical analysis
Results were expressed as mean ± standard deviation (S.D.). Statistical analyses were performed using Student’s t-test and one-way analysis of variance. Statistical probability (p) values <0.05 were considered to denote significant differences.
RESULTS AND DISCUSSION
Physicochemical characterization of in situ gelling systems of roselle extract
Appearance, pH and viscosity
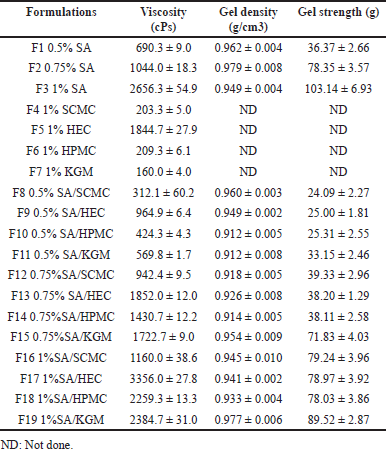
The in situ gelling liquids were dark red/purple due to the color of anthocyanin in the roselle extract (Fig. 1A). In order to maintain the stability of rosella extract and to confirm the formation of the gel after contact with acidic medium, the pH of liquid formulations were measured. The pH was in the range of 4.52–4.89 and no significant difference was found between the formulations, indicating no effect of the type or concentration of the polymer excipients.
The viscosity of liquid formulations based on SA alone increased significantly from 690 to 2,656 cPs with increasing SA concentration from 0.5% to 1% w/v (F3 > F2 > F1, p < 0.001). Liquid formulations prepared using only the release rate-controlling polymers (1% w/v) without SA exhibited significantly higher viscosity for HEC (1,844 cPs), compared with very low, similar values for SCMC (203 cPs), HPMC (209 cPs), and KGM (160 cPs). The viscosity of liquid formulations prepared by combining SA gel-forming polymer and release rate-controlling polymers (0.25% w/v) was also investigated. At low SA conc (0.5% w/v), the inclusion of SCMC, HPMC, or KGM tended to reduce the viscosity (F1, F8, F10, F11) (Table 2), indicating disruption of the entangled chain network of the alginate phase. However, the combination of the higher viscosity HEC solution with SA increased the viscosity of the mixture to 965 cPs (F1, F9, p < 0.001). At intermediate SA concentration (0.75%w/v), the addition of HEC, HPMC, or KGM generally resulted in a large increase in viscosity (F2, F13, F14, F15), suggesting an interaction between the gel-forming and release-controlling components, resulting in resistance to flow. In this study, the addition of SCMC to all concentrations of SA lowered the viscosity of the resulting solution. SCMC is an anionic, hydrophilic cellulose polymer synthesized by partial substitution of the cellulose hydroxyl groups by carboxymethyl groups. SCMC has been reported to induce change in the interaction between alginate chain [28]. Thereby, the viscosity reduction might result from the less interaction between alginate molecules. The combination of SA with HPMC or KGM showed a similar pattern for increasing the viscosity of the liquid formulations. The enhancing viscosity can be affected by the hydrogen bond formation between SA and HPMC or KGM led to the tightening of the formulations [29,30]. In the case of HEC, it is a non-ionic of cellulose derivative polymer. HEC has many OH group in the structure that can interact with other combine polymers [31]. Hence, the formulations F9, F13, and F17 showed a dramatic increase in viscosity. At a high SA concentration (1% w/v), the addition of HEC caused a great increase in viscosity from 2,656 to 3,356 cPs (F3, F17).
 | Table 2. Physicochemical properties of in situ gelling liquid formulations. [Click here to view] |
The viscosity of all liquid formulations decreased with increasing shear rate, indicating pseudoplastic or shear thinning behavior (Fig. 2). Shear thinning is regarded as an advantageous feature of liquid pharmaceutical formulations since it assists pouring of viscous medication and the dispersion of sedimented particulates [25].
