INTRODUCTION
The drug delivery system can deliver and release active pharmaceutical ingredients at the desired location at an appropriate rate, which can produce optimal therapeutic effects [1]. Nowadays, the most preferred route of administration is oral due to numerous advantages, such as easy administration, high patient compliance, and an inexpensive manufacturing process [2]. However, oral drug administration also has some drawbacks, including the slow onset of action, first-pass metabolism, the possibility of degradation, stability of the drug in the gastrointestinal tract, and is influenced by gastric emptying time [3]. To overcome the drawbacks of oral administration, the colon is one of the potential drug absorption sites in the oral delivery system. The colon has a longer transit time, around 20 to 30 hours, compared to the upper gastrointestinal tract. Furthermore, the pH condition in the colon is around 6.0 to 7.5 which is a neutral pH for drug absorption [4]. In addition, a low enzyme activity in the colon may prevent the degradation of the drug [5]. High drug concentrations can be achieved in the colon, therefore increasing efficacy and reducing side effects [6].
Drug delivery systems via the oral route targeted to the colon are currently being developed to deliver drugs with low bioavailability that can be administered both systemically and locally [7]. The colonic drug delivery system is an alternative for drugs that have low bioavailability, instability to pH, enzymes, low solubility, short half-life, and poor absorption [8]. Several developments of oral drug delivery systems targeted to the colon include those for delivering insulin [9,10], proteins [11], hormones [12], colorectal cancer [13,14], and drugs for inflammatory bowel diseases (IBDs) [15,16].
Several colon-targeted drug delivery systems (CDDSs) have been developed to control drug release so the drug will not be released at the upper gastrointestinal tract and improve drug efficacy [17], including pH-sensitive systems [18,19], time control systems [20], degradation systems by microflora in the colon [21], prodrugs [22], and osmotic systems [23]. From several approaches to CDDS, an excipient with unique characteristics is needed to protect drug release at the upper gastrointestinal tract and enhance drug release in the colon.
Polysaccharides have been widely used for CDDS through swelling and degradation by microflora present in the colon [4]. When the drug delivery system reaches the colon, polysaccharide-digesting enzymes are secreted by the colonic microflora to degrade the polysaccharides so that the drug can be released [24]. In addition, polysaccharides can also be used as prodrug carriers by targeting ligands and activation by enzymes in the colon so that the drug is not released before the drug reaches the colon [22]. Furthermore, polymers can be used to protect, delay, and control drug release [25]. The polymers used have pH-sensitive characteristics, which are resistant or insoluble at acidic pH but can be degraded when the pH becomes alkaline [26]. Polymers are also used to release drugs based on time so that drugs can be released in the colon [20,27].
Combining polysaccharides with water-insoluble polymers can control the development and/or solubility of polysaccharides so that drug release does not occur in the stomach and small intestine [17]. The combination of polysaccharides with water-insoluble polymers is often used to control the development and/or solubility of polysaccharides so that drug release does not occur in the upper gastrointestinal tract [17]. The use of a combination of excipients such as polysaccharides with enteric polymers can prevent burst effects in the stomach and small intestine and can increase the delay time and effectiveness of targeted drug delivery systems to the colon.
This review article will discuss the excipients that can be used for drug delivery systems targeted to the colon. The excipients to be discussed are classified based on the type or class of these excipients, including polymers and polysaccharides.
CHARACTERISTICS OF EXCIPIENTS USED FOR COLON DRUG DELIVERY SYSTEM
CDDS should control drug release and maintain drug concentration at the colon site [8]. Therefore, CDDS requires excipients that can protect the drug in the large intestine from degradation, release, and absorption before reaching the colon [4].
Polysaccharides
Polysaccharides are macromolecular compounds with large molecular weight, consisting of long chains of monosaccharide units (more than 10 monosaccharides) linked by glycosidic linkages. Polysaccharides can be used in CDDS because they can be degraded by enzymes from various microflora colonization in the colon [28]. Some of the microflora in the colon include Bacteroides, Bifidobacterium, Eubacterium, Enterobacteriaceae, Lactobacillus, Bacillus subtilis, Peptococcus, Clostridium, and Escherichia coli [29,30]. These microflora produce reductive and hydrolytic enzymes that can degrade polysaccharides, such as β-d-glucosidase, β-d-galactosidase, amylase, pectinase, xylanase, β-d-xylosidase, dextranase, glucuronidase, xylosidase, and arabinosidase [30,31].
When the drug reaches the colon, the glycosidic bonds of the polysaccharides will be hydrolyzed by enzymes secreted by the colonic microflora, and the drug can be released in the colon [29]. Some mechanisms of polysaccharide degradation by microflora include a Starch utilization system (Sus)-like transport, ATP-binding cassette (ABC)-transport system, and cellulosome-like scaffolded enzyme system. The enzymes in the Sus-like transport system are encoded by the polysaccharide utilization loci (PUL) which are found in about 18% of the genome of Bacteroides, especially Bacteroides thetaiotaomicron. Lipoproteins SusD, SusE, and SusF degrade polysaccharides into maltooligosaccharides through SusG which is then carried into the cytoplasm through the TonB-dependent transporter SusC. Maltooligosaccharides will be degraded into maltose and glucose by α-glucosidase and neopululanase. Another mechanism of polysaccharide degradation is through the ABC-transport system in Eubacterium rectale which will degrade polysaccharides into maltooligosaccharides by the amylase enzyme on the cell surface. Maltooligosaccharides will be recognized by glycoside hydrolases and then transported to the cytoplasm in the form of maltose and glucose. The cellulosome-like scaffolded enzyme system in Ruminococcus unites cellulose and enzymes together on the cell surface through the dokerin protein to degrade cellulose into monosaccharides [32]. When the polysaccharides that coat the drug are degraded by enzymes produced by the microflora in the colon, the drug will be released from the system.
Another mechanism of drug release is that polysaccharide-based hydrogel particles can swell when water is absorbed with the increased size of the porous structure. The encapsulated drug can be released from the matrix system [33]. However, high polysaccharide concentrations form a thicker gel layer around the tablet when the polysaccharide expands, which may inhibit drug release [34]. Excipients of polysaccharides used for the CDDS can be seen in Table 1.
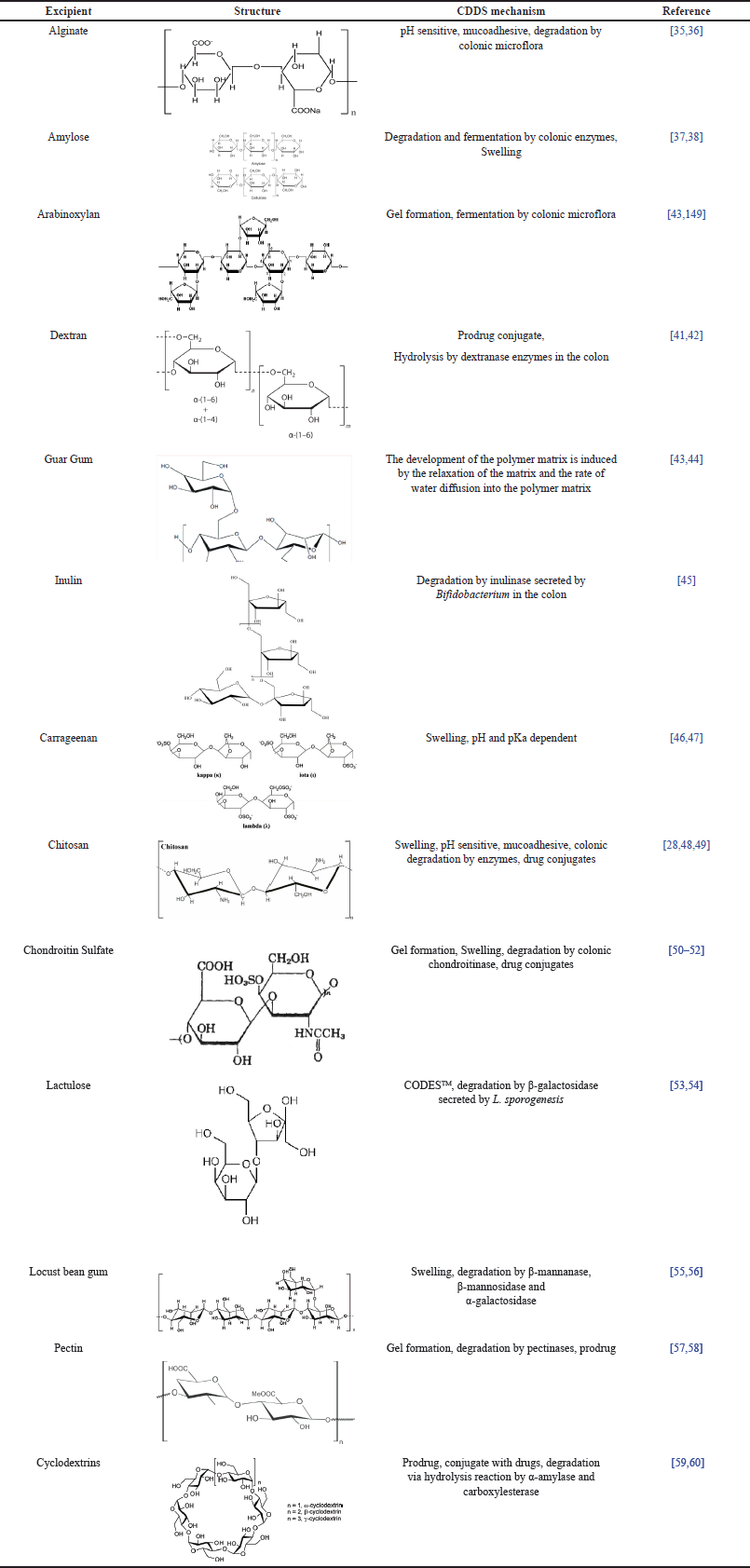 | Table 1. Excipients of polysaccharide used for CDDS. [Click here to view] |
Alginate
Alginate is an anionic polysaccharide that contains negatively charged carboxylic groups at pH 5 and above. The alginate structure consists of α-1,4-l-guluronic (G) units and β-1,4-d-manuronic acid (M) from Laminaria hyperborea, Macrocystis pyrifera, and Ascophyllum nodosum. Alginate is available in the form of salts such as sodium alginate, and calcium alginate. Alginates can form a stable gel in aqueous media, and with the addition of multivalent cations, such as calcium chloride, they can form cross-links to make a strong chemical bonding [61,62]. Alginates have low solubility in the stomach and small intestine but can dissolve and expand at alkaline pH in the colon. Alginates also have a mucoadhesive ability which allows for adherence to the colon. Under acidic pH, carboxylic acid groups do not dissociate, but at alkaline pH, carboxylic acid groups are converted into negatively charged carboxylic ions. This will produce electrostatic repulsion and swelling that cause drug release [30]. Alginate and its derivative compounds have been utilized for CDDS with pH-dependent and enzyme degradation mechanisms. Iswandana et al. [63] conducted a drug release study from tetrandrine-coated calcium alginate and cellulose acetate phthalate (CAP) which showed that 1.23% and 67.68% of tetrandrine have been released in pH 1.2 and 6.8, respectively. Hence, this formulation can protect against burst release in acid pH and enhance drug release in colon conditions. The hydration of the hydroxyl groups of alginates influences calcium alginate swelling studies. The more ion Ca2+ bound to the alginate can increase the swelling degree [63]. In addition, Agarwal et al. [30] have successfully employed a combination of calcium alginate and carboxymethyl cellulose for the delivery of 5-Fluorouracil beads, which reported that the beads remained attached to the mucosal surface of the large intestine for 24 hours, but only 2–3 hours at acidic pH. The combination of calcium alginate and carboxymethyl cellulose exhibits sustained drug release during transit time in the gastrointestinal tract before reaching the colon, and higher adhesion to the colonic mucosa, whose degradation is controlled by colonic microflora [30].
Amylose
Amylose is a linear polysaccharide that is not branched from glucopyranose units (D-1, 4-D-glucose) obtained from starch [64]. Amylose is resistant to pancreatic amylase, so it cannot be digested in the small intestine but can be degraded by Bacteroides, and Bifidobacterium in the colon [65]. Amylose can maintain its structure against amylase enzyme degradation throughout in-vitro test, so drug release can be controlled [66]. Amylose will swell when in contact with water because it has an elasticity degree. The qualitative testing of corn starch for CDDS using scanning electron microscopy (SEM) showed that hydrolysis of starch can swell due to the formation of pores and enzymatic degradation by α-amylase [67].
Arabinoxylan
Arabinoxylan is a hetero-polysaccharide consisting of linear xylose with arabinose substitution and cross-linking with ferulic acid [68]. Arabinoxylan is fermented and degraded by enzymes produced by the colonic microflora, especially by Bacteroides and Bifidobacterium [39]. These enzymes include β-xylosidase, xylanase, α-arabinofuranosidase, and β-glucosidase [69]. Arabinoxylan can form covalent gels when there is an enzymatic reaction of ferulic acid, which is influenced by changes in pH, temperature, and ionic strength. When insulin and insulin-glutamic acid are mixed with arabinoxylan, there is an increase in gelation time, which may be due to changes in pH and increased viscosity. In addition, arabinoxylan can form covalent cross-linking, which strengthens the gel. This excipient has been widely used for delivering drugs to the colon site. For instance, the successful delivery of the insulin release profile from arabinoxylan-coated microspheres revealed the biphasic release with the initial release of insulin at pH 1.2 followed by gradual drug release at pH 6.8. When microspheres reach the colon, insulin will be released by colonic microbes fermentation [70].
