INTRODUCTION
Zinc deficiency, caused by inadequate dietary intake, remains a common problem in many countries [1–4]. Since zinc deficiency is linked to various diseases, addressing this condition is crucial for improving quality of life and life expectancy. Affecting almost 17% of the world’s population, zinc deficiency impacts many organ systems, leading to impaired humoral and cell-mediated immunity, and increasing susceptibility to infections [5]. The biological role of zinc as a cofactor for many enzymes and its effects on health have been thoroughly described in several reviews [6–8].
The development of more efficient zinc delivery systems could be a solution to the problem of zinc deficiency. The use of inorganic salts for treating zinc deficiency is limited by their irritating effect on the esophagus and low bioavailability [9,10]. Therefore, one option for increasing the bioavailability of zinc is using chelate compounds [11–13]. Studies have focused on zinc complexes with methionine, glycine, threonine, and glutamic acid, but many other ligands can be considered. Notably, in 2019, the EFSA Panel on Additives and Products or Substances Used in Animal Feed (FEEDAP) concluded that zinc chelates of lysine and glutamic acid are less toxic than inorganic zinc sulfate [14]. According to the Panel, this finding can be extrapolated to all species and categories of animals. Additionally, zinc complexes with amino acids (AA) have potential as antimicrobial substances. For instance, zinc methioninate has been shown to disrupt bacterial biofilm formation, and a comparative analysis of the toxicity of zinc chelates with various AAs was conducted using the Spirotox method [15].
Since the thalidomide tragedy, researchers have traditionally paid great attention to the chirality of drugs. Assessing enantiomeric equivalence based on the pharmacodynamic characteristics of drugs has become an integral part of developing biologically active molecules. The spatial structure of the drug can also affect its pharmacokinetics. For example, retro-inverso peptides have longer half-lives due to lower protease substrate specificity [16]. The term “chiral switching” refers to the introduction of a specific enantiomer of a drug to replace the previously used racemate, justified by data showing improved pharmacological characteristics of the particular enantiomer [17].
In this study, we investigated the optical properties of zinc complexes with AA. According to IUPAC, there are six types of stereogenic elements in a molecule that can lead to chirality: chiral center, chiral axis, chiral plane, pseudosymmetric block, cis- and trans-isomerism, and enantiomorphic double bond [18]. The most common geometry in the coordination chemistry of d-elements is the octahedron, relevant for metals with a coordination number of six [19]. In such complexes, the central atom often acts as the chirality center or coordinates ligands to form a helix with the chiral axis.
Earlier, information was provided about chiral zinc complexes. For example, the ability of a zinc complex with a tetradentate ligand to catalyze the Diels-Alder reaction was investigated [20]. Chiral zinc complexes have also been shown to be useful for discrimination between L- and D-AA using optical methods and 1H-NMR spectroscopy [21,22]. In our research, we consider ligand selection as a way of controlling chirality. Given that the most common coordination number for zinc is six, and based on previously obtained data on zinc methioninate [23], we believe it is possible to control the chirality of zinc complexes with AA by varying the configuration of the ligands. Additionally, we aimed to determine how zinc complexes with different configurations can affect biological entities.
In our study, we examined the conditions for the formation, properties, and toxicity of optically active zinc complexes with various ligands, specifically L- and D-α-AA.
MATERIALS AND METHODS
Chemicals
L-glutamic acid (98.0%, Leap Chem Co., Ltd, Hong Kong, China), L-aspartic acid (98.0%, Sigma-Aldrich Co., Darmstadt, Germany), D-aspartic acid (99.0%, Sigma-Aldrich Co., Seoul, South Korea), LD-aspartic acid (99.0%, Sigma-Aldrich Co., Hong Kong, China), L-, LD-isoleucine (99.0%, Sigma-Aldrich Co., Hong Kong, China), L-valine (98.0%, Sigma-Aldrich Co., Massachusetts, United States), D-valine (99.0%, Sigma-Aldrich Co., Tokyo, Japan), LD-valine (99.0%, Sigma-Aldrich Co., Dorset, United Kingdom), glycine anhydride (99.0%, Alfa Aesar, Kandel, Germany), L-methionine (pure, pharma grade, AppliChem, Barcelona, Spain), and zinc sulfate heptahydrate (99.5%–103.0%, Sigma-Aldrich, Steinheim, Germany). The remaining reagents employed, including potassium hydroxide, ethanol, and sodium chloride, were of analytical quality. For the dilution process, we utilized highly purified water obtained through the Milli-Q® purification system, provided by Merck, a company based in Darmstadt, Germany.
Optical activity
The optical activity of aqueous solutions of zinc chelate complexes with AA was studied for L-, D-, LD-valine, L-, D-, LD-aspartic acid, L-, LD-isoleucine with different ratios of Zn2+: AA = 1:20, 1:10, 1:5, 1:2, 1:1 under the conditions: 1) [AA] = const, zinc concentration in solution gradually increased, where [L-, D-, LD-Val] = [L-, LD-Ile] = 100 mM, [L-, D-, LD-Asp] = 35 mM; 2) pH = 6.00 ± 0.05.
Aqueous solution of AA without Zn2+ was prepared under the same conditions, 100 mM solution glycine and 100 mM solution glycine with zinc sulfate (in a molar ratio of 1:2) were used as a control. It is a challenging task to determine the concentration of the final compound in the resulting mixture when combining solutions of Zn2+ and AA in varying proportions. Consequently, the findings of the polarimetric analysis are presented as a correlation between the angle of optical rotation and the concentration of Zn2+ in the solution.