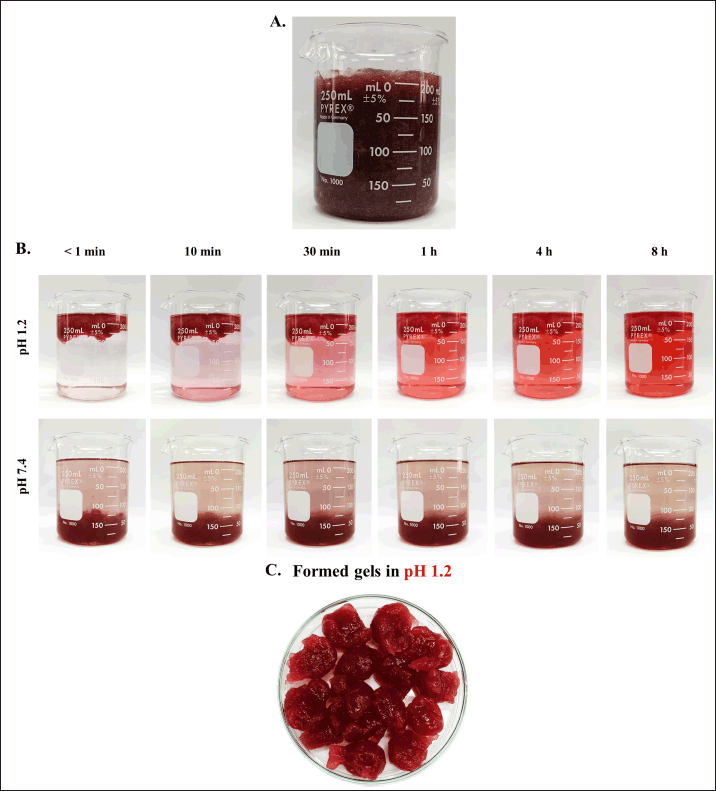 | Figure 1. (A) Appearances of in situ gelling liquid formulation prepared from 1% w/v SA, 0.25% KGM and containing roselle extract, (B) Gel floating behavior in SGF (pH 1.2) and PBS (pH 7.4) and (C) Gel formed at pH 1.2. [Click here to view] |
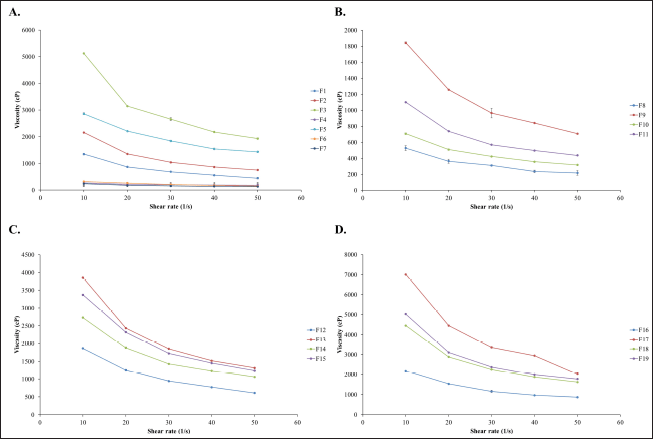 | Figure 2. Rheograms of in situ gelling liquid formulations containing roselle extract. (A) 0.5, 0.75, and 1% w/v SA, (B) 0.5% w/v SA combined with 0.25% SCMC, HEC, HPMC or KGM, (C) 0.75% w/v SA combined with 0.25% SCMC, HEC, HPMC or KGM and (D) 1% w/v SA combined with 0.25% SCMC, HEC, HPMC or KGM. [Click here to view] |
Gel properties
Gels were formed instantaneously on exposure of liquid formulations to SGF (pH 1.2) due to the formation of alginic acid [18], but not on exposure to PBS (pH 7.4) (Fig. 1B). pH-responsive gelation of SA has been exploited widely for extending drug release in the stomach [31]. Bharati et al. [31] for example, prepared pH-responsive raft-gel forming delivery system for controlled release of famotidine to treat gastric ulcers.
Gel floatation was induced by the entrapment of CO2 bubbles within the gel matrix following a reaction between sodium bicarbonate and SGF. Gels formed by liquid formulations based on SA alone (0.5% and 0.75% w/v) exhibited extremely short floating lag times below 3 seconds (Table 2), whereas all other formulations floated in around 5 seconds. All formulations remained afloat for more than 8 hours.