Dextran
Dextran is a polysaccharide with a linear chain consisting of α-1,6-glucopyranose and α-1,3-glucopyranose, which has a molecular weight of approximately 40 kDa [41,71]. Dextran cannot be digested by glycosidase but degraded by dextranase derived from microflora in the colon [72]. Dextran is a polysaccharide that has been developed for CDDS as a prodrug conjugate because it has good water solubility, low toxicity and immunogenicity, and low polydispersity [73]. The drug-dextran conjugate does not release the drug before the colon because of the steric barrier of the polymer matrix preventing enzymatic degradation in the upper gastrointestinal tract. Depolymerization of matrix dextran by endo dextranase, secreted by bacteria in the colon, produces oligomers that reduce steric resistance and break the dextran-drug bond which can lead to the drug release [72]. Succinic acid and glutamic acid are commonly used as a link between drugs and dextrans to increase drug release in the colon [74]. Shrivastava et al. [73] have reported the use of dextran in a CDDS tablet [34]. Their study found that the release of 5-ASA-dextran-conjugated observed in rat cecal for 24 hours showed that the percentage of drug released in the colon was 51.25% ± 1.48%. The other study also supported that an in vivo test in rat cecal of celecoxib-dextran-glutamic acid conjugate showed that 55% of the drug was released at 10 hours and 75% at 24 hours [72].
Guar Gum
Guar gum is a polysaccharide that can be used for CDDS. Guar gum can control drug release due to its hydrophilic properties and is susceptible to microbial degradation in the colon [75]. Guar gum has a linear galactomannan chain consisting of β (1→4) mannose which is bound to 1→6 with galactose units [43]. The mechanism of drug release using guar gum is swelling of a polymer matrix which is influenced by the relaxation properties of the matrix and the rate of diffusion of water into the polymer matrix. The mechanism of drug release from the polymer can be divided into three stages. They are the penetration of water molecules from outside the system into the matrix, polymer swelling, drug dissolution in the polymer, and drug diffusion through the swollen matrix [43,76]. One study conducted by Seeli et al.. found that the release of ibuprofen from beads coated with guar gum succinate-sodium alginate showed that 20% of the drug was released at pH 1.2 within 3 hours and 50% of the drug was released at pH 7.4 after 3 hours. This can be caused by the electrostatic repulsion of the negatively charged guar gum succinate-sodium alginate group in a buffer solution of pH 7.4 [75]. The 5-ASA was fabricated using guar gum and then evaluated for the percentage of 5-ASA release. The result showed a higher 5-ASA release in the media of pH 6.8 and 7.4 than that in the media of pH 1.2. This can be caused by the hydrophilic group being protonated at pH 1.2 to prevent the interaction between water and the polymer system, while at alkaline pH, the hydrophilic group will form hydrogen bonds with water to allow water penetration. When water penetrates the polymer system, the polymer will swell, and the drug in the matrix diffuses out [43].
Inulin
Inulin is a fructant consisting of oligomers and polymer mixtures that have 2 to 60 β (2-1) D-fructose monomers linked to the α (2-1) D-glucose group [45]. Inulin can be used as an excipient for colonic targeted drug release because it protects against degraded drugs in the stomach and small intestine. The stomach and small intestine have no inulinase that can degrade inulin. Inulin is only significantly hydrolyzed by inulinase produced by Bifidobacterium in the colon and not by digestive enzymes in the upper gastrointestinal tract [77]. When it reaches the colon, inulin can be degraded in the presence of the inulinase [78]. The swelling test showed that the addition of 30% inulin to the formulation significantly increased matrix polymer development compared to the one formulated with polymethacrylate only. This can be due to the inulinase in the medium, which diffuses into the polymer chain, hydrolyzing the inulin fructose, thereby increasing polymer swelling [79]. In vitro drug release of ibuprofen-coated inulin and Shellac showed that using Shellac as an outer enteric coating could prevent premature drug release in acidic media [78]. Another study by Akhgari et al. found that In vitro degradation of inulin hydrogel using inulinase showed that inulinase could degrade inulin in the colon and functioned well at pH 5.0 in sodium acetate buffer [79].
Carrageenan
Carrageenan is an anionic polysaccharide containing sulfate, D-galactose, and 3,6-anhydrogalactose units connected by α-1,3 and β-1,4 glycosidic bonds. There are 3 main types of carrageenan based on its bonds, such as λ-carrageenan with three sulfate groups repeating unit of disaccharide; k-carrrageenan which has one sulfate group and ι-carrrageenan that has two sulfate groups [46]. Kappa-carrageenan is a type of carrageenan that has the highest gelling strength compared to other types of carrageenan [80]. The mechanism of drug release by kappa-carrageenan is swelling which is influenced by pH conditions. At pH 1.2, the kappa-carrageenan swelling ratio was higher than pH 6.8 due to electrostatic repulsion between sulfate anions through deprotonation of –SO3H groups with pKa of 4.9. When the pH is increased, the K+ cation charge interferes with the electrostatic repulsion between the sulfate anions thereby reducing the swelling rate [47]. A high concentration of kappa-carrageenan will increase its swelling ratio [81]. A study of carrageenan conducted by Leong et al. revealed that the release study of Fluorescein isothiocyanate-labeled dextran (FD-4) coated carboxymethylated kappa-Carragenan beads showed that 23% ± 2% and 90% ± 3% of FD-4 was released in simulated gastric and colonic fluid, respectively [80]. In another study, the release of tramadol coated with UiO-66 and kappa-carrageenan showed the lowest drug release at pH 1.2 and the highest drug release at pH 7.4 or colon condition [47].
Chitosan
Chitosan is an amino polysaccharide consisting of β-D-glucosamine and N-acetyl-D-glucosamine repeating units linked by 1 → 4 glycosidic bonds [82]. Chitosan is included in the polycation type polymer because it has an ionizable amine group [83]. The mechanism for drug release from chitosan is swelling, which depends on pH conditions. The higher polymer concentration and stronger hydrogen bonds may inhibit the penetration of the acid solution into the matrix. The –COOH groups of the polymer are not ionized in the acidic solution and this induced electrostatic repulsion. It is inversely proportional to the condition of pH 7.4, where the swelling index increases with increasing polymer concentration. The presence of carboxyl groups in ionized form induces electrostatic repulsion at base condition [84]. Another mechanism of drug release is mucoadhesive properties. The amine groups that have a positive charge on chitosan will interact with mucin ions (sulfonic acid and sialic acid residues), which are negatively charged [85]. The release study of tetrandine beads coated with chitosan tripolyphosphate showed that 5.45% and 1.72% of tetrandine release at pH 1.2 when combined with 10% hydroxypropyl methylcellulose phthalate (HPMCP) and chitosan 10% CAP, respectively. The increase in drug release occurred significantly at pH 6.8 media in 315 minutes caused by the polymer layer being eroded so that the matrix could be hydrated and swollen [86]. Another research presented the release study of curcumin coated with chitosan which showed low release at pH 1.2 media (approximately 5.6%) and increased at pH 6.8 to 7.2 (approximately 10.8%). This could be due to the presence of cross-links which strengthen the integrity in acidic conditions [87].
Chondroitin sulfate
Chondroitin sulfate is a negatively charged polysaccharide that has a sulfate group consisting of repeating disaccharide units of β-1,3 N-acetyl galactosamine and β-1,4 D-glucuronic acid [88]. Chondroitin sulfate is a water-soluble polysaccharide and can be degraded by B. thetaiotaomicron and B. ovatus in the colon [50]. The drug delivery mechanism of chondroitin sulfate is by gelling and swelling. The percentage of the swelling index will increase when the chondroitin sulfate concentration is increased. Chondroitin sulfate has sulfonate (–SO3), hydroxyl (–OH), and carboxylate (–COOH) groups at higher pH (7.4) undergo ionization or deprotonation to become negatively charged that produce electrostatic repulsion which increases the swelling ratio [51]. Ramasamy et al. [88] conducted a release study of aceclofenac coated with chondroitin sulfate and enteric polymer [Eudragit S-100 and L-100) which showed that 10% of the drug was released in medium pH 1.2 for 2 hours and increased at pH 7.4 added with an enzyme to 102, 34% ± 0.36% for 8 hours. This is due to the continuous swelling to form a viscoelastic matrix so that the drug can be released [50]. Similarly, another study showed that the release of oxaliplatin from the chondroitin sulfate-acrylic acid hydrogel depended on the pH, namely 19% at pH 1.2 and 94% at pH 7.4 for 36 hours [51].
Lactulose
Lactulose is a disaccharide obtained from isomeration of lactose which consists of fructose and galactose. Lactulose cannot be digested in the upper gastrointestinal tract but can be degraded by Lactobacillus and Bifidobacterium in the colon [89,90]. Lactulose is used in the Novel Colon Targeted Delivery System (CODESTM) formulation to induce specific drug release in the colon by combining pH-dependent release mechanisms and the enzymatic activity of the colonic microflora [91]. CODESTM consists of a tablet core, an acid-soluble polymer (Eudragit® E100), and an enteric polymer (Eudragit® L100). When the drug reaches the colon, lactulose will be converted into lactic and short-chain organic acids through β-galactosidase secreted by microflora in the colon. The presence of this organic acid can decrease the pH so that it can dissolve the acid-soluble polymer and the drug can be released [53,92]. The release of CODES-based budesonide with a concentration of 50% lactulose, 30% Eudragit E and 12% Eudragit FS30D can reach 82.5% on a pH 6.8 medium containing rat cecal [54]. In addition, the release of etoricoxib from CODES demonstrated that drug release in pH 1.2 medium ranged from 0.1%–0.5% and pH 6.8 medium with Lactobacillus sporogenesis ranged from 63.671 ± 1.62% to 98.929 ± 1.69% [53].
Locust bean gum
Locust bean gum is a linear polysaccharide consisting of β-(1,4)-D-mannose units and each fourth or fifth mannose unit is replaced by a 1,6 α-galactose side chain. Locust bean gum is degraded by enzymatic activity in the colon. These enzymes are β-mannanase, β-mannosidase, and α-galactosidase secreted by Bacteroides and Ruminococci [93]. Locust bean gum swelling tests show that it can absorb up to 130% water after 7 hours of hydration. When the polymer is in contact with water, water will penetrate and reach the core of the tablet, which makes the polymer swell so that it can control drug diffusion [94]. Ibuprofen coated with locust bean gum was not released at pH 1.2 or 7.4 but it can release 94.46% ± 0.92% after 26 hours at pH 6.8 [55]. Similarly, the release study of 5-FU coated with locust bean gum and HPMC stated that the drug release ranged from 2.59% to 7.52% for 5 hours at pH 1.2 and 85.94% to 99.51% at pH 6.8 with 4% rat cecal [95]. Furthermore, the drug release of mesalamine coated with locust bean gum-Eudragit S100 proved that 0% of the drug was released at pH 1.2, and the drug release increased when pH increased. The highest percentage of cumulative drug release for 24 hours can reach 96.73% in colon conditions [96].
Pectin
Pectin is a polygalacturonic acid that has a negative charge at neutral pH with a pKa value of 3.5 [97]. Low pectin concentrations will produce rapid degradation of pectin and premature release of the drug. By increasing the concentration of pectin, a stiff gel that cannot be degraded by enzymes will be formed and this can reduce the rate of drug release [57]. The use of enteric polymers and cross-linking agents can be employed for obtaining delayed release of drugs and increasing drug release in the colon [98]. The initial release of the drug can be prevented by using a combination of pectin and ethyl cellulose with a ratio of 1:2. The 5-FU released 4.1% ± 0.4% in the first 5 hours in the simulated stomach and intestines media, while in the simulated colon media with the added rat cecal can release 85.0% ± 0.3% drug for 24 hours [99]. Another pectin-drug delivery mechanism is the prodrug system. The presence of enzymatic degradation by bacteria in the colon causes drug release from its conjugate bonds. The prodrug of metronidazole with pectin released 1% to 5% of the drug in medium pH 1.2 for 2 hours and pH 6.8 for 6 hours and increased at pH 7.0 with rat cecal content of 67.9% for 8 hours. This strongly indicates that the presence of enzymatic degradation by bacteria in the colon causes the release of drugs from their conjugate bonds [100].
Cyclodextrins
Cyclodextrins are α-1,4-linked cyclic oligosaccharides consisting of several glucopyranose units [101]. The targeted colonic drug delivery system approach using β-cyclodextrins is through the formation of drug conjugates with β-cyclodextrins. These conjugates will form esters or amide bonds from drug carboxyl groups and hydroxyl groups on β-cyclodextrins to improve chemical and physical stability, solubility, and biocompatibility. Degradation of β-cyclodextrins in the colon is due to hydrolysis by α-amylase, an enzyme secreted by Bacteroides, into saccharide conjugates, such as maltose and ester conjugates. The ester conjugate is then hydrolyzed by the carboxyl-esterase so that the drug can be released [59]. Madan et al. [102] demonstrated that the dissolution test of the cyclodextrin-bromonoscapine complex coated with guar gum at pH 7.4 showed that only 7.9% of the drug was released. Meanwhile, at pH 6.8, the cyclodextrin-bromonoscapine complex coated with guar gum showed that 30.4% of the drug was released. In simulated colonic media with a pH of 7.0 and a content of 6% rat cecal, 55.6% of the drug was released. Drug release can be prolonged because of the cross-links between glutaraldehyde and the hydroxyl groups of galactose and mannose thereby preventing water absorption. When it reaches the colon, drug release is induced by enzymatic reactions by colonic bacteria. Another study by Arulraj et al. [60] confirmed that the release study of celecoxib-coated guar gum-cyclodextrin matrix showed 1.6%, 7.2%, and 88.4% of the drug was released at pH 1.2 for 2 hours, pH 7.4 for 6 hours and pH 6.8 for 2 hours, respectively.