The optical activity was measured using the Atago POL-1/2 polarimeter (manufactured by Atago Co., Ltd., Tokyo, Japan) in a 1-dm cell with a wavelength of 589.3 nm and a measurement accuracy of ±0.002° and a resolution of 0.0001°. A Peltier electronic module was used for setting the required temperature of t = 20°C ± 0.5°C. The angle of optical rotation was recorded for 1 minute in 5 consecutive measurements.
For L-, D-Val, and L-, D-Asp test solutions the optical activity was also measured using PerkinElmer Model 341 Polarimeter (PerkinElmer Inc., Massachusetts, USA) in a 1 dm cell, λ = 546.0 nm at 20°C ± 0.5°C. The angle of optical rotation was measured for 1 minute in 5 repetitions.
Dynamic light scattering (DLS)
A Zetasizer Nano ZSP (Malvern Panalytical, Worcestershire, UK) based on DLS was used to measure the size of nanoparticles in the aqueous solutions of zinc chelate complexes with AA prepared for the study of optical activity. The disposable polystyrene cuvettes were filled with a 1 ml sample. To determine the size, three measurements were taken for each sample, and the average size was calculated. Each measurement consisted of 12 runs.
X-ray crystallography
X-ray diffraction analysis was used to confirm the formation of a chelate structure in zinc compounds with AA at pH 6.00. This analysis was performed on the synthesized zinc glutamate complex. Zinc glutamate was synthesized according to an improved method described in the literature [24]: an aqueous solution of L-glutamic acid (2 mmol) was added to 20 ml of Zn(OH)2 suspension (2 mmol) and stirred for 30 minutes at 50°C. The Zn2+ content in the zinc hydroxide suspension was controlled by complexometric titration in accordance with the methodology of the European Pharmacopoeia [25]. The resulting colorless and clear aqueous solution of zinc glutamate with a pH of 3.80 was divided into two parts. The first solution was adjusted to pH 5.60 with 20% KOH. The precipitate was filtered out, and the filtrate was placed in a chamber saturated with acetone vapor. The second solution, without pH adjustment, was also placed in the chamber under the same conditions. Colorless, clear zinc glutamate single crystals were isolated from the solutions within 2 weeks.
The crystal structure was determined by X-ray structural analysis using an automatic four-circle area-detector diffractometer, Bruker KAPPA APEX II with MoKα radiation (Bruker AXS GmbH, Karlsruhe, Germany). The cell parameters were refined over the entire data set, together with data reduction using SAINT-Plus software [26]. Absorption corrections were introduced using the SADABS program [27]. The structures were solved using the SHELXT-2018/2 program [28] and refined by full-matrix least squares on F2 in the anisotropic approximation for all non-hydrogen atoms (SHELXL-2018/3 [29]). The C-H bonded hydrogen atoms were placed in geometrically calculated positions and refined in an idealized geometry with isotropic temperature factors equal to 1.2Ueq(C). The N-H and O-H bonded hydrogen atoms were objectively located from the difference Fourier synthesis and refined with isotropic temperature factors equal to 1.2Ueq(N) and 1.5Ueq(O). The structure was refined as inversion twins. A table for structure was generated using Olex2 [30].
Cellular biosensor Spirostomum ambiguum for testing the toxicity
Spirostomum ambiguum is the protozoan ciliate that is widely used as a test organism for toxicological and pharmacological studies of the biological activity of individual and combined medicines [31]. This model is advantageous because the sensitivity of the eukaryotic cell allows for the interpretation of toxicity results in relation to multicellular organisms and humans. Under favorable conditions in a low-mineralized environment, S. ambiguum cells can survive for a period exceeding their cell cycle (about 20 hours). However, when exposed to chemical compounds, the cells die within a time interval that depends on concentration and temperature [32].
The experiment was conducted at temperatures ranging from 22°C to 28°C (in increments of 2°C). The equipment consisted of a plate with five thermostatically controlled holes (Lauda Alpha A6 thermostate, Göttingen, Germany) and an MBS-10 binocular. To provide additional illumination, low-power fluorescent daylight lamps (10 W) were employed.
The toxicity of zinc chelate compounds with tridentate ligands was examined using a 0.1 mM solution of L-aspartic acid with zinc sulfate and a 0.1 mM solution of D-aspartic acid with zinc sulfate (both solutions in a molar ratio of 1:1). The ionic strength of L- and D-aspartic acid solutions was increased to 4 mM by adding sodium chloride. The pH values of all prepared solutions were in the range of 6.00 ± 0.05.
A single S. ambiguum test organism and 250 µl of the test solution were placed in each well of the plate. At each test temperature, five measurements were performed for each solution. The cell lifetime (tL) was recorded as the time from the introduction of the solution until the cell death. Cell death was determined by immobilization with no contractile response to mechanical stimulation or by the rupture of the cell membrane, resulting in the release of the cell contents. To calculate the observed activation energy for the ligand-induced cell transition (obsEa), the results were plotted on Arrhenius coordinates, with the logarithm of the cell death rate (y = ln(1/tL)) plotted against the inverse temperature (x = 1/T) [31,32].
Molecular modeling and data processing
Calculations, statistical processing, and visualization of measurements were performed using the OriginPro 2021 software (OriginLab, USA). The Mercury 2024.1.0 program was used to process and visualize the results of X-ray crystallography [33].
RESULTS AND DISCUSSION
Conditions for the formation of zinc coordination compounds with biogenic AA
Biogenic α-AA, except glycine, are chiral compounds. Quality control of these pharmaceutical substances includes determining their enantiomeric purity using polarimetry. Interestingly, national pharmacopoeias recommend measuring the rotation angle of active pharmaceutical ingredient solutions under highly acidic conditions. In such conditions, AA exists in the cationic form RCH(NH3+)COOH [34], where the specific rotation value is at its maximum, according to the Clough, Lutz, and Jirgensons (CLJ) rule [35–37].