The density of gels was measured in the range of 0.912 to 0.979 g/cm3 which is lower than the density of SGF (1.004 g/cm3) [32]. Thus, the formulations are potentially suitable for application as gel-forming, gastroretentive delivery devices.
Gel strength
The practical functioning of floating gels containing roselle extract as a gastroretentive drug delivery system is governed by their ability to withstand the pressure generated during gastric contraction. The strength of gels formed on contact with the SGF is presented in Table 2. Formulations prepared using only the release rate-controlling polymers (SCMC, HEC, HPMC, and KGM) were not tested since they did not form gels in SGF). Gels formed from liquid formulations containing only the gel-forming polymer SA, showed an almost three-fold increase in strength from 36.4 to 103.1 g with increasing SA concentration from 0.5% to 1% w/v (Table 2), reflecting the higher density of the gel network.
The addition of release rate controlling polymers (SCMC, HEC, or HPMC) to low concentration (0.5% w/v) SA solution reduced the gel strength by around 35%, whereas KGM had only a minor effect. In the case of liquid formulations based on intermediate concentration SA (0.75% w/v), gel strength was reduced significantly by a factor of gelation disturbance on the introduction of SCMC, HEC, or HPMC, indicating severe disruption and weakening of the gel structure [25]. In contrast, KGM resulted in only a 9% loss of strength. At high SA concentration (1% w/v), the gel strength was reduced by around 20% when all release rate-controlling polymers were introduced into the formulation. As can be seen from the data, the gel strength depended on the concentration of SA that formed the gel after contact with an acidic solution. While added controlling polymers tend to reduce the gel strength. These results indicated that the controlling polymers interfere with the gel formation by hindering the formation of the gel network between SA and the ion. Usually, the cellulose derivatives polymers will form a gel after heating the polymer solution. However, in our process, the formulations were performed without boiling process [33]. However, compared among the polymers, KGM had less effect. These results evidenced that the combination of KGM and SA promotes dense gel formation [29].
Release behavior of anthocyanin from formulations in SGF
The anthocyanin content of all formulations was found to be above 95% (95.3–97.5) which is within accepted pharmacopeia limits. The stomach and small intestine are the principal sites of anthocyanin absorption [19,20]. Thus, the prolonged release of anthocyanin from the floating gels in the stomach is anticipated to be of utility for improving systemic bioavailability or local action against gastric disease.
The process of gradual release of anthocyanin from the gel formed by liquid formulation F19 (1% SA/0.25% KGM) is illustrated by the color change of the SGF medium in Figure 1. The composition of the liquid formulation was found to exhibit a significant effect on anthocyanin release (Fig. 3); in general release of anthocyanin was impeded by high SA concentration (Fig. 3A). Formulations prepared using low SA concentration (0.5% w/v), without release rate controlling polymers, exhibited an initial burst release characteristic with more than 90% of the anthocyanin content delivered within 60 minutes., Increasing the SA concentration to 0.75% and 1% w/v significantly reduced the initial burst release to around 60% and 50%, respectively. Almost the total release of anthocyanin, occurred in 8 hours. Formulations based on 0.75% w/v SA confined burst release to below 70% and delivered over 90% of the anthocyanin load in 8 hours. Those prepared using higher SA concentration (1%) restricted the burst release phase to below 50% while maintaining high delivery efficiency. The retard release of drug molecules was related to the concentration of SA. This could be explained by the increment of polymer matrix density that affected to the diffusion process [34].