Polymer
Polymers can be classified according to their constituent units as homopolymers consisting of one type of repeating unit and copolymers consisting of two or more types of repeating units. Copolymers can be classified according to the arrangement of the units as alternating, random, block, or graft. Polymers are widely used in the formulation of targeted delivery systems to the colon because of their ability to dissolve, swell, and change their conformation due to protonation or deprotonation caused by pH changes in the gastrointestinal tract. Polymers can also be classified into two types based on their ionization properties, namely polyanions and polycations, illustrated in Figure 1 [83]. Polyanions have ionizable acid groups, such as carboxylic acid (–COOH), sulfonic acid (–SO3H), or phosphonic acid (–PO3H2), which bind to the polymer. When there is an excess of protons in acidic pH, the acid group will experience protonation to form polymer bonds. Conversely, the acidic groups will be ionized in an alkaline pH and release protons, causing the development of the polymer due to electrostatic repulsion from negatively charged groups [83]. The carboxylic group will accept protons at low or acidic pH and release protons at high pH values [103]. The release of the drug from the system occurs when polymers swell due to pH and ion exchange [104]. The polycation has an ionizable base group, such as an amine (–NH2), which binds to the polymer. Polycation will accept protons in an acidic pH environment, resulting in a positive charge, which leads to the swelling of the polymer system due to the electrostatic repulsion. Some examples of synthetic polycation are polyvinyl amine, poly (2-aminoethyl methacrylate), and polyethylene imine (PEI). One example of natural polycation is chitosan. Excipients of polymer used for colon-targeted drug delivery system can be seen in Table 2.
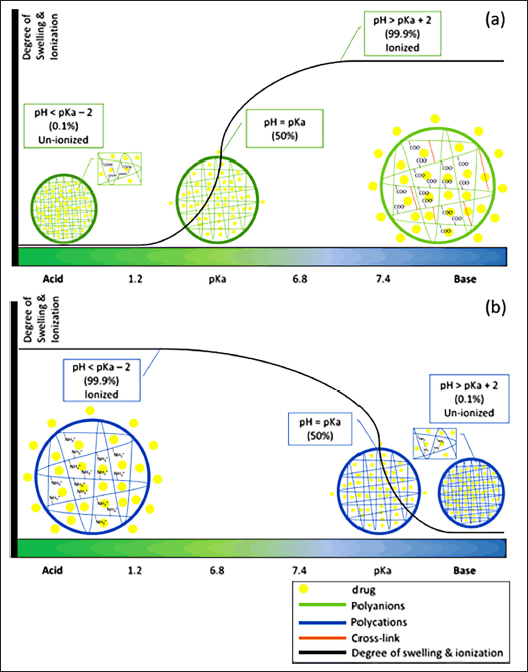 | Figure 1. Relationship of pH, swelling, and ionization of polymer matrix of (a) polyanions and (b) polycations. [Click here to view] |
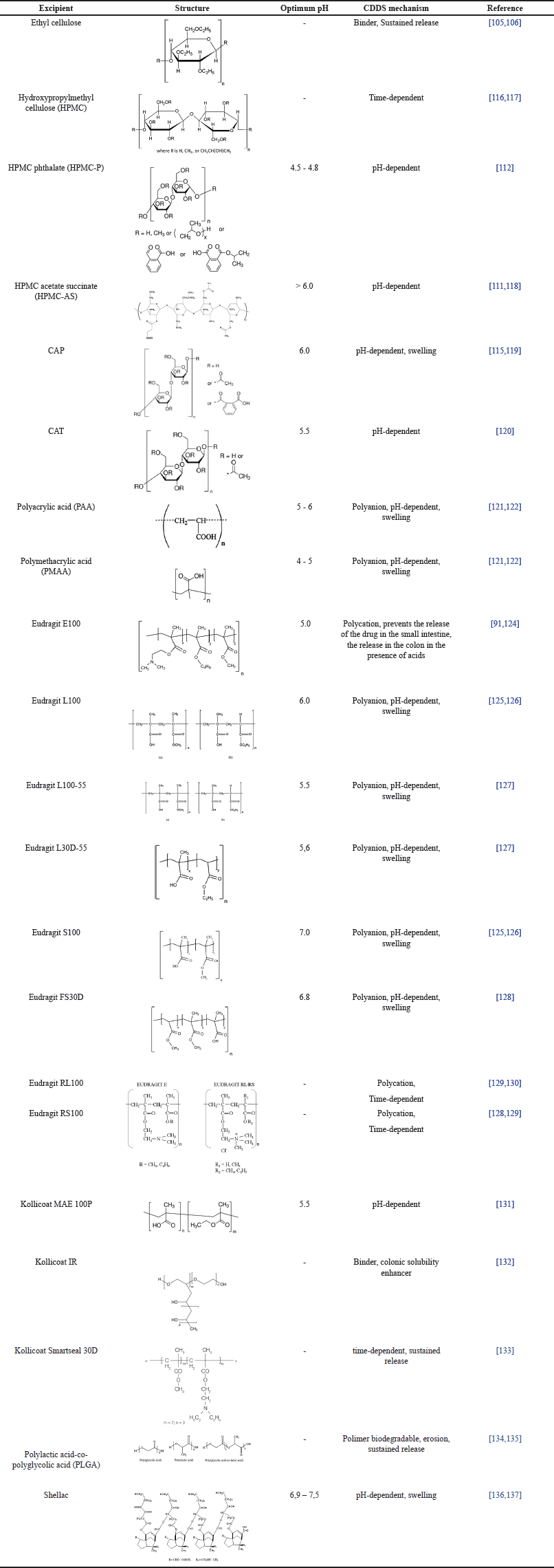 | Table 2. Excipients of polymer used for CDDS. [Click here to view] |
Cellulose derivatives
The cellulose group polymers that are often used for CDDS are ethyl cellulose, hydroxypropyl methylcellulose (HPMC) and their derivatives, and cellulose acetate derivatives. Ethyl cellulose is a linear polysaccharide with a hydrophobic β-anhydroglucose ring which has a hydroxyl group that is substituted with an ethoxy group [105]. Ethyl cellulose is often used as a polymer for the sustained release of drugs. The combination of enteric polymer (Kollicoat MAE 100DP) and ethyl cellulose as a binder can protect the release of the drug in an acidic medium for 2 hours. When it reaches a pH of 6.8, the enteric polymer will dissolve first and ethyl cellulose, which dissolves only at a lower pH, can be used to protect the drug released in the small intestine [106]. In terms of HPMC, it is a hydrophilic polymer that can be utilized for sustained release systems with gel formation [56] so that the drug release can be prolonged [107,108]. Increasing the HPMC concentration can reduce drug release because this polymer tends to form a gel at high concentrations, thereby reducing drug diffusion [37]. One of the modified HPMCs is hydroxypropyl methylcellulose phthalate (HPMCP). It is an enteric polymer consisting of phthalate esters linked to HPMC. Curcumin pellets coated with Pectin and HPMC (1:3) showed a minimum release at pH 1.2 of 0.19%, pH 7.2 of 19.72%, and pH 6.8 of 89.91% during 2 hours [108]. In addition, flurbiprofen loaded in an HPMC matrix and coated with Eudragit S showed a release of 18.41% ± 0.68% after 5 hours in simulated gastric and small intestine medium and increased to 100.43% ± 3.33% in colonic simulation media after 24 hours [107].
HPMCP is often used as an enteric polymer to prevent drug release in the stomach [109]. Another polymer that is widely used for preventing drug release in acidic environments is hydroxypropyl methylcellulose acetate succinate (HPMCAS). The addition of acetyl and succinyl groups to HPMC makes this polymer undissolvable at low/acidic pH. The release of metoprolol succinate coated with the combination of Eudragit-P4135F and HPMC-AS nanoparticles showed that 0% drug was released at pH 1.2 and 94.34% at pH 6.8 [110]. Another study also supported that the release of mesalazine coated with HPMCAS showed that 0.98% ± 1.28%, 6.451% ± 2.14%, and 98.47% ± 1.34% of the drug was released at pH 1.2 for 2 hours, pH 6.0 for 3 hours and pH 7.2 for 5.5 hours, respectively [111]. Additionally, the release of metronidazole coated with Eudragit and Chitosan-HPMCP showed a minimum release at pH 1.2 of 5.57% ± 1.34% and 8.46% ± 2.49% in 2 hours and 5 hours, respectively. The release of metronidazole increased in colon simulation media due to degradation by colonic microbes [112].
CAP is another enteric polymer that dissolves at a pH above 6.0 [113]. CAP is a cellulose derivative that has a hydroxyl group which is acetylated and esterified with phthalic acid [114]. The in vitro study of the release of ketorolac tromethamine capsules coated with guar gum and CAP showed no drug release in the first 2 hours in a pH 1.2 medium and 21.29% of the drug released at pH 7.4 for 3 hours. Meanwhile, the cumulative release can reach 99.18% for 25 hours in colonic conditions at pH 6.8 [115]. Different from CAP, cellulose acetate trimeliate (CAT) is an enteric polymer derived from cellulose which in the hydroxyl group undergoes acetylation and esterification with melicic acid [114]. Microspheres containing indomethacin coated with CAT experienced a 10–15% release in the first 30 minutes. This release can occur due to incomplete entrapment of indomethacin in the microsphere. At a pH of 6.8, there was an increase in drug release, and it reached 100% in one hour. This is due to the increasing solubility of CAT at pH 6.8 where CAT has an optimum solubility above pH 5.5 [120]. These enteric polymers are frequently used in combination with other polymers, such as polysaccharides, to increase the effectiveness of colonic delivery [111].
Poly(acrylic acid) (PAA) and poly (methacrylic acid) (PMAA)
PAA is an acrylic acid polymer that has a carboxylic group in each monomer unit or every two carbon atoms of the main chain [138]. This polymer contains free carboxylic acid which functions as a pH-sensitive delivery system. These carboxylic groups do not ionize at acidic pH and ionize at neutral or weak alkaline pH [139]. In acidic pH, the polymer will form hydrogen bonds in the carboxylic group, thus releasing the drug from the polymer matrix. On the other hand, at pH 7.6 (above the pKa of PAA), the carboxyl group will be ionized to form COO- which is negatively charged, causing electrostatic repulsion. This results in the polymer swelling and the drug can diffuse out of the matrix. The release study of doxorubicin without using PAA showed 78% and 10% at pH 2.0 and 7.6. However, the release of doxorubicin coated with PAA showed 20% and 64% drug release at pH 2.0 and 7.6 for 12 hours, respectively [121].
Poly (methacrylic acid) (PMAA) or poly (2-methyl-2-propenoic acid) is a polymer of methacrylic acid having a methyl group substituted at carbon position 2 [140]. PMAA has a pKa value ranging from 4 to 5, so PMAA can maintain its structure at gastric pH and will swell at neutral pH due to the ionization of carboxylic groups and electrostatic repulsion [123]. The release of ibuprofen-coated GGOg-PMAc hydrogel showed that the drug could be released for 12%, 38%, and 50% at pH 1.2 for 3 hours, pH 7.4 for 4 hours and pH 6.8 for 21 hours, respectively [141].
Eudragit®
Eudragit® is a pH-dependent copolymer of methacrylic acid which is a registered trademark of Rohm Pharmaceuticals, Darmstadt, Germany [142]. Eudragit® E is a cationic polymer that dissolves at an acidic pH of about 5.0 and is used to prevent drug release in the small intestine. The lag time of capsules coated with Eduragit E-100 increased when the pH of the system also increased. The solubility of Eudragit® E-100 increases in the pH below 6.5. Eudragit® E-100 can prevent drug release as they are not dissolved at neutral pH in the small intestine [124].
On the other hand, Eudragit® L and Eudragit® S are polyanion polymers that can be used to prevent drug release in the stomach and dissolve at the pH of the intestine. Eudragit® L is a copolymer of methacrylic acid and methyl methacrylate with a ratio of 1: 1 having a solubility at pH 5.5 to 6 whereas Eudragit® S is a combination polymer of methacrylic acid and methyl methacrylate (1:2), has a solubility at pH 6.5 [139]. Metronidazole-coated chitosan microspheres release study with Eudragit L-100 showed 1.4% ± 0.04% released at pH 1.2 for 3 hours. Eudragit L-100 dissolves at pH above 6.0 so that at pH 6.8 it is 6.7% ± 0.25% for 4 hours. The drug release after 12 hours on Eudragit L-100 was 69.3% ± 3.12%. The addition of rat cecal content increased drug release to 82.4% ± 3.28%. The study of metronidazole release in chitosan microspheres coated with Eudragit S-100 showed that there was no drug release at pH 1.2 during the first 3 hours. Meanwhile, at pH 6.8, it showed 56.9% ± 2.65% for 12 hours. The addition of rat cecal contents increased drug release to 69.8% ± 3.17% [125]. Similarly, the release of aceclofenac from a chitosan matrix coated with Eudragit L-100 and S-100 showed no drug release at pH 1.2 and 9.2% ± 0.62% of the drug released at pH 7.4. The condition of pH 7.4 added with rat cecal contents showed drug release of 102.24% ± 1.2% due to the presence of enzymes that penetrate the gel layer which can lead to gel erosion and increased drug release [126].
Eudragit® RS and Eudragit® RL are the other types of polymer that can be used for CDDS. Drug release from Eudragit® RL and Eudragit® RS is time-dependent or sustained release. Eudragit® RS and Eudragit® RL are copolymers of acrylic and methacrylic acid esters, each containing a quaternary ammonium group between 4.5% and 6.8% (Eduragit RS), and 8.8% and 12% (Eudragit RL) [127]. The release study of Capecitabine microspheres indicated that 6.79% ± 1.85% to 3.59% ± 1.23%, 7.07% ± 1 .23% to 3.22% ± 1.56%, and 3.86% ± 0.74% to 2.99% ± 0.82% at pH 1.2 in the first 4 hours when coated with Eudragit RL-100, Eudragit RS-100, and Eudragit RL/RS-100, respectively. The cumulative drug percentage at pH 6.8 of 96.80%–89.17% for Eudragit RL-100, 94.49%–71.82% for Eudragit RS-100, and 97.07%–83.66% for Eudragit RL/RS-100 [129]. Based on the different properties of Eudragit®, EUDRACOL is a combination system for CDDS depending on pH and time by combining Eudragit® RL or Eduragit® RS (depending on time) with Eudragit® FS30D (depending on pH) [143].