In the previous study, we demonstrated the effect of a complexing metal on the chirality of aqueous solutions of L-methionine compared to a solution of free AA at pH 5.74 [23]. We hypothesized that the change in the optical activity of L-methionine solutions is associated with the formation of new chirality axes resulting from coordination bonds between the free AA and metal cations. We examined the change in optical activity of solutions of bidentate and tridentate AA when zinc sulfate was added across a wide range of pH values at the wavelength of the D-line of the sodium spectrum (λ = 589.3 nm) (Fig. 1). L-valine was chosen as a bidentate AA, and L-aspartic acid was chosen as a tridentate AA.
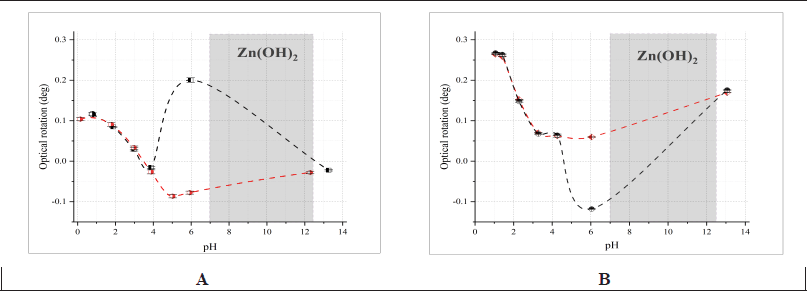 | Figure 1. pH-dependent change in the rotation angle of 35 mM L-aspartic acid (red) and 35 mM L-aspartic acid with Zn2+ in molar ratio 1:1 (black) (A). pH-dependent change in the rotation angle of 100 mM L-valine (red) and 100 mM L-valine with Zn2+ in molar ratio 2:1 (black) (B). Measurement conditions: l=1 dm, λ = 589.3 nm. The error bars indicate the standard deviation of measurements (n = 5). [Click here to view] |
The obtained dependences for L-valine and L-aspartic acid showed similar trends: in the pH range from 0 to 4, there was no difference in the optical activity between the free AA and the mixture with zinc. However, in the pH range from 4 to 6, the values were significantly different. Working with zinc salts is limited by the medium acidity; zinc hydroxide precipitates at approximately pH 6.5 and dissolves only at pH = 12.5. The rotation angle values of AA solutions with a complexing metal at pH>12.5 were close to those of free AA solutions. As expected, the rotation angle of the control solution of 35 mM ZnSO4 was zero (α = 0.00° ± 0.00, L = 1 dm, n = 5).
It is crucial to control the formation of colloidal solutions since measurements are made in a pH range conducive to zinc hydroxide formation. Using the DLS method to study the dispersed composition of AA solutions with Zn2+ at pH 6.00, we did not find a stable signal from nano- or submicron particles, indicating no colloidal Zn(OH)2 formation.
The trends observed for L-valine and L-aspartic acid align with the CLJ rule and confirm the findings of other researchers [38], who showed that the rotation angle of AA solutions depends on the pH and reaches minimum values for L-AA at pH ≈ 6. It is evident that some processes occur in AA and zinc sulfate solutions in the pH range from 4 to 6 that directly affect their optical properties. We believe this phenomenon is due to the chelation of zinc ions with AA, forming optically active complexes. The pH ranges for the formation of zinc aspartate and valinate complexes, described in the literature, are 5.4–8.4 and 6.0–7.8, respectively [39]. However, those studies used potentiometric measurements at increased ionic strength, which may account for discrepancies with our pH values.
In a previous study, we characterized a zinc chelate compound with a bidentate AA (L-methionine) synthesized at pH 5.74 using X-ray powder diffraction [23]. To confirm the formation of zinc chelate compounds with AA in the pH range from 4 to 6, we synthesized complexes of Zn2+ with a tridentate AA at pH 3.80 and 5.60. L-glutamic acid, a tridentate dicarboxylic L-AA similar to L-aspartic acid, was chosen as a chelating ligand. The resulting crystal structures of the synthesized products were characterized by X-ray structural analysis and are presented in Figure 2 and Table 1.