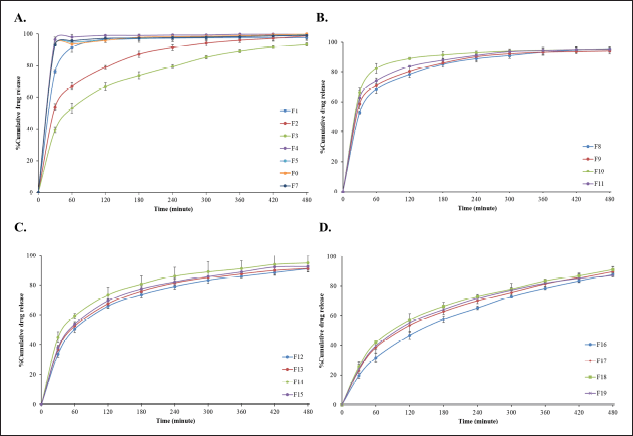 | Figure 3. In vitro release profiles of anthocyanin from in situ gelling, liquid formulations in 0.1 N hydrochloric acid (pH 1.2) at 37?C. (A) SA concentration 0.5, 0.75 and 1% w/v, (B) 0.5% w/v SA combined with 0.25% SCMC, HEC, HPMC or KGM, (C) 0.75% w/v SA combined with 0.25% SCMC, HEC, HPMC or KGM and (D) 1% w/w SA combined with 0.25% SCMC, HEC, HPMC or KGM. Data reported as mean ± S.D. (n = 3). [Click here to view] |
The inclusion of SCMC, HEC, HPMC, or KGM in the liquid formulation with SA resulted in a transition from burst release to gradual release of anthocyanin, reflecting the significant influence of the release-rate controlling component (Fig. 3B–D). When comparing various concentrations of SA mixed with each concentration in the same ratio of SCMC, HEC, HPMC, and KGM, indicating that the release delaying effect was HPMC < KGM < HEC < SCMC, respectively. HPMC, a medium-viscosity polymer showed less impact to the release profiles of anthocyanin. This might be due to the faster erosion of the polymer [35]. The slow-release behavior of anthocyanin from SA combined HEC or SCMC had a similar pattern. It has been reported that the combination of SA and cellulose derivatives such as HEC improved the mechanical properties of the blended polymer [36].
In case of SA combined KGM, KGM exhibits a delayed release of drug in SGF by diffused release from a porous gel matrix [37] and related to its characteristics such as viscosity and swelling behavior of KGM [38]. Formulation containing KGM exhibited the burst release (around 30%) followed by gradual release of anthocyanin throughout 8 hours. Hence, the formualtion contain KGM , F19 was selected as the suitable formulation for further testing because it exhibited good physicochemical properties, strong gel hardness, and sustained anthocyanin release.
Gel degradation and break down in SGF
Mass loss and breakdown behavior is important for gastroretentive dosage forms to ensure clearance from the stomach once drug delivery is complete. Liquid formulations containing SA gel-forming polymer alone at concentrations of 1, 0.75%, and 0.5% w/v resulted in 70%, 65%, and 30% mass remaining after 8 hours exposure to SGF (Fig. 4A). Interestingly, the addition of release rate modifying polymers gave rise to weight remaining profiles (Fig. 4B–D) which were essentially inversions of the anthocyanin release profiles (Fig. 3). Liquid formulations containing SA and SCMC generally exhibited the highest mass loss. Liquid formulations which combined SA at low concentration (0.5%) with SCMC, HEC, HPMC, or KGM exhibited rapid mass loss of 50% to 70% in 1 hour before plateauing (Fig. 4B). Formulations based on higher SA concentrations (0.75% and 1%) resulted in lower mass loss of 30%–50% and 30%–40% in 8 hour, probably reflecting the higher density of the gel network (Fig. 4C–D).