Kollicoat®
Kollicoat® is an excipient produced by BASF, Germany. Several types of Kollicoat® that can be used for CDDS are Kollicoat® IR, Smartseal 30D, and MAE 100P. Kollicoat® IR is a copolymer of polyvinyl alcohol and polyethylene glycol. The addition of Kollicoat® IR to the formulation aims to increase the rate of drug dissolution in the colon The prednisolone coated with Kollicoat IR and Eudragit S-100 release study showed no release at pH 1.2 for the first 5 hours and then a sharp increase in pH 7.4 at 6 hours [132].
Different from Kollicoat® IR, Kollicoat® Smartseal 30D is a copolymer dispersion of methyl methacrylate and diethylaminoethyl methacrylate with a ratio of 6:4. The uses of Kollicoat® Smartseal 30D showed an increase in the drug released after 3 hours. This suggests that increasing Kollicoat® Smartseal 30D will increase the delay time and inhibit drug diffusion. The release study of the combination of chitosan and Kollicoat Smartseal 30D (25:75) indicated that drug release of 0.1% ± 0.04% at pH 1.2 in 2 hours, 9.1% ± 0.7% of drug released after 310 ± 6.0 minutes and in colon simulation liquid with the addition of microflora there was a sharp increase at 562 minutes [133].
Kollicoat® MAE 100P is a copolymer of methacrylic acid and ethyl acrylate with a ratio of 1:1 [144]. Kollicoat® MAE 100P showed that enteric polymers could protect against drug release at pH 1.2. Kollicoat® MAE 100 P has optimal solubility at pH above 5.5. Kollicoat MAE 100P begins to dissolve at pH 4.6 and releases 10% of prednisolone. Moreover, the drug release of prednisolone can reach 80% at pH 6.8 and 10% of the residual drug at pH 7.4 [131].
Poly (lactic-co-glycolic acid) (PLGA)
PLGA is a linear aliphatic copolymer consisting of lactic acid and glycolic acid monomers linked by ester bonds. PLGA can be used for drug delivery systems and drug encapsulation for controlled release. PLGA can be degraded by the hydrolysis of ester groups [145]. The combination of 5 mg Ibuprofen nanoparticles and 95 mg PLGA showed a time-dependent release [134]. PLGA can be used as a nanoparticle polymer because it is biodegradable and biocompatible. The release of the drug from the PLGA polymer was caused by erosion and degradation [139]. An in vitro release study of budesonide nanoparticles coated with PLGA at pH 6.8 for 72 hours showed a burst release of 52% in the first 1 hour followed by a final release of 58% in 72 hours. The burst effect may be due to the presence of drug molecules not encapsulated in the polymer core or drugs on the surface so that they can be released quickly [146]. The release study of curcumin by PLGA showed that 10% of the drug was released at 1,2 and 6,8 in 5 hours. The release increased by 56%, and 78% in the first 24 and 48 hours at pH 7.4, respectively. This is also influenced by the higher solubility of curcumin at neutral pH than under acidic conditions [147].
Shellac
Shellac is a resin secreted by the insect Laccifer lacca that prevents drug release in the upper gastrointestinal tract [148]. Shellac is a biodegradable hydrophobic polymer with a pKa of 5.6 to 6.6 so Shellac does not dissolve in gastric pH [136]. However, it is unsurprising that this type of polymer will dissolve in neutral pH [149]. The release study of anthocyanin-pectin beads coated with 15% Shellac showed 46%, 46%, and 88% of the drug released at pH 2.0, pH 6.3, and pH 6.2 with the addition of enzymes, respectively. This result strongly indicates that increasing the Shellac concentration will decrease the rate of drug release [150].
MECHANISM OF EXCIPIENTS IN COLON DRUG DELIVERY SYSTEM
The various mechanisms of excipients in CDDS can be described in Figure 2.
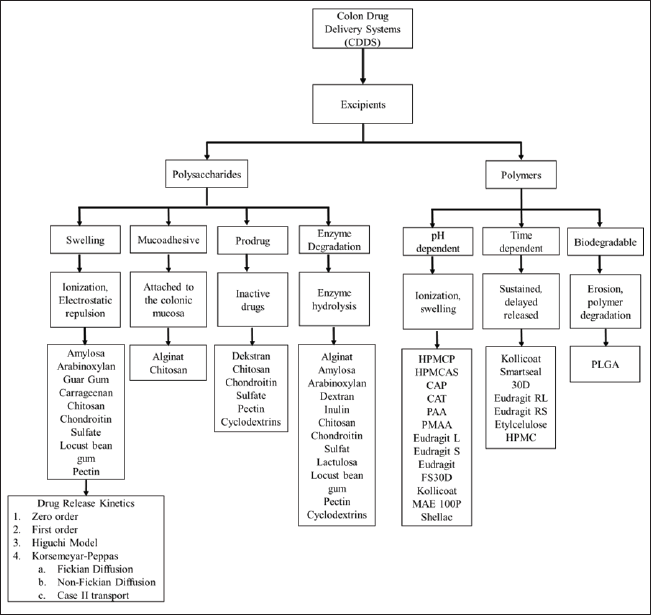 | Figure 2. Various mechanisms of excipients for CDDS. [Click here to view] |
pH-dependent system
This pH-dependent delivery system uses an approach to pH differences in the gastrointestinal tract. The pH-dependent drug delivery systems are essential for targeted drug release in the colon because they utilize the distinct pH variations throughout the gastrointestinal tract to deliver the drug only to the colon site. In the stomach, under normal conditions, the pH is between 1 and 3.5 and can increase to 4.6 after eating. Meanwhile, the pH of the small intestine ranges from 5.5 to 7.5, and in the colon, it is around 5.7 to 6.7 [151]. The pH conditions in the colon are slightly lower compared to the small intestine due to acidification of the colonic contents due to fermentation products from bacteria in the colon [152,153]. Based on the differences in physiological pH conditions of the gastrointestinal tract, excipients that are insoluble at acidic pH can be used to protect the drug from being released at the gastric site but can dissolve at alkaline pH when the drug reaches the intestine and colon [153]. The excipient used has special characteristics, namely that it does not dissolve under acidic conditions, but the dissolution rate of the excipient will increase when the pH shifts towards alkaline conditions [154]. Excipients that can protect drugs from acidic pH are enteric polymers or polyanions. Examples of enteric polymers include HPMC phthalate (HPMC-P), hydroxypropyl methylcellulose acetate succinate (HPMC-AS), CAP, cellulose acetate trimeliat (CAT), PAA, Poly methacrylate, Eudragit L, Eudragit S, Eudragit FS30D, Kollicoat MAE 100P, and Shellac. Enteric polymers play a crucial role in pH-dependent drug delivery systems by preventing drug release in the acidic environment of the stomach. These polymers are designed to remain insoluble at low pH values, thereby protecting the drug from early release. The enteric polymers will dissolve in the more alkaline conditions of the colon, which can allow the drug to be released.
The mechanism of drug release from this matrix is based on the pKa of the polymer and the pH of the medium or system. In general, using polyanion polymers is an ideal excipient to prevent drug release at lower pH values and promote the release of higher pH drugs [83]. Based on the Henderson-Hasselbalch equation (Equation 1), the polymer will be fully ionized at a pH above pKa + 2 and almost not ionized at a pH below pKa – 2. Meanwhile, when the pH of the system is the same as the pKA of the polymer, the ionization value is 50% [20].
where pH is the pH value of the solution, pKa is the acid-ionization constant of the polymer, [A-] is the concentration of the conjugate base or the ionized form of the polymer, and [HA] is the concentration of the weak acid or the non-ionized form of the polymer. This ionization behavior affects the polymer’s solubility and swelling properties, which in turn influences drug release. For instance, a polymer that is non-ionized and less soluble at acidic pH will protect the drug from release in the stomach but will dissolve and release the drug in the alkaline environment of the colon.
Figure 1 shows the drug-released mechanism of polymers, both polyanion and polycation, in several pH conditions [83]. The pH-dependent system can be affected by the swelling properties of polymers. The swelling ratio is the ability of the polymer to expand when hydrated so that it can trigger the release of drugs that are in the matrix. The swelling ratio is influenced by the presence of acid group ionization, hydrogen bonds, cross-linking of polymers, and the concentration of coatings with other polymers. In polyanions, acidic groups such as carboxylic (COO–) will undergo protonation to become COOH under acidic conditions. This protonation forms hydrogen bonds which prevent the polymer from swelling. Conversely, at alkaline pH, the carboxyl group will be deprotonated or lose protons so that it ionizes and becomes negatively charged (COO–). The presence of this negatively charged group causes electrostatic repulsion of similar ionic charges which can lead to polymer swelling and trigger the release of the drug from the polymer matrix [83].
Time dependent
The time-dependent targeted delivery system to the colon uses a non-biodegradable polymer that can coat the drug core where the excipient can be dissolved during the drug transit to the colon so that the new drug core is released at the colonic site. This system relies on the transit time of the drug from the gastrointestinal tract where the drug is expected to be released when the drug reaches the colon [20]. Transit time is crucial for drug release in time-dependent targeted delivery systems because it affects how long the drug remains in the gastrointestinal tract before reaching its intended site of action, such as the colon. The time-dependent system ensures that the drug is released only after a specific period, which aligns with the average transit time to the colon. By adjusting the release mechanism to match these transit times, the system can precisely time drug release in the colon.
The average transit time for the drug to reach the ileocecal junction is 3 to 4 hours and will slow down when it reaches the large intestine, which is around 20 to 35 hours. Increasing residence time in the colon will increase the contact time of the preparation with the microflora in the colon so that this delivery system can be an approach to deliver drugs to the colon [155,156]. Examples of excipients that have a time-dependent delivery mechanism include Kollicoat Smartseal 30D, Eudragit RL, Eudragit RS, Ethyl Cellulose, and Hydroxypropyl Methylcellulose. This system can be combined with enteric coating to prevent premature drug release in the stomach [4]. Enteric coatings protect the drug from being released in the acidic environment of the stomach and upper gastrointestinal tract. This ensures that the drug remains intact until it reaches the more alkaline environment of the small intestine and colon. Once the enteric coating is dissolved in the intestine, the time-dependent polymer mechanism takes over. This polymer coating then dissolves according to a set time frame, allowing the drug to be released specifically when it reaches the colon and preventing premature release in the small intestine.
Mucoadhesive system
Mucoadhesive is the property of the drug to be able to attach to the colonic mucosa. The mechanism of mucoadhesive polymer is that the excipient will swell and bind to the mucous membrane to stick to the mucosa. The mechanism of the mucoadhesive system can be illustrated in Figure 3. Mucoadhesion is crucial in colon drug delivery as it enhances drug retention in the colonic mucosa, leading to prolonged contact time and potentially improved drug absorption. Upon administration, the mucoadhesive drug formulation reaches the colonic mucosa. The formulation comes into direct contact with the mucus layer lining the colon. Mucoadhesive polymers in the formulation swell upon contact with the mucosal surface. This swelling increases the surface area of the polymer in contact with the mucus. The swollen mucoadhesive polymer forms physical and chemical bonds with the mucous membrane. These bonds can include hydrogen bonds, van der Waals forces, and ionic interactions. The binding mechanism slows down the movement of the drug formulation, leading to prolonged residence time in the colon. This extended contact enhances drug absorption and efficacy. As the formulation remains in contact with the mucosal surface, the drug is gradually released. The prolonged interaction facilitates sustained drug release in the targeted area.
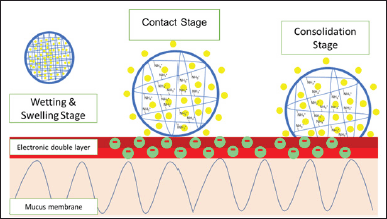 | Figure 3. Mucoadhesive properties mechanism using chitosan (polycation). [Click here to view] |
This mucoadhesive property can increase drug release because it can slow down the retention time of the drug in the colon. At neutral pH, the charge of polysaccharides can interact electrostatically with the mucous membrane so that it has higher mucoadhesive properties than at acidic pH [85]. At neutral pH, the charges on polysaccharides interact more effectively with the charged groups on the mucosal surface, enhancing adhesion. Moreover, neutral pH conditions can facilitate better hydration and swelling of mucoadhesive polymers, improving their ability to form strong bonds with the mucus.
Enzymatic degradation
The drug delivery system targeted to the colon uses a degradation mechanism by the microflora in the colon, requiring coating with polysaccharides. The mechanism of this delivery is that polysaccharides will expand and be degraded by enzymes secreted by microflora in the colon. When the drug reaches the colon, the microflora in the colon will secrete enzymes to break down the excipients so that the drug can be released from the system [4]. Examples of excipients that have a mechanism for delivering degradation by microflora in the colon include alginate, amylose, arabinoxylan, dextran, guar gum, inulin, carrageenan, chitosan, chondroitin sulfate, lactulose, locust bean gum, pectin, and cyclodextrin.
The mechanism of CDDS using polysaccharide class excipients is enzymatic degradation by microflora in the colon. The polysaccharides used cannot be degraded in the upper gastrointestinal tract but these polymers are degraded by specific enzymes produced by the colonic microflora. Polysaccharides will be hydrolyzed by enzymatic reactions in the colon and the glycosidic bonds in polysaccharides are broken into saccharides so that the drug can be released from the polysaccharide matrix [30,31]. Some of the polysaccharides that are degraded by enzymes secreted by the colonic microflora can be seen in Table 3.