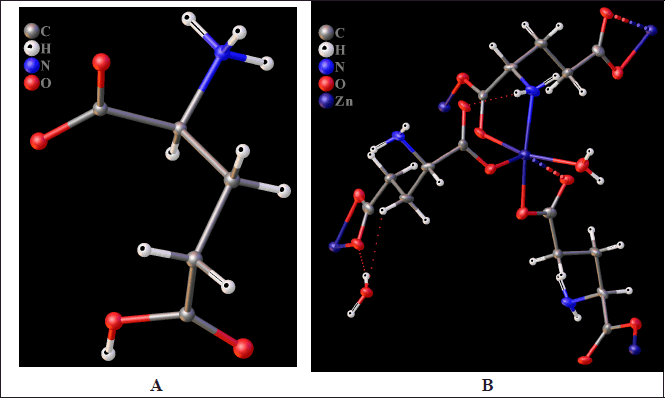 | Figure 2. Molecular structure of zinc glutamate synthesis products according to single crystal X-ray diffraction analysis at reaction mixture pH 3.80 (A) and 5.60 (B). The coordination of only one zinc atom is shown, the environment of other zinc atoms is omitted for clarity. The dotted line shows the extended Zn–O coordination bond. Displacement ellipsoids are drawn at the 50% probability level. [Click here to view] |
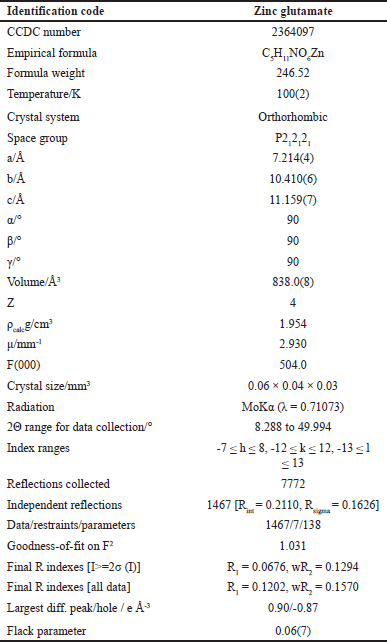 | Table 1. Crystal data and structure refinement for zinc glutamate (Fig. 2B). [Click here to view] |
The atomic coordinates were deposited at the Cambridge Crystallographic Data Centre [40]. The X-ray diffraction analysis confirmed the hypothesis about the possible formation of zinc chelate complexes with AA at pH = 5.60. The structure formed under these conditions is polymeric, and its component is shown in Figure 2B. The coordination number of zinc in this polymer is six, with 2 to 4 glutamic acid residues per zinc cation and 1 or 2 water molecules. Five of the six coordination bonds around the zinc atom are of similar length (2.06 Å on average). One of the six coordination bonds, Zn–O, is slightly longer than the others (shown as a dotted line) and is equal to 2.76 Å. As shown in Figure 2A, at pH 3.80, a zinc chelate is not formed, and the substance released as single crystals is glutamic acid. The polymer structure includes zinc atoms chelated in the distorted octahedral geometry. The formation of chelates at pH 5.60, combined with polarimetric measurements, indicates the formation of new optically active molecules with OC-6 geometry [19]. In such complexes, chirality can arise as both central and helical (similar to hexahelicenes).
Concentration dependences of the rotation angle of AA solutions upon addition of zinc sulfate
Earlier, we showed that the optical activity of an L-methionine solution can be altered by adding zinc sulfate at pH 5.74 and at the wavelength of the D-line of the sodium spectrum (λ = 589.3 nm) [23] (Fig. 3). An inverse exponential relationship was found between the optical activity of the resulting solutions and the concentration of zinc ions: as the concentration of Zn2+increases, the rotation angle becomes more negative. The ionic strength of the solutions increased with the zinc concentration, from 0.03 M in a methionine solution to 0.54 M in the zinc methionate solution with maximum metal content. To study the effect of ionic strength on the optical rotation angle, control solutions were prepared under the following conditions: 1) [Met] = const=134 mM (2%); 2) pH = pI(Met) = 5.74; 3) I(control) = I (test solution), obtained by mixing Zn2+ and Met in molar ratios of 1:20 and 1:2 (I = 0.13 M and 0.37 M, respectively); 4) the ionic strength of the solution was increased by adding sodium chloride, a strong electrolyte that does not form coordination compounds with ligands.
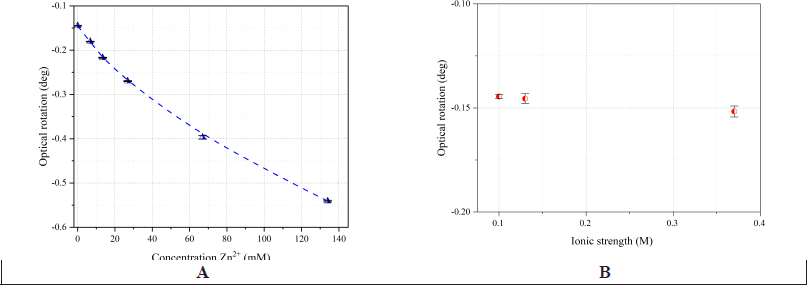 | Figure 3. The change in the optical rotation of 134 mM L-methionine at different concentrations of Zn2+ at pH 5.74 (A). The ionic strength influence on the optical activity of 134 mM L-methionine at pH 5.74: the change in the optical rotation from the sodium chloride concentration in solution (B). Measurement conditions: l=1 dm, λ = 589.3 nm. The error bars indicate the standard deviation of measurements (n = 5). [Click here to view] |
The results (Fig. 3B) showed that ionic strength does not significantly affect the rotation angle of the plane of polarized light in the solution. No colloidal Zn(OH)2 particles were detected when mixing Zn²+ and AA solutions in different ratios. The chelate complex concentration could not be precisely calculated, so polarimetric analysis results are presented as rotation angle values (Figs. 1,3–7).