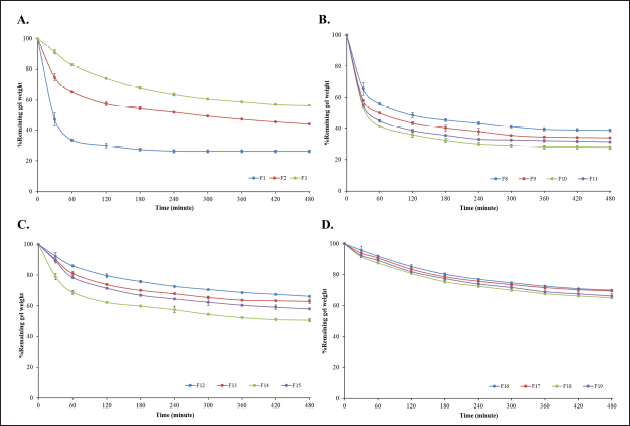 | Figure 4. Remaining gel weight following exposure of in situ gelling, liquid formulations to 0.1 N hydrochloric acid (pH 1.2) at 37?C. (A) 0.5, 0.75 and 1% w/v SA, (B) 0.5% w/v of SA combined with 0.25% SCMC, HEC, HPMC or KGM, (C) 0.75% w/v SA combined with 0.25% SCMC, HEC, HPMC or KGM and (D) 1% w/v SA combined with 0.25% SCMC, HEC, HPMC or KGM. Data reported as mean ± S.D (n = 3). [Click here to view] |
Mass loss behavior may be explained by diffusion of anthocyanin and erosion and dissolution of the polymer components of the hydrogel in SGF. The gastric environment in vivo is expected to support both mechanisms, accompanied by chemical/enzymatic breakdown of the polymer chains in the formulation [39].
Antioxidant activity of in situ gelling formulations containing roselle extract
Anthocyanin has been reported to display free radical scavenging properties, which are a broad mechanism of antioxidants. Anthocyanins can provide hydrogen bonds with radicals, which can then be reduced by free radicals [40]. Measurement of DPPH free radical scavenging activity was employed to investigate the potential antioxidant activity of in situ gelling liquid formulations containing roselle extract. Formulation, F19 (1% SA/0.25% KGM) was selected for evaluation due to good in vitro anthocyanin release and high gel strength. The IC50 values of DPPH radical scavenging activity for non-formulated roselle extract, F19, and unloaded (blank) F19 formulation were measured as 1.30, 1.31, and >500 μg/ml, respectively (Table 3). Radical scavenging BHT and ascorbic acid standards resulted in IC50 values of 11.72 and 4.72 μg/ml, respectively. These findings indicate that in situ gel formulations containing roselle extract produce an antioxidant effect with no activity arising due to the vehicle.
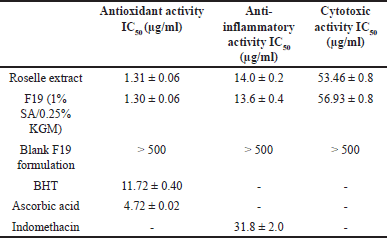 | Table 3. Antioxidant, anti-inflammatory and cytotoxic activity of in situ gelling liquid formulations containing roselle extract. [Click here to view] |
In vitro cytotoxic behavior
The anticancer activity on AGS cells of roselle extract is considered to result of two main signaling mechanisms that induce apoptosis mediated by via p53 phosphorylation and p38 MAPK/FasL cascade pathway [8] and via JNK/p38 signaling [9].
The MTT assay was employed to assess the cytotoxic effect of formulations containing roselle extract (F19) against AGS cells. Non-formulated roselle extract, F19 formulation, and F19 blank gave rise to IC50 values of 53.46, 56.93, and > 500 μg/ml, respectively (Table 3). These findings support earlier reports of H. sabdariffa L’s anticancer activity through induction of cell cycle arrest and apoptosis [7–9].
Anti-inflammatory effect
The cell viability of RAW264.7 cells following exposure to formulation (F19) and non-formulated roselle extract is presented in Figure 5. Cell viability remained above 80% for RAW264.7 cells exposed to anthocyanin concentrations below 10 μg/ml. AGS cells retained >80% viability when exposed to concentrations < 25 μg/ml). These limits were applied when testing the anti-inflammatory effect of formulations.
F19 formulation and non-formulated roselle extract inhibited NO production by LPS-activated RAW264.7 cells with presented IC50 value of 14.0 and 13.6 μg/ml, respectively, whereas the blank F19 formulation showed very weak activity with IC50 > 500 μg/ml. The NSAID, indomethacin, utilized as the control resulted in an IC50 value of 31.8 μg/ml (Table 3). Anthocyanin from roselle extract and F19 showed greater decreased NO production that is directly related to the effect of lower pH from organic acids in extracts [2] which has an influence on the action of anthocyanin on inhibiting NO production [6]. In addition, anthocyanin inhibits the nuclear translocation of nuclear factor kappa B that suppressing of pro-inflammatory expression of cycloxygenase-2, TNF-α, and inducible nitric oxide synthase [41,42] which are the enzymes related to NO production.