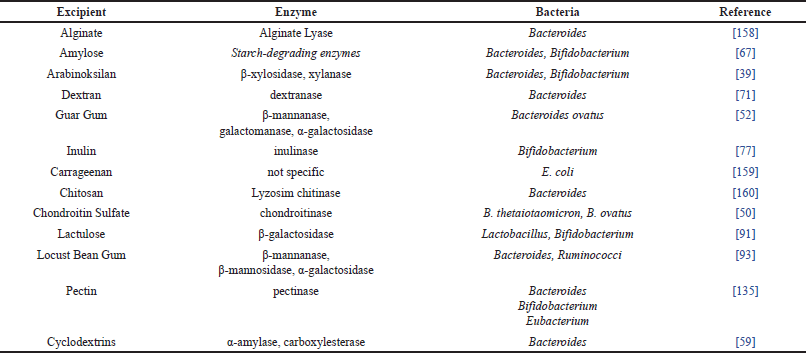 | Table 3. Colonic enzyme degradation of polysaccharide excipients. [Click here to view] |
Prodrug system
A prodrug is an inactive form of a drug that has no pharmacological action but can be converted to its active form in the gastrointestinal tract. When it reaches the colon, the prodrug will be degraded by specific enzymes secreted by bacteria in the colon, such as dextran by dextranase, pectin by pectinase, and cyclodextrin by α-amylase and carboxylesterase. Following the degradation by bacterial enzymes in the colon, the hydrogen bonds between the drug and the conjugate will be hydrolyzed into saccharide conjugates, then the drug can be released in its active form [41,59,73,100]. The prodrug system can be used as a drug delivery system targeted to the colon by a metabolism mechanism or activation by enzymes in the colon. Prodrug is another form of drug that has a lower pharmacological effect than its original form but can be activated into its original form. Prodrugs can be formed by encapsulating the drug with a conjugate so that a bond occurs between the drug and the conjugate [157]. Examples of excipients that have a mechanism for delivering degradation by microflora in the colon include dextran, chitosan, chondroitin sulfate, pectin, and cyclodextrin.
For instance, Metronidazole made as a prodrug with pectin released 1% to 5% of the drug in pH 1.2 media for 2 hours and pH 6.8 for 6 hours and increased at pH 7.0 with a rat cecal content of 67.9% for 8 hours. The presence of enzymatic degradation by bacteria in the colon causes the release of the drug from its conjugate bond [100]. In another study, the release of 4-amino salicylic acid prodrug conjugated with β-cyclodextrin showed that there was a release of 20%–23% in the stomach and small intestine and 92% release in the colon for 8 hours. This shows that the prodrug is not able to deliver the drug completely to the colon even though the highest percentage of drug release is in the colon. Therefore, it is necessary to add other excipients such as enteric polymers to prevent drug release before reaching the colon [161].
DRUG RELEASE KINETICS FROM POLYMER MATRIX
The factors of drug release can be related to the dissolution and diffusion processes. The coating concentration can affect the release of the drug from the polymer matrix [139]. Based on the Noyes-Whitney/Nernst-Brunner equation (Equation 2), higher coating concentrations increase the thickness of the diffusion layer around the drug. According to the Noyes-Whitney/Nernst-Brunner equation, a thicker diffusion layer reduces the dissolution rate of the drug, as the drug should diffuse through a greater thickness of coating material. Moreover, as coating concentration increases, the dissolution rate decreases due to the increased barrier for drug diffusion. This can be advantageous for controlling the release rate. Furthermore, achieving sink conditions, where the drug concentration in the solution is maintained at a lower level, can help facilitate continuous drug release by maintaining a concentration gradient [20].
where dW/dt is the dissolution rate (g/sec), W is the mass of the drug dissolved in time (t), D is the diffusion coefficient (cm2/second), S is the surface area (cm2), h is the membrane thickness (cm), Cs is the saturated drug concentration (g/cm3), and C is the drug concentration in solution at time t (g/cm3).
The release of drugs from the targeted delivery system to the colon will protect or prevent the premature release of drugs in the stomach and small intestine as well as increase the release of drugs in the colon. The ideal drug release profile for a CDDS is to have a time lag. This time delay is due to the presence of drug coating so the drug takes time to diffuse through the polymer matrix. The ideal delay for CDDS is when the drug reaches the colon in the range of 6 to 8 hours after drug administration. But in reality, drug release in the targeted drug delivery system to the colon can also have a burst effect. The burst effect that occurs at acidic pH is an indicator that there is drug release in the stomach so that the drug delivery system fails to deliver the drug to the colon [139]. This often indicates that the coating is insufficient or not properly designed to withstand the acidic conditions of the stomach, leading to failure in targeting the colon effectively. Therefore, the Noyes-Whitney/Nernst-Brunner equation helps quantify this balance, highlighting the trade-offs between coating thickness and dissolution rates, which directly affect the effectiveness of drug delivery to the colon.
CONSIDERATION FACTORS IN COLON DRUG DELIVERY SYSTEM
Selection of appropriate excipients for CDDS
The choice of excipients affects drug release profiles, stability, and effectiveness. Excipients must be compatible with the active drug to ensure drug stability and avoid undesirable interactions. The selection of appropriate excipients for CDDS can also help in designing systems that can deliver drugs specifically to the colon, overcoming challenges such as premature drug release and variable gastrointestinal conditions. Therefore, the selection of appropriate excipients for CDDS should consider the physicochemical properties of active substances and excipients, the approach to delivery mechanisms, drug and excipient stability, and interactions between drugs and excipients.
Polysaccharide group excipients have several main mechanisms, such as swelling, mucoadhesive, prodrug, and degradation by colonic microflora enzymes. In general, the main mechanism of drug release using polysaccharide excipients occurs due to degradation by enzymes secreted by microflora in the colon. Apart from that, polysaccharide excipients have a swelling or swelling mechanism, such as alginate, amylose, guar gum, pectin, and others, allowing the drug to be released by the principle of diffusion or relaxation of the polymer matrix. Polysaccharides that have a mucoadhesive mechanism, such as alginate and chitosan, are often used to formulate drugs for IBD or local drugs to increase the retention time of drugs in the colon. Polysaccharides often form prodrugs such as dextran, chitosan, chondroitin sulfate, pectin, and cyclodextrin. The formation of a prodrug aims to minimize drug absorption at other sites before it reaches the colon by increasing the hydrophilicity of the drug and/or the size of the drug molecule.
Polymer group excipients have several main mechanisms, such as pH-dependent, time-dependent, and biodegradable. The use of pH-dependent excipients such as HPMCP, HPMCAS, CAP, CAT, Eudragit L, Eudragit S, and others aims to protect and prevent drug release in acidic pH (stomach) so that drug absorption does not occur in the upper gastrointestinal tract. The use of excipients intended for time-dependent releases, such as Kollicoat Smartseal 30D, Eudragit RL, Eudragit RS, Ethyl Cellulose, and HPMC, aims to increase the delay time or time lag of drug release and regulate drug release gradually. Biodegradable excipients such as PLGA have a release mechanism, namely erosion or degradation of the polymer, which aims to control the release of the drug so that there is no sudden large release.
Selection of the concentration of excipients used in the formulation for CDDS
Furthermore, the concentration of excipients used is very influential in drug release. According to the Noyes-Whitney/Nernst-Brunner equation (Equation 2), the increased concentration of excipients can prolong drug release time and even inhibit drug release. Nevertheless, the excipient concentration that is not sufficient will cause a burst effect. The burst effect can also be caused by an imperfect coating process so that there is no drug trapped in the polymer matrix [139]. The Noyes-Whitney/Nernst-Brunner equation is crucial for understanding how excipient concentration affects drug release, as it relates the rate of dissolution to the thickness of the diffusion barrier and the concentration gradient. Key parameters include the diffusion coefficient, surface area, and thickness of the coating, where a thicker barrier (from a higher excipient concentration) can slow drug release, while a thinner barrier (from a lower concentration) may lead to premature release. If the concentration of excipients is too high, it can create a thicker diffusion barrier. This often results in slower or incomplete drug release because the thicker barrier slows down the diffusion of the drug through the coating. Conversely, if the concentration is too low, the coating may be inadequate. This can lead to premature or burst release of the drug, as the insufficient coating fails to retain the drug within the matrix effectively.
In addition, several factors should be considered in preparing the coating solution for CDDS, which are the type of solvent, the concentration of excipients in the coating solution, and the concentration of the coating solution used in the formulation. The choice of solvent can affect the solubility and dispersion of excipients. Solvents must dissolve the excipients adequately and ensure a uniform coating. Moreover, the concentration in the coating solution determines the thickness and effectiveness of the coating. Higher concentrations can produce thicker coatings, which might delay drug release, while lower concentrations might result in inadequate coatings. Furthermore, the concentration of the final coating solution used during the coating process affects the uniformity and adherence of the coating.
Utilization of a combination of excipients for CDDS
Additionally, the use of a combination of excipients, such as polysaccharides with enteric polymers, can prevent a burst effect in the stomach and small intestine and can increase the delay time and effectiveness of CDDS. The combination of polysaccharides and polymers is currently widely developed to overcome some of the drawbacks of each excipient. The use of a combination of polysaccharides and polymers is used to protect early drug release in the stomach [162]. In addition, the use of this combination also prevents early drug release that occurs in the small intestine before reaching the colon where the small intestine has a slightly more alkaline pH condition compared to the colon [163].
Some polysaccharides cannot be used alone for a targeted colonic drug delivery system because they release the drug in the upper gastrointestinal tract before it reaches the colon, although perhaps the highest percentage of drug released is in the colon [59,79,60,164]. Several studies using a combination of polysaccharides and enteric polymers can be seen in Table 4. In addition, the combination of polymers with polymers can also be utilized, such as combinations of time-dependent polymers (Eudragit RS or RL) with pH-dependent polymers (Eudragit L or S or FS). This is used to prevent premature release of drugs in both the stomach and small intestine, thereby increasing the effectiveness of the drug delivery system to the colon. The mechanism of the combination of enteric and sustained polymers or enteric polymer and polysaccharide can be seen in Figure 4(a) and (b), respectively.
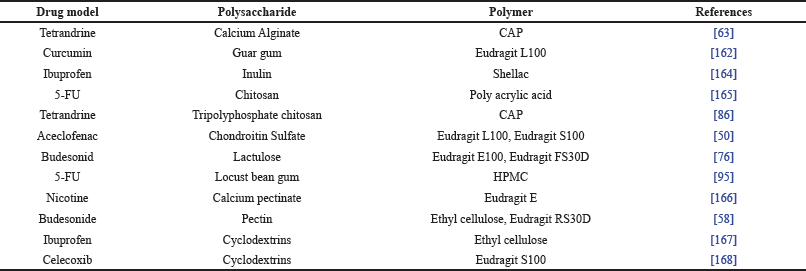 | Table 4. Polysaccharide and polymer combination for CDDS. [Click here to view] |
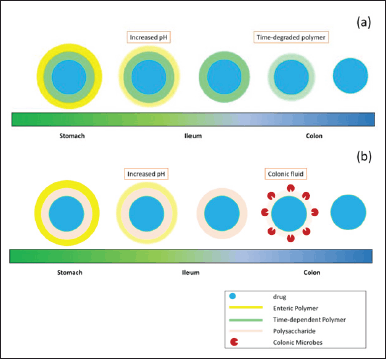 | Figure 4. Mechanism of combined excipients for CDDS using (a) enteric and sustained polymers, (b) enteric polymer and polysaccharide. [Click here to view] |
One of the drug delivery systems to the colon using a combination of polymers and polysaccharides is the Novel CODESTM. This system consists of polysaccharides (lactulose), acid-soluble polymers (Eudragit E), and enteric polymers (Eudragit L). Enteric polymers will protect the drug from being released in the stomach, but when it reaches the small intestine, the enteric polymer will dissolve rapidly. In the small intestine, acid-soluble polymers will prevent the release of the drug until it reaches the colon. When the drug is in the colon, the microflora in the colon will degrade lactulose into lactic acid and dissolve the acid-soluble polymer so that the drug can be released [4, 169].
Excipient modification for CDDS
Several modifications to the excipient used in targeted colonic drug delivery systems can be utilized to increase the effectiveness of CDDS. Modification of these excipients can improve their functional properties and/or improve other properties. Esterification involves reacting excipients with acids to modify their chemical properties. For example, hydroxypropyl methylcellulose acetate succinate (HPMCAS) is created by esterifying hydroxypropyl methylcellulose (HPMC) with anhydrous acetic acid and succinic anhydride. This modification results in a polymer with improved enteric properties, allowing it to withstand acidic conditions in the stomach and dissolve in the more alkaline environment of the intestine [111]. Another modification, the ionic Gelation uses ionic interactions to form a gel network within excipients. For instance, chitosan triphosphate-tetrandrine beads utilize ionic gelation to create a polyelectrolyte complex. This method enhances gel stability and swelling properties, which can improve drug retention and release profiles in the colon. Iswandana et al.. have developed Chitosan Triphosphate-Tetrandrine beads using ionic gelation to increase polyelectrolyte bonds that intensify the gel formed [86].
Evaluation for CDDS
The evaluation should be conducted to prove the effectiveness of CDDS such as development or swelling studies [79,81], and drug release both in vitro [54] and in vivo evaluation [66,73]. In vitro studies measure drug release and kinetics under controlled conditions, which can be utilized to optimize the formulation. Moreover, in vivo studies confirm how the CDDS performs within a complex gastrointestinal environment. In vitro-in vivo correlation (IVIVC) can show an association between drug release both in vitro and in vivo or absorption of drugs in the systemic circulation [58,86,95]. One of the IVIVC studies was carried out using prednisolone core tablets and tablets that had been coated with carboxymethyl xanthan gum and sodium alginate as a delivery system to the colon. There was a good linear correlation (r = 0.997) between the cumulative percentage of drug released in vitro from the core tablet and the percentage of drug absorbed in vivo. Meanwhile, tablets that had been coated for delivery to the colon had a poor correlation (r = 0.842) between the cumulative percentage of drug released and the percentage of in vivo drug absorbed. However, the cumulative percentage of colon-targeted drug released in vitro with the percentage of in vivo drug absorbed has a good correlation after a delay time of 360 minutes indicating drug release in the colon [152]. IVIVC can correlate between in vitro drug release data with in vivo drug absorption to predict clinical outcomes. A strong IVIVC, such as the one observed with prednisolone tablets, indicates that in vitro results can reliably forecast in vivo performance. This correlation aids in predicting plasma drug concentrations and therapeutic effectiveness.