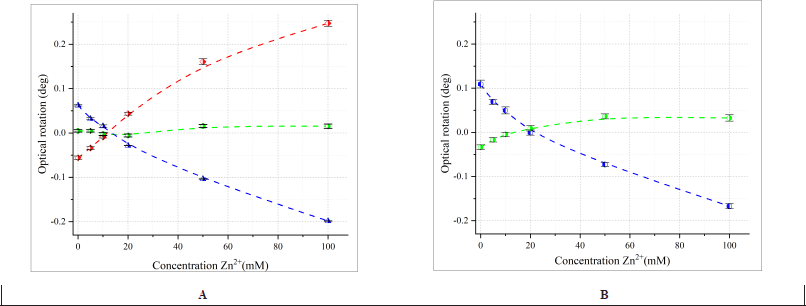 | Figure 4. The change in the optical rotation of 100 mM L- (blue), LD- (green) and D-valine (red) (A) and 100 mM L- (blue), LD-isoleucine (green) (B) at different concentrations of Zn2+ at pH 6.00. Measurement conditions: l=1 dm, λ = 589.3 nm. The error bars indicate the standard deviation of measurements (n = 5). [Click here to view] |
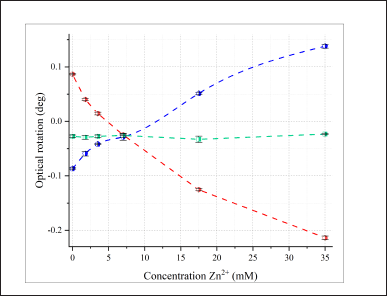 | Figure 5. The change in the optical rotation of 35 mM L- (blue), LD- (green) and D-aspartic acid (red) at different concentrations of Zn2+ at pH 6.00, l=1 dm, λ = 589.3 nm. The error bars indicate the standard deviation of measurements (n = 5). [Click here to view] |
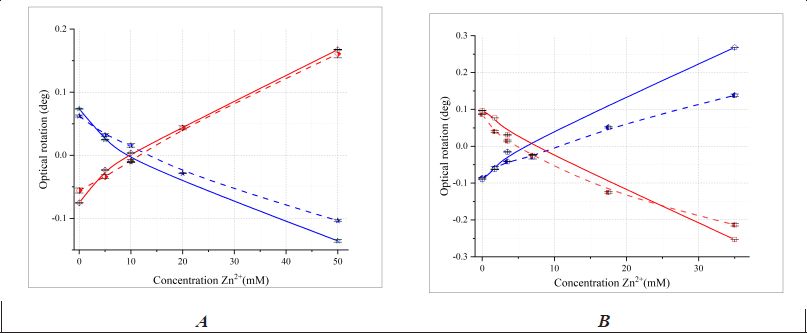 | Figure 6. The change in the optical rotation of 100 mM L- (blue), D-valine (red) (A) and 35 mM L- (blue), D-aspartic acid (red) (B) at different concentrations of Zn2+ recorded at λ = 589.3 nm – dotted lines and λ = 546.0 nm – solid lines and at pH 6.00, l=1 dm. The error bars indicate the standard deviation of measurements (n = 5). [Click here to view] |
 | Figure 7. Optical activity of 100 mM glycine (yellow), 100 Mm L-valine (red) and 35 mM L-aspartic acid (blue) in the free state and in the bound state with Zn2+ at pH 6.00. Measurement conditions: l=1 dm, λ = 589.3 nm. The error bars indicate the standard deviation of measurements (n = 5). [Click here to view] |
The optical rotation values for zinc sulfate mixtures with bidentate valine and tridentate aspartic acid (Fig. 1) differ significantly in both magnitude and sign at pH 6. After determining the pH range for complex formation, we studied their optical properties in more detail. Data on the rotation angle were obtained at the wavelength of the D-line of the sodium spectrum (λ = 589.3 nm) for solutions of enantiomers of bidentate (Fig. 4) and tridentate (Fig. 5) AA, varying the zinc ion concentration. For bidentate L-valine, L-isoleucine, and L-methionine, the rotation angle decreased exponentially with increasing zinc concentration. For bidentate D-valine, the dependence was mirrored. This difference, due to the configuration of the optically active center, allows control over the chirality of zinc complexes with AA, a phenomenon known as “predetermined chirality” [41–44]. The dependencies were exponential for individual enantiomers, but no pronounced dependence was observed for a mixture of enantiomers, likely due to the formation of a racemic mixture. Considering that LD-AA substances are not declared by the manufacturer to be racemic ones, for some AA (LD-isoleucine) some optical activity is observed both with and without a complexing agent (Fig. 4B). The ionic strength of the solution is determined by the content of zinc sulfate in the sample and varies within the range from 0.02 to 0.40 M for the presented dependences.
We also examined tridentate aspartic acid solutions (Fig. 5). For tridentate ligands, an exponential increase in the rotation angle was typical for L-AA, and an exponential decrease for D-AA, opposite to the properties of bidentate AA. Again, no dependence of optical activity on the complexing agent concentration was observed for the enantiomer mixture. The ionic strength of these solutions varied from 0.007 to 0.14 M.
Zinc can have a coordination number of up to six, leading to various stereoisomers of its chelate complexes with L- or D-AA. Racemic forms of chelates do not form because the chirality of the ligands sets the diastereomeric nature of the synthesis. Diastereomeric structures form in solutions with ligands of a single configuration, but this does not hold for L- and D- AA mixtures.
We also studied the optical activity of bidentate and tridentate AA solutions when adding zinc sulfate at pH 6 and at the mercury spectrum wavelength (λ = 546.0 nm). The dependencies were reproduced (Fig. 6) with some differences in absolute values, which were statistically insignificant (t-test, p < 0.05).
To assess the possible effect of solvent interactions on the optical activity of zinc chelate solutions, known as the “Sergeants-and-Soldiers” effect [45,46], we performed control polarimetric measurements for an optically inactive ligand, glycine, under identical conditions. Control tests compared the optical properties of free glycine and its Zn2+ complex (1:2 ratio, pH 6) with those of other optically active AA (Fig. 7). The zinc-glycinate complex solution had an ionic strength of 0.40 M. The results showed that zinc-glycine chelate complexes do not exhibit optical activity, confirming the absence of the “Sergeants-and-Soldiers” effect, as achiral ligands form racemates in coordination compounds [19].
Chiral chelate compounds with optically active central metal atoms are known [47,48], with both organic and inorganic ligands, such as those in A. Werner’s complexes [49]. In our study, identifying the absolute configuration of the complexes was challenging due to their polymeric nature. Despite this, polarimetric analysis indicated the presence of predetermined chirality.