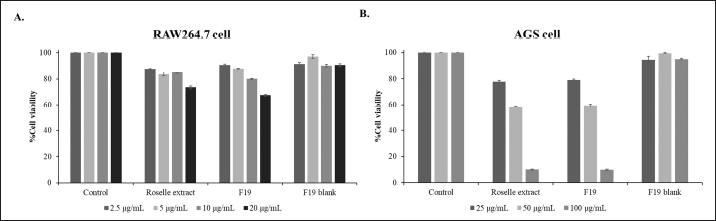 | Figure 5. Cell viability of (A) RAW264.7 cells and (B) AGS cells following incubation with Formulation F19 (1% SA/0.25% KGM), F19 blank and roselle extract for 24 hours. Results expressed as a percentage of cell viability (n = 3). [Click here to view] |
CONCLUSION
Dietary fiber-based floating in situ gel formulations containing roselle extract were developed as gastroretentive devices. Formulation based on 1% SA as the gel-forming polymer combined with 0.25% SCMC, HEC, HPMC, or KGM as the release rate modifier floated rapidly on contact with SGF in less than 5 seconds and over 80% of the anthocyanin content was gradually released in SGF over 8 hours. Formulations containing 1% w/v SA combined with 0.25% KGM displayed antioxidant activity using the DPPH assay, anti-inflammatory activity against LPS-activated RAW264.7 cells and cytotoxic behavior against AGS cells. These findings reveal the capable of KGM combined with SA as an alternative carrier to prolong the release of water-soluble natural compounds such as anthocyanin in the stomach. The formulations also demonstrate the potential benefits for the treatment of gastric diseases such as gastric ulcers and gastric cancer.
ACKNOWLEDGMENTS
Financial support from National Science, Research and Innovation Fund (NSRF), and Prince of Songkhla University (Grant No. PHA6601187S).
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took Fart in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Iglesias I, Echeverría G, Lopez M. Fruit color development, anthocyanin content, standard quality, volatile compound emissions and consumer acceptability of several ‘Fuji’apple strains. Sci Hortic. 2012;137:138–47. CrossRef
2. Izquierdo-Vega JA, Arteaga-Badillo DA, Sánchez-Gutiérrez M, Morales-González JA, Vargas-Mendoza N, Gómez-Aldapa CA, et al. Organic acids from roselle (Hibiscus sabdariffa L.)-a brief review of its pharmacological effects. Biomedicines. 2020;8:100. CrossRef
3. Da-Costa-Rocha I, Bonnlaender B, Sievers H, Pischel I, Heinrich M. Hibiscus sabdariffa L.-a phytochemical and pharmacological review. Food Chem. 2014;165:424–43. CrossRef
4. Wu HY, Yang KM, Chiang PY. Roselle anthocyanins: antioxidant properties and stability to heat and pH. Molecules. 2018;23:1357. CrossRef
5. Janson B, Prasomthong J, Malakul W, Boonsong T, Tunsophon S. Hibiscus sabdariffa L.calyx extract prevents the adipo-genesis of 3T3-L1 adipocytes, and obesity-related insulin resistance in high-fat diet-induced obese rats Hibiscus sabdariffa L. calyx extract prevents the adipogenesis of 3T3-L1 adipocytes, and obesity-related insulin resistance in high-fat diet-induced obese rats. Biomed Pharmacother. 2021;138:111438. CrossRef
6. Pinsirodom P, Parinyapatthanaboot H, Taprap R, Kaewthong P. The in vitro scavenging ability of anthocyanin extracts from roselle calyces against reactive nitrogen species and their potential use for nitrite reduction in meat. Curr Res Nutr. 2019;7:340–49. CrossRef
7. Laskar YB, Mazumder PB. Insight into the molecular evidence supporting the remarkable chemotherapeutic potential of Hibiscus sabdariffa L. Biomed Pharm. 2020;127:110153. CrossRef
8. Lin HH, Huang HP, Huang CC, Chen JH, Wang CJ. Hibiscus polyphenol-rich extract induces apoptosis in human gastric carcinoma cells via p53 phosphorylation and p38 MAPK/FasL cascade pathway. Mol Carcinog. 2005;43:86–99. CrossRef