Based on the IVIVC, the plasma concentration over time can be predicted which can be clinically meaningful from in vitro studies. However, in the IVIVC prediction, there may be some variations that can be caused by dissolution testing not clearly describing conditions in the gastrointestinal tract, such as transit time, pH, content, fasting conditions, number of microbes, enzymes, enterohepatic cycle, and first-pass metabolism. IVIVC predictions can also be affected by variations in gastrointestinal conditions, such as pH and enzyme activity, and individual physiological differences. These factors can lead to discrepancies between in vitro and in vivo results, highlighting the need for improved testing conditions and integration of both data types for accurate predictions. Therefore, the improved in vivo testing models that more accurately mimic human gastrointestinal conditions can help in better evaluating the performance of CDDS formulations. This can lead to more effective and safer drug delivery systems.
Future Perspective
CDDS has a very promising strategy for enhancing the oral drug delivery system which can increase the bioavailability of drug via targeting delivery to the colon. However, to enhance the effectiveness of CDDS, novel excipients with improved properties should be discovered and synthesized that offer better performance in diverse gastrointestinal environments and can lead to more effective CDDS formulations. Furthermore, the advanced drug delivery system, including the use of advanced nanocarriers and smart polymers, could offer improved precision in drug delivery. In addition, CDDS could also have a potential for personalized medicine approaches tailored to individual patient needs by considering genetic, physiological, and lifestyle factors that can optimize therapeutic outcomes. Personalized approaches could involve designing excipients that respond to specific biomarkers or conditions unique to individual patients. Some techniques such as 3D printing and microencapsulation may provide more customized and efficient drug delivery systems.
CONCLUSION
CDDS requires excipients that have specific characteristics to protect the drug released in the stomach and small intestine and increase the release of the drug in the colon. Polysaccharides have mechanisms including swelling and can be degraded by enzymes produced by colonic microflora which can facilitate the drug release in the colon site. Meanwhile, polymer-based excipients typically release the drug in the colon site through pH-dependent, time-dependent, or biodegradable mechanisms via diffusion release. Effective CDDS requires careful selection and optimization of these excipients. However, the implementation of these excipients presents challenges, including the complexity of formulation and the need for multiple excipients to address various biopharmaceutical needs. Therefore, future research should focus on refining excipient combinations, exploring novel polymers and polysaccharides, and addressing practical challenges in formulation by using advanced drug delivery systems.
ACKNOWLEDGMENTS
Maxius Gunawan would like to acknowledge the Faculty of Pharmacy, Universitas Indonesia which facilitates the completion of this review study.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
CONSENT FOR PUBLICATION
All authors have read and agreed with the content of this manuscript.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Chaubet F, Paris U. Pharmacology?: drug delivery. Encyclopedia of biomedical engineering. Amsterdam, The Netherlands: Elsevier Inc.; 2017. pp 1–14.
2. Kaur G, Grewal J, Jyoti K, Jain UK, Chandra R, Madan J. Oral controlled and sustained drug delivery systems: concepts, advances, preclinical, and clinical status. Drug targeting and stimuli sensitive drug delivery systems. Amsterdam, The Netherlands: Elsevier Inc.; 2018. pp 567–626.
3. Kohrs NJ, Liyanage T, Venkatesan N, Najarzadeh A, Puleo DA, States U. Drug delivery systems and controlled release. Encyclopedia of biomedical engineering. Amsterdam, The Netherlands: Elsevier Inc.; 2018. pp 1–14
4. Ray S. Advanced colon-specific delivery systems for treating local disorders. Polysaccharide carriers for drug delivery. Amsterdam, The Netherlands: Elsevier Ltd.; 2019. pp 737–62.
5. Lee SH, Bajracharya R, Min JY, Han J won, Park BJ. Strategic approaches for colon targeted drug delivery?: an overview of recent advancements. Pharmaceutics 2020;12(1):68.
6. Ma Z, Ma R, Wang X, Gao J, Zheng Y, Sun Z. Enzyme and PH responsive 5-fl urouracil ( 5-FU ) loaded hydrogels based on olsalazine derivatives for colon-speci fi c drug delivery. Eur Polym J. 2019;118(November 2018):64–70.
7. Iswandana R. Colon specific drug delivery systems: the importance. 2017;3–5.
8. Gaon D, Nagar S. A review on colon targeted drug delivery system. Int J Pharm Sci Res. 2019;10(1):47–56.
9. Patel MM. Colon targeting: an emerging frontier for oral insulin delivery. Expert Opin Drug Deliv. 2013;731–9.
10. Morales-Burgos AM, Carvajal-millan E, Rascón-chu A, Martínez-lópez AL, Lizardi-mendoza J, López-franco YL, et al. Tailoring reversible insulin aggregates loaded in electrosprayed arabinoxylan microspheres intended for colon-targeted delivery. J Appl Polym Sci. 2019;47960:1–8.
11. Deb PK, Al-attraqchi O, Chandrasekaran B, Paradkar A, Tekade RK. Protein/peptide drug delivery systems?: practical considerations in pharmaceutical product development. Basic fundamentals of drug delivery. Amsterdam, The Netherlands: Elsevier Inc.; 2019. pp 651–84.
12. Gadalla HH, Mohammed FA, El-Sayed AM, Soliman GM. Colon-targeting of progesterone using hybrid polymeric microspheres improves its bioavailability and in vivo biological e ffi cacy. Int J Pharm. 2020;577(November 2019):119070.
13. Zhu J, Zhong L, Chen W, Song Y, Qian Z, Cao X, et al. Preparation and characterization of pectin/chitosan beads containing porous starch embedded with doxorubicin hydrochloride: a novel and simple colon targeted drug delivery system. Food Hydrocoll. 2019;95:562–70.
14. Wang R, Huang J, Chen J, Yang M, Wang H, Qiao H, et al. Enhanced anti-colon cancer efficacy of 5-fluorouracil by epigallocatechin-3-gallate co-loaded in wheat germ agglutinin-conjugated nanoparticles. Nanomedicine. 2019;21:102068.
15. El-hady SM, Aboughaly MHH, El-ashmoony MM, Helmy HS, El-gazayerly ON. Colon targeting of celecoxib nanomixed micelles using pulsatile drug delivery systems for the prevention of inflammatory bowel disease. Int J Pharm. 2020;576(October 2019):118982.
16. Iwao Y, Tomiguchi I, Domura A, Mantaira Y, Minami A. Inflamed site-specific drug delivery system based on the interaction of human serum albumin nanoparticles with myeloperoxidase in a murine model of experimental colitis. Euro J Pharm Biopharm. 2018;125(October 2017):141–7.
17. Shahdadi H, Akhgari A, Afrasiabi H, Sadeghi F. Screening of different polysaccharides in a composite film based on Eudragit RS for subsequent use as a coating for delivery of 5-ASA to colon. Int J Pharm. 2019;568(June):118527.
18. Zeeshan M, Ali H, Khan S, Ahmad S, Weigmann B. Advances in orally-delivered pH-sensitive nanocarrier systems; an optimistic approach for the treatment of inflammatory bowel disease. Int J Pharm. 2019;558(October 2018):201–14.
19. Asfour MH, Mohsen AM. Formulation and evaluation of pH-sensitive rutin nanospheres against colon carcinoma using HCT-116 cell line. J Adv Res. 2018;9:17–26.
20. Song L, Liang L, Shi X, Chen H. Optimizing pH-sensitive and time-dependent polymer formula of colonic pH-responsive pellets to achieve precise drug release. Asian J Pharm Sci 2019;14(1):413–22.
21. Sharma N, Sharma A, Bhatnagar A, Nishad D, Karwasra R. Novel gum acacia based macroparticles for colon delivery of Mesalazine: development and gammascintigraphy study. J Drug Deliv Sci Technol. 2019;54(August):101224.
22. Sharma A, Kim EJ, Shi H, Yong J, Geun B, Seung J. Biomaterials Development of a theranostic prodrug for colon cancer therapy by combining ligand-targeted delivery and enzyme-stimulated activation. Biomaterials. 2018;155:145–51.
23. Chaudhary A, Tiwari N, Jain V, Singh R. Microporous bilayer osmotic tablet for colon-specific delivery. Euro J Pharm Biopharm. 2011;78(1):134–40.
24. Kaur R, Gulati M, Singh SK. Role of synbiotics in polysaccharide assisted colon targeted microspheres of mesalamine for the treatment of ulcerative colitis. Int J Biol Macromol. 2017;95:438–50.
25. Mastropietro D, Park K, Omidian H. Polymers in oral drug delivery. Amsterdam, The Netherlands: Elsevier Inc.; 2017. Vol. 4, pp 430–44.
26. Sunoqrot S, Abujamous L. pH-sensitive polymeric nanoparticles of quercetin as a potential colon cancer-targeted nanomedicine. J Drug Deliv Sci Technol. 2019;52(May):670–6.
27. Elbatanony RSE. Modified pH independent/time controlled explosion system (TES) for targeted drug delivery in the lower intestinal tract: formulation and pharmacokinetic evaluation in healthy volunteers. J Drug Deliv Sci Technol. 2019;50(November 2018):163–73.
28. Chen S, Song Y, Wang C, Tao S, Yu F, Lou H, et al. Chitosan-modified lipid nanodrug delivery system for the targeted and responsive treatment of ulcerative colitis. Carbohydr Polym. 2020;230(July 2019):115613.
29. Sinha VR, Kumria R. Microbially triggered drug delivery to the colon. Euro J Pharm Sci. 2003;18(1):3–18.
30. Agarwal T, Narayana SNGH, Pal K, Pramanik K, Giri S, Banerjee I. Calcium alginate-carboxymethyl cellulose beads for colon-targeted drug delivery. Int J Biol Macromol. 2015;75:409–17.
31. Elkhodairy KA, Elsaghir HA, Al-Subayiel AM. Formulation of indomethacin colon targeted delivery systems using polysaccharides as carriers by applying liquisolid technique. Biomed Res Int. 2014;1(2014):704362.
32. Zhang T, Yang Y. Beneficial effect of intestinal fermentation of natural polysaccharides. Nutrients 2018;10(8):1055.
33. Miao T, Wang J, Zeng Y, Liu G, Chen X. Polysaccharide-based controlled release systems for therapeutics delivery and tissue engineering: from bench to bedside. Advanced Sci 2018;5:1700513.
34. Sharma N, Srivastava P, Sharma A, Nishad DK, Karwasra R, Khanna K, et al. Potential applications of Abelmoschus moschatus polysaccharide as colon release tablets-Rheology and gamma scintigraphic study. J Drug Deliv Sci Technol. 2020;57(February):101632.
35. Maity S, Kundu A, Karmakar S, Sa B. In vitro and in vivo correlation of colon-targeted compression-coated tablets. J Pharm (Cairo). 2016;2016:1–9.
36. Zhang B, Yan Y, Shen Q, Ma D, Huang L, Cai X, et al. A colon targeted drug delivery system based on alginate modificated graphene oxide for colorectal liver metastasis. Mater Sci Eng C. 2017;79:185–90.
37. Xu W, Gao Q, Xu Y, Wu D, Sun Y. pH-controlled drug release from mesoporous silica tablets coated with hydroxypropyl methylcellulose phthalate. Mater Res Bull. 2009;44(3):606–12.
38. Bisharat L, Barker SA, Narbad A, Craig DQM. In vitro drug release from acetylated high amylose starch-zein films for oral colon-specific drug delivery. Int J Pharm. 2019;556:311–9.
39. Li S, Sun Y, Hu X, Qin W, Li C, Liu Y, et al. Effect of arabinoxylan on colonic bacterial metabolites and mucosal barrier in high—fat diet—induced rats. Food Sci Nutr. 2019;7:3052–61.
40. Martínez-lópez AL, Carvajal-millan E, Miki-yoshida M, Alvarez-contreras L, Rascón-chu A, Lizardi-mendoza J, et al. Arabinoxylan microspheres: structural and textural characteristics. Molecules 2013;18(4):4640.
41. Shrivastava PK, Shrivastava A, Sinha SK, Shrivastava SK. Dextran carrier macromolecules for colon-specific delivery of 5-aminosalicylic acid. Indian J Pharm Sci. 2013 May;75(3):277–83.
42. Kiani M, Sadat F, Tekie M, Dinarvand M, Soleimani M. Thiolated carboxymethyl dextran as a nanocarrier for colon delivery of hSET1 antisense?: in vitro stability and ef fi ciency study. Mater Sci Eng C. 2016;62:771–8.
43. Elkhodairy KA, Afifi SA, Zakaria AS. A promising approach to provide appropriate colon target drug delivery systems of vancomycin HCL: pharmaceutical and microbiological studies. BioMed Res Int 2014;2014:182197.
44. Seeli DS, Prabaharan M. Guar gum succinate as a carrier for colon-specific drug delivery. Int J Biol Macromol. 2016;84:10–5.
45. Mensink MA, Frijlink HW, van der Voort K, Hinrichs WLJ. Inulin, a flexible oligosaccharide. II?: Review of its pharmaceutical applications. Carbohydr Polym. 2015;134:418–28.
46. Kusumaningrum IK, Wijaya AR, Marfuah S, Fadilah MN. Optimation of alkoxide formed step on carboxymethyl kappa carrageenan synthesis. IOP Conf Ser Earth Environ Sci. 2019;299(1):012008.
47. Mahdaviniaa GR, Rahmania Z, Karamia S, Pourjavadi A. Magnetic/pH-sensitive κ-carrageenan/sodium alginate hydrogel nanocomposite beads: preparation, swelling behavior, and drug delivery. J Biomater Sci Polym Ed. 2014;25(17):1891–1906.