Study of the toxicity of zinc complexes with L- and D-AA using the example of the cellular biosensor S. ambiguum
In our previous work, it was demonstrated that zinc complexes with AA tend to exhibit antibacterial activity [15]. Previous results did not take into consideration any issues of chiral purity of complexes. In this study, the biological effect on eukaryotic cell, defined by the optical configuration of zinc complexes with L- and D-AA, was a priority. An eukaryotic unicellular organism—S. ambiguum (Protozoa) – served for research purposes [50].
As an obligate oligotrophic inhabitant of natural water bodies, S. ambiguum is sensitive to even small changes in the concentration of dissolved substances. The Spirotox method, which involves studying the kinetics of death of this organism in Arrhenius coordinates, leverages this sensitivity [51,52]. The interest in this ciliate species stems from the proven correlation between activation energy and LD50 for various substances with different pharmacological activities [53].
In this study, we use the Spirotox method to investigate the influence of the steric effects of zinc chelate complexes on their toxicity. Zn2+ chelates with L- and D-aspartic acids were chosen for the biological experiment. These chelates were obtained by mixing solutions of zinc sulfate with the corresponding AA at pH 6. The concentrations of aspartic acid and zinc sulfate were selected to optimize the lifespan of the cellular biosensor. At a concentration of 2 mM for both the complexing ion and ligand (1:1 molar ratio), the lifespan of the ciliates at 22°C averaged 40–50 seconds. However, since increasing temperature decreases lifespan and increases error, this concentration was deemed too high. Therefore, the observed activation energy values were obtained for solutions with a concentration of 0.1 mM (Table 2). Under these conditions, the ciliates lived on average 160 seconds in the presence of the zinc chelate with L-aspartic acid and 130 seconds in the presence of the zinc chelate with D-aspartic acid at 22°C. Control tests were also conducted for solutions L- and D-aspartic acid enantiomers, with ionic strength adjusted by adding sodium chloride. Notably, the ciliates were resistant to L- and D-aspartic acids at a concentration of 0.1 mM and remained alive for more than 30 minutes.
 | Table 2. The obtained observation activation energy (obsEa) of the cell death kinetics of cellular biosensor S. ambiguum for 0.1 mM solution L-aspartic acid with Zn2+ and 0.1 mM solution D-aspartic acid with Zn2+ in a molar ratio of 1:1 (n = 5, p < 0.05 t-test). [Click here to view] |
The obtained values of the observed activation energy indicate the higher toxicity of the levorotatory zinc complex with D-aspartic acid. This result demonstrates the selective sensitivity of S. ambiguum to stereoisomers of the complexes, which can be useful in assessing the enantiomeric equivalence of various substances.
CONCLUSION
The study demonstrated that chelate compounds of zinc with AA form in an aqueous medium with specific chirality within the pH range of 5.50–6.00. The stereoisomerism of these complexes depends directly on the configuration and concentration of the chosen ligand. Therefore, when describing the interaction of zinc ions with L- or D-AA under these conditions, we can refer to the occurrence of predetermined chirality at the metal center. The resulting complexes have different effects on biological entities depending on their stereoisomerism. For instance, the zinc complex with D-aspartic acid was found to be more toxic than the zinc chelate with L-aspartic acid. This work continues the study of predetermined chirality in metal complexes. Based on the hypothesis of the non-equivalent toxicity of enantiomers, we propose to consider the use of chiral complexes with real or potential biological activity in terms of chiral purity.
ACKNOWLEDGMENTS
This publication has been supported by the RUDN University Scientific Projects Grant System, project No. 033323-2-000.
AUTHOR CONTRIBUTIONS
All authors contributed significantly to the development of the concept and design, the collection of data, or analysis and interpretation of the data. They also participated in the drafting or revision of the article, providing critical feedback on its intellectual content. They approved the final version for publication and agreed to be accountable for all aspects of the work. All authors meet the requirements and guidelines set by the International Committee of Medical Journal Editors (ICMJE).
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Gupta S, Brazier AKM, Lowe NM. Zinc deficiency in low- and middle-income countries: prevalence and approaches for mitigation. J Hum Nutr Diet. 2020 Oct;33(5):624–43. CrossRef
2. Wessells KR, Jorgensen JM, Hess SY, Woodhouse LR, Peerson JM, Brown KH. Plasma zinc concentration responds rapidly to the initiation and discontinuation of short-term zinc supplementation in healthy men. J Nutr. 2010 Dec;140(12):2128–33. CrossRef
3. Kim J, Paik HY, Joung H, Woodhouse LR, King JC. Plasma zinc but not the exchangeable zinc pool size differs between young and older Korean women. Biol Trace Elem Res. 2011 Aug;142(2):130–6. CrossRef
4. Foster M, Hancock D, Petocz P, Samman S. Zinc transporter genes are coordinately expressed in men and women independently of dietary or plasma zinc. J Nutr. 2011 Jun;141(6):1195–201. CrossRef
5. Chasapis CT, Ntoupa PA, Spiliopoulou CA, Stefanidou ME. Recent aspects of the effects of zinc on human health. Arch Toxicol. 2020 May;94(5):1443–60. CrossRef
6. King JC. Zinc: an essential but elusive nutrient. Am J Clin Nutr. 2011 Aug;94(2):679S–84S. CrossRef
7. Plum LM, Rink L, Haase H. The essential toxin: impact of zinc on human health. Int J Environ Res Public Health. 2010 Apr;7(4):1342–65. CrossRef
8. Roohani N, Hurrell R, Kelishadi R, Schulin R. Zinc and its importance for human health: an integrative review. J Res Med Sci. 2013 Feb;18(2):144–57.