9. Lin HH, Chen JH, Kuo WH, Wang CJ. Chemopreventive properties of Hibiscus sabdariffa L. on human gastric carcinoma cells through apoptosis induction and JNK/p38 MAPK signaling activation. Chem Biol Interact. 2007;165:59–75. CrossRef
10. Yin Z, Wang Z, He Z, Zeng M, Qin F, Chen J. Effect of particle size and microstructure on the physical properties of soybean insoluble dietary fiber in aqueous solution. Food Biosci. 2021;41:100898. CrossRef
11. Sinha AK, Kumar V, Makkar H, Boeck GD, Becker K. Non-starch polysaccharides and their role in fish nutrition-a review. Food Chem. 2011;127:1409–26. CrossRef
12. Guo L, Yokoyama W, Chen M, Zhong F. Konjac glucomannan molecular and rheological properties that delay gastric emptying and improve the regulation of appetite. Food Hydrocoll. 2021;120:106894. CrossRef
13. Jones J. Update on defining dietary fiber. Cereal Foods World. 2000;45:219–20.
14. Brownlee IA, Allen A, Pearson JP, Dettmar PW, Havler ME, Atherton MR, et al. Alginate as a source of dietary fiber. Crit Rev Food Sci Nutr. 2005;45:497–510. CrossRef
15. Kumar S, Maliviya R. Dietary fibers and their derivatives for drug delivery applications: advances and prospective. J Drug Deliv Sci Technol. 2023;89:105084. CrossRef
16. Vrettos NN, Roberts CJ, Zhu Z. Gastroretentive technologies in tandem with controlled-release strategies: a potent answer to oral drug bioavailability and patient compliance implications. Pharmaceutics. 2021;13:1591. CrossRef
17. Prajapati VD, Jani GK, Khutliwala TA, Zala BS. Raft forming system—an upcoming approach of gastroretentive drug delivery system. J Controlled Release. 2013;168:151–65. CrossRef
18. Ayarza J, Coello Y, Nakamatsu J. SEM–EDS study of ionically cross-linked alginate and alginic acid bead formation. Int J Polym Anal Charact. 2016;22:1–10. CrossRef
19. Talavera S, Felgines C, Texier O, Besson C, Lamaison JL, Remesy C. Anthocyanins are efficiently absorbed from the stomach in anesthetized rats. J Nutr. 2003;133:4178–82. CrossRef
20. Felgines C, Texier O, Besson C, Lyan B, Lamaison JL, Scalbert A. Strawberry pelargonidin glycosides are excreted in urine as intact glycosides and glucuronidated pelargonidin derivatives in rats. Br J Nutr. 2007;98:1126e1131. CrossRef
21. Fungfoung K, Praparatana R, Issarachot O, Wiwattanapatapee R. Development of oral in situ gelling liquid formulations of garcinia extract for treating obesity. Gels. 2023;9:660. CrossRef
22. Issarachot I, Bunlung S, Kaewkroek K, Wiwattanapatapee R. Superporous hydrogels based on blends of chitosan and polyvinyl alcohol as a carrier for enhanced gastric delivery of resveratrol. Saudi Pharm J. 2023;31:335–47. CrossRef
23. Lee J, Durst RW, Wrolstad RE. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: collaborative study. J AOAC Int. 2005;88:1269–78. CrossRef
24. Kaewkroek K, Tewtrakul S, Wiwattanapatapee R. Development of expandable, gastro-retentive films for delivery of resveratrol and evaluation of cytotoxic and anti-inflammatory activity. Lat Am J Pharm. 2019;38:691–700. CrossRef
25. Bunlung S, Nualnoi T, Issarachot O, Wiwattanapatapee R. Development of raft-forming liquid and chewable tablet formulations incorporating quercetin solid dispersions for treatment of gastric ulcers. Saudi Pharm J. 2021;29:1143–54. CrossRef