48. Nalinbenjapun S, Ovatlarnporn C. Chitosan-5-aminosalicylic acid conjugates for colon-specific drug delivery: methods of preparation and in vitro evaluations. J Drug Deliv Sci Technol. 2019;57:101397.
49. Xu J, Tam M, Samaei S, Lerouge S, Barralet J, Stevenson MM, et al. Mucoadhesive chitosan hydrogels as rectal drug delivery vessels to treat ulcerative colitis. Acta Biomater. 2017;48:247–57.
50. Barkat K, Ahmad M, Minhas MU, Khalid I, Malik NS. Chondroitin sulfatebased smart hydrogels for targeted delivery of oxaliplatin in colorectal cancer: preparation, characterization and toxicity evaluation. Polymer Bulletin. 2019;77:6271–97.
51. Algieri F, Rodr A, Garrido-mesa N, Vezza T, Garrido-mesa J, Utrilla MP, et al. Intestinal anti-inflammatory effects of oligosaccharides derived from lactulose in the trinitrobenzenesulfonic acid model of rat colitis. J Agric Food Chem. 2014;62(19):4285–97.
52. Onishi H, Ikeuchi-takahashi Y, Kawano K, Hattori Y. Preparation of chondroitin sulfate-glycyl-prednisolone conjugate nanogel and its efficacy in rats with ulcerative colitis. Biol Pharm Bull. 2019;42(7):1155–63.
53. Mahzouni P. Development of novel budesonide pellets based on CODES TM technology?: in vitro/in vivo evaluation in induced colitis in rats. DARU: J Faculty Pharm Tehran Univ Med Sci. 2011;19(2):107.
54. Dionísio M, Grenha A. Locust bean gum?: exploring its potential for biopharmaceutical applications. J Pharm Bioallied Sci. 2012;4(3):175–86.
55. Chickpetty SM, Raga BV. Formulation, in vitro drug release and in vivo human X-ray investigation of polysaccharide based drug delivery systems for targeting 5-fluorouracil to the colon. Braz J Pharm Sci. 2013;49:263–73.
56. Vemula SK. Colon specific drug delivery: effect of Eudragit enteric coating on hydroxypropyl methylcellulose matrix tablets of flurbiprofen. J Young Pharm. 2015;7(4):373–83.
57. Wong TW, Colombo G, Sonvico F. Pectin matrix as oral drug delivery vehicle for colon cancer treatment. Aaps Pharm Sci Tech. 2011;12(1):201–14.
58. Varshosaz J, Emami J, Tavakoli N, Minaiyan M. Pectin film coated pellets for colon-targeted delivery of budesonide: in-vitro/in-vivo evaluation in induced ulcerative colitis in rat. Iran J Pharm Res 2012;11:733–45.
59. Madan J, Gundala SR, Baruah B, Nagaraju M, Yates C, Turner T, et al. Cyclodextrin complexes of reduced bromonoscapine in guar gum microspheres enhance colonic drug delivery. Mole Pharm 2014;11(12):4339–49.
60. Almeida H, Amaral MH, Lobão P. Temperature and pH stimuli-responsive polymers and their applications in controlled and selfregulated drug delivery. J Appl Pharm Sci. 2012;2(6):01–10.
61. Dias ML, Agüero L, Zaldivar-Silva D, Pe L. Alginate microparticles as oral colon drug delivery device: a review. Carbohydrate Polym 2017;168:32–43.
62. Sohail R, Abbas SR. Evaluation of amygdalin-loaded alginate-chitosan nanoparticles as biocompatible drug delivery carriers for anticancerous efficacy. Int J Biol Macromol. 2020;153:36–45.
63. Iswandana R, Sari K, Putri S, Wulandari FR, Najuda G, Sari SP. Preparation of calcium alginate-tetrandrine beads using ionic gelation method as colon-targeted dosage form. J Appl Pharm Sci. 2018;8(05):68–74.
64. Rajpurohit H, Sharma P, Sharma S, Bhandari A. Polymers for colon targeted drug delivery. Indian J Pharm Sci. 2010;72:689–96.
65. Neha S, Harikumar SL. Polymers for colon targeted drug delivery: a review. Int J Drug Dev Res. 2013;5(1):21–31.
66. Nabais T, Brouillet F, Kyriacos S, Mroueh M. High-amylose carboxymethyl starch matrices for oral sustained drug-release?: in vitro and in vivo evaluation. Euro J Pharm Biopharm, 2007;65:371–8.
67. Freire AC, Fertig CC, Podczeck F, Veiga F, Sousa J. Starch-based coatings for colon-specific drug delivery. Part I: the influence of heat treatment on the physico-chemical properties of high amylose maize starches. Eur J Pharm Biopharm. 2009;72(3):574–86.
68. Hald S, Schioldan AG, Moore ME, Dige A. Effects of arabinoxylan and resistant starch on intestinal microbiota and short-chain fatty acids in subjects with metabolic syndrome: a randomised crossover study. PLoS One 2016;11:1–18.
69. Rivière A, Selak M, Lantin D, Leroy F, De Vuyst L. Bifidobacteria and butyrate-producing colon bacteria: importance and strategies for their stimulation in the human gut. Front Microbiol. 2016;7:979.
70. Kim W, Kim D. Conjugation of metronidazole with dextran: a potential pharmaceutical strategy to control colonic distribution of the anti-amebic drug susceptible to metabolism by colonic microbes. Drug Des Devel Ther 2017;11:419–29.
71. Lee Y, Kim J, Kim W, Yoo JW. Celecoxib coupled to dextran via a glutamic acid linker yields a polymeric prodrug suitable for colonic delivery. Drug Des Devel Ther 2015;9:4105–13.
72. Varshosaz J, Emami J, Ahmadi F, Tavakoli N, Minaiyan M. Preparation of budesonide—dextran conjugates using glutarate spacer as a colon-targeted drug delivery system?: in vitro/in vivo evaluation in induced ulcerative colitis. 2011;19:140–53.
73. Shrivastava PK, Shrivastava SK. Dextran carrier macromolecule for colon specific delivery of celecoxib. Curr Drug Deliv. 2010;7(2):144–51.
74. Seeli DS, Dhivya S, Selvamurugan N, Prabaharan M. Guar gum succinate-sodium alginate beads as a pH-sensitive carrier for colon-specific drug delivery. Int J Biol Macromol. 2016;91:45–50.
75. Mahto A, Mishra S. Design, development and validation of guar gum based pH-sensitive drug delivery carrier via graft copolymerization reaction using microwave irradiations. Int J Biol Macromol. 2019;138:278–91.
76. Wang D, Sun F, Lu C, Chen P, Wang Z, Qiu Y, et al. Inulin based glutathione-responsive delivery system for colon cancer treatment. Int J Biol Macromol. 2018;111:1264–72.
77. Imran S, Gillis RB, Kok SM, Harding SE, Adams G, Imran S, et al. Application and use of inulin as a tool for therapeutic drug delivery Application and use of Inulin as a tool for therapeutic drug delivery. 2013;8725.
78. Akhgari A, Farahmand F, Garekani HA, Sadeghi F, Vandamme TF. Permeability and swelling studies on free films containing inulin in combination with different polymethacrylates aimed for colonic drug delivery. Eur J Pharm Sci. 2006;8:307–14.
79. Khan AK, Saba AU, Nawazish S, Akhtar F, Rashid R, Mir S, et al. Carrageenan based bionanocomposites as drug delivery tool with special emphasis on the influence of ferromagnetic nanoparticles. Oxid Med Cell Longev. 2017;2017:8158315.
80. Javanbakht S, Shadi M, Mohammadian R, Shaabani A, Amini MM, Pooresmaeil M, et al. Facile preparation of pH-responsive k-Carrageenan/tramadol loaded UiO-66 bio-nanocomposite hydrogel beads as a nontoxic oral delivery vehicle. J Drug Deliv Sci Technol. 2019;54(June):101311.
81. Tong X, Pan W, Su T, Zhang M, Dong W, Qi X. Recent advances in natural polymer-based drug delivery systems. React Funct Polym. 2020;148(December 2019):104501.
82. Ghaffar A, Yameen B, Latif M, Malik MI. pH-sensitive drug delivery systems. Metal nanoparticles for drug delivery and diagnostic applications. Amsterdam, The Netherlands: Elsevier Inc.; 2020. pp 259–78.
83. Sinha P, Udhumansha U, Rathnam G, Ganesh M, Jang HT. Capecitabine encapsulated chitosan succinate-sodium alginate macromolecular complex beads for colon cancer targeted delivery: in vitro evaluation. Int J Biol Macromol. 2018;117:840–50.
84. Cerchiara T, Abruzzo A, Cagno M, Bigucci F, Bauer-brandl A, Parolin C, et al. Chitosan based micro- and nanoparticles for colon-targeted delivery of vancomycin prepared by alternative processing methods. Eur J Pharm Biopharm. 2015;92:112–9.
85. Iswandana R, Sari K, Putri S, Dwiputra R, Yanuari T, Purna S, et al. Formulation of Chitosan tripolyphosphate-tetrandrine beads using ionic gelation method: in vitro and in vivo evaluation. J Biomater Sci Polym Ed. 2017;9(5):1891–906.
86. Jyoti K, Kaur R, Martis EAF, Coutinho EC, Kumar U, Chandra R, et al. Soluble curcumin amalgamated chitosan microspheres augmented drug delivery and cytotoxicity in colon cancer cells?: in vitro and in vivo study. Colloids Surf B Biointerfaces. 2016;148:674–83.
87. Zu M, Ma L, Zhang X, Xie D, Kang Y, Xiao B. Chondroitin sulfate-functionalized polymeric nanoparticles for colon cancer- targeted chemotherapy. Colloids Surf B Biointerfaces. 2019;177(November 2018):399–406.
88. Ramasamy T, Khandasamy US, Shanmugamb S, Ruttala H. Formulation and evaluation of chondroitin sulphate tablets of aceclofenac for colon targeted drug delivery. Iran J Pharm Res IJPR 2012;11(May 2010):465–79.
89. Shpak M, Ryabtseva S, Bratsikhin A. Lactulose effect on viability of starter cultures. J Hygienic Eng Des. 2019;2–7.
90. Suganya G. CODESTM drug delivery: capecitabine in the management of colorectal cancer. 2017.
91. Katsuma M, Watanabe S, Kawai H. Studies on lactulose formulations for colon-specific drug delivery. Int J Pharm. 2002;249:33–43.
92. Trivedi HD, Puranik PK. Formulation and development of colon specific etoricoxib CODES TM tablet?: statistical optimization and in vivo roentgenography. Int J Pharm Pharm Res. 2016;7(1):185–215.
93. Sanjeevani D, Kranti T, Ajinath S, Aparna B, Padm S, Patil D. Development of colon targeted delivery of ketoprofen using natural gums as carrier. Res J Pharm Technol. 2009;2(4):771–6.
94. Milani PZ. Physicochemical characterization and dissolution study of Ibuprofen compression-coated tablets using locust bean gum. Dissolution Technol. 2013;20:38–43.
95. Kumar M, Kaushik S, Saini V. Formulation development and evaluation of colon targeted beads of mesalamine. J Drug Des Res. 2018;5:1067.
96. Shukla S, Jain D, Verma K, Verma S. Pectin-based colon-specific drug delivery. Chron Young Sci. 2011;2(2):83–90.
97. Butte K, Momin M, Deshmukh H. Optimisation and in vivo evaluation of pectin based drug delivery system containing curcumin for colon. Int J Biomater. 2014;2014:924278.
98. Wei H, Qing D, De-Ying C, Bai X, Fanli-Fang. Pectin/ethylcellulose as film coatings for colon-specific drug delivery: preparation and in vitro evaluation using 5-fluorouracil pellets. PDA J Pharm Sci Technol. 2007 Mar 1;61(2):121 LP–130.
99. Vaidya A, Jain S, Agrawal RK, Jain SK. Pectin—metronidazole prodrug bearing microspheres for colon targeting. J Saudi Chem Soc. 2012:19(3):257–64.
100. Tian B, Hua S, Liu J. Cyclodextrin-based delivery systems for chemotherapeutic anticancer drugs?: a review. Carbohydr Polym. 2020;232(December 2019):115805.
101. Shahiwala A. Cyclodextrin conjugates for colon drug delivery. J Drug Deliv Sci Technol. 2020;55(December 2019):101448.
102. Arulraj P, Gopal V, Jeyabalan G, Kandasamy CS, Chander SJJU, Venkatanarayanan R. Studies on formulation development and in vitro evaluation of celecoxib matrix tablet containing combination of equal ratio of natural gums guargum and cyclodextrin, amylose and pectin for colon specific drug delivery. Int J Pharm Sci Res. 2015;6(12):5273–8.
103. Jiang HL, Zhu KJ. Polyanion/gelatin complexes as pH-sensitive gels for controlled protein release. J Appl Polym Sci. 2001;80(9):1416–25.
104. Adeleke OA. Premium ethylcellulose polymer based architectures at work in drug delivery. Int J Pharm X. 2019;1:100023.
105. Godge GR, Hiremath SN. Development and evaluation of colon targeted drug delivery system by using natural polysaccharides/polymers. Dhaka Univ J Pharm Sci. 2014;13(1):105–13.
106. Joshi SC. Sol-gel behavior of hydroxypropyl methylcellulose (HPMC) in ionic media including drug release. Materials. 2011;4(10):1861–905.
107. Sureshkumar R, Munikumar M, Ganesh GNK, Jawahar N, Nagasamyvenkatesh D, Senthil V, et al. Formulation and evaluation of pectin-hydroxypropyl methylcellulose coated curcumin pellets for colon delivery. Asian J Pharm. 2009;3(2):138–42.
108. Kshirsagar SJ, Bhalekar MR, Shewale NS, Godbole VP, Jagdale PK, Mohapatra SK. Development of enzyme-controlled colonic drug delivery using amylose and hydroxypropyl methylcellulose?: optimization by factorial design. 2011;18:385–93.