9. Kim YR, Park JI, Lee EJ, Park SH, Seong NW, Kim JH, et al. Toxicity of 100 nm zinc oxide nanoparticles: a report of 90-day repeated oral administration in Sprague Dawley rats. Int J Nanomed. 2014 Dec 15;9(Suppl 2):109–26. CrossRef
10. Chang Y, Wang K, Wen M, Wu B, Liu G, Zhao H, et al. Organic zinc glycine chelate is better than inorganic zinc in improving growth performance of cherry valley ducks by regulating intestinal morphology, barrier function, and the gut microbiome. J Anim Sci. 2023 Jan 3;101:skad279. CrossRef
11. Farhadi Javid S, Moravej H, Ghaffarzadeh M, Esfahani MB. Comparison of zinc sulfate and zinc threonine based on Zn bioavailability and performance of broiler chicks. Biol Trace Elem Res. 2021 Jun;199(6):2303–11. CrossRef
12. Chen X, He C, Zhang K, Wang J, Ding X, Zeng Q, et al. Comparison of zinc bioavailability in zinc-glycine and zinc-methionine chelates for broilers fed with a corn-soybean meal diet. Front Physiol. 2022 Nov 17;13:983954. CrossRef
13. Boerboom GM, Busink R, Smits CH, Hendriks WH, Martín-Tereso J. Efficacy of l-glutamic acid, N,N-diacetic acid to improve the dietary trace mineral bioavailability in broilers. J Anim Sci. 2020 Dec 1;98(12):skaa369. CrossRef
14. EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP); Bampidis V, Azimonti G, Bastos ML, Christensen H, Dusemund B, et al. Safety and efficacy of zinc chelates of lysine and glutamic acid as feed additive for all animal species. EFSA J. 2019 Jul 25;17(7):e05782. CrossRef
15. Marukhlenko AV, Morozova MA, Mbarga AMJ, Antipova NV, Syroeshkin AV, Podoprigora IV, et al. Chelation of zinc with biogenic amino acids: description of properties using Balaban index, assessment of biological activity on Spirostomum ambiguum cellular biosensor, influence on biofilms and direct antibacterial action. Pharmaceuticals (Basel). 2022 Aug 9;15(8):979. CrossRef
16. Doti N, Mardirossian M, Sandomenico A, Ruvo M, Caporale A. Recent applications of retro-inverso peptides. Int J Mol Sci. 2021 Aug 12;22(16):8677. CrossRef
17. Cižmáriková R, Cižmárik J, Valentová J, Habala L, Markuliak M. Chiral aspects of local anesthetics. Molecules. 2020 Jun 12;25(12):2738. CrossRef
18. Favre HA, Powell WH. IUPAC recommendations and preferred names 2013. London, UK: Royal Society of Chemistry.
19. Knof U, von Zelewsky A. Predetermined chirality at metal centers. Angew Chem Int Ed Engl. 1999 Feb 1;38(3):302–22. CrossRef
20. Endo K, Liu Y, Ube H, Nagata K, Shionoya M. Asymmetric construction of tetrahedral chiral zinc with high configurational stability and catalytic activity. Nat Commun. 2020;11:6263. CrossRef
21. Li G, Zhao X, Wang L, Weisheng L. Chiral zinc complexes used as fluorescent sensor for natural amino acids. Inorg Chem. 2019;4:9317–21. CrossRef
22. Shirbate M, Nandhakumar R, Kim Y, Kim SJ, Kim SK, Kim K. Discrimination of the chirality of the α-amino acids in Zn(II) complexes of DPA-appended binaphthyl imine. Eur J Org Chem. 2018;2018:4959–64. CrossRef
23. Marukhlenko AV, Tumasov VN, Butusov LA, Shandryuk GA, Morozova MA. Comparative analysis of physical and chemical properties of differently obtained Zn—methionine chelate with proved antibiofilm properties (Part II). Pharmaceutics. 2023;15:590. CrossRef
24. Abendrot M, Checinska L, Kusz J, Lisowska K, Zawadzka K, Felczak A, et al. Zinc(II) complexes with amino acids for potential use in dermatology: synthesis, crystal structures, and antibacterial activity. Molecules. 2020;25:951. CrossRef
25. Council of Europe. 01/2008:20511 complexometric titrations. In: The European pharmacopoeia. 8th edition. Strasbourg, France: Council of Europe; 2013. Volume 1.
26. Bruker AXS. SAINT, Version 8.40B. Madison, WI: Bruker AXS Inc.,; 2020.
27. Krause L, Herbst-Irmer R, Sheldrick GM, Stalke D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J Appl Crystallogr. 2015;48:3–10. CrossRef
28. Sheldrick GM. SHELXT-integrated space-group and crystal-structure determination. Acta Crystallogr Sect A Found Crystallogr. 2015;71:3–8. CrossRef
29. Sheldrick GM. Crystal structure refinement with SHELXL. Acta Crystallogr Sect C Struct Chem. 2015;71:3–8. CrossRef
30. Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JAK, Puschmann H. OLEX2: a complete structure solution, refinement, and analysis program. J Appl Crystallogr. 2009;42:339–41. CrossRef
31. Levitskaya OV, Syroeshkin AV, Pleteneva TV. Arrhenius kinetics as a bioactivity assessment criterion for drug substances and excipients. Pharm Chem J. 2016;49:779–781. CrossRef
32. Goncharuk VV, Syroeshkin AV, Zlatskiy IA, Uspenskaya EV, Orekhova AV, Levitskaya OV, et al. Quasichemical description of the cell death kinetics of cellular biosensor Spirostomum ambiguum for testing the biological activity of aqueous solutions. J Water Chem Technol. 2017;39:178–87. CrossRef
33. Mercury 2024.1.0. Available from: https://www.ccdc.cam.ac.uk/solutions/software/free-mercury/
34. Council of Europe. 01/2008:20207 optical rotation. In: The European pharmacopoeia. 8th edition. Strasbourg, France: Council of Europe; 2013. Volume 1.