26. Siripruekpong W, Issarachot O, Kaewkroek K, Wiwattanapatapee R. Development of gastroretentive carriers for curcu-min-loaded solid dispersion based on expandable starch/chitosan films. Molecules. 2023;28:361. CrossRef
27. Wiwattanapatapee R, Yaoduang T, Bairaham M, Pumjan S, Leelakanok N, Petchsomrit A. The development of expandable films based on starch and chitosan for stomach-specific delivery of quercetin solid dispersions. J Drug Deliv Sci Technol. 2024;95:105631. CrossRef
28. Zheng J, Kan J. Effects of sodium carboxymethyl cellulose on rheological properties and gelation behaviors of sodium alginate induced by calcium ions. LWT Food Sci Technol. 2019;103:131–13. CrossRef
29. Jiang S, Shang L, Liang H, Li B, Li J. Preparation of konjac glucomannan/xanthan gum/sodium alginate composite gel by freezing combining moisture regulation. Food Hydrocoll. 2022;127:107499. CrossRef
30. Amjadi S, Nouri S, Yorghanlou RA, Roufegarinejad L. Development of hydroxypropyl methylcellulose/sodium alginate blend active film incorporated with Dracocephalum moldavica L. essential oil for food preservation. J Thermoplast Compos Mater. 2020;35:2354–70. CrossRef
31. Al-Mamari A, Shahitha F, Al-Sibani M, Al-Saadi A, Al-Harrasi A, Ahmad A. Novel antibacterial wound healing hydrogels based on HEC/SA/HA using green chemistry approach. Lett Appl NanoBioSci. 2023;12:69. CrossRef
32. Tripathi J, Thapa P, Maharjan R, Jeong SH. Current state and future perspectives on gastroretentive drug delivery systems. Pharmaceutics. 2019;11:193. CrossRef
33. Jain S, Sandhu PS, Malvi R, Gupta B. Cellulose derivatives as thermoresponsive polymer: an overview. J Appl Pharm Sci. 2013;3:139–44. CrossRef
34. Debnath S, Niranjan Babu M, Kusuma G, Saraswathi K, Sramika NR, Reddy AK. Formulation and evaluation of floatable in situ gel as carrier for stomach-specific drug delivery of metoclopramide Hcl. IJPFR. 2011;1:53–64.
35. Sahoo ACK, Rao SRM, Sudhakar M. HPMC a biomedical polymer in pharmaceutical dosage forms. J Chem Pharm Sci. 2015;8:875–81.
36. Russo R, Abbate M, Malinconico M, Santagata G. Effect of polyglycerol and the crosslinking on the physical properties of a blend alginate-hydroxyethylcellulose. Carbohydr Polym. 2010;82:1061–7. CrossRef
37. Dao L, Chen S, Sun X, Pang W, Zhang H, Liao J, et al. Construction and sustained release of konjac glucomannan/naringin composite gel spheres. Front Nutr. 2023;19(9):1123494. CrossRef
38. Wang K, He Z. Alginate-konjac glucomannan-chitosan beads as controlled release matrix. Int J Pharm. 2002;244:117–26. CrossRef
39. Qiu Y, Parkn K. Superporous IPN hydrogels having enhanced mechanical properties. AAPS Pharm Sci Tech. 2003;4:406–12. CrossRef
40. Ge Q, Ma X. Composition and antioxidant activity of anthocyanins isolated from Yunnan edible rose (An ning). Food Sci Hum Wellness. 2013;2:68–74. CrossRef
41. Li L, Wang L, Wu Z, Yao L, Wu Y, Huang L, et al. Anthocyanin-rich fractions from red raspberries attenuate inflammation in both RAW264.7 macrophages and a mouse model of colitis. Sci Rep. 2014;4:6234. CrossRef
42. Kim MJ, Rehman SU, Amin FU, Kim MO. Enhanced neuroprotection of anthocyanin-loaded PEG-gold nanoparticles against Abeta1-42-induced neuroinflammation and neurodegeneration via the NF-KB /JNK/GSK3beta signaling pathway. Nanomedicine. 2017;13:2533–44. CrossRef