109. Mishra S. Formulation and evaluation of pH sensitive nanoparticles for colon targeted drug delivery system. In: 3rd International Conference and Exhibition on Pharmaceutics and Novel Drug Delivery Systems. 2013.
110. Lone KD, Dhole JA. An in vitro investigation of suitability of press-coated tablets with hydroxypropyl methylcellulose acetate succinate (HPMCAS) and sodium alginate in outer shell for colon targeting. Int J Pharm Sci Res. 2013;4(6):2244–51.
111. Kaur S, Narang RK, Aggarwal G. Formulation and development of colon-targeted mucopenetrating metronidazole nanoparticles. Trop J Pharm Res. 2017;16(5):967–73.
112. Sinha VR, Kumria R. Coating polymers for colon specific drug delivery: a comparative in vitro evaluation. Acta Pharm. 2003;53(1):41–7.
113. Rathore VS, Tanwar YS, Rathore GS, Ahmed JA. a review on enteric coating technology. Int J Chem Pharm Sci. 2014;2(9):1155–9.
114. Neekhra M, Shrivastav S, Sharma S, Molvi KI, Kumar V. Development and evaluation of oral colon targeted ketorolac tromethamine capsule. Int J Pharm Sci Res. 2010;1(5):61–7.
115. Giunchedi P, Ltorre M, Maggi L, Conti B, Conte U. Cellulose acetate trimellitate microspheres containing NSAIDS. Drug Dev Ind Pharm. 1995 Jan 1;21(3):315–30.
116. Sirisha VNL, Eswariah MC, Rao AS. A novel approach of locust bean gum microspheres for colonic delivery of mesalamine. Int J Appl Pharm. 2018;10(1):86–93.
117. Tung NT, Pham TMH, Nguyen TH, Pham TT, Nguyen TQ. Pectin/HPMC dry powder coating formulations for colon specific targeting tablets of metronidazole. J Drug Deliv Sci Technol. 2016;33:19–27.
118. Jeganathan B, Prakya V, Deshmukh A. Preparation and evaluation of diclofenac sodium tablet coated with polyelectrolyte multilayer film using hypromellose acetate succinate and polymethacrylates for pH-dependent, modified release drug delivery. AAPS Pharm Sci Tech. 2016;17(3):578–87.
119. Jagdale S, Chandekar A. Optimization of chitosan and cellulose acetate phthalate controlled delivery of methylprednisolone for treatment of inflammatory bowel disease. Adv Pharm Bull. 2017;7(2):203–13.
120. Terao K. Poly(acrylic acid) (PAA). Encyclop Polym Nanomater. 2014.
121. National Center for Biotechnology Information. PubChem Database. 2020 [cited 2020 May 21]. Methacrylic acid, CID=4093. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Methacrylic-acid
122. Khan S, Anwar N. Highly porous ph-responsive carboxymethyl chitosan- grafted -poly (acrylic acid) based smart hydrogels for 5-fluorouracil controlled delivery and colon targeting. Int J Polym Sci. 2019;2019:6579239.
123. Seeli DS, Prabaharan M. Guar gum oleate-graft-poly(methacrylic acid) hydrogel as a colon-specific controlled drug delivery carrier. Carbohydr Polym. 2017;158:51–7.
124. Chourasia MK, Jain SK. Design and development of multiparticulate system for targeted drug delivery to colon. Drug Deliv J Deliv Target Therapeutic Agents. 2004;11(3):201–7.
125. Devi SKU, Thiruganesh R, Suresh S. Preparation and characterization of chitosan tablets of aceclofenac for colon targeted drug delivery. Asian J Pharm Res Health Care 2010;2(1):46–65.
126. Nguyen CA, Konan-Kouakou YN, Allémann E, Doelker E, Quintanar-Guerrero D, Fessi H, et al. Preparation of surfactant-free nanoparticles of methacrylic acid copolymers used for film coating preparation of surfactant-free nanoparticles of methacrylic acid copolymers used for film coating. Aaps Pharm 2006;7:E54–60.
127. Agrawal D, Ranawat MS. Formulation and charecterisation of colon targeted time dependent microspheres of capecitabine for colorectal cancer. Asian J Pharm Res Dev. 2013;1(5):152–61.
128. Choi M, Cao J, Lee Y, Ikram M. Colon-targeted delivery of budesonide using dual pH- and time-dependent polymeric nanoparticles for colitis therapy. Drug Des Devel Ther. 2015;3789–99.
129. Soni P, Gp C, Lk S. Enzyme specific drug delivery system: a potential approach for colon targeting. Curr Res Pharm Sci. 2015;05(02):28–50.
130. Ofokansi KC, Kenechukwu FC. Formulation development and evaluation of drug release kinetics from colon-targeted ibuprofen tablets based on Eudragit RL 100-Chitosan interpolyelectrolyte complexes. Int Scholar Res Notices 2013;2013:838403.
131. Sun X, Xu C, Wu G, Ye Q, Wang C. Poly(lactic-co-glycolic acid): applications and future prospects for periodontal tissue regeneration. Polymers (Basel). 2017;9(6):1–19.
132. Drechsler M, Garbacz G, Thomann R, Schubert R. Development and evaluation of chitosan and chitosan/Kollicoat® Smartseal 30 D film-coated tablets for colon targeting. Eur J Pharm Biopharm. 2014;88(3):807–15.
133. BASF. Excipients and actives for pharma. Ludwigshafen, Germany: BASF Aktiengesellschaft; 2002.
134. Ali H, Weigmann B, Collnot EM, Khan SA, Windbergs M, Lehr CM. Budesonide loaded PLGA nanoparticles for targeting the inflamed intestinal mucosa—pharmaceutical characterization and fluorescence imaging. Pharm Res. 2016;33(5):1085–92.
135. Zhang W, Mahuta KM, Mikulski BA, Harvestine JN, Zachary J, Lee JC, et al. Novel pectin-based carriers for colonic drug delivery. Pharm Devel Technol 2014;7450:1–4.
136. Liu Z, Yu D, Wang K. Colon-specific pulsatile drug release provided by electrospun shellac nanocoating on hydrophilic amorphous composites. Int J Nanomed 2018;13:2395–404.
137. Naeem M, Bae J, Oshi MA, Kim MS, Moon HR, Lee BL, et al. Colon-targeted delivery of cyclosporine a using dual-functional eudragit® FS30D/PLGA nanoparticles ameliorates murine experimental colitis. Int J Nanomedicine. 2018;13:1225–40.
138. Perrie Y, Rades T. FASTtrack pharmaceutics: Drug Delivery and Targeting. London, UK: Pharmaceutical Press; 2012. (Fast Track Pharmacy Series).
139. Tian B, Liu S, Wu S, Lu W, Wang D, Jin L, et al. pH-responsive poly (acrylic acid)-gated mesoporous silica and its application in oral colon targeted drug delivery for doxorubicin. Colloids Surf B Biointerfaces 2017;154:287–96.
140. Tian B, Liu S, Lu W, Jin L, Li Q, Shi Y, et al. Construction of pH-responsive and up-conversion luminescent NaYF 4:Yb3+/Er3+ @SiO2 @PMAA nanocomposite for colon targeted drug delivery. Sci Rep. 2016;6:1–11.
141. Vibhooti P, Rajan G, Seema B. Eudragit and Chitosan—the two most promising polymer for colon drug delivery. J Med Sci Clin Res. 2013;1:61–83.
142. Kathiravan P. Formulation and evaluation of colon specific drug delivery system of capecitabine containing polymer coated capsule dosage form. Int J Drug Dev Res. 2015;7(3):26–31.
143. Alkazzaz SZM, Ali WK. Design and in-vitro evaluation of colon targeted prednisolone solid dispersion tablets. UK J Pharm Biosci. 2015;3(6):30.
144. Nguyen MNU, Tran PHL, Tran TTD. A single-layer film coating for colon-targeted oral delivery. Int J Pharm. 2019;559:402–9.
145. Lozoya-Agullo I, Araújo F, González-Álvarez I, Merino-Sanjuán M, González-Álvarez M, Bermejo M, et al. PLGA nanoparticles are effective to control the colonic release and absorption on ibuprofen. Eur J Pharm Sci. 2018;115:119–25.
146. Akl MA, Kartal-Hodzic A, Suutari T, Oksanen T, Montagner IM, Rosato A, et al. Real-time label-free targeting assessment and in vitro characterization of curcumin-loaded poly-lactic-co-glycolic acid nanoparticles for oral colon targeting. ACS Omega. 2019;4(16):16878–90.
147. Reddy PS, Subhash P, Bose C, Saritha D, Sruthi V. Formulation and evaluation of colon targeted matrix tablet using natural tree gums. Int J Pharm Pharm Sci 2018;10(9):92–7.
148. Wang X, Yu DG, Li XY, Bligh SW, Williams GR. Electrospun medicated shellac nanofibers for colon-targeted drug delivery. Int J Pharm. 2015 Jul 25;490(1–2):384–90. doi: 10.1016/j.ijpharm.2015.05.077.
149. Oehme A, Valotis A, Krammer G, Zimmermann I. Preparation and characterization of shellac-coated anthocyanin pectin beads as dietary colonic delivery system. Mole Nutr Food Res 2011;55:75–85.
150. Wahlgren M, Axenstrand M, Håkansson Å, Marefati A, Pedersen BL. In vitro methods to study colon release: state of the art and an outlook on new strategies for better in-vitro biorelevant release media. Pharmaceutics. 2019;11(2):95.
151. Ahmadi F, Varshosaz J, Emami J, Tavakoli N, Minaiyan M, Mahzouni P, et al. Preparation and in vitro/in vivo evaluation of dextran matrix tablets of budesonide in experimental ulcerative colitis in rats. Drug Deliv. 2011;18(2):122–30.
152. Rajpoot K, Jain SK. Oral delivery of pH-responsive alginate microbeads incorporating folic acid-grafted solid lipid nanoparticles exhibits enhanced targeting effect against colorectal cancer: a dual-targeted approach. Int J Biol Macromol [Internet]. 2020; Available from: https://doi.org/10.1016/j.ijbiomac.2020.02.132
153. Asikainen S, Seppälä J. Photo-crosslinked anhydride-modified polyester and—ethers for pH- sensitive drug release. Eur J Pharm Biopharm [Internet]. 2020; Available from: https://doi.org/10.1016/j.ejpb.2020.02.015
154. Sinko PJ, Martin AN. Martin’s physical pharmacy and pharmaceutical sciences: Physical chemical and biopharmaceutical principles in the pharmaceutical sciences. Philadelph, PA: Lippincott Williams & Wilkins; 2006.
155. Jain V, Shukla N, Mahajan SC. Polysaccharides in colon specific drug delivery. J Transl Sci. 2015;1(1):3–11.
156. Wilson C, Wang Lee W, Mukherji G. Time-dependent systems for colonic delivery. Boca Raton, FL: CRC Press; 2002. pp 243–8.
157. Kumar V, Kumar B, Deeba F, Bano S, Kulshreshtha A. Lipophilic 5-fluorouracil prodrug encapsulated xylan-stearic acid conjugates nanoparticles for colon cancer therapy. Int J Biol Macromol [Internet]. 2019;128:204–13. Available from: https://doi.org/10.1016/j.ijbiomac.2019.01.101
158. Pinto DC. Seaweeds Secondary metabolites: successes in and/or probable therapeutic applications. Basel, Switzerland: MDPI AG; 2020.
159. Necas J, Bartosikova L. Carrageenan?: a review. Veter Med 2013;2013(4):187–205.
160. Guarino V, Caputo T, Altobelli R, Ambrosio L. Degradation properties and metabolic activity of alginate and chitosan polyelectrolytes for drug delivery and tissue engineering applications. AIMS Mater Sci. 2015;2:497–502.
161. Vadnerkar G, Dhaneshwar S. Macromolecular prodrug of 4-aminosalicylic acid for targeted delivery to inflamed colon. Curr Drug Discov Technol 2013;10:16–24.
162. Kumar VS, John R, Sabitha M. Guargum and Eudragit® coated curcumin liquid solid tablets for colon specific drug delivery. Int J Biol Macromol. 2018;110:318–27.
163. Oshi MA, Naeem M, Bae J, Kim J, Lee J. Colon-targeted dexamethasone microcrystals with pH-sensitive chitosan/alginate/Eudragit S multilayers for the treatment of in fl ammatory bowel disease. Carbohydr Polym. 2018;198:434–42.
164. Rachmawati H, Mudhakir D, Kusuma J. Combination of inulin-shellac as a unique coating formulation for design of co-lonic delivery dosage form of ibuprofen. Int J Res Pharm Sci. 2012;3(1):17–23.
165. Amini-Fazl MS, Mohammadi R, Kheiri K. 5Fluorouracil loaded chitosan/polyacrylic acid/Fe3O4 magnetic nanocomposite hydrogel as a potential anticancer drug delivery system. Int J Biol Macromol. 2019;132:506–13.
166. Penhasi A, Gomberg M, Shalev DE. A novel nicotine pectinate salt formulated in a specific time-controlled delivery system: a new approach for colon-targeted nicotine release. J Drug Deliv Sci Technol. 2020;56:101583.
167. Rehman K, Amin MCIM, Muda S. Influence of beta-cyclodextrin and chitosan in the formulation of a colon-specific drug delivery system. Drug Res 2013;63:657–62.
168. Maqbool I, Akhtar M, Ahmad R, Sadaquat H, Noreen S, Batool A, et al. Novel multiparticulate pH triggered delayed release chronotherapeutic drug delivery of celecoxib-β-cyclodextrin inclusion complexes by using Box-Behnken design. Eur J Pharm Sci. 2020;146:105254.
169. Katsuma M, Watanabe S, Takemura S, Sako K, Sawada T. Scintigraphic evaluation of a novel colon-targeted delivery system (CODES TM) in healthy volunteers. J Pharm Sci. 2004;93(5):1287–99.