35. Clough G. The relationship between the optical rotatory powers and the relative configurations of optically active compounds. The influence of certain inorganic haloids on the optical rotatory powers of alpha-hydroxy-acids, alpha-amino-acids, and their derivatives. J Chem Soc Trans. 1918;113:526–54. CrossRef
36. Lutz O, Jirgensons B. Ueber eine neue Methode der Zuteilung optisch-aktiver alpha-amino-saeuren zur Rechts- oder Linksreihe (I. Mitteil.). Ber Dtsch Chem Ges[Abt] B: Abh. 1930;63:448–60.
37. Lutz O, Jirgensons B. Ueber eine neue Methode der Zuteilung optisch-aktiver alpha-amino-saeuren zur Rechts- oder Linksreihe (II. Mitteil.). Ber Dtsch Chem Ges[Abt] B: Abh. 1931;64:1221–32.
38. Syroeshkin AV, Pleteneva TV, Uspenskaya EV, Levitskaya OV, Tribot-laspiere MA, Zlatsky IA, et al. Polarimetric research of pharmaceutical substances in aqueous solutions with different water isotopologues ratio. Int J Appl Pharm. 2018;10:243.
39. Chernova SP, Trubachova LV. Potentiometric study of the behavior of Zn(II) in solutions of amino acids and komplexones. Analytics ontrol. 2006;10(3-4):336–41. (In Russ.)
40. Groom CR, Bruno IJ, Lightfoot MP, Ward SC. The Cambridge structural database. Acta Crystallogr Sect B Struct Sci Cryst Eng Mater. 2016;72:171–9. CrossRef
41. Larionov VA, Feringa BL, Belokon YN. Enantioselective “organocatalysis in disguise” by the ligand sphere of chiral metal-templated complexes. Chem Soc Rev. 2021 Sep 7;50(17):9715–40. CrossRef
42. Crassous J. Chiral transfer in coordination complexes: towards molecular materials. Chem Soc Rev. 2009 Mar;38(3):830–45. CrossRef
43. Mamula O, von Zelewsky A, Bark T, Stoeckli-Evans H, Neels A, Bernardinelli G. Predetermined chirality at metal centers of various coordination geometries: a chiral cleft ligand for tetrahedral (T-4), square-planar (SP-4), trigonal-bipyramidal (TB-5), square-pyramidal (SPY-5), and octahedral (OC-6) complexes. Chemistry. 2000 Oct 2;6(19):3575–85. CrossRef
44. Hayoz P, von Zelewsky A, Stoeckli-Evans H. Stereoselective synthesis of octahedral complexes with predetermined helical chirality. J Am Chem Soc. 1993;115(12):5111–4. CrossRef
45. Nagata Y, Nishikawa T, Suginome M. Abnormal sergeants-and-soldiers effects of poly(quinoxaline-2,3-diyl)s enabling discrimination of one-carbon homologous n-alkanes through a highly sensitive solvent-dependent helix inversion. Chem Commun (Camb). 2018 Jun 19;54(50):6867–70. CrossRef
46. Nagata Y, Nishikawa T, Suginome M. Solvent effect on the sergeants-and-soldiers effect leading to bidirectional induction of single-handed helical sense of poly(quinoxaline-2,3-diyl)s copolymers in aromatic solvents. ACS Macro Lett. 2016 Apr 19;5(4):519–22. CrossRef
47. Ehnbom A, Ghosh SK, Lewis KG, Gladysz JA. Octahedral Werner complexes with substituted ethylenediamine ligands: a stereochemical primer for a historic series of compounds now emerging as a modern family of catalysts. Chem Soc Rev. 2016 Dec 21;45(24):6799–811. CrossRef
48. Parada J, Larrazábal G, Aguirre P, Zolezzi S, Vega C, Garrido C. The stereoselective synthesis of the Werner complex with substoichiometric sugars. J Chil Chem Soc. 2008;53(1):1390–2. CrossRef
49. Werner A. Zur Kenntnis des asymmetrischen Kobalt atoms I. Ber Dtsch Chem Ges. 1911;44:1887–98.
50. Schoch CL, Ciufo S, Domrachev M, Hotton CL, Kannan S, Khovanskaya R, et al. NCBI taxonomy: a comprehensive update on curation, resources and tools. Database (Oxford). 2020 Jan 1;2020:baaa062. CrossRef
51. Syroeshkin AV, Antipova NV, Zlatska AV, Zlatskiy IA, Skylska MD, Grebennikova TV, et al. The effect of the deuterium depleted water on the biological activity of the eukaryotic cells. J Trace Elem Med Biol. 2018 Dec;50:629–33. CrossRef
52. Syroeshkin AV, Uspenskaya EV, Pleteneva TV, Morozova MA, Zlatskiy IA, Koldina AM, et al. Mechanical transformation of compounds leading to physical, chemical, and biological changes in pharmaceutical substances. Sci World J. 2018 Dec 13;2018(4):1–8. CrossRef
53. Uspenskaya EV, Pleteneva TV, Hanh P, Kazimova I. Assessment of biology activity of the peeling substances by the physicochemical approaches on the Spirostomum ambiguum cell model. Int J Pharm Pharm Sci. 2021 Jul;13(7):82–6. CrossRef